Abstract
Background
Fertility preservation is an important concern in breast cancer patients. In the present investigation, we set out to create a specific protocol of controlled ovarian stimulation (cos) for oocyte cryopreservation in breast cancer patients.
Methods
From November 2014 to December 2016, 109 patients were studied. The patients were assigned to a specific random-start ovarian stimulation protocol for oocyte cryopreservation. The endpoints were the numbers of oocytes retrieved and of mature oocytes cryopreserved, the total number of days of ovarian stimulation, the total dose of gonadotropin administered, and the estradiol level on the day of the trigger.
Results
Mean age in this cohort was 31.27 ± 4.23 years. The average duration of cos was 10.0 ± 1.39 days. The mean number of oocytes collected was 11.62 ± 7.96 and the mean number of vitrified oocytes was 9.60 ± 6.87. The mean estradiol concentration on triggering day was 706.30 ± 450.48 pg/mL, and the mean dose of gonadotropins administered was 2610.00 ± 716.51 IU. When comparing outcomes by phase of the cycle in which cos was commenced, we observed no significant differences in the numbers of oocytes collected and vitrified, the length of ovarian stimulation, and the estradiol level on trigger day. The total dose of follicle-stimulating hormone and human menopausal gonadotropin administered was statistically greater in the group starting cos in the luteal phase than in the group starting in the late follicular phase.
Conclusions
Our results suggest that using a specific protocol with random-start ovarian stimulation for oocyte cryopreservation in breast cancer patients is effective and could be offered to young women undergoing oncologic treatment.
Keywords: Fertility preservation, breast cancer, ovarian stimulation, oocyte cryopreservation
INTRODUCTION
Given improved cure rates for cancer in young patients, greater attention has been paid to fertility preservation procedures. Hematologic cancers and other malignancies that affect young people can have 90%–95% 5-year survival rates1. Breast cancer is the most common malignancy in adult women, and in the United States, 5%–7% of patients with invasive breast cancer (approximately 11,000 annually) are less than 40 years of age at diagnosis2. With the advent of earlier breast cancer diagnosis and effective treatments, survival rates after breast cancer are increasing, with a 5-year survival rate exceeding 80%3–5. That survival rate justifies concerns about chemotherapy-related gonadal toxicity in women with reproductive goals.
Chemotherapy treatment can have deleterious effects on the ovarian reserve, affecting the resting pool of primordial follicles or the growing follicle population4,6. Moreover, about two thirds of women less than 40 years of age have a hormone receptor–positive cancer and are candidates to receive 10 years of treatment with tamoxifen7. To preserve quality of life for those women, fertility preservation procedures should be offered. A consideration of early referral to fertility specialists for a discussion of fertility preservation procedures is therefore important8.9. Among those procedures, medical ovarian protection, ovarian tissue cryopreservation, and oocyte or embryo cryopreservation are the most common strategies7,10. Embryo and oocyte cryopreservation are the most preferred methods of fertility preservation, although ovarian tissue cryopreservation has been demonstrated to be an useful option11.
For embryo or oocyte cryopreservation, controlled ovarian stimulation (cos) is the first step to be considered. In patients with breast cancer, concerns about cos can emerge, such as delay in starting chemotherapy and exposure to high levels of estradiol consequent to multiple follicle development. Oocyte retrieval requires cos, which might delay oncologic treatment given that conventional cos, initiated at the beginning of the follicular phase, can require up to 6 weeks to conclude.
Random-start ovarian stimulation, which means initiating cos immediately and independently of the menstrual cycle, has become a well-established approach in fertility preservation strategies, allowing oocyte retrieval in no more than 2 weeks in most cases. Moreover, the outcome of random-start ovarian stimulation seems to be similar no matter the phase of the menstrual cycle at the initiation of stimulation12,13.
