Abstract
Background
Colorectal Cancer Canada, in partnership with a Scientific Advisory Committee, is developing a Canadian Patient Group Pathway to Accessing Cancer Clinical Trials (“Pathway”). A central element of the Pathway is presented here—namely, a set of recommendations and tools aimed at each stakeholder group.
Methods
A summary of the peer-reviewed and grey literature informed discussions at a meeting, held in June 2017, in which a cross-section of stakeholders reached consensus on the potential roles of patient groups in the cancer clinical trials process, barriers to accessing cancer clinical trials, best practice models for patient-group integration, and a process for developing the Pathway. Canadian recommendations and tools were subsequently developed by a small working group and reviewed by the Scientific Advisory Committee.
Results
The major output of the consensus conference was agreement that the Clinical Trials Transformation Initiative (ctti) model, successfully applied in the United States, could be adapted to create a Canadian Pathway. Two main differences between the Canadian and American cancer clinical research environments were highlighted: the effects of global decision-making and systems of regulatory and funding approvals. The working group modified the ctti model to incorporate those aspects and to reflect Canadian stakeholder organizations and how they currently interact with patient groups.
Conclusions
Developing and implementing a Canadian Pathway that incorporates the concepts of multi-stakeholder collaboration and the inclusion of patient groups as equal partners is expected to generate significant benefits for all stakeholders. The next steps to bring forward a proposed Pathway will involve engaging the broader cancer research community. Clinical trial sponsors will be encouraged to adopt a Charter recognizing the importance of including patient groups, and to support the training of patient groups through an independent body to ensure quality research partners. Integration of patient groups into the process of developing “real world” evidence will be advanced by a further consensus meeting being organized by Colorectal Cancer Canada for 6–7 November 2018.
Keywords: Cancer patient groups, cancer clinical trials, clinical research, patient engagement, advocacy, recruitment, Clinical Trials Transformation Initiative
INTRODUCTION
Colorectal Cancer Canada believes that access to clinical trials of new drugs, medical technologies, and other cancer treatments should be a standard of care for all Canadian patients, regardless of age, residence, or income. (In this paper, the term “patients” refers to people who have been diagnosed with cancer and their caregivers.)
Need for Improved Access to Cancer Clinical Trials
The positive effects of cancer clinical trials are widely acknowledged, but participation rates by adult cancer patients in Canada remain significantly low. In 2014, fewer than 7% of adult cancer patients in Canada were enrolled in a clinical trial, compared with fewer than 5% in the United States and 12% in the United Kingdom1. Rates of patient recruitment and retention are unsatisfactory. In the United States, for example, estimates suggest that 85% of clinical trials fail to retain the patient numbers needed to continue; 80% fail to finish on time; and half the investigation sites enrol 1 or no patients2. Of the overall pharmaceutical budget for clinical trials, 40% has been reported to be spent on recruitment, with 30% of patients dropping out of a study3. Those discouraging statistics suggest that opportunities for potentially life-saving improvements in patient outcomes might be missed, leading to a devastating loss of hope for many cancer patients.
Part of the answer to improving the system of cancer clinical research and development lies in reducing barriers that dissuade or prevent patients from participating in studies. For too many patients, those deterrents are significant and have been shown to contribute to low rates of enrolment in cancer clinical trials4.
Emerging Role of Patients in Clinical Research and Development
Patient input has proved effective in identifying and resolving barriers to participation in cancer clinical trials5,6. Moreover, the patient voice is emerging in importance across the spectrum of cancer clinical research. For example, patients in many countries now advise on setting research agendas7 and on the design, planning, and implementation of trials8–12. Patient-focused strategies such as patient-reported outcomes13 have gathered momentum and contribute to “real-world evidence,” which is increasingly valued by stakeholders. Multi-stakeholder efforts, such as those of the American Society of Clinical Oncology, the Friends of Cancer Research, and the U.S. Food and Drug Administration, have successfully furthered the goal of improving clinical trials accrual rates14.
Patient groups (a term encompassing patient advocacy organizations, disease advocacy organizations, voluntary health agencies, health charities, nonprofit research foundations, and public health organizations) can play a critical role by facilitating the patient voice and by organizing the involvement of patients in a systematic way, ensuring consistency and quality throughout the process.
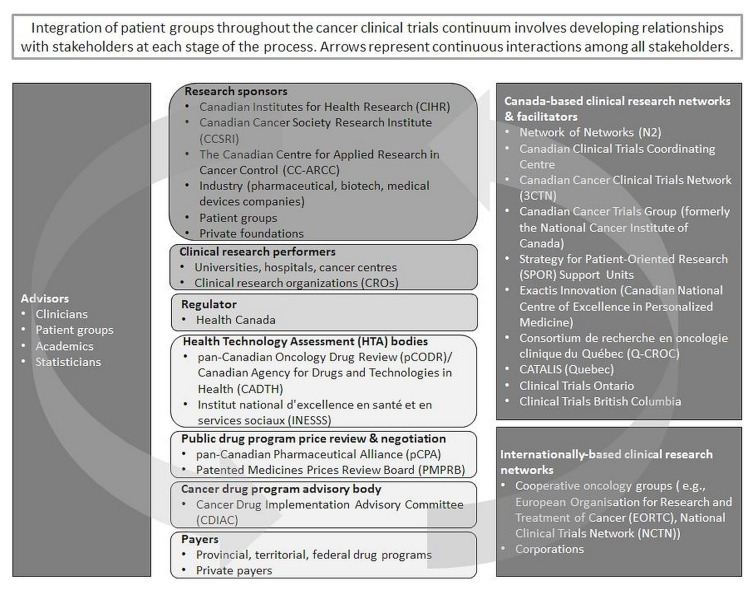
Figure 1 illustrates the Canadian cancer clinical trials “ecosystem,” including examples of organizations in each stakeholder group. Integration of patient groups across the cancer clinical research and development continuum involves developing relationships with stakeholders at each stage of the process.
FIGURE 1.
Canadian cancer clinical trials “ecosystem.”
