Abstract
Background
Little is known about the benefits of adjuvant chemotherapy (adj) in the older population with locally advanced rectal cancer (larc). We evaluated use of adj, survival outcomes, and adj-related toxicity in older patients with larc.
Methods
Our retrospective review included 286 patients with larc (stages ii and iii) diagnosed between January 2010 and December 2013 in Nova Scotia who underwent curative-intent surgery. Baseline patient, tumour, and treatment characteristics were collected. The survival analysis used the Kaplan–Meier method and Cox regression statistics.
Results
Of 286 identified patients, 152 were 65 years of age or older, and 92 were 70 years of age or older. Median follow-up was 46 months, and 163 patients (57%) received neoadjuvant chemoradiation. Although adj was given to 81% of patients (n = 109) less than 65 years of age, only 29% patients (n = 27) 70 years of age and older received adj. Kaplan–Meier analysis suggested a potential survival advantage for adj regardless of age. In multivariate Cox regression analysis, Eastern Cooperative Oncology Group performance status, T stage, and adj were significant predictors of overall survival (p < 0.04); age was not. Similarly, N stage, neoadjuvant chemoradiation, and adj were significant predictors of disease-free survival (p < 0.01). Poor Eastern Cooperative Oncology Group performance status was the most common cause of adj omission. In patients 70 years of age and older, grade 1 or greater chemotherapy-related toxicities were experienced significantly more often by those treated with adj (85% vs. 68% for those not treated with adj, p < 0.05).
Conclusions
Regardless of age, patients with larc seem to experience a survival benefit with adj. However, older patients are less likely to receive adj, and when they do, they experience more chemotherapy-related toxicities.
Keywords: Rectal cancer, locally advanced; adjuvant chemotherapy; toxicity; older adults; survival
INTRODUCTION
The standard-of-care treatment for locally advanced rectal cancer (larc) involves neoadjuvant chemoradiotherapy followed by total mesorectal excision plus adjuvant chemotherapy1,2. Despite the fact that rectal cancer occurs more commonly in the older population, many trials of rectal cancer treatments underrepresent older patients3–5.
Since the end of the 1990s, treatment for rectal cancer has changed dramatically, with the use of neoadjuvant chemoradiotherapy now being routine3,6. The adoption of total mesorectal excision as the standard surgical technique has also substantially lowered the rate of local recurrence to less than 10% from 25%–50%6. Currently, guidelines published by the U.S. National Comprehensive Cancer Network recommend either neoadjuvant chemoradiation or neoadjuvant radiation alone, followed by surgery and adjuvant chemotherapy, in select patient populations at high risk of distant recurrence1. Available evidence supports the use of adjuvant chemotherapy in all patients with pT3–4 or node-positive disease1,2,7,8.
Older patients with larc are generally less aggressively treated because of a potentially higher risk of treatment complications and poorer outcome; however, the basis of that approach is not well documented9,10. Given an aging population and an overall increase in life expectancy, there is an urgency to reduce disparities in health outcomes and quality of life, which are often inferior in older patients11. Several oncology studies have recently shown that, compared with younger populations, older populations are likely to experience similar survival outcomes when treated per standard guidelines8,11–15, although the degree to which the chemotherapy completion rate and toxicity profile might be affected is unclear16. However, a recent large meta-analysis showed that adjuvant 5-fluorouracil (5fu)–based therapy after neoadjuvant treatment did not improve survival, suggesting ongoing controversy17.
The aim of the present retrospective study was to assess the use and effect of adjuvant chemotherapy on clinical outcomes, focusing on toxicity, rate of adjuvant chemotherapy completion, and survival in older adult patients (65–69 years and ≥70 years) and in adult patients (<65 years) with larc treated with curative-intent surgery.
METHODS
Study Design and Study Population
This retrospective provincial cohort analysis considered all patients diagnosed with larc (stages ii and iii) during 2010–2013, inclusive, who underwent curative-intent surgery and treatment at the Nova Scotia Cancer Centre, which serves the largest population in Atlantic Canada. Patients were included if they were 18 years of age or older, had rectal adenocarcinoma confirmed with a pathology diagnosis, and had completed curative-intent surgery. Patients were excluded if they had synchronous colon cancer or recurrent or metastatic disease at diagnosis or if they were treated with palliative intent. Patients were identified through the Nova Scotia Cancer Registry, an electronic provincial dataset operated by Nova Scotia Cancer Care. Using International Classification of Diseases (version 10) diagnosis codes, 286 eligible patients were identified.
