Abstract
Introduction
Radiotherapy (rt) plays an important role in the treatment of lung cancer. One of the most common comorbidities in patients with lung cancer is pulmonary emphysema. The literature offers conflicting data about whether emphysema increases the occurrence and severity of radiation pneumonitis (rp). As a result, whether high doses of rt (with curative intent) should be avoided in patients with emphysema is still unclear.
Objective
We measured the documented incidence of rp in patients with and without emphysema who received curative radiation treatment.
Methods
This retrospective cohort study considered patients in the lung cancer clinical database of the Peter Brojde Lung Cancer Centre. Data from the database has been used previously for research studies, including a recent publication about emphysema grading, based on the percentage of lung occupied by emphysema on computed tomography (ct) imaging.
Results
Using previously published methods, chest ct imaging for 498 patients with lung cancer was scored for the presence of emphysema. The analysis considered 114 patients who received at least 30 Gy radiation. Of those 114 patients, 64 (56%) had emphysema, with approximately 23% having severe or very severe disease. The incidence of rp was 34.4% in patients with emphysema (n = 22) and 32.0% in patients with no emphysema (n = 16, p = 0.48). No difference in the incidence of rp was evident between patients with various grades of emphysema (p = 0.96). Similarly, no difference in the incidence of rp was evident between the two treatment protocols—that is, definitive rt 17 (37%) and combined chemotherapy–rt 21 (31%, p = 0.5).
Conclusions
In our cohort, the presence of emphysema on chest ct imaging was not associated with an increased risk of rp. That finding suggests that patients with lung cancer and emphysema should be offered rt when clinically indicated. However, further prospective studies will be needed for confirmation.
Keywords: Lung cancer, emphysema, radiation pneumonitis
INTRODUCTION
Radiotherapy (rt) plays an important role in the treatment of lung cancer1–5, but its benefits must be balanced against the risk of lung injury. Radiation pneumonitis (rp) often develops insidiously and becomes clinically evident 2–3 months after completion of rt. The diagnosis of acute rp can be challenging6; it requires exclusion of allergic, infective, and chemical causes of pneumonitis. In the longer term, rt side effects typically present as well-defined areas of pulmonary fibrosis confined to the field of radiation.
There is consensus that poor pulmonary function is a risk factor and a relative contraindication in patients undergoing radical rt7,8. A recent survey about recommendations by radiation oncologists for the treatment of hypothetical patients with stage iiib non-small-cell lung cancer and comorbid pulmonary illness demonstrated that most radiation oncologists would not recommend any radiation therapy in patients with severe pulmonary comorbidities9. Furthermore, guidelines from the American College of Chest Physicians state that a patient with a forced expiratory volume in 1 s (fev1) less than 1 L, is unlikely to be able to tolerate rt therapy of 60 Gy or more1,5. In reality, one of the most common lung pathologies in patients with lung cancer is pulmonary emphysema. The few available published data are conflicting about whether emphysema increases the occurrence and severity of rp. As a result, it is still unclear whether the use of higher doses of rt (with curative intent) should be avoided in patients with emphysema7,10.
The purpose of the present study was to measure the documented incidence of rp in patients with and without emphysema who received curative radiation treatment.
METHODS
Study Design
Data were extracted from the Peter Brojde Lung Cancer Centre’s clinical database, which contains prospectively collected data for all patients diagnosed and treated for lung cancer. Patient information is obtained by specialized oncology data managers from clinical charts, weekly tumour board meetings, and patient interviews. Data from the database have previously been used for research studies, including a recent publication on emphysema11.
Study Subjects and Methods
In a previous study11, chest ct imaging for 498 patients with lung cancer was scored for the presence of emphysema using previously published methods12,13. Emphysema was assigned as present or absent, and if present, was graded based on the percentage of lung occupied by emphysema on ct: none (0%), mild (1%–10%), moderate (11%–25%), severe (26%–50%), or very severe (>50%). Data concerning rt treatment, including dose, site of radiation, and complications, were also collected. According to institutional standards of treatment, the dose of radiation was based on a V20 calculation, which is the percentage of the lung volume (with subtraction of the volume involved by the lung cancer) treated with a radiation dose of 20 Gy or more. For the purposes of the present analysis, a patient was deemed to have experienced rp if high-dose steroid treatment (prednisone 40 mg daily minimum) was started to address symptoms in a patient who received at least 30 Gy radiation to the lung. Treatment for rp had to be initiated within 6 months of rt start after all infectious, cardiac, and allergic causes for symptoms were excluded. In the identified cohort, 114 patients had received at least 30 Gy radiation and were included in the analysis (Figure 1). The study was approved by the institutional ethics review board.
