Abstract
Background
Nivolumab was the first immuno-oncology agent available for the treatment of lung cancer in Canada. In the present study, we evaluated the real-world benefit of nivolumab in Canadian patients with lung cancer.
Methods
Patients included in the cohort were identified from a registry of patients treated through expanded access to nivolumab before and after Health Canada approval. Demographics were collected from the application forms. Outcome data for the duration of treatment and survival were collected retrospectively.
Results
In contrast to the randomized clinical trial populations, our study cohort included patients who were older (median age: 66 years; range: 36–92 years) and who had an Eastern Cooperative Oncology Group performance status of 2 (8.9%). Despite the poorer-prognosis cohort, median overall survival was 12.0 months, which is comparable to the survival demonstrated in the randomized phase iii trials of nivolumab in lung cancer. Median time to treatment discontinuation was 3.45 months and was similar for all patient subgroups, including poorer-prognosis groups such as those with a performance status of 2, those 75 years of age and older, and those with brain metastases.
Conclusions
Nivolumab given in a real-world clinical setting was associated with results similar to those reported in the phase iii clinical trial setting.
Keywords: Nivolumab, non-small-cell lung cancer, real-world data, duration of treatment, overall survival
INTRODUCTION
Worldwide, lung cancer is the most common cause of death from cancer among both men and women1. Each year, more people die of lung cancer than of colon, breast, and prostate cancers combined2,3. In Canada, as in the rest of the world, the mortality rate for lung cancer patients is the highest of all cancers; more than 20,000 people die each year2. Despite offering only modest improvements in survival since the end of the 1990s, chemotherapy has remained the initial standard-of-care therapy in patients with advanced non-small-cell lung cancer (nsclc) whose tumours do not harbour mutations sensitive to targeted therapies4. Recently, significant progress has been seen with the introduction of immuno-oncology agents2. Two anti–PD-1 agents have received regulatory approval in Canada for previously treated advanced or metastatic nsclc: nivolumab, approved for all patients regardless of PD-L1 status, and pembrolizumab, approved for patients whose tumours express PD-L15–7.
Nivolumab is a monoclonal antibody that blocks the interaction of the PD-1 protein with its ligands PD-L1 and PD-L28,9. The binding of PD-L1 and PD-L2 to the PD-1 receptor on T cells inhibits the immune response by limiting T cell proliferation and cytokine production10. This inhibition of PD-1 activation might thus prevent PD-1–mediated inhibition of the antitumour immune response10. Compared with docetaxel, nivolumab was shown, in two pivotal phase iii clinical trials, to be associated with significantly longer overall survival (os) and a favourable safety profile in patients with metastatic squamous and nonsquamous nsclc who experienced disease progression during or after platinum-based chemotherapy. Median os was 9.2 months compared with 6.0 months in squamous nsclc (CheckMate 017) and 12.2 months compared with 9.4 months in nonsquamous nsclc (CheckMate 057)11,12. Data about the clinical effectiveness and safety of nivolumab in patients with a poor performance status (ps) and those 70 years of age and older are more limited, because such patients were underrepresented in the foregoing randomized clinical trials. Two studies that included those patient groups, CheckMate 153 and CheckMate 169, provided evidence that nivolumab tolerability is comparable in all patient groups and that effectiveness is comparable in all age groups, but that patients with an Eastern Cooperative Oncology Group (ecog) ps of 2 experience shorter os13,14.
Here, we report the Canadian real-world experience of the early use of nivolumab, which was the first immunooncology agent available to treat nsclc patients in Canada outside of clinical trials. Nivolumab was provided by Bristol–Myers Squibb in a compassionate use program from May 2015 until Health Canada approval in February 2016. Post approval, nivolumab was available through a patient access program. We describe treatment outcomes, including time to treatment discontinuation (ttd) and os, for patients who received nivolumab through those two programs at 53 institutions across Canada.
METHODS
Patients and Treatment
Patients eligible to receive compassionate nivolumab between May 2015 and February 2016 were those with advanced or metastatic nsclc who had progressed during or after at least 1 line of systemic therapy, including 1 line of platinum-containing chemotherapy. They were required to have an ecog performance status of 0–2 and adequate laboratory values. They were excluded if they had a history of autoimmune disease, if they required steroids at a dose equivalent to more than 10 mg prednisone daily, if they had carcinomatous meningitis or known hiv infection. They were also excluded if they had interstitial lung disease that was symptomatic or could interfere with the detection or management of suspected drug-related pulmonary toxicity, or if they did not have a minimum life expectancy of 6 weeks. Patients with brain metastases were eligible if treated and stable for at least 2 weeks. Patients enrolled in the patient access program after marketing authorization (February 2016) were eligible per the Health Canada indication for metastatic nsclc5. Nivolumab was administered at the approved dose of 3 mg/kg every 2 weeks.