Data Collection
Data were collected from baseline (time of the oncology consultation) to the end of the observation period (that is, the patient’s last visit or 30 November 2016). Sociodemographic, tumour, radiographic, and chemotherapy details were extracted from patient charts. Baseline patient (age, sex, comorbidities, Eastern Cooperative Oncology Group performance status, and body mass index), tumour (pathology and radiography reports concerning stage, differentiation, margins, lymphovascular invasion, and nodal status), and treatment (surgery, radiation, chemotherapy) characteristics were also collected. Comorbidities included cardiac conditions (any hypertension, coronary artery disease, arrhythmias, or congestive heart failure), type 2 diabetes (with and without complications), other malignancies (excluding non-melanoma skin cancers diagnosed at any time in the past), and respiratory illnesses (any obstructive lung disease, asthma, interstitial lung disease). Treatment information, including details of surgical and neoadjuvant treatment, and any change or omission of adjuvant treatment were extracted directly from oncologic progress note dictated by the treating oncologist. Patients were grouped by age category: less than 65 years, 65–69 years, and 70 years and older. In situations in which the disease stage changed after pathology assessment, the highest T and N stages were used. Full approval from the Nova Scotia Health Authority research ethics board was obtained.
Outcomes Measured
The primary outcomes were use, completion rate, and toxicities of adjuvant chemotherapy. Completion rate was further measured by discontinuation of adjuvant chemotherapy at any cycle postoperatively, dose reduction (defined as a reduction of 10% or more, which is what is deemed clinically significant at our centre), delay in treatment (defined as a delay of 1 week or more, which again is what is deemed clinically significant at our centre), and switch of treatment regimen at any point during adjuvant chemotherapy. Toxicity profiles were captured using standardized nursing scoring sheets at each visit in clinic before chemotherapy, with grading assessed using the Common Terminology Criteria for Adverse Events, version 4. Secondary outcomes included overall survival (os), disease-free survival (dfs), and cancer-specific survival. The os was calculated from the date of the pathology diagnosis of larc (biopsy or definitive surgery, whichever was first) to the date of death from any cause or the last visit if still living. The dfs was defined as the number of months between the initial diagnosis and disease recurrence as evidenced by radiography or pathology findings, or death from any cause. Finally, cancer-specific survival was defined as time from diagnosis until cancer-related death.
Statistical Analysis
Data were collected in Microsoft Excel (Microsoft Corporation, Redmond, WA, U.S.A.) and imported into the IBM SPSS Statistics software package (version 22.0 for Windows: IBM, Armonk, NY, U.S.A.) for statistical analysis. The statistical significance of between-cohort differences in categorical variables was determined by chi-square test. Continuous data were compared using the two-sample t-test and one-way analysis of variance (with Tukey post hoc testing) when the three age groups were being compared. All tests were 2-tailed with a significance level of p < 0.05.
Kaplan–Meier curves were constructed to compare patients treated with and without adjuvant chemotherapy, by the number of months until death or study end. A multivariate Cox proportional hazards regression analysis was undertaken to assess time to death, while controlling for known risk factors, including age, sex, stage at diagnosis, differentiation (well, poorly, or moderately differentiated), and pathologically positive lymph nodes. All statistical analyses were performed by a biostatistician.
RESULTS
Patient Demographics and Clinical Characteristics
Table I summarizes the patient demographics. For the 286 eligible patients identified, median follow-up was 45.8 months (range: 2.1–79.2 months). Mean and median age were, respectively, 65 years and 66 years, with a range of 31–92 years. Compared with patients less than 65 years of age, those 70 years of age and older, who constituted 32.2% of the patient population, had, as expected, higher comorbidity rates (cardiovascular conditions: 32.6% vs. 11.2%; p < 0.01; other malignancies: 25.0% vs. 10.4%; p < 0.01). Node-positive disease was found in 188 patients (65.7%), with no significant differences between the age groups (p = 1.0).