Another concern might be the estradiol level consequent to ovarian stimulation. To keep estradiol concentrations low, adjuvant therapy with an aromatase inhibitor, letrozole, is recommended throughout cos. Ovarian stimulation combined with aromatase inhibitors has proved to be an efficient procedure14–16. In neoadjuvant chemotherapy for hormone receptor–positive cancers, we propose the administration of tamoxifen in addition to letrozole during ovarian stimulation. To prevent thromboembolic complications, patients are given a prophylactic dose of low molecular weight heparin. With the aim of preventing ovarian hyperstimulation syndrome, which is an important complication of ovarian stimulation, avoidance of human menopausal gonadotropin for triggering final follicular maturation is strongly recommended; a gonadotropin-releasing hormone (gnrh) agonist is therefore used for that purpose17,18.
The foregoing approaches to ovarian stimulation for breast cancer patients could make the procedure more efficient and safer when the aim is cryopreservation of oocytes. In the present study, we report the outcome of a specific protocol of cos for breast cancer patients, and we assess the outcomes of a random-start approach to initiation of stimulation, no matter the phase of the menstrual cycle.
METHODS
The breast cancer patients reported here were undergoing cos for oocyte vitrification in a tertiary public hospital. From November 2014 to December 2016, we studied 109 patients who underwent random-start cos to retrieve oocytes for fertility preservation. We divided the cycles of cos into 3 groups, according to the phase of the menstrual cycle at cos initiation:
-
▪ Initial follicular phase group (ifp, n = 42)
In these patients, cos was initiated at the beginning of the follicular phase, in which no dominant follicle greater than 10 mm was observed.
-
▪ Late follicular phase group (lfp, n = 20)
In these patients, cos was initiated in the late follicular phase, in the presence of a dominant follicle greater than 10 mm.
-
▪ Luteal phase group (lp, n = 47)
In these patients, cos was initiated in the lp, with either or both of ultrasound evidence of follicular rupture and an endometrium of secretory pattern.
Specifically, cos was performed using either recombinant follicle-stimulating hormone (fsh) or urinary human menopausal gonadotropin, in a daily dose of 150–300 IU. In the follicular phase, ovarian stimulation was performed using urinary human menopausal gonadotropin, and in the lp, the gonadotropin choice was recombinant fsh so as to avoid luteinizing hormone activity. The gonadotropin starting dose was chosen according to the antral follicle count: 150 IU daily with 15 or more antral follicles, 225 IU daily with fewer than 15 but 10 or more antral follicles, and 300 IU daily with fewer than 10 antral follicles. Letrozole was started concomitantly with the gonadotropins, at a dose of 5 mg once daily, independently of the immunohistochemistry of the tumour.
Pituitary suppression to prevent a premature luteinizing hormone surge was performed using 0.25 mg of a gnrh antagonist daily. When cos was initiated in the late follicular phase in the presence of a follicle larger than 10 mm, the gnrh antagonist was introduced concomitantly with the gonadotropin; otherwise, the antagonist was administered in the presence of a follicle 13 mm or larger in size. In cases of neoadjuvant chemotherapy, patients also daily received oral tamoxifen 20 mg and a prophylactic dose of low molecular weight heparin (enoxaparin 40 mg) administered subcutaneously to prevent thromboembolic complications.
During ovarian stimulation, ultrasonography imaging was performed every 48 hours. Final follicular maturation was achieved using 0.2 mg triptorelin in the presence of follicles 19 mm or larger in size, and oocyte retrieval was performed transvaginally under ultrasound guidance, 35–36 hours later.
The endpoints of the study were the number of oocytes retrieved and the number of mature oocytes cryopreserved, total number of days of ovarian stimulation, total dose of gonadotropin administered, and estradiol level on the day of the trigger. Outcomes were also analyzed according to the phase of menstrual cycle in which ovarian stimulation was initiated.
Inclusion Criteria
Patients were included if they had been diagnosed with breast cancer, with an indication for neoadjuvant or adjuvant chemotherapy; if they had plans for reproduction after cancer treatment; and if they were 40 years of age or younger.
Exclusion Criteria
Patients with advanced or metastatic disease and those more than 40 years of age were not included in the fertility preservation program.
Ethics Approval
This research was approved by the Committee of Ethics in Research of the Women’s Health Reference Center, Sao Paulo, 29 October 2014, under the number 848.880.
Statistical Analysis
A hypothesis test was applied to evaluate the statistical differences between the groups. The Kruskal–Wallis test was used to compare the results between groups: ifp compared with lfp, ifp compared with lp, and lfp compared with lp. The level of statistical significance was considered to be a p value less than 0.05.