FIGURE 1.
Lung cancer patients enrolled in the analysis. aEmphysema on computed tomography imaging (CT). RP = radiation pneumonitis.
Analysis
Means and medians are used to summarize patient characteristics. Data were analyzed using the IBM SPSS Statistics software application (version 20: IBM, Armonk, NY, U.S.A.) for Windows (Microsoft Corporation, Redmond, WA, U.S.A.). Simple descriptive statistics (means with standard deviation, or proportions) are used to summarize the demographic, clinical, and tumour characteristics of the cohort. Comparisons between patients with emphysema and those without emphysema used the chi-square test. To assess characteristics that predict the occurrence of rp, 4 variables (emphysema presence, sex, smoking, lung cancer type) were assessed using logistic regression modelling. A 2-sided p value of 0.05 was considered statistically significant. Because of missing data, pulmonary function (fev1%) was not included in the analysis.
RESULTS
Of the 114 patients who received rt with curative intent between 2001 and 2015, 68 (60%) received definitive rt, and 46 (40%) received combined chemotherapy–rt. Table I presents the characteristics of the study patients. In this cohort, 56% (n = 64) had emphysema, with 23% having severe or very severe disease.
TABLE I.
Clinical characteristics of 114 lung cancer patients who received curative-dose radiotherapy
| Characteristic | Value |
|---|---|
| Age (years) | |
| Median | 69 |
| IQR | 60–76 |
|
| |
| Sex [n (%) women] | 50 (44) |
|
| |
| Histology [n (%)] | |
| Small-cell carcinoma | 12 (10) |
| Adenocarcinoma | 64 (56) |
| Squamous carcinoma | 18 (16) |
| Large-cell and neuroendocrine tumour | 8 (7) |
| Pleomorphic or sarcomatoid carcinoma | 1 (1) |
| Undifferentiated carcinoma | 11 (10) |
|
| |
| Emphysema [n (%)] | |
| Absent | 50 (44) |
| Present | 64 (56) |
| Grade (n=64) | |
| Mild | 29 (45.3) |
| Moderate | 20 (31.3) |
| Severe | 9 (14.1) |
| Very severe | 6 (9.4) |
|
| |
| Smoking [n (%)] | |
| Never-smoker | 11 (10) |
| Ever-smoker | 103 (90) |
| Pack–year history | |
| <30 Pack–years | 35 (31) |
| 30–44 Pack–years | 27 (23) |
| 45–59 Pack–years | 28 (25) |
| 60–74 Pack–years | 15 (13) |
| ≥75 Pack–years | 9 (8) |
|
| |
| Type of radiation therapy | |
| Definitive radiation alone | 68 (60) |
| Combined chemoradiation | 46 (40) |
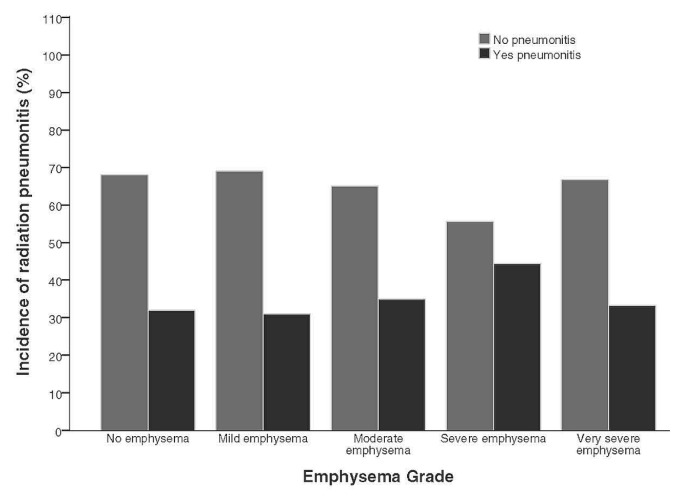
The median total rt dose delivered was 50.2 Gy (range: 30–66 Gy). Radiation was delivered in fractionated doses of 2 Gy daily for intensity-modulated rt and 10–15 Gy for stereotactic rt. The 38 symptomatic patients with presumed rp (33.3%) were treated with high-dose steroids. Only 6 of those patients required oxygen. The rp was defined as grade 2 in 84% of the symptomatic group and grade 3 in 16%. The rates of any-grade emphysema in patients treated with rt were no different than the rates in patients excluded from the study (Figures 1 and 2). The rp incidence was 34.4% in patients with ct imaging–documented emphysema (n = 22) and 32.0% in patients with no emphysema (n = 16, p = 0.48). No statistical difference in the incidence of rp was evident between patients with the various grades of emphysema (p = 0.96, Figure 3). Similarly, the incidence of rp was not different for the two treatment protocols—that is, definitive rt 17 (37%) and combined chemotherapy–rt 21 (31%, p = 0.5).