TABLE I.
Baseline patient characteristics
| Characteristic | Age group | p Valuea | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Overall | <65 Years | 65–69 Years | ≥70 Years | 65–69 Years | ≥70 Years | |
| Patients (n) | 286b | 134b | 60b | 92b | — | — |
|
| ||||||
| Sex [n (%)] | ||||||
| Men | 188 (65.7) | 88 (65.6) | 39 (65.0) | 61 (66.3) | 1.0 | 1.0 |
| Women | 98 (34.3) | 46 (34.3) | 21 (35.0) | 31 (33.7) | ||
|
| ||||||
| ECOG PS [n (%)] | 279 | |||||
| 0 | 181 (64.9) | 101 (75.4) | 39 (65.0) | 41 (44.6) | ||
| 1 | 64 (22.9) | 29 (21.6) | 14 (23.3) | 21 (22.8) | 0.568 | 0.113 |
| ≥2 | 34 (12.2) | 3 (2.2) | 7 (11.7) | 24 (26.1) | 0.010 | <0.001 |
|
| ||||||
| Comorbidities [n (%)] | ||||||
| Cardiac | 60 (21.0) | 15 (11.2) | 15 (25.0) | 30 (32.6) | 0.018 | <0.001 |
| Respiratory | 44 (15.4) | 15 (11.2) | 12 (20.0) | 17 (18.5) | 0.118 | 0.173 |
| Cancer | 43 (15.0) | 14 (10.4) | 6 (10.0) | 23 (25.0) | 1.0 | 0.005 |
| Type 2 diabetes | 45 (15.7) | 24 (17.9) | 9 (15.0) | 12 (13.0) | 0.684 | 0.360 |
|
| ||||||
| Stage [n (%)] | 1.0 | 1.0 | ||||
| II | 98 (34.3) | 46 (34.3) | 20 (33.3) | 32 (34.8) | ||
| III | 188 (65.7) | 88 (65.7) | 40 (66.7) | 60 (65.2) | ||
|
| ||||||
| Differentiation [n (%)] | 1.0 | 1.0 | ||||
| Well differentiated | 60 (21.0) | 26 (19.4) | 12 (20.0) | 22 (23.9) | ||
| Moderately well differentiated | 153 (53.5) | 71 (53.0) | 33 (55.0) | 49 (53.2) | ||
| Poorly differentiated | 30 (10.5) | 11 (8.2) | 6 (10.0) | 13 (14.1) | ||
| Unknown | 43 (15.0) | 26 (19.4) | 9 (15.0) | 8 (8.7) | ||
|
| ||||||
| Positivity for [n (%)] | ||||||
| Lymphovascular invasion | 85 (34.4) | 38 (28.3) | 13 (21.7) | 34 (36.9) | 0.380 | 0.192 |
| Margins | 33 (12.8) | 10 (7.4) | 2 (3.3) | 21 (22.8) | 0.348 | 0.001 |
|
| ||||||
| T Stage [n (%)] | 1.0 | 1.0 | ||||
| T1–2 | 18 (6.4) | 9 (6.7) | 5 (8.3) | 4 (4.3) | ||
| T3–4 | 266 (93.6) | 125 (93.3) | 54 (90.0) | 87 (94.5) | ||
|
| ||||||
| N Stage [n (%)] | 1.0 | 1.0 | ||||
| N0 | 98 (34.3) | 46 (34.3) | 20 (33.3) | 32 (34.8) | ||
| N1 | 151 (52.8) | 72 (53.7) | 33 (55.0) | 46 (50.0) | ||
| N2–3 | 37 (12.9) | 16 (11.9) | 7 (11.6) | 14 (15.2) | ||
|
| ||||||
| Surgery [n (%)] | 0.509 | 0.775 | ||||
| Lower anterior resection | 177 (61.9) | 86 (64.1) | 34 (56.7) | 57 (62.0) | ||
| Abdominal perineal resection | 101 (35.3) | 45 (33.6) | 23 (38.3) | 33 (35.9) | ||
| Other | 8 (2.8) | 3 (2.2) | 3 (5.0) | 2 (2.2) | ||
|
| ||||||
| RT and CTx [n (%)] | ||||||
| Neoadjuvant CtxRT | 163 (57.0) | 94 (70.1) | 40 (66.7) | 29 (31.5) | 0.619 | <0.001 |
| Adjuvant CTx | 177 (61.9) | 109 (81) | 41 (68) | 27 (29) | 0.063 | 0.001 |
| Both | 126 (44.1) | 82 (61.2) | 30 (50) | 14 (15.2) | 0.124 | <0.001 |
Comparing the older age group with the group less than 65 years of age. Significant values appear in boldface type.
Varies within the characteristic categories because of missing information.
ECOG PS = Eastern Cooperative Oncology Group performance status; RT = radiation therapy; CTx = chemotherapy; CtxRT = chemoradiation.
Treatment Information and Adverse Events
All 286 patients underwent surgical resection (177 low anterior, and 101 abdominal perineal). The age groups did not differ with respect to the type of surgery received (p = 0.5, p = 0.7). Postoperative complications occurred in 137 patients, most of which were immediate complications requiring prolonged hospitalizations (n = 74). The most common type of complication was infection (n = 52), followed by pain (n = 47) and ileus (n = 38). The complication rate did not differ between the age groups (p = 0.88).
Neoadjuvant chemoradiotherapy was given to 163 patients, and 19 patients received neoadjuvant radiotherapy only. The neoadjuvant chemotherapy regimen consisted of either capecitabine (n = 81) or infusion or bolus 5fu (n = 82). In 165 patients, standard pelvic neoadjuvant radiotherapy was completed at a dose of 50.4 Gy in 25 or 28 fractions. Notably, 43 patients completed radiation in the adjuvant setting because of an upfront need for surgical resection. Neoadjuvant chemotherapy was not completed by 40 patients (24.5%) because of side effects, and 12 patients (7.4%) required a dose reduction of at least 10%. Radiation-related complications occurred in 53.4% of the patients (n = 87), with no difference between the age groups (p = 0.11). Neoadjuvant chemoradiotherapy was associated with a significant effect on dfs, but not on os, but age was not a factor within that subgroup of patients (data not shown).