Data Management
Baseline characteristics were collected from drug request forms completed by the treating physician. Treatment outcome data (start and end date of treatment and date of death, if applicable) were collected retrospectively by chart review led by participating physicians. Data were anonymized for all statistical analyses. The research was reviewed and approved by the research ethics boards at the institutions where the data were collected, and subjects gave informed consent to the work, as applicable. No safety data were collected as part of this study.
Statistics
Survival was calculated as the time from initiation of nivolumab until death from any cause or last follow-up per patient chart review. The ttd was calculated as the time from initiation of nivolumab to the last dose administered. Survival data were censored when patients had not experienced an event at the predefined cut-off date of 15 May 2017 or when the date of death was unknown (patient lost to follow-up). The os and ttd were plotted in Kaplan–Meier curves and compared between subgroups by log-rank test15. Hazard ratios (hrs) and corresponding confidence intervals (cis) were estimated in multivariate analyses using Cox proportional hazards models16. A Grambsch–Therneau correlation test was used to assess the validity of the proportional hazards assumption17. The statistical analysis was generated using the R statistical software package (The R Foundation, Vienna, Austria).
RESULTS
Patients and Treatment
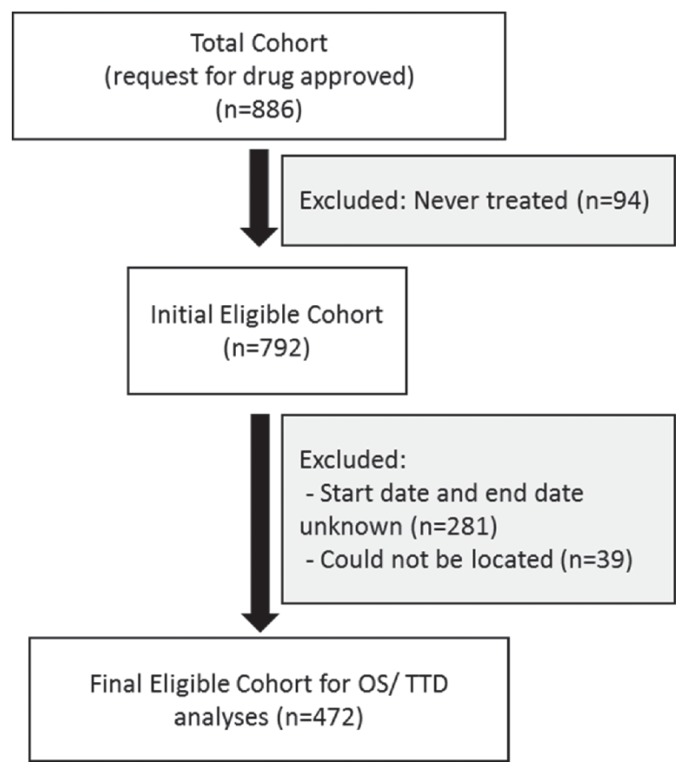
From a total cohort of 886 patients, 94 patients were excluded from all analyses because they did not start nivolumab therapy. A further 320 patients were excluded because treatment start and end dates were undetermined (n = 281) or patient charts could not be located with the patient identifier used (n = 39, Figure 1). All analyses were performed based on the final eligible cohort of 472 patients, which represents patients from most Canadian provinces, including Quebec (n = 176), Ontario (n = 143), British Columbia (n = 52), Alberta (n = 31), Saskatchewan (n = 34), New Brunswick (n = 24), Manitoba (n = 9), Prince Edward Island (n = 2), and Nova Scotia (n = 1). Most patients (94%) received compassionate nivolumab; the remaining 6% received nivolumab through the patient access program after marketing authorization. Median follow-up for os was 9.3 months (95% ci: 7.52 months to 11.0 months; range: 0.03–24.5 months).
FIGURE 1.
Flow diagram of the study population. OS = overall survival; TTD = time to treatment discontinuation.
Complete descriptive statistics for patient and disease characteristics were obtained for the initial eligible cohort who received nivolumab (n = 792) and for the final eligible cohort. All baseline characteristics were balanced between the cohorts (Table I). In the final eligible cohort, patients had a median age of 66 years, with 42% of the patients being between 65 and 75 years of age, and 13% being 75 years or age and older. Most patients were current or former smokers; 9% had an ecog ps of 2; 13% had central nervous system (cns) metastases; and 27% and 73% had squamous and nonsquamous nsclc respectively. EGFR mutations were identified in 5% of the cohort; a confirmed ALK translocation was present in fewer than 1%. Because the provision of smoking status or tumour mutation status was not mandatory to receive compassionate nivolumab, a high proportion of the patients had unknown values in those fields. Patients with unknown values were excluded from the relevant subgroup analyses. In terms of prior therapy, most patients had received 1 or 2 prior lines of therapy; 26% of patients had received 3 or more lines of therapy (Table I). No information about PD-L1 status was available for this patient cohort, because a positive PD-L1 test was not required to access nivolumab.
TABLE I.