RESULTS
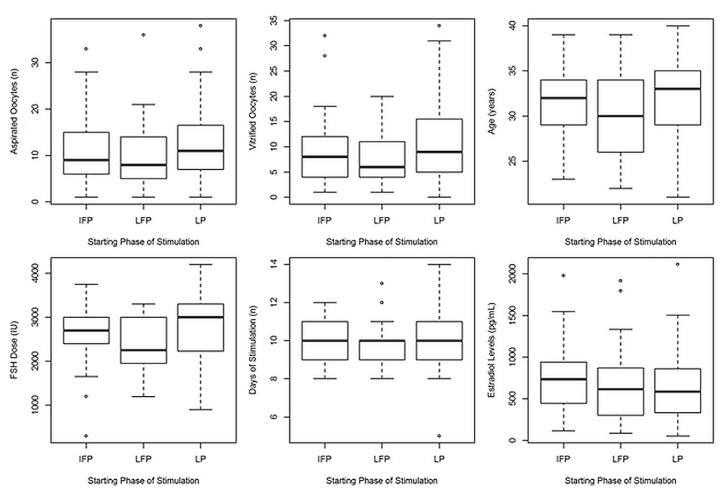
Of the 109 breast cancer patients included in the study, 42 commenced cos in the lp; 20, in the ifp, and 47, in the lfp. Mean age of the patients was 31.27 ± 4.23 years. The average duration of cos was 10.0 ± 1.39 days. The mean number of collected oocytes was 11.62 ± 7.96, and the mean number of vitrified oocytes was 9.60 ± 6.87. The mean estradiol concentration on triggering day was 706.30 ± 450.48 pg/mL, and the mean dose of fsh administered was 2610.00 ± 716.51 IU (Table I). When comparing outcomes according to the phase of the menstrual cycle in which cos was initiated, we observed no significant differences in the number of oocytes collected and vitrified, in the ovarian stimulation duration, and in the estradiol level on the trigger day. A statistically significant increase in the total dose of fsh administered was observed in the group starting cos in the lp compared with the group starting in the lfp. In Figure 1, the box plots show overall mean values, standard deviations, and outliers of the mean values.
TABLE I.
Outcomes in 109 breast cancer patients undergoing controlled ovarian stimulation for fertility preservation
| Variable | Patient group | p Value | |||
|---|---|---|---|---|---|
| Overall | IFP | LFP | LP | ||
| Patients (n) | 109 | 41 | 21 | 47 | |
| Mean aspirated oocytes (n) | 11.62±7.96 | 10.95±7.23 | 10.38±8.0 | 12.77±8.54 | NS |
| Mean vitrified oocytes (n) | 9.60±6.87 | 8.927±6.75 | 7.952±5.38 | 10.94±7.43 | NS |
| Age (years) | 31.27 ± 4.23 | 31.37±3.48 | 29.76±4.94 | 31.85±4.41 | NS |
| Mean FSH or hMG dose (IU) | 2610±716.51 | 2577±670.19 | 2387±615.31 | 2738±780.9 | 0.04457a |
| Mean days of stimulation (n) | 10±1.39 | 9.854±1.33 | 9.714±1.31 | 10.26±1.45 | NS |
| Mean serum estradiol (pg/mL) | 706.3±450.48 | 761±439.93 | 677.8±503.39 | 671.2±440.1 | NS |
Statistically significant difference between the LFP and LP groups.
IFP = initial follicular phase; LFP = late follicular phase; LP = luteal phase; NS = statistically nonsignificant; FSH = follicle-stimulating hormone; hMG = human menopausal gonadotropin.
FIGURE 1.
Box plots showing the results of random-start ovarian stimulation. The inner black line marks the median; the box delimits the upper and lower quartiles; and small circles mark outliers. IFP = initial follicular phase; LFP = late follicular phase; LP = luteal phase; FSH = follicle-stimulating hormone.