Adjuvant chemotherapy was given to 177 patients: 109 of the group were less than 65 years of age, 41 were 65–69 years of age, and 27 were 70 years of age and older (Table II). Of the 109 patients for whom adjuvant chemotherapy was omitted, 84 were 65 years of age or older. The rate of adjuvant chemotherapy omission was significantly higher in patients 70 years of age and older than in younger patients (p < 0.001). The reasons for omission included poor performance status (n = 31), patient preference (n = 30), surgical complications (n = 27), cardiac contraindication (n = 16), and progression of disease between surgical resection and time of oncologic consultation (n = 5). The most common adjuvant chemotherapy was capecitabine monotherapy (n = 79), followed by 5fu bolus with or without folinic acid (n = 63), oxaliplatin-based chemotherapy with either 5fu or capecitabine (n = 23), and raltitrexed (n = 9).
TABLE II.
Adjuvant chemotherapy: use, completion rate, and toxicity
| Variable | Age group | p Valuea | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Overall | <65 Years | 65–69 Years | ≥70 Years | 65–69 Years | ≥70 Years | |
| Patients (n) | 134 | 286 | 60 | 92 | — | — |
|
| ||||||
| Adjuvant CTx [n (%)] | 177 (62) | 109(81) | 41 (68) | 27 (29) | 0.063 | <0.001 |
|
| ||||||
| CTx regimen [n (%)] | ||||||
| Capecitabine | 79 (45) | 45 (41) | 24 (59) | 10 (37) | ||
| FUFA, 5FUb | 63 (36) | 38 (35) | 12 (29) | 13 (48) | ||
| XELOX | 11 (6) | 7 (6) | 3 (7) | 1 (4) | ||
| FOLFOX | 12 (7) | 10 (9) | 2 (5) | 0 (0) | ||
| Other | 17 (10) | 12 (11) | 1 (2) | 4 (15) | ||
|
| ||||||
| Completion [n (%)] | 116 (66) | 76 (70) | 26 (65) | 14 (52) | 0.089 | <0.001 |
|
| ||||||
| Dose reduction [n (%)] | 71 (40) | 40 (38) | 14 (35) | 17 (68) | 0.390 | 0.062 |
|
| ||||||
| Delay [n (%)] | 58 (33) | 40 (38) | 11 (28) | 7 (28) | 0.112 | <0.001 |
|
| ||||||
| Regimen change [n (%)] | 10 (6) | 9 (8) | 0 (0) | 1 (4) | 0.059 | 0.051 |
|
| ||||||
| Toxicity [n (%)] | 125 (71) | 74 (68) | 28 (70) | 23 (85) | 0.281 | <0.001 |
| Mucositis | 31 | 20 | 5 | 6 | ||
| Diarrhea | 39 | 20 | 11 | 8 | ||
| Anorexia | 27 | 13 | 6 | 8 | ||
| Nausea, vomiting, or both | 23 | 18 | 2 | 3 | ||
| Hand–foot | 31 | 17 | 12 | 2 | ||
| Fever | 13 | 7 | 3 | 3 | ||
|
| ||||||
| Toxicity grade [n (%)] | ||||||
| 1 | 44 (35) | 33 (45) | 5 (18) | 6 (26) | ||
| 2 | 59 (47) | 29 (39) | 19 (68) | 11 (48) | 0.008 | 0.274 |
| ≥3 | 22 (18) | 12 (16) | 4 (14) | 6 (26) | 0.424 | 0.165 |
|
| ||||||
| Hospitalization | 18 (10) | 11 (10) | 3 (7) | 4 (14) | 0.752 | 0.750 |
Comparing the older age group with the group less than 65 years of age. Significant values appear in boldface type.
CTx = chemotherapy; FUFA= 5-fluorouracil–folinic acid; 5FUb= 5-fluorouracil bolus; XELOX = capecitabine–oxaliplatin; FOLFOX= 5-fluorouracil–oxaliplatin.
Adjuvant Chemotherapy
The rate of adjuvant chemotherapy completion was 65.5%, with 61 patients unable to complete adjuvant chemotherapy because of toxicity (n = 46), preference (n = 10), and progression or death (n = 5). The rate of adjuvant chemotherapy completion was significantly lower in patients 70 years of age and older (52%) than in those less than 65 years of age (70%, p < 0.01). The average treatment completion percentage for patients who did not complete chemotherapy was 84.6%. In 71 patients (40.1%), dose reduction by at least 10% was required, and 58 patients (32.8%) required a delay of at least 1 week in the start of adjuvant treatment. The rate of delay was significantly higher in patients 70 years of age and older (p < 0.001). Finally, 10 patients (5.6%) required a change of regimen mid-cycle because of toxicity, the most common regimen change being from infusional 5fu to raltitrexed (60%).
Toxicity related to adjuvant chemotherapy occurred in 70.6% of patients (n = 125). In patients treated with adjuvant chemotherapy, the rate of toxicity was significantly higher in those 70 years of age and older than in those less than 65 years of age (85% vs. 68%, p < 0.01). The most common type of toxicity was diarrhea (n = 39), followed by hand–foot syndrome (n = 31), mucositis (n = 31), anorexia (n = 24), and nausea and vomiting (n = 23). The rate of grade 2 toxicities was significantly higher in patients 65–69 years of age than in those less than 65 years of age (p = 0.008). Finally, 10.2% of the patients (n = 18 of 177) required hospitalization because of chemotherapy-related toxicity.