Population characteristics
| Characteristic | Present study cohort | CheckMate studies (nivolumab arm) | ||
|---|---|---|---|---|
|
|
|
|||
| Eligiblea | Initial | 01712 | 05711 | |
| Patients (n) | 472 | 792 | 135 | 292 |
|
| ||||
| Age (years) | ||||
| Median | 66.0 | 66.0 | 62 | 61 |
| Range | 36–92 | 33–92 | 39–85 | 37–84 |
|
| ||||
| Age category [n (%)] | ||||
| <65 Years | 213 (45.1) | 363 (45.8) | 79 (59) | 184 (63) |
| 65–75 Years | 199 (42.2) | 336 (42.4) | 45 (33) | 88 (30) |
| >75 Years | 60 (12.7) | 93 (11.7) | 11 (8) | 20 (7) |
|
| ||||
| Sex [n (%)] | ||||
| Men | 203 (43.0) | 354 (44.7) | 111 (82) | 151 (52) |
| Women | 214 (45.3) | 350 (44.2) | 24 (18) | 141 (48) |
| Not reported | 55 (11.7) | 88 (11.1) | ||
|
| ||||
| Histology [n (%)] | ||||
| Non-squamous | 345 (73.1) | 576 (72.7) | (0) | (100) |
| Squamous | 124 (26.3) | 206 (26.0) | (100) | (0) |
| Others | 3 (0.6) | 8 (8) | ||
| Unknown | 2 (0.3) | |||
|
| ||||
| Known driver mutations [n (%)]b | ||||
| EGFR-positive | 25 (6.9) | 45 (7.9) | 44 (15) | |
| EGFR-negative | 229 (63.6) | 355 (62.3) | 168 (58) | |
| EGFR unknown | 106 (29.4) | 170 (29.8) | 80 (27) | |
| ALK-positive | 4 (1.1) | 5 (0.8) | 13 (4) | |
| ALK-negative | 231 (64.3) | 374 (63.9) | 113 (39) | |
| ALK unknown | 124 (34.5) | 206 (35.2) | 166 (57) | |
|
| ||||
| CNS metastases [n (%)] | ||||
| Present | 62 (13.1) | 117 (14.8) | 9 (7) | 34 (12) |
| Absent | 384 (81.4) | 624 (78.8) | 126 (93) | 258 (88) |
| Not reported | 26 (5.5) | 51 (6.4) | ||
|
| ||||
| ECOG PS [n (%)] | ||||
| 0 or 1 | 404 (85.6) | 661 (83.5) | 133 | 292 (100) |
| ≥2 | 42 (8.9) | 80 (10.1) | 0 | |
| Not reported | 26 (5.5) | 51 (6.4) | 2 (1) | |
|
| ||||
| Smoking status [n (%)] | ||||
| Never | 32 (6.8) | 55 (7.0) | 10 (7) | 58 (20) |
| Former | 213 (45.1) | 328 (41.4) | 121 (90) | 231 (79) |
| Current | 41 (8.7) | 53 (6.7) | ||
| Unknown | 186 (39.4) | 356 (45) | 4 (3) | 3 (1) |
| Line of therapy [n (%)] | ||||
| 2 | 209 (44.3) | 332 (42) | 135 (100) | 256 (88) |
| 3 | 138 (29.2) | 232 (29.3) | 35 (12) | |
| 4 | 77 (16.3) | 150 (19.0) | ||
| ≥5 | 48 (10.2) | 78 (9.9) | ||
|
| ||||
| Response to last therapy (before nivolumab) [n (%)] | ||||
| Progressive disease | 199 (42.2) | 334 (42.2) | 44 (33) | 111 (38) |
| Stable disease | 89 (18.9) | 136 (17.2) | 33 (24) | 103 (35) |
| Response | 101 (21.4) | 153 (19.3) | 48 (36) | 73 (25) |
| Unknown | 83 (17.6) | 169 (21.3) | 10 (7) | 5 (2) |
The eligible cohort of patients for the purposes of the present analysis.
Excluding squamous cell carcinoma (not tested).
CNS = central nervous system; ECOG PS = Eastern Cooperative Oncology Group performance status.