FIGURE 2.
Rate of emphysema in patients with lung cancer who did or did not receive radiotherapy.
FIGURE 3.
Rate of radiation pneumonitis in patients with lung cancer by grade of emphysema.
Most of the primary tumours (76%, n = 87) were located in the upper lobes. A slightly higher, but statistically non-significant incidence of rp was observed for patients with a tumour in the lower lobes (37% vs. 32%)
Because of a transition to electronic from paper records in 2007 and the unavailability of paper records on-site, dose–volumetric parameters (V20) were available for only 46 of the 114 patients. The mean V20 for that subset was 15.7% (range: 1.4%–40.9%). The V20 was no different in the 17 patients who developed rp than in the 29 who did not develop rp: 16.3% ± 10.4% and 15.4% ± 10.5 respectively. However, the V20 was slightly higher (18.9%) in patients with rp and emphysema (n = 10) than in patients (n = 6) with rp and no emphysema (12.0%, p > 0.05).
Unfortunately, fev1 data were available for only 56 patients (49%): 23 with no emphysema (41%) and 33 with mild or moderate grades of emphysema (57%) on ct imaging. Although ct imaging showed lower pulmonary function in patients with emphysema than in other patients (mean fev1% predicted: 68.5% vs. 78.9%), the difference was not statistically significant (p = 0.15). In addition, no statistically significant difference in fev1 was observed between 18 patients with rp (mean fev1: 69.8%) and 38 patients without rp (mean fev1: 75.2%; p = 0.47).
Although precise details about the risk factors for developing rp are unclear, it is thought that patient factors (age, smoking), type of cancer (small-cell vs. non-small-cell lung cancer), pre-existing lung disease, and radiation dose are all potentially important. We assessed whether those factors (excluding radiation dose) were related to the occurrence of rp. Univariate analyses for sex, smoking status, emphysema, and type of lung cancer were not predictive for rp. Furthermore, none of those factors were predictive for rp in multivariate analysis (Table II)
TABLE II.
Multivariate analysis of factors potentially predictive of radiation pneumonitis in lung cancer patients
| Variable | Comparison | Exp(B) | 95% CI for Exp(B) | p Value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Emphysema | Absent vs. present | 1.141 | 0.482 | 2.700 | 0.764 |
| Sex | Women vs. men | 1.275 | 0.566 | 2.872 | 0.557 |
| Smoking | Never vs. ever | 1.507 | 0.355 | 6.396 | 0.578 |
| Histology | SCLC vs. NSCLC | 0.495 | 0.142 | 1.726 | 0.270 |
| RT variant | RT alone vs. chemoRT | 1.194 | 0.520 | 2.742 | 0.675 |
CI = confidence interval; SCLC = small-cell lung cancer; NSCLC = non-small-cell lung cancer; RT = radiotherapy; chemoRT = chemoradiation.
DISCUSSION
Our study describes the relationship between the incidence of rp and emphysema in a cohort of patients with radiologically confirmed emphysema graded by severity. Existing recommendations have suggested that pulmonary emphysema is a risk factor for rp and that rt treatment should be restricted or avoided in patients with emphysema8–10. Our results demonstrate an overall incidence of rp of 33%, with rates of rp being similar in patients with and without emphysema. Furthermore, we observed no difference in the rp rate for patients receiving rt alone and those receiving combined chemotherapy–rt. The location of the primary tumour also had no effect on the incidence of rp.