Value of a Systematic Framework
Although the inclusion of patient groups in cancer clinical research has advanced in recent years, particularly in the area of health technology assessment, in which formal mechanisms are now well integrated, policies and practices in other aspects of the Canadian cancer clinical trials process remain inconsistent. An evidence-based framework could help to integrate the patient voice in a coherent and meaningful manner15.
Of the models that currently exist internationally, the Clinical Trials Transformation Initiative [ctti (https://www.ctti-clinicaltrials.org/)] offers the most comprehensive guidelines for patient involvement in cancer clinical trials. The ctti is a public–private partnership cofounded in 2007 by Duke University and the U.S. Food and Drug Administration to deal with the inefficiency and high costs of clinical trials and the need to generate strong evidence that answers therapeutic questions. The ctti uses a collaborative approach to develop and drive adoption of best practices that will increase the quality and efficiency of clinical trials. Since its inception, the initiative has grown to include more than 80 member organizations representing academia, clinical investigators, government and regulatory agencies, industry, institutional review boards, patient advocacy groups, and other groups16.
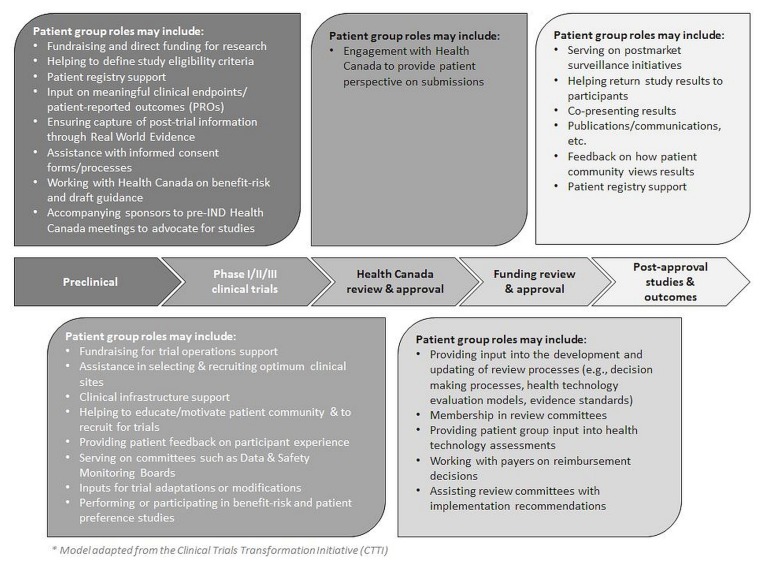
Figure 2 depicts the roles that patient groups could potentially play across the research and development continuum in Canada.
FIGURE 2.
Potential engagement of patient groups in the Canadian cancer clinical trials process.
Adoption of a Canadian Patient Group Pathway to Accessing Cancer Clinical Trials (“Pathway”) that incorporates the concepts of multi-stakeholder collaboration and the inclusion of patient groups as equal partners is expected to generate significant benefits for all stakeholders. For cancer patients, the intended outcomes are expected to include faster access to innovative treatments, a greater understanding of new cancer therapies, and improvements in the overall standard of care. Clinical trial sponsors and investigators could see improved cancer research and development strategies, shorter development timelines, lower costs, and higher approval rates for new drugs and other treatments. Society at large might eventually benefit from lower costs as treatments are better understood and targeted to patients’ needs. An early example of a positive impact in that regard comes from a recent publication by ctti citing significant cost reductions to clinical trials sponsors as a result of implementing its recommendations17.
The developers of new therapies could also gain a better understanding of unmet medical needs and advance their knowledge of real-world outcomes. Health technology assessment bodies and public and private insurers might be able to determine the value of new treatments with increased confidence. (To further advance patient involvement in developing real-world outcome measures, Colorectal Cancer Canada is convening a multi-stakeholder consensus conference for 6–7 November 2018 as a next step.) Canada could be better positioned to attract cancer research opportunities, resulting in greater funding flows and increased utilization of the country’s research infrastructure. Finally, all participants are expected to benefit from improved relationships between stakeholder groups.
The impact of the Pathway could be measured initially by increased participation rates in cancer clinical trials. Statistics presented in future issues of the Canadian Partnership Against Cancer’s Cancer System Performance Report could be reported at 5-year intervals for the next 20 years. Further metrics could be developed as implementation of the Pathway progresses.
METHODS
The development and implementation of the Pathway is an initiative of Colorectal Cancer Canada (formerly the Colorectal Cancer Association of Canada). A cross-sectoral Scientific Advisory Committee continues to provide guidance on all aspects of the Pathway development and is central to its implementation.
The components of the Pathway are
■ a set of recommendations and tools aimed at each stakeholder group;
■ a Charter, signed by clinical trial sponsors, that commits them to implementing the recommendations; and
■ a guide for operationalizing the recommendations, including training of patient groups through an independent body to ensure quality research partners.
Step 1: Literature Review
In advance of the consensus development meeting, a literature review was prepared to inform the discussions. Peer-reviewed publications and the grey literature identified by the Scientific Advisory Committee were examined and summarized. Subject areas were
■ assessments of cancer clinical trial performance in Canada and internationally,
■ barriers to patient participation in cancer clinical trials,
■ roles of patient groups in cancer clinical research and development, and
■ patient engagement models.
Step 2: Consensus Meeting
A meeting with a cross-section of stakeholders was held in June 2017 to develop a consensus about
■ the role of patient groups in cancer clinical trials,
■ barriers related to accessing cancer clinical trials,
■ best-practice models,
■ a process for developing a Pathway, and
■ identification of relevant stakeholders.
Details of the consensus meeting are available on Colorectal Cancer Canada’s Web site (https://www.colorectalcancercanada.com/).
Step 3: Canadianizing the Model
After the meeting, the selected international model was “Canadianized” to address key factors that distinguish the Canadian reality from that in the United States.
RESULTS
Meeting participants agreed that the ctti model, which has demonstrated success in the United States, could be adapted for use in Canada. Two major areas of difference between the cancer clinical research and development systems in the two jurisdictions—global decision-making processes, and systems of approval for new cancer treatments—were identified for adaptation and are described in detail in the subsections that follow. Also, key Canadian stakeholder organizations were identified, and their interactions with patient groups were characterized. Those inputs were also incorporated into the Canadianized recommendations and tools.