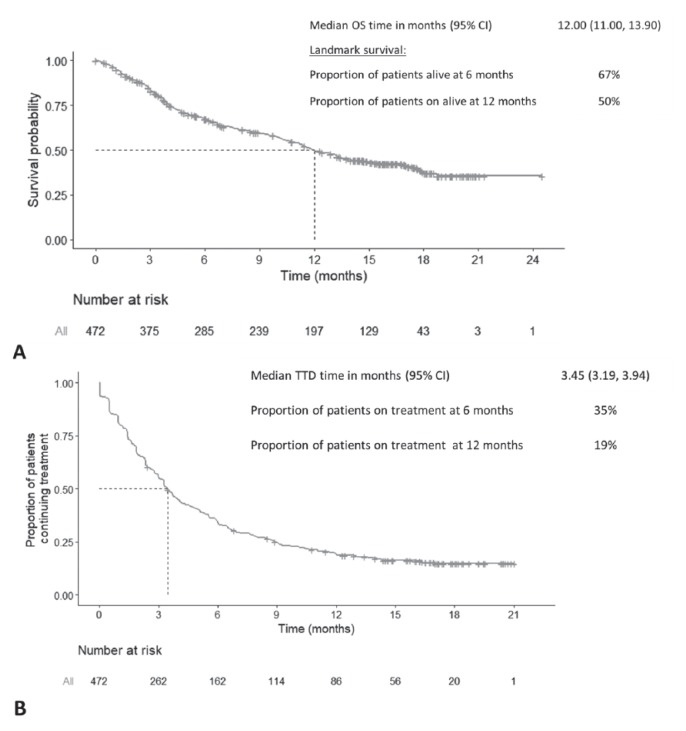
OS
The median os was 12.0 months [95% ci: 11.0 months to 13.9 months; Figure 2(A)]. The os rate at 6 and 12 months was 67% and 50% respectively. At the cut-off date of 15 May 2017, 179 patients (38%) were still alive.
FIGURE 2.
(A) Overall survival (OS) and (B) time to treatment discontinuation (TTD) in the overall patient population. The data are based on the cut-off date of 15 May 2017. Crosses indicate censored observations. Dotted lines indicate the median OS and TTD. CI = confidence interval.
TTD
The median ttd was 3.5 months [95% ci: 3.2 months to 4.0 months; Figure 2(B)], with 35% of patients remaining on treatment at 6 months, and 20%, at 12 months. At the time of data cut-off, 76 patients (16%) were continuing treatment.
Subgroup Analyses
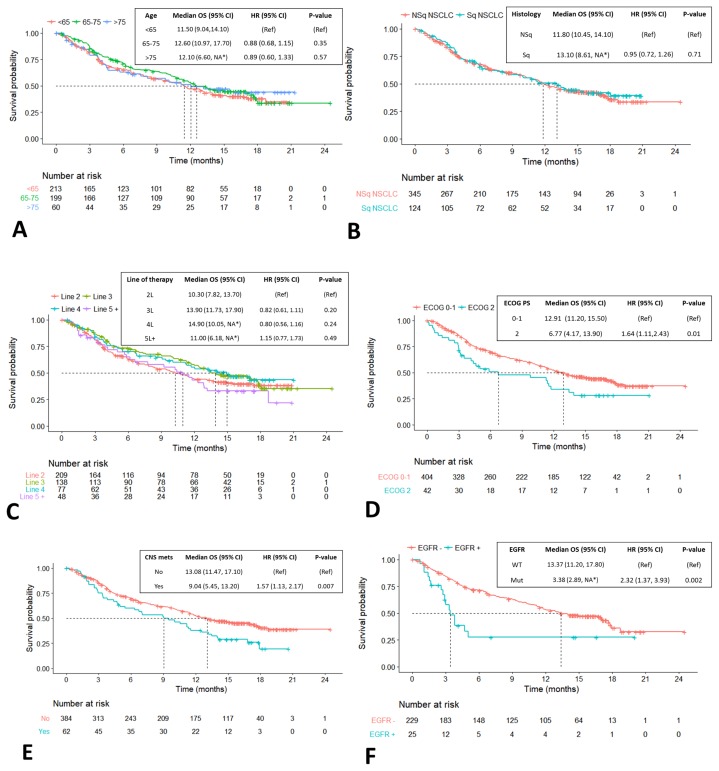
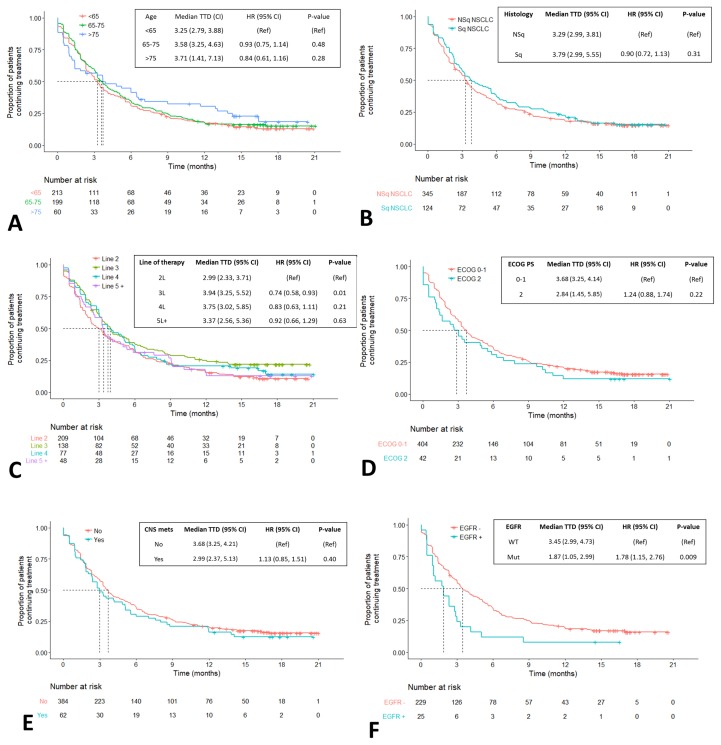
No statistically significant differences in os or ttd were observed in subgroups defined by age, smoking status, tumour histology, or line of therapy (Figures 3 and 4). Compared with patients having an ecog ps of 0–1, those with an ecog ps of 2 experienced significantly shorter median os [6.8 months (95% ci: 4.2 months to 13.9 months) vs. 12.9 months (95% ci: 11.2 months to 15.5 months)], with a hr of 1.64 (95% ci: 1.11 to 2.43), p = 0.01 [Figure 3(D)]; however, ttd was not significantly different between the subgroups defined by ps [Figure 4(D)]. Compared with patients having no cns metastases, those with cns metastases experienced significantly shorter os, but not ttd [9.0 months (95% ci: 5.5 months to 13.3 months) vs. 13.1 months (95% ci: 11.5 months to 17.1 months); hr: 1.57; 95% ci: 1.13 to 2.17; p = 0.007; Figure 3(E)]. The third subgroup in which outcomes were significantly worse was the group of 25 patients whose tumours had a confirmed EGFR mutation. In those patients, median os and median ttd were 3.4 months (95% ci: 2.9 months to unavailablea) and 1.9 months (95% ci: 1.1 months to 3.0 months) compared with 13.4 months (95% ci: 11.2 months to 17.8 months) and 3.5 months (95% ci: 3.0 months to 4.7 months) in patients with confirmed absence of an EGFR mutation. The hr for os was 2.3 (95% ci: 1.4 to 3.94), p = 0.003, and the hr for ttd was 1.8 (95% ci: 1.2 to 2.8), p = 0.009 [Figures 3(E) and 4(E)].