Survival Analysis
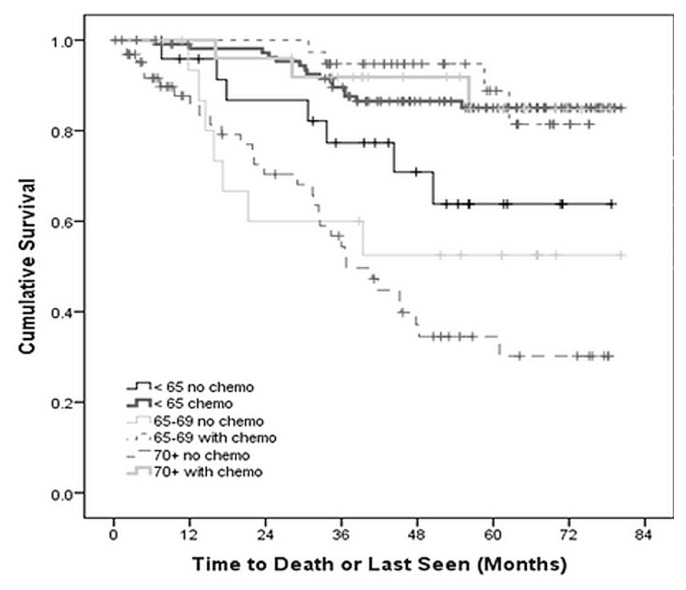
Within the follow-up period, 69 patients died. No death was attributable to chemotherapy adverse events. The 5-year os for patients treated without adjuvant chemotherapy was 70.8%, 58.8%, and 52.3% for the groups less than 65 years of age, 65–69 years of age, and 70 years of age or older respectively (p < 0.05). For patients who received adjuvant chemotherapy, the 5-year os was 86.2%, 90.2%, and 88.9% respectively for the same groups (p < 0.05, Figure 1). Findings from the cancer-specific survival analysis were similar (Table III). In all patients treated with adjuvant chemotherapy, age was not a significant predictor of survival (p = 0.6).
FIGURE 1.
Overall survival of patients with locally advanced rectal cancer, by age group and use of adjuvant chemotherapy (ADJ), p < 0.001.
TABLE III.
Overall survival (OS), cancer-specific survival (CSS), and disease-free survival (DFS) with or without adjuvant treatment, by age
| Age group | Adjuvant treatment? | Survival outcome | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 5-Year OS | CSS | Median DFS | |||||
|
|
|
|
|||||
| Pts (n) | (%) | Pts (n) | (%) | Pts (n) | (months) | ||
| <65 Years | |||||||
| No | 24 | 70.8 | 21 | 71.4 | 25 | 34.2 | |
| Yes | 109 | 86.2 | 103 | 90.3 | 109 | 49.9 | |
| (p=0.076) | (p=0.028) | (p=0.002) | |||||
|
| |||||||
| 65–69 Years | |||||||
| No | 17 | 58.8 | 16 | 62.5 | 19 | 35.7 | |
| Yes | 41 | 90.2 | 37 | 94.6 | 41 | 46.1 | |
| (p=0.001) | (p=0.007) | (p=0.094) | |||||
|
| |||||||
| ≥70 Years | |||||||
| No | 65 | 52.3 | 33 | 45.4 | 64 | 23.1 | |
| Yes | 27 | 88.9 | 24 | 87.5 | 27 | 49.7 | |
| (p=0.008) | (p=0.009) | (p<0.001) | |||||
Pts = patients.
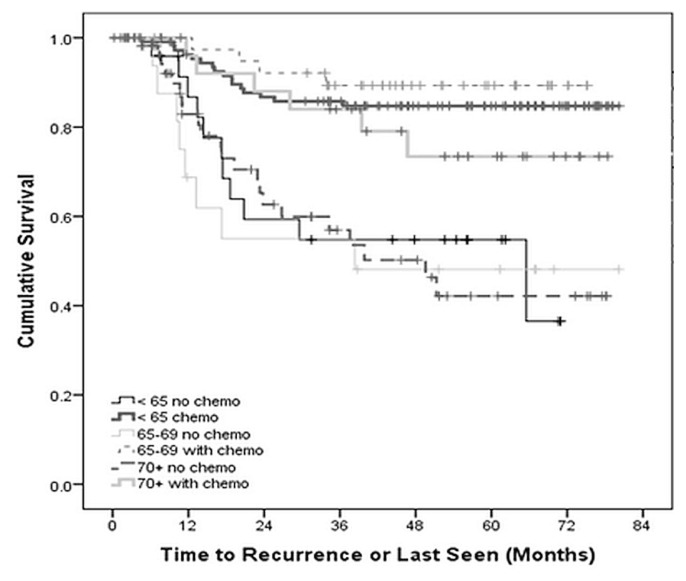
Of the recurrences experienced by 70 patients (24.5%), 11 were locoregional, and 59 were metastatic. The most common sites of metastasis were liver (n = 28) and lung (n = 28), and 64% of those patients underwent further treatment. Median dfs in patients treated without adjuvant chemotherapy was 34.2, 35.7, and 23.1 months for the groups less than 65 years of age, 65–69 years of age, and 70 years of age or older respectively (p < 0.05). For patients who received adjuvant chemotherapy, the median dfs was 49.9, 46.1, and 49.7 months respectively for the same groups (p < 0.05, Table III). Kaplan–Meier analysis concurred that, compared with patients not treated with chemotherapy, treated patients experienced improved dfs (Figure 2).