The incidence of symptomatic rp in the present study was similar to the 15%–40% reported in a Radiation Therapy Oncology Group randomized controlled trial of concurrent chemoradiotherapy14,15 and congruent with the 29.8% rate reported in a meta-analysis by Palma et al.16. The higher incidence of pneumonitis in an unselected group was demonstrated in small cohort studies by Kimura et al.7 and Ishijima et al.10.
As mentioned earlier, poor lung function or other lung pathology has been suggested to increase the risk of rp. Kimura et al.7 suggested that patients with reduced lung function had greater propensity to develop rp. They reported an overall 88% incidence of grades 1 and 2 rp, with significant correlation between the rate of rp and emphysema grade. In contrast, Ishjima et al.10 reported the incidence of rp to be 43% in patients with early lung cancer undergoing stereotactic rt. Those authors found that the risk of rp was lower in patients with severe emphysema than in patients with no underlying lung disease. The authors reasoned that the emphysematous tissue around the tumour decreased the risk of rp developing despite high local doses of radiation. The results of our study stand somewhere between those two extremes: that is, we observed no significant difference in the rate of rp between patients with and without emphysema, and we observed no correlation between the incidence of rp and the severity of emphysema.
In addition, some authors have reported that chemotherapy increases the risk of rp, especially when used concurrently17,18. Those reports contrast with our study results, in which about 40% of patients received radiation in combination with chemotherapy and showed no evidence of an increased risk of rp compared with their counterparts who received comparable doses of rt alone.
We recognize a number of deficiencies in our study. First, the rp was established retrospectively as a diagnosis of exclusion, based on the use of steroids in the absence of other causes within 6 months of treatment completion. Although we believe that the most severe cases of rp (grades 2 and 3) would have been captured in our study cohort, it is possible that, as a result of the foregoing criteria, a somewhat higher proportion of the more mild cases of rp might have been excluded from the analysis because of differences in clinician documentation of such events and because of our definition of rp. Also, high proportions of the cohort lacked data for pulmonary function tests and V20, which could potentially have been used for risk-stratification.
As in any retrospective cohort study, eliminating the possibility of selection bias is hard. However, it appears that the presence of emphysema did not play a decisive role in selection of patients for rt, because the rate of emphysema was no different for patients treated with rt than for patients who were not so treated (Figure 1). We also had no data about other potential confounding factors such as circulating pro-inflammatory cytokines in the study patients.
It is plausible that the malignancy-related systemic inflammatory response might exacerbate the local damaging effects of radiation-induced cytokine release that is thought to have a major role in development of lung toxicity19,20. Like other authors, we have not attempted to grade the extent of lung involvement with rp or to include data about the outcome of rp in our cohort. It is still possible that, although the incidence of rp might not be affected by the presence of emphysema, emphysema might have a role in determining the patient’s response—for example, resolution without symptoms compared with progressive respiratory failure. Thus, to paint a more nuanced picture of the effect of rp in various patients with lung cancer, future studies should perhaps, in addition to simple incidence rates, include data about the extent of lung involvement and the severity and effect of any symptoms.
CONCLUSIONS
In our study, the presence of emphysema on chest ct imaging was not associated with an increased risk of rp. Those findings suggest that patients with lung cancer and emphysema should be offered rt when clinically indicated. However, further prospective studies will be needed for confirmation and should include data about other clinically relevant outcomes such as effect on respiratory function and delays in subsequent anticancer treatment.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none. We have full control of all primary data and agree to allow review of our data if requested.