FIGURE 3.
Overall survival (OS) in select patient subgroups. Crosses indicate censored observations. Dotted lines indicate the median OS. *Insufficient power to detect the upper confidence limit because of sample size. CI = confidence interval; HR = hazard ratio; Ref = reference; NA = the sample size did not provide power sufficient to detect the upper confidence limit; NSq = nonsquamous; NSCLC = non-small-cell lung cancer; Sq = squamous; ECOG PS = Eastern Cooperative Oncology Group performance status; CNS mets = central nervous system metastases; WT = wild-type; Mut = mutated.
FIGURE 4.
Time to treatment discontinuation (TTD) in select patient subgroups. Crosses indicate censored observations. Dotted lines indicate the median TTD. *Insufficient power to detect the upper confidence limit because of sample size. CI = confidence interval; HR = hazard ratio; Ref = reference; NSq = nonsquamous; NSCLC = non-small-cell lung cancer; Sq = squamous; ECOG PS = Eastern Cooperative Oncology Group performance status; CNS mets = central nervous system metastases; WT = wild-type; Mut = mutated.
Descriptive statistics for the 76 patients who were continuing treatment at the time of data cut-off (median duration of treatment: 16.8 months; range: 2.4–21 months) showed that 56 patients (74%) had nonsquamous histology; 2 (3%) had a confirmed EGFR mutation; 8 (11%) had cns metastases; and 5 (7%) had an ecog ps of 2. In 25 of those patients (33%), nivolumab was given as second-line therapy; in 31 (41%), as third-line therapy; in 13 (17%), as fourth-line therapy; and in 7 (9%), as fifth-line therapy or beyond.
DISCUSSION
Real-world evidence of the effectiveness of newly approved and funded therapies is being increasingly requested by funding bodies in countries with publicly funded health care systems. Clinical trials have strict eligibility criteria for participation. After regulatory approval, broader use of new agents outside the clinical trial eligibility criteria is inevitable, especially when the new agent demonstrates less toxicity than the standard of care. Patients with a poorer performance status or other comorbidities that would have precluded clinical trial participation are treated. Feedback about the effectiveness of the agent in such real-world scenarios is not often formally collected.