DISCUSSION
Modern treatments for breast cancer, including surgery, chemotherapy, and radiotherapy, have improved cure rates, and the decline in mortality is remarkable in women less than 50 years of age10. Nevertheless, gonadal toxicity consequent to cancer therapy can lead to impaired reproductive function in younger patients, such that procedures aiming to preserve reproductive potential are necessary. Quality of life is an important issue to be considered in cancer survivors, and compromised fertility is a concern, particularly for young women.
We can emphasize that reproductive concerns are not meaningless for young women diagnosed with breast cancer, and the demand for fertility preservation techniques by those patients has increased with improved cure rates. The techniques used for fertility preservation include reducing the effect of chemotherapy on the ovaries, cryopreservation of oocytes and embryos, and cryopreservation of ovarian tissue10. Given that protection of ovarian function with gnrh analogs is controversial and that cryopreservation of ovarian tissue is still an experimental procedure, cryopreservation of oocytes or embryos is the most important procedure indicated for fertility preservation19.
Considering the availability of in vitro fertilization (ivf) as a technique to treat infertile couples, embryo cryopreservation has become a very efficient procedure, and studies suggest a “freeze all” policy, even in conventional cycles of ivf20. Because many young women with breast cancer do not have a male partner at the moment of treatment, because concerns could arise about the destiny of embryos should the disease progress, and because ivf outcomes with vitrified oocytes are comparable to those with fresh oocytes21, oocyte cryopreservation has become the option of choice.
The method called vitrification, which implies ice-free cryopreservation, represents significant progress in oocyte cryopreservation, being associated with satisfactory rates of pregnancy22. Among established methods, oocyte cryopreservation has been postulated to be the preferred option in postpubertal women; in contrast, ovarian tissue cryopreservation is the only possibility for prepubertal girls23. Regardless, cos is mandatory for optimization of embryo or oocyte cryopreservation. The procedure normally require 2–6 weeks, depending on the current day of the patient’s menstrual cycle, potentially leading to an undesirable delay in initiating chemotherapy.
Given that a receptive endometrium is not necessary in fertility preservation procedures, random-start stimulation is an interesting option. Our specific protocol proposes random-start ovarian stimulation, and our results demonstrate that outcomes are comparable to those obtained during conventional ivf cycles, which accords with other observations12,13,24. Currently, random-start ovarian stimulation is routinely and successfully used for emergency ivf25–27. In the present study, results (number of days of stimulation, maturity rate of the oocytes collected) were similar for the initiation of stimulation in women at all three phases of the menstrual cycle, although a statistically significantly higher dose of gonadotropins was administered in the lp group than in the lfp group. Those results accord with the results obtained by von Wolff et al.13, who reported a significant increase in the total dose of gonadotropin when cos was initiated in the lp. However, that finding was not clinically significant. For a few patients, we cryopreserved embryos, and fertilization rates were similar to those obtained with conventional cos (data not shown).
Our data confirm that the concomitant use of an aromatase inhibitor during ovarian stimulation is efficient for preventing the high levels of estradiol commonly observed in conventional cos. The aromatase inhibitor used most often in ovarian stimulation protocols is letrozole, which has proved to be more efficient than anastrozole for this purpose28. Ovarian stimulation combined with letrozole at a daily dose of 5 mg has proved to be efficient in this context14. On the other hand, some reports suggest that the concomitant use of letrozole with gonadotropins significantly lowers the number of oocytes available for cryopreservation29. In our investigation, the mean numbers of oocytes collected and cryopreserved (11.62 ± 7.96 and 9.60 ± 6.87 respectively) were considered adequate, given that recent data suggest that at least 8–10 metaphase ii vitrified oocytes are necessary to achieve reasonable success22. Recent publications also corroborate that adjuvant therapy with letrozole throughout cos is a safe and efficient approach15,16,30.
In our patient series, the mean estradiol concentration on triggering day was 760.30 ± 450.48 pg/mL (median: 655 pg/mL). Given that serum estradiol levels during cos are increased by a factor of 10 compared with levels during natural cycles31, estradiol concentrations on triggering day are expected to reach 2500 pg/mL, which are much higher than the levels observed in our study.