FIGURE 2.
Disease-free of patients with locally advanced rectal cancer, by age group and use of adjuvant chemotherapy (ADJ), p < 0.001.
Cox proportional hazards analysis identified Eastern Cooperative Oncology Group performance status (p = 0.04), T stage (p = 0.046), and adjuvant chemotherapy (p = 0.002) as significant predictors of survival; age was not a predictor (p = 0.413, Table IV). Similarly, N stage (p = 0.002), neoadjuvant treatment (p = 0.006), and adjuvant chemotherapy (p = 0.007) were significant predictors of time to relapse, but again, age was not a predictor (p = 0.94, Table IV). Notably, comorbidities were found to be statistically nonsignificant in univariate analysis and were not incorporated into the multivariate analysis.
TABLE IV.
Cox multivariate regression analysis
| Variable | Comparator | For overall survival | For disease-free survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| HR | 95.0% CI | p Valuea | HR | 95.0% CI | p Valuea | ||||
|
|
|
||||||||
| Lower | Upper | Lower | Upper | ||||||
| Age | <65 Years | 0.413 | 0.945 | ||||||
| 65–69 Years | 1.412 | 0.619 | 3.221 | 0.412 | 1.099 | 0.493 | 2.453 | 0.817 | |
| ≥70 Years | 1.660 | 0.763 | 3.611 | 0.201 | 0.939 | 0.429 | 2.055 | 0.876 | |
|
| |||||||||
| Sex | |||||||||
| Female | Male | 0.823 | 0.413 | 1.641 | 0.580 | 0.624 | 0.312 | 1.249 | 0.183 |
|
| |||||||||
| ECOG PS | |||||||||
| 1–2 | 0 | 1.781 | 1.026 | 3.094 | 0.040 | 1.654 | 0.967 | 2.829 | 0.066 |
|
| |||||||||
| T Stage | |||||||||
| T3–4 | T2 | 2.088 | 1.015 | 4.297 | 0.046 | 1.481 | 0.756 | 2.902 | 0.252 |
|
| |||||||||
| N Stage | |||||||||
| N1–2 | N0 | 1.229 | 0.745 | 2.028 | 0.420 | 2.124 | 1.304 | 3.459 | 0.002 |
|
| |||||||||
| Differentiation | Well differentiated | 0.690 | 0.919 | ||||||
| Unknown | 0.544 | 0.157 | 1.889 | 0.338 | 0.747 | 0.246 | 2.266 | 0.606 | |
| Poorly differentiated | 1.163 | 0.362 | 3.738 | 0.800 | 1.157 | 0.382 | 3.504 | 0.797 | |
| Moderately well differentiated | 0.991 | 0.414 | 2.373 | 0.984 | 0.948 | 0.430 | 2.090 | 0.894 | |
|
| |||||||||
| Neoadjuvant chemoradiation | |||||||||
| Yes | No | 0.752 | 0.359 | 1.574 | 0.449 | 0.381 | 0.191 | 0.761 | 0.006 |
|
| |||||||||
| Adjuvant chemotherapy | |||||||||
| Yes | No | 0.321 | 0.157 | 0.656 | 0.002 | 0.379 | 0.187 | 0.769 | 0.007 |
Significant values shown in boldface type.
DISCUSSION
This provincial retrospective study suggests that older patients with larc are less likely to undergo adjuvant chemotherapy. When patients did receive adjuvant treatment, chemotherapy completion rates were lower and chemotherapy-related toxicity was significantly worse for the older patients (≥70 years) than for their younger counterparts. We also found that patients who receive adjuvant chemotherapy seem to have an os advantage regardless of age. The cancer-specific outcomes observed also suggest that the disparity in improved cancer outcomes is associated with adjuvant chemotherapy.
Our results are consistent with those from several studies in the literature that considered outcomes in older patients with larc. A recent large study by Xu et al.18 reviewed 14,742 patients with stages ii and iii rectal cancers from the U.S. National Cancer Database (2006–2011) and found that adjuvant therapy was an independent predictor of os regardless of patient factors (including age and comorbidities), stage of disease, and pathologic response. Similarly, those authors found age to be a significant factor for non-receipt of adjuvant therapy. Although completion rate was not considered in that study, our results suggest that, despite a higher rate of non-completion of adjuvant therapy, older patients still derive survival benefit with the addition of adjuvant treatment alone. That observation accords with findings in the literature of treatment efficacy in older patients with other malignancies, such as stage iii colon cancer19.