The present study was conducted in an effort to assess the real-world effectiveness of nivolumab in Canada. Patients included in the study represented all provinces except for Newfoundland and Labrador and the territories. Submission of data for this project by treating physicians at the various sites was voluntary, and as a result, we do not have outcomes data for the full cohort. In an effort to assess for ascertainment bias between the initial and final analyzed cohort, we compared the required demographic data for the full cohort and for the cohort analyzed for os and ttd (Table I). The demographic characteristics of the final analyzed cohort and the initial cohort showed no clinically significant differences. We also compared the demographic characteristics of our cohort with the demographics of the participating patients in the phase iii clinical trials of nivolumab that were conducted in the platinum-refractory setting11,12. Although no formal statistical comparisons were made, our cohort was older: 55% of the patients were more than 65 years of age, compared with 38% in the clinical trials. In our cohort, 9% of the patients had an ecog ps of 2, an exclusion criterion in the clinical trials. Our cohort also included patients who had received multiple prior lines of therapy. The Check-Mate 057 trial allowed use of an egfr inhibitor in addition to a prior platinum doublet in patients with a known EGFR sensitizing mutation, and such patients constituted 10% of the combined CheckMate 017 and 057 trial participants. Excluding from our cohort the patients with EGFR and ALK mutations, all of whom received nivolumab in the third-line setting or beyond, 54% of our patients received nivolumab in the third line or beyond.
Despite an overall poorer ps, our cohort experienced a median os of 12.0 months [Figure 2(A)]. That result is comparable to the median survivals seen in the cohorts of the CheckMate 017 and 057 clinical trials, where the median os for the overall populations, including all patients regardless of PD-L1 status, was 9.2 months and 12.2 months respectively11,12. We lack a long-enough duration of follow-up to comment on the tail of the os curve in our real-world cohort, but the observed 1-year os of 50% is comparable to the 42% in CheckMate 017 and the 51% reported in CheckMate 057. Other groups have reported their real-world evidence of nivolumab benefit, showing results similar to those in our Canadian cohort18–24.
Our study did not have access to imaging to estimate progression-free survival (pfs). As an alternative, we therefore analyzed ttd as a measure of benefit duration, with the caveat that no distinction can be made between patients who discontinued treatment because of toxicity or because of disease progression. Notably, a combined analysis of several trials presented at the 2018 American Society of Clinical Oncology annual meeting suggests a high correlation (0.85) between ttd and pfs in patients (n = 2028) treated with immune checkpoint inhibitors, suggesting that ttd might be an appropriate pragmatic endpoint to use in real-world evidence studies25. The median ttd was 3.45 months [Figure 2(B)], which is similar to the reported pfs in the CheckMate 017 study (3.5 months)12 and superior to the reported pfs in the CheckMate 057 study (2.3 months)11. In each of the phase iii studies, treatment beyond progression was allowed and was reported in up to 25% of patients. That additional treatment duration could not be captured in the pfs statistic, but is presumably included in our ttd analysis.
We reviewed our cohort by demographic subtype (Figure 3). Review of os by age strata did not identify any significant differences with advancing age [Figure 3(A)]. No difference in os was seen between patients with squamous and nonsquamous nsclc [Figure 3(B)]. Regardless of treatment, patients with squamous histology generally experience poorer os, as can be seen in comparing the CheckMate 017 and 057 studies11,12.
Our review of demographic characteristics in the present study noted that patients with squamous cell cancer were more likely to receive nivolumab as second-line therapy, which contrasts with the treatment history of patients with nonsquamous tumours, who were more heavily pretreated at the time of nivolumab initiation (Table II). That difference might explain the better-than-expected performance of patients with squamous nsclc in our cohort. Review of os by line of therapy showed a similar benefit in all groups [Figure 3(C)]. The outcome results across therapy lines are descriptive only. The trend of equal or even increasing os with line of therapy might be a result of favourable characteristics and disease process in patients who attain later lines of therapy rather than differences in the effectiveness of nivolumab across those lines. Compared with patients having a performance status of 0 or 1, or no brain metastases, those with an ecog ps of 2, or with brain metastases, experienced poorer os [Figure 3(D,E)]. Those trends likely represent a prognostic feature rather than an issue with treatment toxicity, given that those groups showed no significant difference in ttd [Figure 4(D,E)].
TABLE II.
Baseline characteristics by histology
| Characteristic | Eligible NSCLC cohort (n=472) | |||
|---|---|---|---|---|
|
| ||||
| Squamous (n=124) | Non-squamous (n=345) | |||
|
|
|
|||
| (n) | (%) | (n) | (%) | |
| Sex | ||||
| Men | 73 | 58.87 | 128 | 37.10 |
| Women | 38 | 30.65 | 175 | 50.72 |
| Not reported | 13 | 10.48 | 42 | 12.17 |
|
| ||||
| Smoking status | ||||
| Former | 53 | 42.74 | 157 | 45.51 |
| Current | 10 | 8.06 | 31 | 8.99 |
| Never | 4 | 3.23 | 28 | 8.12 |
| Unknown | 57 | 45.97 | 129 | 37.39 |
|
| ||||
| Line of therapy | ||||
| 2 | 70 | 56.45 | 137 | 39.71 |
| 3 | 38 | 30.65 | 99 | 28.70 |
| 4 | 10 | 8.06 | 67 | 19.42 |
| ≥5 | 6 | 4.84 | 42 | 12.17 |
|
| ||||
| CNS metastases | ||||
| Present | 6 | 4.84 | 55 | 15.94 |
| Absent | 115 | 92.74 | 267 | 77.39 |
| Unknown | 3 | 2.42 | 23 | 6.67 |
|
| ||||
| ECOG PS | ||||
| 0 or 1 | 109 | 87.90 | 292 | 84.64 |
| ≥2 | 12 | 9.68 | 30 | 8.70 |
| Unknown | 3 | 2.42 | 23 | 6.67 |
CNS = central nervous system; ECOG PS = Eastern Cooperative Oncology Group Performance Status.
