This article reports the ROADMAP‐CAT study, which assessed cancer patient‐related risk factors of venous thromboembolism and biomarkers of hypercoagulability.
Keywords: Lung cancer, Venous thromboembolism, Thrombin generation, Cancer‐associated thrombosis, Risk assessment model
Abstract
Background.
The aim of this prospective study was to identify the most clinically relevant hypercoagulability biomarkers in lung adenocarcinoma patients for elaboration of an improved risk assessment model (RAM) for venous thromboembolism (VTE).
Subjects, Materials, and Methods.
One hundred fifty ambulatory patients with lung adenocarcinoma were prospectively enrolled. Thrombin generation, procoagulant phospholipid‐dependent clotting time (Procoag‐PPL), tissue factor activity (TFa), factor VIIa (FVIIa), factor V (FV), antithrombin, D‐Dimers, P‐selectin, and heparanase levels were assessed in platelet‐poor plasma at inclusion (baseline) and at the end of the third chemotherapy cycle (third chemotherapy). Cox regression analysis was used to identify independent VTE predictors.
Results.
At baseline, patients had significantly attenuated thrombin generation, shorter Procoag‐PPL, higher levels of TFa, D‐Dimers, and heparanase, and lower levels of FVIIa and P‐selectin, compared with controls. A significant increase in Procoag‐PPL, FV, and FVIIa and a decrease of P‐selectin levels were observed between baseline and third chemotherapy. Hospitalization within the last 3 months prior to assessment, time since cancer diagnosis less than 6 months, mean rate index (MRI) of thrombin generation, and Procoag‐PPL were independently associated with symptomatic VTE. Accordingly, a prediction model including Procoag‐PPL and MRI showed significant discriminating capacity (area under the curve: 0.84).
Conclusion.
Ambulatory patients with lung adenocarcinoma may display pronounced blood hypercoagulability due to decreased Procoag‐PPL, increased endothelial cell activation, and increased degradation of fibrin. Incorporation of Procoag‐PPL and MRI of thrombin generation may improve the accuracy of a VTE‐RAM in the above setting.
Implications for Practice.
The prospective ROADMAP‐CAT study identified two biomarkers of hypercoagulability, the procoagulant phospholipid‐dependent clotting time (Procoag‐PPL) and the mean rate index (MRI) of the propagation phase of thrombin generation assessed with the Calibrated Automated Thrombinoscope, as being clinically relevant for the classification of ambulatory patients with lung adenocarcinoma receiving a maximum of one cycle of chemotherapy into high and intermediate/low risk for venous thromboembolism. Measurement of Procoag‐PPL and MRI within 1 month after the administration of the first chemotherapy cycle provides significant accuracy of the assessment. Association of the Procoag‐PPL and MRI with the clinical risk assessment model for cancer‐associated thrombosis in ambulatory patients with solid tumors (COMPASS‐CAT RAM) further improved its accuracy.
Introduction
Symptomatic venous thromboembolism (VTE) occurs in about 10% of ambulatory patients with lung cancer and is associated with increased mortality and deterioration of the quality of life [1], [2], [3], [4]. Lung adenocarcinoma is associated with twofold higher risk of VTE compared with other types of lung cancer [5], [6]. The overall VTE risk is heterogeneous and dynamic over the disease course [7]. The risk of cancer‐associated thrombosis (CAT) significantly increases when patient‐related risk factors are present (i.e., personal or family history of VTE, cardiovascular risk factors, obesity, comorbidities, etc.) or when patients are exposed to transient risk factors (i.e., surgery, trauma, acute infection, hospitalization, etc.) [2], [5].
Routine risk assessment for VTE is recommended for cancer outpatients [8], [9], [10], [11], [12]. The most widely known risk assessment model (RAM) for VTE in outpatients with solid tumors has been presented by Khorana et al. [13]. The model was constructed by a post hoc analysis of a database from the “Awareness of Neutropenia in Chemotherapy Study Group” Registry, and patients must be assessed before initiation of chemotherapy. The predictors of the Khorana RAM include the tumor type, body mass index (BMI), and some nonspecific hematological biomarkers (prechemotherapy levels of hemoglobin, platelets, and white blood cells count). However, the accuracy of the Khorana score in real‐life patients is not optimal. For example, the score was unable to predict CAT in about 70% of 3,212 patients enrolled in the SAVE‐ONCO study, which assessed the efficacy and safety of semuloparin in primary prophylaxis of VTE [14], [15]. Furthermore, the Khorana score was unable to predict VTE risk in outpatients with lung cancer [16]. Hence, a reliable RAM for lung cancer patients remains an unmet medical need, and thromboprophylaxis is often either underused or misused [17]. We have recently presented a new improved RAM for CAT derived from a prospective study with 1,355 outpatients receiving chemotherapy for lung, breast, ovarian, or colon cancer [18]. The COMPASS‐CAT RAM is applicable after anticancer treatment initiation and includes VTE predictors related to patients' characteristics and comorbidities and variables related to cancer and its treatment. The COMPASS‐CAT RAM accurately stratifies patients in high or intermediate/low risk for VTE [18].