If cos is performed in the presence of cancer, as occurs when the indication is neoadjuvant chemotherapy, we propose using tamoxifen together with letrozole. It is possible that the different mechanisms of action of those agents are complementary, with the aromatase inhibitor lowering the estrogen level, thus allowing tamoxifen to function more effectively as a competitive inhibitor with estradiol. Given that venous thromboembolism is a major concern in cancer patients32, and considering the risk of administering tamoxifen together with letrozole, our protocol for women undergoing neoadjuvant chemotherapy proposes the addition of daily enoxaparin in a prophylactic dose. Our group recently published a case series in which cos was performed with letrozole, tamoxifen, and enoxaparin for 40 patients who received neoadjuvant chemotherapy (also reported in the present series), obtaining the same outcomes for days of stimulation and number and maturity of oocytes33.
With respect to triggering of the final oocyte maturation, we believe that there is very little place for a human menopausal gonadotropin trigger in cos for fertility preservation. The use of gnrh agonist to trigger final oocyte maturation is strongly recommended to prevent the occurrence of hyperstimulation ovarian syndrome18, which would be an undesirable complication of ovarian stimulation in cancer patients. Moreover, it was recently reported that a gnrh agonist trigger increases the number of mature oocytes available for vitrification in cancer patients34.
Our data confirm that random-start ovarian stimulation is effective and, compared with conventional stimulation, produces mature oocytes in the same proportion. That important fact reassures patients that an oocyte vitrification procedure will not delay their oncologic treatment. Moreover, the concomitant use of letrozole, an aromatase inhibitor, provides a safe option for women with breast cancer, resulting in estradiol levels that are lower than those in conventional stimulation15. The efficacy of cos is not altered by concomitant use of letrozole, and no evidence suggests an increased risk of malformations in newborns conceived while women are taking aromatase inhibitors35. Considering the emotional stress of the diagnosis and treatment of cancer at a young age, reassuring patients, family, and oncologists that the fertility preservation procedure is safe and effective is important for reducing the psychological burden to which the patient is exposed.
With respect to the efficacy and safety of cryopreserved oocytes to produce a normal pregnancy, reports in the medical literature confirm that the procedure is safe and efficient in women with cancer. Cancer patients who undergo oocyte cryopreservation before chemotherapy achieve good ivf performance and good perinatal outcomes36. However, on a cautionary note, it is important to realize that most of the reports assessing the safety and efficacy of oocyte cryopreservation involve healthy women undergoing conventional ivf or participants in ovodonation programs. Whether the results can be extrapolated to cancer patients remains to be better elucidated, even if the data obtained so far are reassuring22,29.
CONCLUSIONS
The results observed in our patient series suggest that oocyte or embryo cryopreservation in a specific protocol based on random-start ovarian stimulation for breast cancer patients is effective and safe, and can be offered to young women undergoing oncologic treatment who have concerns related to their reproductive future.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.The Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in cancer patients. Fertil Steril. 2005;83:1622–8. doi: 10.1016/j.fertnstert.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Kim SS, Klemp J, Fabian C. Breast cancer and fertility preservation. Fertil Steril. 2011;95:1535–43. doi: 10.1016/j.fertnstert.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 4.Morgan S, Anderson RA, Gourley C, Wallace WH, Spears N. How do chemotherapeutic agents damage the ovary? Hum Reprod Update. 2012;18:525–35. doi: 10.1093/humupd/dms022. [DOI] [PubMed] [Google Scholar]