REFERENCES
- 1.Jett JR, Schild SE, Keith RL, Kesler KA on behalf of the American College of Chest Physicians. Treatment of non–small cell lung cancer, stage iiib: accp evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(suppl):266S–76S. doi: 10.1378/chest.07-1380. [DOI] [PubMed] [Google Scholar]
- 2.Kong FS, Cuneo KC, Wang L, et al. Patterns of practice in radiation therapy for non–small cell lung cancer among members of the American Society for Radiation Oncology. Pract Radiat Oncol. 2014;4:e133–41. doi: 10.1016/j.prro.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Rodrigues G, Choy H, Bradley J, et al. Definitive radiation therapy in locally advanced non-small cell lung cancer: executive summary of an American Society for Radiation Oncology (astro) evidence-based clinical practice guideline. Pract Radiat Oncol. 2015;5:141–8. doi: 10.1016/j.prro.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Novello S, Barlesi F, Califano R, et al. on behalf of the esmo Guidelines Committee. Metastatic non-small-cell lung cancer: esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v1–27. doi: 10.1093/annonc/mdw326. [DOI] [PubMed] [Google Scholar]
- 5.Robinson LA, Ruckdeschel JC, Wagner H, Jr, Stevens CW on behalf of the American College of Chest Physicians. Treatment of non–small cell lung cancer—stage iiia: accp evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(suppl):243S–65S. doi: 10.1378/chest.07-1379. [DOI] [PubMed] [Google Scholar]
- 6.Kocak Z, Evans ES, Zhou SM, et al. Challenges in defining radiation pneumonitis in patients with lung cancer. Int J Radiat Oncol Biol Phys. 2005;62:635–8. doi: 10.1016/j.ijrobp.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 7.Kimura T, Togami T, Takashima H, Nishiyama Y, Ohkawa M, Nagata Y. Radiation pneumonitis in patients with lung and mediastinal tumours: a retrospective study of risk factors focused on pulmonary emphysema. Br J Radiol. 2012;85:135–41. doi: 10.1259/bjr/32629867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kong FM, Wang S. Nondosimetric risk factors for radiationinduced lung toxicity. Semin Radiat Oncol. 2015;25:100–9. doi: 10.1016/j.semradonc.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee IH, Hayman JA, Landrum MB, et al. Treatment recommendations for locally advanced, non-small-cell lung cancer: the influence of physician and patient factors. Int J Radiat Oncol Biol Phys. 2009;74:1376–84. doi: 10.1016/j.ijrobp.2008.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishijima M, Nakayama H, Itonaga T, et al. Patients with severe emphysema have a low risk of radiation pneumonitis following stereotactic body radiotherapy. Br J Radiol. 2015;88:20140596. doi: 10.1259/bjr.20140596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith BM, Schwartzman K, Kovacina B, et al. Lung cancer histologies associated with emphysema on computed tomography. Lung Cancer. 2012;76:61–6. doi: 10.1016/j.lungcan.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Wilson DO, Weissfeld JL, Balkan A, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med. 2008;178:738–44. doi: 10.1164/rccm.200803-435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fishman A, Fessler H, Martinez F, et al. on behalf of the National Emphysema Treatment Trial Research Group. Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med. 2001;345:1075–83. doi: 10.1056/NEJMoa11798. [DOI] [PubMed] [Google Scholar]
- 14.Werner-Wasik M, Paulus R, Curran WJ, Jr, Byhardt R. Acute esophagitis and late lung toxicity in concurrent chemoradiotherapy trials in patients with locally advanced non-small-cell lung cancer: analysis of the radiation therapy oncology group (rtog) database. Clin Lung Cancer. 2011;12:245–51. doi: 10.1016/j.cllc.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 15.Werner-Wasik M, Yu X, Marks LB, Schultheiss TE. Normaltissue toxicities of thoracic radiation therapy: esophagus, lung, and spinal cord as organs at risk. Hematol Oncol Clin North Am. 2004;18:131–60. x–xi. doi: 10.1016/S0889-8588(03)00150-3. [DOI] [PubMed] [Google Scholar]
- 16.Palma DA, Senan S, Tsujino K, et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013;85:444–50. doi: 10.1016/j.ijrobp.2012.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta V. Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: pulmonary function, prediction, and prevention. Int J Radiat Oncol Biol Phys. 2005;63:5–24. doi: 10.1016/j.ijrobp.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 18.Graham MV, Purdy JA, Emami B, et al. Clinical dose–volume histogram analysis for pneumonitis after 3D treatment for non–small cell lung cancer (nsclc) Int J Radiat Oncol Biol Phys. 1999;45:323–9. doi: 10.1016/S0360-3016(99)00183-2. [DOI] [PubMed] [Google Scholar]
- 19.Rubin P, Johnston CJ, Williams JP, McDonald S, Finkelstein JN. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys. 1995;33:99–109. doi: 10.1016/0360-3016(95)00095-G. [DOI] [PubMed] [Google Scholar]
- 20.Clark H, Palaniyar N, Hawgood S, Reid KB. A recombinant fragment of human surfactant protein D reduces alveolar macrophage apoptosis and pro-inflammatory cytokines in mice developing pulmonary emphysema. Ann N Y Acad Sci. 2003;1010:113–16. doi: 10.1196/annals.1299.019. [DOI] [PubMed] [Google Scholar]