The incorporation of biomarkers specific for the diagnosis of hypercoagulability may improve the accuracy of the clinical RAM for CAT. The validity of this concept has been tested in an expanded prediction model elaborated for patients with solid tumors and for hematological malignancies. These studies showed that incorporation of biomarkers such as soluble P‐selectin and D‐Dimers into the Khorana model improved its accuracy [19], [20]. However, due to the heterogeneity of the cohorts with respect to tumor types, the predictive value of the most common biomarkers (i.e., platelet and/or white cell count, hemoglobin levels, D‐Dimers, and P‐selectin) for CAT in cohorts of lung cancer patients is rather weak [21].
Aiming to identify biomarkers of hypercoagulability with the highest predictive value for symptomatic VTE, which could improve the accuracy of the COMPASS‐CAT RAM in patients with lung adenocarcinoma, we performed the prospective, observational study ROADMAP‐CAT (pROspective Risk Assessment anD bioMArkers of hyPercoagulability for the identification of patients with lung adenocarcinoma at risk for Cancer‐Associated Thrombosis). Cancer‐ and patient‐related risk factors of VTE and a large number of biomarkers of hypercoagulability were assessed. Outpatients with lung adenocarcinoma receiving the recommended anticancer treatment were followed for 12 months, and symptomatic VTE was the study endpoint.
Subjects, Materials, and Methods
Study Design and Participants
The prospective observational cohort study ROADMAP‐CAT enrolled ambulatory patients with histologically confirmed lung adenocarcinoma. Patients were recruited from the outpatient day clinic of “Sotiria” Athens General Hospital from October 2013 to November 2015. Consecutive patients from the ambulatory anticancer clinic were assessed for eligibility. Eligible patients had not undergone any surgery in the preceding 3 months. They were similar to the cohort included in COMPASS‐CAT study [18] regarding chemotherapy status and before enrollment had either received a maximum of one cycle of chemotherapy or were scheduled to initiate chemotherapy. All patients received standard chemotherapy regimens according to institutional practice. The exclusion criteria were as follows: age younger than 18 years; life expectancy less than 3 months; ongoing pregnancy; major psychiatric disorders; recent (<6 months) episode of VTE or acute coronary syndrome; active anticoagulant treatment (for any indication); administration of two or more cycles of chemotherapy; scheduled open elective curative surgery under general anesthesia for abdominal, pelvic, or lung cancer; and hospitalization due to stroke, acute coronary syndrome, congestive heart failure, or acute respiratory failure.
Follow‐Up and Outcome Monitoring
Clinical evaluation of patients was performed at inclusion and at 3, 6, and 12 months after inclusion. The primary endpoint was symptomatic and objectively confirmed VTE, including deep vein thrombosis (DVT), pulmonary embolism (PE), or both (DVT and PE), central venous catheter (CVC) thrombosis, upper limb vein thrombosis (not related to the CVC), or vein thrombosis at a rare localization (i.e., splanchnic vein or cerebral vein thrombosis). Symptomatic VTE had to be documented by at least one of the recommended methods (color Echo‐Doppler, computed tomography, magnetic resonance imaging angiography, scintigraphy, or computed tomography scan). The investigators confirmed the occurrence of VTE by analysis of the medical records, taking into consideration the results of the imaging methods and the administration of therapeutic doses of anticoagulant drugs. Patients with incidental VTE were not included in the analysis because no consensus currently exists regarding the need to treat this form of thrombosis with anticoagulant therapy.
The evolution of the disease was registered during the follow‐up visits and cross‐checked by analysis of the medical records.
Definitions for Key Predictors for VTE
Eligible patients were interviewed at the inclusion visit using a standardized clinical research form (CRF), which included previously validated risk factors for VTE [22]. The CRF also assessed the status of the disease, the ongoing treatments, the devices and the values of hemogram, and laboratory parameters of liver and renal functions measured within 1 week prior to enrollment. The comorbidities and VTE risk factors nonrelated to the cancer were defined as follows: renal function was considered normal if the estimated creatinine clearance rate using Cockcroft‐Gault formula was ≥60 mL/min/1.73 m2. Liver impairment was defined as transaminase increase twofold higher than the upper normal level. The BMI at the day of the assessment was stratified into three groups: normal weight (BMI <25), overweight (BMI ≥25 but <30), or obese (BMI ≥30).