A recent Canadian multi-institutional retrospective review by Jiang et al.20 compared 1172 older larc patients (≥70 years) with younger patients in the neoadjuvant setting and found similar results for os, dfs, and cancer-specific survival. However, the data in the literature for preoperative treatment in rectal cancer is favourable mainly for local control. In a large Swedish rectal cancer study, the rate of local recurrence was 6% (improved with preoperative treatment), but the rate of distant metastasis was 34%, regardless of whether preoperative radiotherapy was delivered9. Furthermore, a study by van Erning et al.21 looked at 829 patients with stage iii rectal cancer and found that survival benefit, measured by distant recurrence-free survival, was seen only in those who received adjuvant chemotherapy. The 24.5% overall recurrence rate in our study is similar to rates published in the literature and echoes findings that most recurrences are distant rather than local, thereby indicating the potential importance of using adjuvant chemotherapy in patients at highest risk of distant recurrence, such as those with node-positive disease.
The higher toxicity experienced by our older patients was not unexpected, given findings reported in the literature15,16,19. Performance status remains a significant predictor of poor outcomes, including the ability to receive and tolerate chemotherapy. Many older patients have a poorer Eastern Cooperative Oncology Group performance status and more comorbidities that contribute to their poorer survival and higher risk of toxicity. A review by Guimas et al.16 analyzed a healthy older (≥75 years) population of patients (n = 56) with larc and found good tolerance of preoperative chemoradiation, with a rate of adherence to radiation similar to that seen in younger populations. It is therefore imperative to distinguish performance status and medical comorbidities from age alone.
Although every attempt was made to minimize errors, our study has several limitations. First, given the retrospective nature of the study, it has inherent selection biases, and the available information was limited. Our multivariate analysis attempted to control for unrecorded information by including a category called “missing data” for each variable, which did not affect the overall results. Second, our study is relatively small compared with many of the larger multi-institutional studies that include thousands of patients, and so it might be difficult to extrapolate our Atlantic Canada results to the rest of the world. Nonetheless, the study’s results were consistent with many of those larger studies. Third, we did not categorize medical comorbidities using a standard scoring system such as the Charlson comorbidity index, mainly because of the limits on the information available from the database that would be required to calculate the score with accuracy. Instead, we included chronic medical comorbidities that would affect treatment decisions at our centre, finding them to be a significant factor in univariate analysis. Finally, given the poor documentation of pathologic response, that variable was not assessed as a predictor of survival in our study; however, in the literature, it seems to correlate with survival. The review by Xu et al. of the U.S. National Cancer Database for 2006–2011 indicated that the largest survival benefit was found in complete responders18, indicating the potential importance of that variable.
One factor that must be considered is the type of adjuvant chemotherapy used. Our study considered the years 2010–2013, when capecitabine was the standard treatment and what most of our patients received. More long-term results from the adore trial now suggest that, from a survival perspective, oxaliplatin in addition to capecitabine or 5fu is superior to fluoropyrimidine monotherapy in the adjuvant setting for larc22. However, the confidence interval in their subgroup analysis for both os and dfs was wide and crossed 1 in patients more than 65 years of age, calling into question the benefit of oxaliplatin in older patients.
In general, given our study results and data from the literature, it seems imperative to include older patients in clinical trials of rectal cancer treatment so as to provide a high-powered prospective analysis of the effects of treatment in an older population.
CONCLUSIONS
The present study suggests that adjuvant chemotherapy can be associated with a survival benefit in patients with larc regardless of age, but that the use of adjuvant chemotherapy appears to be lower in older patients. However, older patients experience more chemotherapy-related toxicities, leading to higher rates of early treatment discontinuation. Further prospective work is required to confirm those results.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Benson AB, Venook AP, Bekaii-Saab T, et al. Rectal cancer, version 2.2015. J Natl Compr Canc Netw. 2015;13:719–28. doi: 10.6004/jnccn.2015.0087. [DOI] [PubMed] [Google Scholar]
- 2.Schmoll HJ, Van Cutsem E, Stein A, et al. esmo consensus guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol. 2012;23:2479–516. doi: 10.1093/annonc/mds236. [DOI] [PubMed] [Google Scholar]