Global Decision-Making Processes
Meeting participants heard that research programs conducted by pharmaceutical companies are designed and implemented on a worldwide basis. Global and regional academic clinical trials groups can also develop their plans centrally.
Unlike their American counterparts, Canadian stakeholders are generally not involved in strategic decisions made at the earliest stages of the research and development process. By focusing the involvement of Canadian patient groups on the later stages of cancer clinical research, the resources of all stakeholders would be optimized.
Engagement of Canadian patient groups at the Prediscovery phase of the ctti model was therefore removed in the Pathway; patient groups first become involved at the Preclinical phase, as described in Figure 2.
Systems of Approval for New Cancer Treatments
The approval of new cancer therapies includes regulatory and funding systems, both of which differ between the United States and Canada. Health Canada is structured differently from the U.S. Food and Drug Administration with respect to engagement with patient groups. Legislation and policies have been created to facilitate the robust involvement of patient groups with the Food and Drug Administration18,19; Health Canada has only one formal opportunity for the participation of patient groups in oncologyrelated decisions20. Because the speed of marketing approvals for new cancer drugs in Canada lags behind that in the United States by approximately 6 months21,22 (a delay that is highly significant for cancer patients), the Canadian Pathway emphasizes collaborative work with Health Canada and with manufacturers of new drugs and other cancer treatments to reduce submission and review timelines.
Canada and the United States also differ in their funding mechanisms for cancer treatments, in terms of sources of financing and review mechanisms used by public sector payers. A greater proportion of the population in the United States than in Canada is covered by private health insurance (91% vs. 67%)23,24. The greater role played by public sector payers has resulted in a more restrictive environment in Canada. Compared with the United States, where, by legislation, all or substantially all new cancer drugs must be made available to patients through Medicare25, many fewer such drugs are recommended for listing and many that are listed have eligibility restrictions in Canadian public drug programs26–29. In the United States, Medicare reviewers are explicitly prohibited by law from considering evidence relating to the cost or cost-effectiveness of technologies when making coverage determinations30. In contrast, cost-effectiveness criteria are included as part of the deliberative frameworks of the pan-Canadian Oncology Drug Review31 and the Institut national d’excellence en santé et en services sociaux32 (together with evaluations of overall clinical benefit, alignment with patient values, and feasibility of adoption into the health system), and those considerations feature prominently in coverage decisions26. Finally, the funding review process for oncology medications takes much longer in the Canadian public sector: more than 1 year33 compared with just over 1 month in the United States34. Consequently, the Canadian Pathway recommendations were adapted to include a greater focus on public systems of health technology assessment and reimbursement.
RECOMMENDATIONS
The intent of the following recommendations is to enhance, rather than to replace, any existing models of integration of patient groups into the clinical research processes.
Part A: Recommendations for All Stakeholders
-
Engage the “patient voice” by establishing partnerships starting at the preclinical phase of the research and development program to improve trial design and execution.
Include the perspective of patients in the early stages of disease targeting, making full use of input from patient groups to help shape and refine the study protocol while clinical trials are still in the planning phase. Soliciting input from patient groups early in development benefits both sponsors and patients. Table I shows examples of sponsor and patient benefits.
-
From the start, clearly define the expectations, roles, and responsibilities of all partners, including the resources being committed, data being shared, and objectives of the program.
Patient groups and research sponsors often have different backgrounds and perceptions of the value that patient representatives bring to the clinical trials process or the tasks that patient groups will be expected to undertake. At the outset of the development program, it is important to clearly delineate the roles of the partnership and to clarify the goals and objectives of the collaboration. Responsibilities and expectations could be outlined in agreements reflecting the resources being committed, data being shared, or overall nature of the program (for example, early vs. late phase, trial process issues, informed consent forms, patient-reported outcomes vs. clinical endpoints).
Although input from patient groups could be taken into account when determining the objectives of a clinical program or the development of a protocol, it is important for research sponsors to balance that input with scientific understanding and patient, business, and regulatory needs.
-
Build the trust required for successful partnerships by being transparent and trustworthy, following through on commitments, and honouring confidentiality.
Building trust requires all stakeholders to be open and transparent and to honour commitments to the development program. Commitments between partners can be pre-specified and documented in an agreement, including how teams will be formed and how intellectual property and revenue-sharing will be managed. Confidentiality agreements and nondisclosure agreements can be useful tools to allow sharing of sensitive information with patient groups.
-
Solicit the expertise of multiple partners for a broader perspective to mitigate risk and enrich pipeline development.
Engaging with as many organizations as possible across the Canadian cancer clinical trials “ecosystem” (Figure 1) will encourage a broad scope of inputs into the decision-making processes and will maximize efforts to recruit and retain patients in clinical trials, ultimately resulting in more and better therapeutic options.
-
Manage real or perceived conflicts of interest by establishing policies that require full disclosure, transparency, and accountability.
Restrictions that could limit engagement with patient groups have to be understood and followed. For example:
■ Some industry associations (such as Innovative Medicines Canada35) require their members to adhere to codes of ethical practices.
■ Patient groups might adhere to a written code of conduct (such as the Code of Conduct Governing Corporate Funding from the Canadian Cancer Action Network36).
Contractual rules and parameters can increase transparency and accountability. Some common examples are
-
■ patient groups that act as service providers to the company on a contractual basis.
▪ Roles and responsibilities are clarified in the contract.
▪ If the sponsor is retaining the patient group to do certain work with a tangible end product, the patient group might be compensated at fair market value.
-
■ patient groups that receive funding from a company.
▪ The provision of unrestricted funds increases the independence of the patient group.
-
■ patient groups that act as non-compensated collaborators.
▪ Rules of engagement consider legal, regulatory, and research administration requirements applicable to the partners and could include a nondisclosure agreement.
TABLE I.