The predictors “hyperlipidemia,” “hypertension,” “diabetes,” “personal history of acute coronary syndrome,” “stroke,” and “peripheral artery disease” appeared individually in the CRF, were assessed at the inclusion, and refer to objectively diagnosed conditions according to the respective diagnostic criteria.
Total bed rest with bathroom privileges for >3 days was evaluated when occurring within 1 month prior to the inclusion in the study.
The predictor “pulmonary disease” includes any active pulmonary disease (except cancer) requiring treatment and present to the patient at least within 1 month prior to the inclusion in the study.
The “hospitalization” was defined as hospitalization for any nonsurgical reason occurring within the last 3 months before assessment.
Patients were staged according to the 7th American Joint Committee on Cancer TNM staging criteria; histological classification of tumors was based on the latest classification system proposed by the International Association for the Study of Lung Cancer, the American Thoracic Society, and the European Respiratory Society. Treatment response was evaluated according to RECIST, version 1.1. The “stage” of cancer was dichotomized into two categories: “local stage” and “metastatic stage.” The “time since cancer diagnosis” refers to the time between the day of the assessment and the objective first diagnosis of the cancer or the recurrence of the cancer (if the patient was in complete remission).
Blood Sampling
Blood samples were obtained by atraumatic puncture of the antecubital vein, using a 20‐gauge needle without tourniquet into siliconized vacutainer tubes containing 0.105 mol/L trisodium citrate; 1/9 v/v (Becton and Dickinson, Le Pont‐de‐Claix, France). Sampling was performed at two predetermined time points: (a) at the inclusion (baseline) and (b) at the end of the third cycle of chemotherapy. Platelet‐poor plasma (PPP) was obtained by double centrifugation at 2,000 g for 20 minutes at room temperature, and plasma aliquots were stored at −80°C until assayed. Samples were centralized to the Core Lab in Thrombosis Center, Service d'Hématologie Biologique, Tenon University Hospital, Paris, where the measurements of biomarkers were performed.
Molecular and Functional Analysis
Thrombin Generation Assay.
Thrombin generation in plasma was assessed using the Calibrated Automated Thrombogram assay (CAT; Diagnostica Stago, Asnières, France) according to manufacturer's instructions, in the presence of optimal concentrations of tissue factor (TF; 5 pM) and procoagulant phospholipids (4 μM) using the PPP‐Reagent (Diagnostica Stago). The following parameters of thrombogram were analyzed: (a) lag‐time that indicates the initiation phase of thrombin generation, (b) time to reach maximum concentration of thrombin (ttPeak), (c) maximum concentration of thrombin (Peak), (d) mean rate index (MRI) of the propagation phase of thrombin generation calculated by the formula: Peak/(ttPeak – lag‐time) and expressed in nM/min, and (e) endogenous thrombin potential (ETP) that shows the integral enzymatic activity of thrombin. Assay specifications have been published elsewhere [23], [24].
Procoag‐PPL was measured with STA Procoag‐PPL (Diagnostica Stago) according to the manufacturer's instructions, as previously described [25]. The inter‐ and intra‐assay coefficients of variation were 3% and 4%, respectively.
Specific TF Activity.
Tissue Factor activity (TFa) in PPP was measured with a homemade test as previously described [25], [26]. The inter‐ and intra‐assay coefficients of variation were 7% and 5%, respectively.
The levels of factor VIIa (Staclot VIIa‐rTF), factor V (FV), antithrombin (AT), fibrin monomers (FM), and D‐Dimers were measured with commercially available assays (Diagnostica Stago) according to the manufacturer's instructions, on a STA‐R analyzer (Diagnostica Stago). The levels of P‐Selectin and heparanase in plasma were measured with ELISA Kits from Cusabio Biotech (CliniSciences, Nanterre, France) and R&D Systems (Lille, France), respectively.
Control Group
The control group consisted of 30 healthy individuals, without any known hereditary or acquired thrombophilic alterations or personal history of thrombotic or bleeding disorders. Controls had the same mean age as patients. The protocol of the study was in accordance with the commitment of the Helsinki declaration and was approved by the institutional ethics committee. All subjects provided informed written consent before inclusion in the study.
Role of the Funding Source
The study was supported financially by Leo Pharma. Protocol development, construction of the database, data collection, statistical analysis, data interpretation, and manuscript writing were all done by the investigators with no involvement from the funding sources.