- 5.Murk W, Seli E. Fertility preservation as a public health issue: an epidemiological perspective. Curr Opin Obstet Gynecol. 2011;23:143–50. doi: 10.1097/GCO.0b013e3283455270. [DOI] [PubMed] [Google Scholar]
- 6.Reh A, Oktem O, Oktay K. Impact of breast cancer chemotherapy on ovarian reserve: a prospective observational analysis by menstrual history and ovarian reserve markers. Fertil Steril. 2008;90:1635–9. doi: 10.1016/j.fertnstert.2007.09.048. [DOI] [PubMed] [Google Scholar]
- 7.de Pedro M, Otero B, Martin B. Fertility preservation and breast cancer: a review. eCancerMedicalScience. 2015;9:503. doi: 10.3332/ecancer.2015.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Wolff M, Dittrich R, Liebenthron J, et al. Fertility-preservation counselling and treatment for medical reasons: data from a multinational network of over 5000 women. Reprod Biomed Online. 2015;31:605–12. doi: 10.1016/j.rbmo.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Srikanthan A, Amir E, Warner E. Does a dedicated program for young breast cancer patients affect the likelihood of fertility preservation discussion and referral? Breast. 2016;27:22–6. doi: 10.1016/j.breast.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Sonmezer M, Oktay K. Fertility preservation in young women undergoing breast cancer therapy. Oncologist. 2006;11:422–34. doi: 10.1634/theoncologist.11-5-422. [DOI] [PubMed] [Google Scholar]
- 11.Van der Ven H, Liebenthron J, Beckmann M, et al. on behalf of the Fertiprotekt network. Ninety-five orthotopic transplantations in 74 women of ovarian tissue after cytotoxic treatment in a fertility preservation network: tissue activity, pregnancy and delivery rates. Hum Reprod. 2016;31:2031–41. doi: 10.1093/humrep/dew165. [DOI] [PubMed] [Google Scholar]
- 12.Cakmak H, Rosen MP. Random-start ovarian stimulation in patients with cancer. Curr Opin Obstet Gynecol. 2015;27:215–21. doi: 10.1097/GCO.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 13.von Wolff M, Capp E, Jauckus J, Strowitzki T, Germeyer A on behalf of the Fertiprotekt study group. Timing of ovarian stimulation in patients prior to gonadotoxic therapy: an analysis of 684 stimulations. Eur J Obstet Gynecol Reprod Biol. 2016;199:146–9. doi: 10.1016/j.ejogrb.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Reddy J, Oktay K. Ovarian stimulation and fertility preservation with the use of aromatase inhibitors in women with breast cancer. Fertil Steril. 2012;98:1363–9. doi: 10.1016/j.fertnstert.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Turan V, Oktay K. Long-term safety of letrozole and gonadotropin stimulation for fertility preservation in women with breast cancer. J Clin Endocrinol Metab. 2016;101:1364–71. doi: 10.1210/jc.2015-3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pereira N, Hancock K, Cordeiro CN, Lekovich JP, Schattman GL, Rosenwaks Z. Comparison of ovarian stimulation response in patients with breast cancer undergoing ovarian stimulation with letrozole and gonadotropins to patients undergoing ovarian stimulation with gonadotropins alone for elective cryopreservation of oocytes. Gynecol Endocrinol. 2016;32:823–6. doi: 10.1080/09513590.2016.1177013. [DOI] [PubMed] [Google Scholar]
- 17.Humaidan P, Nelson SM, Devroey P, et al. Ovarian hyperstimulation syndrome: review and new classification criteria for reporting in clinical trials. Hum Reprod. 2016;31:1997–2004. doi: 10.1093/humrep/dew149. [DOI] [PubMed] [Google Scholar]
- 18.Thomsen L, Humaidan P. Ovarian hyperstimulation syndrome in the 21st century: the role of gonadotropin-releasing hormone agonist trigger and kisspeptin. Curr Opin Obstet Gynecol. 2015;27:210–14. doi: 10.1097/GCO.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 19.Vu JV, Llarena NC, Estevez SL, Moravek MB, Jeruss JS. Oncofertility program implementation increases access to fertility preservation options and assisted reproductive procedures for breast cancer patients. J Surg Oncol. 2017;115:116–21. doi: 10.1002/jso.24418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roque M, Valle M, Guimarães F, Sampaio M, Geber S. Freezeall policy: fresh vs. frozen-thawed embryo transfer. Fertil Steril. 2015;103:1190–3. doi: 10.1016/j.fertnstert.2015.01.045. [DOI] [PubMed] [Google Scholar]