Benefits of early patient-group input
| Benefits for … | |
|---|---|
|
| |
| Sponsors | Patients |
|
|
Part B: Recommendations for Research Sponsors—Industry and Academia
-
Integrate into your ongoing research and portfolio planning an assessment of patient-group expertise, assets, and value to your program.
Research sponsors can benefit from building awareness within their organizations about the impact of early patient-group engagement on clinical trial success.
Plans can be created for integrating patient groups into local clinical drug development processes at each phase of the process. The plan can serve to
■ include and coordinate activities across all relevant departments,
■ outline how the interactions with patient groups will be managed, and
■ allocate appropriate resources to support patient-group engagement.
-
Match patient-group expertise and assets to the specific needs and phases of research and development programs.
It is important for research sponsors and investigators to recognize differences in the skills, experience, and capabilities of patient groups. Ideally, the selection criteria for patient groups would include
■ excellent relationships with patients and families;
■ experience working with patients and caregivers;
■ experience working with patient registries, trial networks, trial design, trial awareness and recruitment, and dissemination of results; and
■ broad communication platforms.
Tools 1–3 in the Tools section can be used to analyze patient-group skills and strengths. Those tools could also facilitate the assignment of tasks according to the patient group’s strengths and limitations.
-
Ensure that patient groups are essential partners, and not token voices, throughout the research and development process.
Experience has shown that the most successful partnerships with patient groups are those in which both entities are full partners at the outset, working toward the same goals from different perspectives. The patient voice, as communicated by patient groups, is key to understanding the day-to-day effects of the condition and acceptable benefit–risk trade-offs of treatment.
Patient groups can add value during all phases of the cancer clinical research and development continuum. Figure 2 lists some of the potential roles for patient groups at each phase. Engagement with patient groups is optimized when there is a discrete division of labour, in which each group contributes its unique area of expertise.
-
For consistency, establish guiding principles and clear lines of communication to facilitate a fit-for-purpose process for collaborating with patient groups.
Having standard work practices can assist the sponsor in ensuring that all elements of the collaborative partnership are met on each project and in providing a means of measuring the success of the partnership. Elements of a work practice could include a database of previous collaborations, required documents, and clear lines of communication.
Reviewing best practices for engaging with patient groups can help research sponsors to develop their own processes, such as
■ how to approach patient groups,
■ legal requirements for working with patient groups, and
■ a template for master service agreements.
Standard work practices can
■ support the integration of patient-group engagement into clinical program strategies,
■ minimize any perceived burden to incorporating patient perspectives as part of the collaboration,
■ ensure consistency across clinical teams about the approach to and evolution of the work with patient groups,
■ identify parties responsible for relations with the patient groups if multiple people are making contact with the groups,
■ drive transparent communication between the research sponsor and patient groups, and
■ define and implement contracting and communication plans.
-
Measure the impact of patient-group engagement.
Although no standard metrics exist to measure patient-group integration with industry or academic research sponsors, it is recommended that expectations be mutually established up front about how to measure the effectiveness of the partnership. Being that such standards are continually evolving, it is important that sponsors and patient groups agree on critical elements of measurement for each arrangement.
A regular assessment of satisfaction related to objectives, expectations, and success of strategies is recommended. For example, the CTTI assesses reduction in protocol amendments and recruitment times, increase in retention rates, shorter cycle times, and longer patent life during product marketing. Additional measures were related to the development and validation of endpoints and patient-reported outcomes17. In Canada, an initial measure of success could be clinical trial enrolment (as measured in the Canadian Partnership Against Cancer’s annual Cancer System Performance Report) and participant retention.
-
Establish ongoing relationships with patient groups and communicate openly with them on a regular basis.
Early involvement and regular communication by research sponsors throughout the development program would allow sponsors to benefit from mutual education and let patient groups know how their feedback has been incorporated into the program.
Such communications could cover important study events, study modifications or cancellations, redirection of research priorities, enrolment rates, presentations and publications, and study results. It is also important to maintain regular communication with patient groups even when there is no study news.
Part C: Recommendations for Patient Groups
-
Proactively identify, engage, and bring patient voices to stakeholders relevant to the group’s clinical research interests.
It is important that patient groups recognize the limits of what any group can accomplish alone. Development of cancer interventions is a team endeavour, and partnerships are founded on the trust that the patient group has established with its patient community, their families, and the clinicians who provide their care.
Education, awareness and connections between stakeholders can be strengthened by activities such as
■ involving partners in workshops and meetings to advance the science and collaboration;
■ matchmaking between various partners such as academic investigators and government programs, or industry partners and academic investigators;
■ making presentations to industry, government agencies, and academic partners;
■ serving on advisory councils, steering committees, or external oversight boards for industry and academia;
■ conducting periodic state-of-the-science meetings with Health Canada and, where appropriate, accompanying research sponsors to Health Canada meetings focused on priority areas of drug development; and
■ establishing collaborative relationships with organizations involved in health technology assessment and drug programs to promote the integration of patient groups into the cancer drug review and funding decision-making processes.
-
Promote the group’s value as an essential partner by maximizing and articulating its expertise and assets.
Patient groups are better prepared to enter into partnerships when they understand what they can offer to research sponsors and when they have information and metrics that clearly articulate their value proposition. (Tools 1–3 in the Tools section provide a template for collecting that information.) They also benefit from understanding the perspectives of potential partners, such as the economics of drug development and clinical research, and the associated regulatory and contracting processes.
Patient groups have important clinical trial assets that are sought by industry and academic partners. Depending on the patient group, these assets can include
■ a group of educated advocates;
■ a base of knowledge and understanding of the disease mechanisms and natural history;
■ housing, maintaining, and promoting a clinical trials database that would provide patients and health care professionals with knowledge of available research options in real time;
■ financial and organizational support;
■ patient preference or benefit–risk assessments;
■ a willingness and ability to assemble a group consisting of any or all of key opinion leaders, patients, and advocates familiar with the disease; and
■ translational tools to assist in trial design.