One clear and consistent trend is that, compared with patients having wild-type tumours, those with EGFR-mutated tumours experienced shorter median os and treatment duration [Figures 3(F) and 4(F)]. Lesser benefit in the patient population with driver mutations also was observed in the CheckMate 057 trial11. It is notable that patients experiencing durable benefit (beyond 12 months) can be found in all patient subgroups, including those with an ecog ps of 2 [Figure 4(D)], brain metastases [Figure 4(E)], and even in select EGFR-mutated tumours [Figure 4(F)]. In other subgroups such as age, ps, line of therapy, and presence of brain metastases, ttd appeared similar, with no statistically significant differences between the prognostic categories, suggesting tolerability of the agent. Cytotoxic chemotherapy has historically not performed as well in groups with a poorer ps25,26.
Although there is great value and a growing need to report real-world clinical outcomes, this type of retrospective chart review has inherent limitations. First, given that data submission was voluntary, outcomes data were not available for the full cohort, and the data collected were limited. Incomplete data also led to censoring much earlier in our study than in the nivolumab phase iii clinical trials. Additionally, no safety outcomes were available, thus not allowing for the real-life safety profile of nivolumab in a Canadian population to be compared with clinical trial data or other real-world evidence reports.
CONCLUSIONS
In our real-world Canadian lung cancer cohort, nivolumab demonstrated efficacy similar to that seen in the published randomized phase iii clinical trials, despite including patients who were older, who were more heavily pretreated, and who had a poorer ps. Even in poorer prognostic groups, the ttd was similar, suggesting good tolerability of nivolumab. Compared with patients not having an identified driver mutation, patients with an EGFR mutation had a shorter time on treatment and a worse os. In summary, implementation of nivolumab in a real-world setting has demonstrated benefit similar to that seen in clinical trials.
ACKNOWLEDGMENTS
We thank all physicians, nurses, and pharmacists from the participating centres who provided treatment outcome data for their nivolumab-treated patients. We also thank Amine Merghoub for data collection and management. This research did not receive specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Statistical analyses performed by Amaris Conseil Inc. were funded by Bristol–Myers Squibb.
Footnotes
Because of the sample size, power was insufficient to detect the upper confidence limit.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: CL reports personal fees from Bristol–Myers Squibb, Merck, Pfizer, and AstraZeneca, outside the submitted work; CB reports personal fees from Bristol–Myers Squibb, Merck, and AstraZeneca, outside the submitted work; MNR reports personal fees from Bristol–Myers Squibb outside the submitted work; FAS reports personal fees and a grant from Bristol–Myers Squibb; personal fees, stock ownership, and an advisory role with Eli Lilly; personal fees, stock ownership, and an advisory role with Astra-Zeneca; personal fees from Roche/Genentech; personal fees from Merck Sharp and Dohme; personal fees from Merck Serono; and grants from Pfizer, AstraZeneca/MedImmune, Roche Canada, and Merrimack, outside the submitted work; JR reports personal fees from Bristol–Myers Squibb, Roche, Merck, AstraZeneca, Boehringer Ingelheim, and Pfizer, outside the submitted work; SO reports personal fees from Bristol–Myers Squibb outside the submitted work; RAJ reports grants, personal fees, and an advisory role with Bristol-Myers Squibb; grants, personal fees, and an advisory role with Merck Sharp and Dohme; personal fees and an advisory role with Roche Canada; personal fees and an advisory role with Boehringer Ingelheim; grants and personal fees from AstraZeneca/MedImmune; personal fees from Eli Lilly; personal fees and an advisory role with Pfizer; personal fees from Amgen; personal fees and an advisory role with Novartis, outside the submitted work; FP, FR, and JV report being employees of Bristol–Myers Squibb. The remaining authors have no conflicts to disclose.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Melosky B, Chu Q, Juergens R, Leighl N, McLeod D, Hirsh V. Pointed progress in second-line advanced non-small-cell lung cancer: the rapidly evolving field of checkpoint inhibition. J Clin Oncol. 2016;34:1676–88. doi: 10.1200/JCO.2015.63.8049. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara R, Mezquita L, Besse B. Progress in the management of advanced thoracic malignancies in 2017. J Thorac Oncol. 2018;13:301–22. doi: 10.1016/j.jtho.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Canadian Agency for Drugs and Technologies in Health (cadth), pan-Canadian Oncology Drug Review. Opdivo for Non-Small Cell Lung Cancer—Details [Web page] Ottawa, ON: CADTH; 2016. [Available at: https://www.cadth.ca/opdivo-non-small-cell-lung-cancer-details; cited 1 February 2018] [Google Scholar]