- 21.Argyle CE, Harper JC, Davies MC. Oocyte cryopreservation: where are we now? Hum Reprod Update. 2016;22:440–9. doi: 10.1093/humupd/dmw007. [DOI] [PubMed] [Google Scholar]
- 22.Cobo A, García-Velasco JA, Coello A, Domingo J, Pellicer A, Remohí J. Oocyte vitrification as an efficient option for elective fertility preservation. Fertil Steril. 2016;105:755–64. doi: 10.1016/j.fertnstert.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 23.Martínez F, Devesa M, Coroleu B, et al. Cancer and fertility preservation: Barcelona consensus meeting. Gynecol Endocrinol. 2013;29:285–91. doi: 10.3109/09513590.2012.743019. [DOI] [PubMed] [Google Scholar]
- 24.Grynberg M, Poulain M, le Parco S, Sifer C, Fanchin R, Frydman R. Similar in vitro maturation rates of oocytes retrieved during the follicular or luteal phase offer flexible options for urgent fertility preservation in breast cancer patients. Hum Reprod. 2016;31:623–9. doi: 10.1093/humrep/dev325. [DOI] [PubMed] [Google Scholar]
- 25.Rashidi BH, Tehrani ES, Ghaffari F. Ovarian stimulation for emergency fertility preservation in cancer patients: a case series study. Gynecol Oncol Rep. 2014;10:19–21. doi: 10.1016/j.gore.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JH, Kim SK, Lee HJ, et al. Efficacy of random-start controlled ovarian stimulation in cancer patients. J Korean Med Sci. 2015;30:290–5. doi: 10.3346/jkms.2015.30.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robertson DM, Gilchrist RB, Ledger WL, Baerwald A. Random start or emergency ivf/in vitro maturation: a new rapid approach to fertility preservation. Women’s Health (Lond) 2016;12:339–49. doi: 10.2217/whe-2015-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azim AA, Costantini-Ferrando M, Lostritto K, Oktay K. Relative potencies of anastrozole and letrozole to suppress estradiol in breast cancer patients undergoing ovarian stimulation before in vitro fertilization. J Clin Endocrinol Metab. 2007;92:2197–200. doi: 10.1210/jc.2007-0247. [DOI] [PubMed] [Google Scholar]
- 29.Revelli A, Porcu E, Levi Setti PE, Delle Piane L, Merlo DF, Anserini P. Is letrozole needed for controlled ovarian stimulation in patients with estrogen receptor-positive breast cancer? Gynecol Endocrinol. 2013;29:993–6. doi: 10.3109/09513590.2013.819083. [DOI] [PubMed] [Google Scholar]
- 30.Domingo J, Garcia-Velasco JA. Oocyte cryopreservation for fertility preservation in women with cancer. Curr Opin Endocrinol Diabetes Obes. 2016;23:465–9. doi: 10.1097/MED.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 31.Joo BS, Park SH, An BM, Kim KS, Moon SE, Moon HS. Serum estradiol levels during controlled ovarian hyperstimulation influence the pregnancy outcome of in vitro fertilization in a concentration-dependent manner. Fertil Steril. 2010;93:442–6. doi: 10.1016/j.fertnstert.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 32.Brand JS, Hedayati E, Bhoo-Pathy N, et al. Time-dependent risk and predictors of venous thromboembolism in breast cancer patients: a population-based cohort study. Cancer. 2017;123:468–75. doi: 10.1002/cncr.30364. [DOI] [PubMed] [Google Scholar]
- 33.Cavagna F, Pontes A, Cavagna M, et al. A specific controlled ovarian stimulation (cos) protocol for fertility preservation in women with breast cancer undergoing neoadjuvant chemotherapy. Contemp Oncol (Pozn) 2017;21:290–4. doi: 10.5114/wo.2017.72395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pereira N, Kelly AG, Stone LD, et al. Gonadotropin-releasing hormone agonist trigger increases the number of oocytes and embryos available for cryopreservation in cancer patients undergoing ovarian stimulation for fertility preservation. Fertil Steril. 2017;108:532–8. doi: 10.1016/j.fertnstert.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 35.Sharma S, Ghosh S, Singh S, et al. Congenital malformations among babies born following letrozole or clomiphene for infertility treatment. PLoS One. 2014;9:e108219. doi: 10.1371/journal.pone.0108219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinez M, Rabadan S, Domingo J, Cobo A, Pellicer A, Garcia-Velasco JA. Obstetric outcome after oocyte vitrification and warming for fertility preservation in women with cancer. Reprod Biomed Online. 2014;29:722–8. doi: 10.1016/j.rbmo.2014.09.002. [DOI] [PubMed] [Google Scholar]