Through active, continuous engagement in the development program, patient groups could demonstrate a unique value to their academic and industry partners. Outcomes of engagement can include
■ de-risking early-stage development with funding and public–private partnerships for early clinical research;
■ reducing uncertainty in the regulatory process by working closely with the regulators throughout the entire research and development process; and
■ helping to develop efficient trials that are more effective and have a greater chance of success by contributing to better questions and study design, efficient recruitment, improved retention, fewer amendments, procedures that are better-suited to the patient, clinical endpoints that are well-grounded in the natural history of the disease, and potential benefits that are most important to the patient.
-
Deliver expertise and assets to sponsors throughout the entire research and development process.
Patient groups are positioned to deliver maximum value when they have opportunities to express the patient perspective as early as possible and throughout the research and development process—during the preclinical, clinical trials, regulatory, and postapproval phases. Figure 2 summarizes potential patient group activities at each phase.
-
Select sponsors who have a product or program with significant promise for your constituents and who are committed to engaging in a meaningful way.
Patient groups are in a stronger position to contribute when they have a “finger on the pulse” of the preclinical landscape. That focus enables proactive identification of opportunities and reinforces the view of the group as a valuable partner for sponsors.
Having a formal, prospective review process in place enables patient groups to independently evaluate and prioritize potential partners and projects. Potential partners, and the right points of contact with key decision-makers within the organization, could be identified. Advisory boards can be helpful in assisting the patient group to lay out a strategy and action plan for meaningful engagement in the clinical trials process.
-
Manage real or perceived conflicts of interest by establishing policies that require full disclosure, transparency, and accountability.
It is important that patient groups recognize and guard against the dangers of being perceived as marketing instruments or offering exclusive services to a particular organization. At the same time, it behooves patient groups to acknowledge and accept that all trial participants must meet standard eligibility requirements and that their involvement with a sponsor will not result in preferential treatment.
Patient groups should be aware of stakeholder policies about conflicts of interest and might wish to consult guidelines such as those published by the Canadian Cancer Action Network, Innovative Medicines Canada, and Imagine Canada37 to determine how best to manage such situations.
Internal and external conflicts of interest can be managed effectively by
■ having written policies about activities that might be perceived as generating a conf lict, such as accepting funds from industry sponsors and purchasing company stock;
■ fully disclosing relationships with industry sponsors in internal deliberations and external transactions; and
■ being transparent and accountable in publications, communications and reporting.
In addition, to help patient groups navigate the complex web of decisions and opportunities, it is recommended that they prospectively develop a “guiding principles” document that defines how and with whom they will collaborate. The following topics could be covered:
■ Confidentiality
■ Working with competitors
■ Data sharing
■ Expectations for communication
■ Working with regulators (for example, will the group advocate for specific treatments or approvals, or will it advocate only for general principles?)
■ Compensation policy for consulting
■ Expectations for expanded or continued access to research treatments
■ Ethical treatment of research participants
TOOLS
The tools presented here are intended as guides that stakeholders can use to evaluate the assets a patient group could bring to the research-and-development process.
Tool 1
Tool 1 (Table II) summarizes, at a high level, the potential roles that a patient group could play at the various stages of clinical research. Each role can be considered in light of the group’s ability to gather and present the perspective of patients and of the organization’s relationships with patients, families, and caregivers.
TABLE II.
Tool 1: patient-group organizational expertise and assets evaluation toola
| Item | Preclinical | Phase I/II/III clinical trials | Health Canada review and approval | Funding review and approval | Post-approval studies and outcomes |
|---|---|---|---|---|---|
| Input with respect to interest of research question to patient community | X | ||||
| Providing data on unmet need and burden of current therapies | X | X | X | X | |
| Facilitating collaboration with the Canadian Institutes of Health Research and other funding agencies | X | ||||
| Characterizing the disease and relevant mechanisms of action | X | ||||
| Helping to define a study’s eligibility criteria | X | X | X | ||
| Patient registry support | X | X | X | X | X |
| Input on meaningful clinical endpoints or patient-reported outcomes, or both | X | X | X | X | |
| Assistance with respect to the relevance and wording of an informed consent form | X | X | X | ||
| Working with Health Canada on benefit–risk and draft guidance | X | ||||
| Accompanying sponsor to pre-submission meetings with Health Canada to present the patient perspective for the study | X | ||||
| Fundraising and direct funding for research and trial operations support | X | X | |||
| Assistance in selecting and recruiting optimum clinical sites | X | ||||
| Clinical infrastructure support | X | ||||
| Helping to educate and motivate the patient community about research; providing information to community about participating in clinical trials | X | ||||
| Providing patient feedback on participant experience | X | X | |||
| Serving on Data and Safety Monitoring Board | X | ||||
| Input for any trial adaptations or modifications | X | ||||
| Accompanying sponsor to milestone meetings with Health Canada | X | ||||
| Serving on post-market surveillance initiatives | X | ||||
| Helping to return study results to participants | X | ||||
| Co-presenting scientific findings and results | X | X | |||
| Publications or communication of results | X | ||||
| Providing feedback on how the patient community views study results | X | X | |||
| Input to health technology assessment bodies on the patient experience | X | X | |||
| Working with payers (private and public) with respect to understanding a need for reimbursement | X | X |
Cells with an “X” indicate where patient groups can play a role at the given phase of research.
This tool can be used by clinical trial sponsors and patient groups to define the parameters of their relationship within a particular research program. Patient groups could also use the tool as an organizational planning aid, to help define how to most effectively use their finite resources.
Tool 2
Tool 2 (Table III) presents a more detailed guide for analyzing the internal strengths and weaknesses of a patient group as a potential partner. The tool could be adapted by sponsors of clinical trials as a screening tool to help identify patient groups of interest early in the research planning process. It can also be used as the foundation of a discussion with a specific patient group to structure a partnership agreement. Patient groups might also find this tool useful for self-evaluation.
TABLE III.