- 6.Canadian Agency for Drugs and Technologies in Health (cadth), pan-Canadian Oncology Drug Review. Keytruda for Non–Small Cell Lung Cancer (Second Line or Beyond)— Details [Web page] Ottawa, ON: CADTH; 2016. [Available at: https://www.cadth.ca/keytruda-non-small-cell-lung-cancer-second-line-or-beyond-details; cited 1 February 2018] [Google Scholar]
- 7.Canadian Agency for Drugs and Technologies in Health (cadth), pan-Canadian Oncology Drug Review. Pembrolizumab for Non–Small Cell Lung Cancer (First Line)—Details [Web page] Ottawa, ON: CADTH; 2017. [Available at: https://www.cadth.ca/keytruda-advanced-non-small-cell-lungcarcinoma-first-line-details; cited 1 February 2018] [Google Scholar]
- 8.Iafolla MAJ, Juergens RA. Update on programmed death–1 and programmed death–ligand 1 inhibition in the treatment of advanced or metastatic non–small cell lung cancer. Front Oncol. 2017;7:6–67. doi: 10.3389/fonc.2017.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–15. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juergens R, Chu Q, Rothenstein J, et al. CheckMate 169: safety/efficacy of nivolumab in Canadian pretreated advanced nsclc (including elderly and ps 2) patients [abstract P2.07-029] J Thorac Oncol. 2017;12(suppl 2):S2426–7. doi: 10.1016/j.jtho.2017.11.088. [DOI] [Google Scholar]
- 14.Spigel D, Schwartzberg L, Waterhouse D, et al. Is nivolumab safe and effective in elderly and ps2 patients with non–small cell lung cancer (nsclc)? Results of CheckMate 153 [abstract P3.02c-026] J Thorac Oncol. 2017;12(suppl):S1287–8. doi: 10.1016/j.jtho.2016.11.1821. [DOI] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 16.Cox DR. Regression models in life tables. J R Stat Soc Series B Stat Methodol. 1972;34:187–220. [Google Scholar]
- 17.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer; 2000. [DOI] [Google Scholar]
- 18.Brustugun OT, Sprauten M, Helland A. Real-world data on nivolumab treatment of non–small cell lung cancer. Acta Oncol. 2017;56:438–440. doi: 10.1080/0284186X.2016.1253865. [DOI] [PubMed] [Google Scholar]
- 19.Calpe-Armero P, Ferriols-Lisart R, Ferriols-Lisart F, Perez-Pitarch A. Effectiveness of nivolumab versus docetaxel as second-line treatment in non–small cell lung cancer patients in clinical practice. Chemotherapy. 2017;62:374–80. doi: 10.1159/000475803. [DOI] [PubMed] [Google Scholar]
- 20.Dudnik E, Moskovitz M, Daher S, et al. Effectiveness and safety of nivolumab in advanced non–small cell lung cancer: the real-life data. Lung Cancer. 2017 doi: 10.1016/j.lungcan.2017.11.015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Grossi F, Crinò L, Delmonte A, et al. Real-world results in non-squamous non–small cell lung cancer patients: Italian nivolumab expanded access program [abstract MA 10.06] J Thorac Oncol. 2017;12(suppl 2):S1841. doi: 10.1016/j.jtho.2017.09.537. [DOI] [Google Scholar]
- 22.Molinier O, Audigier-Valette C, Cadranel J, et al. ifct-1502 clinivo: real-life experience with nivolumab in 600 patients (Pts) with advanced non–small cell lung cancer (nsclc) [abstract OA 17.05] J Thorac Oncol. 2017;12(suppl 2):S1793. doi: 10.1016/j.jtho.2017.09.430. [DOI] [Google Scholar]
- 23.Schouten RD, Muller M, de Gooijer CJ, Baas P, van den Heuvel M. Real life experience with nivolumab for the treatment of non–small cell lung carcinoma: data from the expanded access program and routine clinical care in a tertiary cancer centre—the Netherlands Cancer Institute. Lung Cancer. 2017 doi: 10.1016/j.lungcan.2017.11.012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Tournoy KG, Thomeer M, Germonpre P, et al. Does nivolumab for progressed metastatic lung cancer fulfill its promises? An efficacy and safety analysis in 20 general hospitals. Lung Cancer. 2018;115:49–55. doi: 10.1016/j.lungcan.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Gong Y, Kenneth KL, Oxnard GR, et al. Time to treatment discontinuation (ttd) as a pragmatic endpoint in metastatic non–small cell lung cancer (mnsclc): a pooled analysis of 8 trials [abstract 9064] J Clin Oncol. 2018. p. 36. [Available online at: https://meetinglibrary.asco.org/record/160867/abstract; cited 3 June 2018]
- 26.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]