- 3.Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German cao/aro/aio-94 randomized phase iii trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–33. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 4.Gérard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3–4 rectal cancers: results of ffcd 9203. J Clin Oncol. 2006;24:4620–5. doi: 10.1200/JCO.2006.06.7629. [DOI] [PubMed] [Google Scholar]
- 5.De Paoli A, Chiara S, Luppi G, et al. Capecitabine in combination with preoperative radiation therapy in locally advanced, resectable, rectal cancer: a multicentric phase ii study. Ann Oncol. 2006;17:246–51. doi: 10.1093/annonc/mdj041. [DOI] [PubMed] [Google Scholar]
- 6.Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, Gunnarsson U. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol. 2005;23:5644–50. doi: 10.1200/JCO.2005.08.144. [DOI] [PubMed] [Google Scholar]
- 7.Loree JM, Kennecke HF, Lee-Ying RM, et al. Impact of postoperative adjuvant chemotherapy following long-course chemoradiotherapy in stage ii rectal cancer. Am J Clin Oncol. 2018;41:643–64. doi: 10.1097/COC.0000000000000342. [DOI] [PubMed] [Google Scholar]
- 8.Tay RY, Jamnagerwalla M, Steel M, et al. Survival impact of adjuvant chemotherapy for resected locally advanced rectal adenocarcinoma. Clin Colorectal Cancer. 2017;16:e45–54. doi: 10.1016/j.clcc.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Townsley C, Pond GR, Peloza B, et al. Analysis of treatment practices for elderly cancer patients in Ontario, Canada. J Clin Oncol. 2005;23:3802–10. doi: 10.1200/JCO.2005.06.742. [DOI] [PubMed] [Google Scholar]
- 10.Aparicio T, Navazesh A, Boutron I, et al. Half of elderly patients routinely treated for colorectal cancer receive a sub-standard treatment. Crit Rev Oncol Hematol. 2009;71:249–57. doi: 10.1016/j.critrevonc.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Thiels CA, Bergquist JR, Meyers AJ, et al. Outcomes with multimodal therapy for elderly patients with rectal cancer. Br J Surg. 2016;103:e106–14. doi: 10.1002/bjs.10057. [DOI] [PubMed] [Google Scholar]
- 12.Dobie SA, Warren JL, Matthews B, Schwartz D, Baldwin LM, Billingsley K. Survival benefits and trends in use of adjuvant therapy among elderly stage ii and iii rectal cancer patients in the general population. Cancer. 2008;112:789–99. doi: 10.1002/cncr.23244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francois E, Azria D, Gourgou-Bourgade S, et al. Results in the elderly with locally advanced rectal cancer from the accor12/prodige 2 phase iii trial: tolerance and efficacy. Radiother Oncol. 2014;110:144–9. doi: 10.1016/j.radonc.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 14.Bergquist JR, Thiels CA, Shubert CR, et al. Is chemotherapy or radiation therapy in addition to surgery beneficial for locally advanced rectal cancer in the elderly? A National Cancer Data Base (ncdb) study. World J Surg. 2016;40:447–55. doi: 10.1007/s00268-015-3319-7. [DOI] [PubMed] [Google Scholar]
- 15.Shan JL, Li Q, He ZX, Ren T, Zhou SF, Wang D. A population-based study elicits a reverse correlation between age and overall survival in elderly patients with rectal carcinoma receiving adjuvant chemotherapy. Clin Exp Pharmacol Physiol. 2015;42:752–65. doi: 10.1111/1440-1681.12420. [DOI] [PubMed] [Google Scholar]
- 16.Guimas V, Boustani J, Schipman B, et al. Preoperative chemoradiotherapy for rectal cancer in patients aged 75 years and older: acute toxicity, compliance with treatment, and early results. Drugs Aging. 2016;33:419–25. doi: 10.1007/s40266-016-0367-0. [DOI] [PubMed] [Google Scholar]
- 17.Breugom AJ, Swets M, Bosset JF, et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol. 2015;16:200–7. doi: 10.1016/S1470-2045(14)71199-4. [DOI] [PubMed] [Google Scholar]
- 18.Xu Z, Mohile SG, Tejani MA, et al. Poor compliance with adjuvant chemotherapy use associated with poorer survival in patients with rectal cancer: an ncdb analysis. Cancer. 2017;123:52–61. doi: 10.1002/cncr.30261. [DOI] [PubMed] [Google Scholar]
- 19.Hung A, Mullins CD. Relative effectiveness and safety of chemotherapy in elderly and nonelderly patients with stage iii colon cancer: a systematic review. Oncologist. 2013;18:54–63. doi: 10.1634/theoncologist.2012-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang DM, Raissouni S, Mercer J, et al. Clinical outcomes of elderly patients receiving neoadjuvant chemoradiation for locally advanced rectal cancer. Ann Oncol. 2015;26:2102–6. doi: 10.1093/annonc/mdv331. [DOI] [PubMed] [Google Scholar]
- 21.van Erning FN, Rutten HJT, van den Berg HA, Lemmens VE, van Halteren HK. Effect of adjuvant chemotherapy on recurrence-free survival varies by neo-adjuvant treatment in patients with stage iii rectal cancer. Eur J Surg Oncol. 2015;41:1630–5. doi: 10.1016/j.ejso.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Hong YS, Kim SY, Lee JS, et al. Long-term results of the adore trial: adjuvant oxaliplatin, leucovorin, and 5-fluorouracil (folfox) versus 5-fluorouracil and leucovorin (fl) after preoperative chemoradiotherapy and surgery for locally advanced rectal cancer [abstract 3501] J Clin Oncol. 2018;36 [Available online at: https://meetinglibrary.asco.org/record/158736/abstract; cited 22 September 2018] [Google Scholar]