Tool 2: assessment of patient-group internal aspects: focus
| Item | Yes | No | NA | Notes |
|---|---|---|---|---|
| Vision or areas of focus: Are the patient group’s vision, mission, goals, and areas of focus clearly stated and reasonable? | ||||
| Do these statements seem to reflect sound judgment regarding the disease space and state of the science? | ||||
| Is commitment to these statements demonstrated in the patient group’s activities and performance? | ||||
|
| ||||
| Operations: Are the patient group’s operational programs well structured, performing well, and demonstrating measurable impact? | ||||
| If the patient group awards grants, are awards made via a credible application and peer-review process, and do the awards reflect the vision, mission, goals, and areas of focus? | ||||
| Does the patient group have and make good use of solid scientific or medical professional staff and advisors? | ||||
| Does the patient group have an effective fundraising and budgeting process adequate to its vision, mission, goals, and areas of focus? | ||||
| Does the patient group receive good ratings from charity monitors such as Imagine Canada? | ||||
| Does the patient group’s collaborative model include partnering options for sponsors outside of grant-based options? | ||||
|
| ||||
| Budget and fundraising: Do the patient group’s budget and fundraising programs seem adequate to its needs or show signs of being able to become so? | ||||
| Are the sources of the patient group’s fundraising transparently disclosed (donors, industry, government grants, etc.)? | ||||
| Has the patient group been able to marshal the resources required to establish important assets for development (for example, patient registry, clinical network)? | ||||
| Does the patient group devote a healthy percentage of its budget to its operational program compared with its overhead (for example, administrative and fundraising costs)? | ||||
| Does the patient group’s budget over the last 5 years demonstrate a fundraising capacity that is steady or growing and diverse in sources? | ||||
|
| ||||
| Communications: Does the patient group have the communications systems needed to facilitate development across the full continuum? | ||||
| Does the patient group have sufficient online presence, including social media? | ||||
| Does the patient group issue a variety of publications to various audiences? | ||||
| Does the patient group use these communications effectively to educate, motivate, and engage its patient community and its medical, scientific, industry, and government partners? | ||||
| Does the patient group use these communications effectively across all phases of clinical development in which it is engaged? | ||||
Tool 3
Like Tool 2, Tool 3 (Table IV) evaluates patient-group assets in a detailed way, but with a focus on external relationships.
TABLE IV.
Tool 3: assessment of patient-group external relationships
| Item | Yes | No | NA | Notes |
|---|---|---|---|---|
|
Relationships with other patient groups Does the patient group engage collaboratively with other patient groups of interest?
|
||||
|
Relationships with academia Does the patient group engage collaboratively with academic and other research institutions, centres of excellence, and so on?
|
||||
|
Relationships with industry Does the patient group accept funding from industry to conduct its business?
|
||||
|
Relationships with patients Does the patient group’s relationships with patients and their families enable the patient group to
|
||||
|
Relationships with drug plan sponsors Does the patient group engage collaboratively with private payers, governments, and government agencies that influence access to cancer therapies? |
||||
|
Relationships with governments and government agencies Does the patient group maintain dialog with public drug program officers and appropriate offices of special interest at the provincial and federal levels (for example, chief health innovation officers and translational or clinical staff)? Does the patient group participate in advisory boards to provincial or federal drug programs, or both? Does the patient group have patient representatives designated as liaisons with governments or government agencies, or both? |
||||
|
Relationships with health technology assessment review bodies Does the patient group provide patient input to health technology assessment reviews related to their specific disease area or areas or generally to calls for input (for example, to general patient input submission templates, etc.)? Does the patient group respond to calls for input on strategic or operational issues (for example, patient input submission templates)? Does the patient group attend and participate in symposia or forums organized by health technology assessment bodies? |
||||
|
Relationships with Health Canada Does the patient group engage collaboratively with the appropriate centres and offices of Health Canada (for example, Health Products and Food Branch, Therapeutic Products Directorate)? Does the patient group help to educate Health Canada personnel about the disease, its unmet medical needs, benefit–risk evaluations, and so on (for example, include Health Canada personnel in the patient group’s scientific conferences, brief Health Canada personnel at Health Canada workshops and events)? Does the patient group work with its academic and industry partners in preparing investigational new drug submissions and participating in pre-submission and other milestone meetings? |
||||
|
Relationships with elected representatives Does the patient group encourage its community members to engage their elected representatives in support of legislation beneficial to them? Does the patient group collaborate with other patient groups in organizations aimed at concerted efforts to work with government in support of beneficial budgets and policies? |
CONCLUSIONS
Developing and implementing a Pathway incorporating the concepts of multi-stakeholder collaboration and inclusion of patient groups as equal partners is expected to generate significant benefits for all stakeholders. During a consensus meeting, stakeholders from across the cancer clinical research and development continuum concluded that the ctti model, successfully deployed in the United States, could be adapted for use in Canada as the basis for a comprehensive framework for patient-group engagement. Canadianization of the ctti recommendations and tools involved adapting them to the Canadian cancer clinical research and development landscape, identifying the relevant stakeholder organizations and processes, and modifying engagement approaches to suit the Canadian context.
Recommendations are presented for each broad stakeholder group, accompanied by a set of tools that clinical research sponsors can use to assess the readiness and capacity of patient groups to engage with them.
Further steps in the development of the Pathway will be undertaken. The broader cancer research community will be invited to participate in the process. A Charter, in which clinical trials sponsors commit to involve patient groups in all stages of cancer clinical research, will be developed collaboratively. As part of an operationalization plan, clinical research sponsors will be encouraged to support training of patient groups so that they are able to participate as equal partners. The integration of patient groups into the development of “real world” evidence will be advanced through a further consensus meeting being organized by Colorectal Cancer Canada for 6–7 November 2018 in Montreal.
ACKNOWLEDGMENTS
Support for this initiative was generously provided by BioCanRx: Biotherapeutics for Cancer Treatment, Boehringer Ingelheim, Bristol–Myers Squibb, the Canadian Partnership Against Cancer, Coalition Priorité Cancer au Québec, Innovative Medicines Canada, Eli Lilly Canada, Merck Canada, Hoffman–La Roche, and Novartis. We thank all the meeting participants and members of the Scientific Advisory Committee for their active and insightful input into the development of the recommendations and for their leadership in promoting the engagement of Canadian patient groups in cancer clinical trials. Also, we are grateful to the ctti for their inspiration in serving as the basis for the Canadian Pathway.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conf licts of interest, and we declare the following interests: Colorectal Cancer Canada has received support from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim Canada, Bristol–Myers Squibb, Eli Lilly Canada, Hoffmann–La Roche, Janssen, Merck Frosst Canada and Company, Novartis Pharma Canada, Pfizer Canada, Pharmascience; GB has contracted with multiple biopharma companies in clinical research programs and has collaborated with many in matching funds for major peer-reviewed grants, including Hoffman–La Roche, Merck, Pfizer, Bristol–Myers Squibb, Eli Lilly Canada, Esperas Pharma, Chorus, AstraZeneca, Novartis, and Amgen; DPR has accepted consulting fees, speaker fees, and honoraria from multiple biopharma companies, including AbbVie, Amgen, Janssen, Eli Lilly Canada, Merck, Novartis, Novo Nordisk, Pfizer, and Hoffman–La Roche; BioCanRx has received support from Turnstone Biologics, Merck, EMD Serono, Roche Pharma, General Electric, PeproTech, Immudex, Immunovaccine, Pall, Zymeworks, Beckman Coulter, AstraZeneca, Roche Diagnostics, GlaxoSmithKline, Affymetrix, Blueline Bioscience, Caprion Biosciences, PerkinElmer, BioLegend, PeproTech, and NanoString Technologies.
REFERENCES
- 1.Canadian Partnership Against Cancer (cpac) The 2017 Cancer System Performance Report. Toronto, ON: CPAC; 2017. [Google Scholar]
- 2.Hargreaves B. Clinical trials and their patients: the rising costs and how to stem the loss. Pharmafocus. 2016;November:18–20. [Available online at: http://edition.pagesuite-professional.co.uk/html5/reader/production/default.aspx?pubname=&edid=c64556ab-bf8d-4389-82d1-3006925cdb70; cited 8 February 2018] [Google Scholar]
- 3.Nuttall A. Considerations for improving patient recruitment into clinical trials [online white paper] Clinical Leader Newsletter. 2012. [Available at: http://vertassets.blob.core.windows.net/download/64c39d7e/64c39d7e-c643-457b-aec2-9ff7b65b3ad2/rdprecruitmentwhitepaper.pdf; cited 9 November 2017.
- 4.Unger JM, Cook E, Tai E, Bleyer A. The role of clinical trial participation in cancer research: barriers, evidence, and strategies. Am Soc Clin Oncol Educ Book. 2016;35:185–98. doi: 10.1200/EDBK_156686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swartz LJ, Callahan KA, Butz AM, et al. Methods and issues in conducting a community-based environmental randomized trial. Environ Res. 2004;95:156–65. doi: 10.1016/j.envres.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Shah SG, Robinson I. Benefits of and barriers to involving users in medical device technology development and evaluation. Int J Technol Assess Health Care. 2007;23:131–7. doi: 10.1017/S0266462307051677. [DOI] [PubMed] [Google Scholar]
- 7.Oliver S, Clarke-Jones L, Rees R, et al. Involving consumers in research and development agenda setting for the nhs: developing an evidence-based approach. Health Technol Assess. 2004;8:1–148. doi: 10.3310/hta8150. [DOI] [PubMed] [Google Scholar]
- 8.Brett J, Staniszewska S, Mockford C, et al. Mapping the impact of patient and public involvement on health and social care research: a systematic review. Health Expect. 2014;17:637–50. doi: 10.1111/j.1369-7625.2012.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.United Kingdom, National Health Service (nhs), National Institute for Health Research. The James Lind Alliance [Web page] London, UK: NHS; n.d.. [Available at: https://www.nihr.ac.uk/research-and-impact/research/the-james-lindalliance; cited 9 November 2017. [Google Scholar]
- 10.Crowe S, Fenton M, Hall M, Cowan K, Chalmers I. Patients’, clinicians’ and the research communities’ priorities for treatment research: there is an important mismatch. Res Involv Engagem. 2015;1:2. doi: 10.1186/s40900-015-0003-x. [Erratum in: Res Involv Engagem 2015;1:14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esmail L, Moore E, Rein A. Evaluating patient and stakeholder engagement in research: moving from theory to practice. J Comp Eff Res. 2015;4:133–45. doi: 10.2217/cer.14.79. [DOI] [PubMed] [Google Scholar]
- 12.Domecq JP, Prutsky G, Elraiyah T, et al. Patient engagement in research: a systematic review. BMC Health Serv Res. 2014;14:89. doi: 10.1186/1472-6963-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Medicines Agency (ema), Committee for Medicinal Products for Human Use. Guideline on the Evaluation of Anticancer Medicinal Products in Man. London, UK: EMA; 2016. Appendix 2: The Use of Patient-Reported Outcome (pro) Measures in Oncology Studies. [Google Scholar]
- 14.Kim ES, Bruinooge SS, Roberts S, et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research Joint Research Statement. J Clin Oncol. 2017;35:3737–44. doi: 10.1200/JCO.2017.73.7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoos A, Anderson J, Boutin M, et al. Partnering with patients in the development and lifecycle of medicines: a call for action. Ther Innov Regul Sci. 2015;49:929–39. doi: 10.1177/2168479015580384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical Trials Transformation Initiative (ctti) 2017 Annual Report. Durham, NC: CTTI; 2017. [Available online at: https://www.ctti-clinicaltrials.org/annual-reports/2017-annual-report; cited 26 July 2018] [Google Scholar]
- 17.Levitan B, Getz K, Eisenstein EL, et al. Assessing the financial value of patient engagement. A quantitative approach from ctti’s Patient Groups and Clinical Trials Project. Ther Innov Regul Sci. 2018;52:220–9. doi: 10.1177/2168479017716715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.United States, Department of Health and Human Services, Food and Drug Administration (fda) Enhancing benefit–risk assessment in regulatory decision-making [Web page] Silver Spring, MD: FDA; 2017. [Available at: https://www.fda.gov/ForIndustry/UserFees/PrescriptionDrugUserFee/ucm326192.htm; cited 8 March 2018] [Google Scholar]
- 19.United States, Department of Health and Human Services, Food and Drug Administration (fda) FDA and European Medicines Agency Patient Engagement Cluster [Web page] Silver Spring, MD: fda; 2017. [Available at: https://www.fda.gov/ForPatients/PatientEngagement/ucm507907.htm; cited 8 March 2018] [Google Scholar]
- 20.Health Canada. Scientific advisory committee on oncology therapies – summary of expertise, experience and affiliations and interest [Web page] Ottawa, ON: Health Canada; 2010. [Available at: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/scientific-expert-advisory-committees/oncology-therapies/summary-expertise-experience-affiliations-interest.html; cited 11 February 2018] [Google Scholar]
- 21.Samuel N, Verma S. Cross-comparison of cancer drug approvals among international regulatory bodies. Ann Oncol. 2014;25:v1–41. doi: 10.1093/annonc/mdu438.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rawson NSB. Access to New Oncology Drugs in Canada Compared with the United States and Europe. Vancouver, BC: Fraser Institute; 2012. [Available online at: https://www.fraserinstitute.org/sites/default/files/access-to-new-oncology-drugs-in-canadarev.pdf; cited 9 March 2018] [Google Scholar]
- 23.Barnett JC, Berchick ER. Current Population Reports, P60–260. Health Insurance Coverage in the United States: 2016. Washington, DC: U.S. Government Printing Office; 2017. [Available online at: https://www.census.gov/content/dam/Census/library/publications/2017/demo/p60-260.pdf; cited 12 March 2018] [Google Scholar]
- 24.Canadian Life and Health Insurance Association (clhia) Canadian Life and Health Insurance Facts, 2016 Edition. Toronto, ON: CLHIA; 2016. [Google Scholar]
- 25.National Council on Aging (ncoa) Medicare Part D Drug Plans: What They Must, May, and Cannot Cover. Arlington, VA: NCOA; 2017. [Available online at: https://www.ncoa.org/wp-content/uploads/part-d-drug-coverage-rules.pdf; cited 9 March 2018] [Google Scholar]
- 26.Rawson N. Has pCODR Improved Access to Oncology Drugs? Timeliness and Provincial Acceptance of pan-Canadian Oncology Drug Review Recommendations. Vancouver, BC: Fraser Institute; 2014. [Available online at: https://www.fraserinstitute.org/sites/default/files/has-pCODR-improved-access-to-oncology-drugs-rev.pdf; cited 9 March 2018] [Google Scholar]
- 27.QuintilesIMS Institute. Global Oncology Trends 2017. Parsippany, NJ: QuintilesIMS Institute; 2017. [Available online at: https://morningconsult.com/wp-content/uploads/2017/06/QuintilesIMS-Institute-Oncology-Report.pdf; cited 9 March 2018] [Google Scholar]
- 28.IMS Institute for Healthcare Informatics. Global Oncology Trend Report. A Review of 2015 and Outlook to 2020. Parsippany, NJ: IMS Institute for Healthcare Informatics; 2016. [Available online at: https://morningconsult.com/wp-content/uploads/2016/06/IMS-Institute-Global-Oncology-Report-05.31.16.pdf; cited 8 March 2018] [Google Scholar]
- 29.Millson B, Thiele S, Zhang Y, Dobson-Belaire W, Skinner B. Access to New Medicines in Public Drug Plans: Canada and Comparable Countries. 2016 Annual Report. Ottawa, ON: Innovative Medicines Canada; 2016. [Available online at: http://innovativemedicines.ca/wp-content/uploads/2016/05/20160524_Access_to_Medicines_Report_EN_Web.pdf; cited 9 March 2018] [Google Scholar]
- 30.Sullivan SD, Watkins J, Sweet B, Ramsey SD. Health technology assessment in health-care decisions in the United States. Value Health. 2009;12(suppl 2):S39–44. doi: 10.1111/j.1524-4733.2009.00557.x. [DOI] [PubMed] [Google Scholar]
- 31.Canadian Agency for Drugs and Technologies in Health (cadth) pCODR Expert Review Committee Deliberative Framework. Ottawa, ON: CADTH; 2016. [Available online at: https://www.cadth.ca/sites/default/files/pcodr/The%20pCODR%20Expert%20Review%20Committee%20%28pERC%29/pcodr_perc_deliberative_frame.pdf; cited 13 March 2018] [Google Scholar]
- 32.Institut national d’excellence en santé et en services sociaux (inesss) Médicaments d’exception [Web page, French] Québec, QC: Gouvernement du Québec; 2018. [Available at: https://www.inesss.qc.ca/index.php?id=41; cited 13 March 2018] [Google Scholar]
- 33.Srikanthan A, Mai H, Penner N, et al. Impact of the pan-Canadian Oncology Drug Review on provincial concordance with respect to cancer drug funding decisions and time to funding. Curr Oncol. 2017;24:295–301. doi: 10.3747/co.24.3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mycka J, Dellamano R, Lobb W, et al. Regulatory approval to patient access: an evaluation of EU5 and US national timing differences—an update. Value Health. 2014;17:A427–8. doi: 10.1016/j.jval.2014.08.1075. [DOI] [PubMed] [Google Scholar]
- 35.Innovative Medicines Canada. Code of Ethical Practices. Ottawa, ON: Innovative Medicines Canada; 2016. [Available online at: http://innovativemedicines.ca/wp-content/uploads/2015/06/IMC_Code_EN.pdf; cited 13 March 2018] [Google Scholar]
- 36.Canadian Cancer Action Network (ccan) CCAN Code of Conduct Governing Corporate Funding. Toronto, ON: CCAN; 2012. [Available online at: http://www.canceradvocacy.ca/images/CCAN-code-of-conduct.pdf; cited 19 October 2018] [Google Scholar]
- 37.Imagine Canada. Standards Program [Web page] Ottawa, ON: Imagine Canada; n.d.. [Available at: http://www.imaginecanada.ca/our-programs/standards-program; cited 13 March 2018] [Google Scholar]