Statistical Analysis
Continuous variables are described by mean and standard deviation (SD) and categorical variables by frequency and percentage. Descriptive statistics for relevant baseline characteristics are provided with the corresponding frequency and standard deviation or interquartile range (depending on a Gaussian or a skewed distribution).
A comparison of quantitative variables between two groups was performed using the Student's t test for independent samples. In view of the deviation from normality (as evidenced by the Shapiro‐Wilk test), the comparison of biomarker levels between cases and controls was performed using the Mann‐Whitney‐Wilcoxon text for independent samples. Paired t test and analysis of variance test were applied to compare changes in continuous variables at inclusion and at the follow‐up time point. Regarding the associations between VTE and biomarkers, the latter were converted to binary variables through receiver operating characteristic (ROC) curve analysis; the selection of cutoff levels was based on the maximization of Youden's index.
The upper normal limit (UNL) and the lower normal limit (LNL) for each studied biomarker were defined in the control group as follows: UNL = mean + 2 SD, and LNL = mean − 2 SD. The UNL and LNL of the studied biomarkers were compared with the corresponding normal reference range used by our laboratory. The normal ranges have been established according to the requirements for the good quality of laboratory practice by performing the tests in healthy individuals that are representative of the general population with regard to age, sex, ethnicity, BMI, and socioeconomic status. Because VTE treatment may, directly or indirectly, influence biomarkers of blood coagulation activation, values measured after VTE occurrence were not considered in the analysis. The modelization of the predicted values for the biomarkers was done by defining symptomatic documented index VTE events as dependent variables. The first step for model development was the univariate analysis that identified the variables associated with VTE risk. The selection of independent variables was done at the level of 5% using the stepwise procedure.
Multivariate logistic regression analysis was performed with VTE as the dependent variable; the clinical factors and biomarkers proven significant at the univariate logistic regression analysis were examined as possible independent variables. The selection of independent variables was performed at the level of 5% using the stepwise procedure. The Fine and Gray regression model was applied to control if competing risk related to the mortality affects the relative risk and 95% confidence intervals (95% CI) of the predictors for VTE. Subdistribution hazard ratio (sHR) were calculated and compared with the odds ratio (OR; 95% CI) obtained from the univariate and multivariate analysis. To prevent erroneous inclusion of predictors into the model, the rule of thumb, events per variable 1:10 was applied: one candidate predictor per 10 outcome events were included in the data set [27], [28]. The same method was applied for the identification of clinical predictors for VTE. The number of enrolled patients was defined according to this rule. The discrimination capacity of the model was tested with ROC analysis and the area under the curve (AUC) was calculated. The individual ability of biomarkers to improve the AUC of the ROC analysis was analyzed. Calibration of the models was controlled with the Hosmer‐Lemeshow test. At the last part of the study, the COMPASS‐CAT RAM (described elsewhere [18]) was applied and the effect of the incorporation of the clinically relevant biomarkers was analyzed. Model discrimination performance was evaluated by calculating sensitivity, specificity, positive predictive value, and negative predictive value for both cohorts. Two‐sided p values <.05 were considered statistically significant. Data were analyzed using the STATA/SE version 13 statistical software (Stata Corp., College Station, TX).
Results
Study Population
A total of 150 patients (110 males and 40 females) were enrolled in the study. No patients were lost on follow‐up or excluded from analysis due to missing data. The demographics and clinical characteristics of the patients at the time of inclusion are summarized in Table 1. Mean age of patients was 65 ± 10 years (range: 20–85 years) and was not significantly different compared with the mean age of the controls (60 ± 10 years; p = .08). The BMI was normal for 43.4% of patients, and 44.0% and 12.6% of patients were overweight and obese, respectively. The majority of patients (68.7%) were diagnosed within 6 months prior to study entry. Most patients (88.6%) had metastatic disease, and 11 patients (7.3%) had recurrent disease. The Eastern Cooperative Oncology Group performance status was <3 in 83.3% of patients. At inclusion, 12.7% of patients were chemotherapy naïve. In the remaining 87.3% of the patients, chemotherapy was initiated within 1 month before inclusion. At 3‐months follow‐up, 6.4% of patients were in complete remission, 3.2% were in partial remission, 24.2% had stable disease, and 41.9% had progressive disease. At 6‐months follow‐up, 7.7% of patients were in complete remission, 3.8% were in partial remission, 28.8% had stable disease, and 37.5% had progressive disease. Among 104 patients, 23.0% were hospitalized between the 3‐ and 6‐months follow‐up visits. The 1‐year mortality rate was 30.0%.
Table 1. Demographic data, clinicopathological features and associated treatments, co‐morbidities, and risk factors for VTE unrelated to cancer in the cohort of patients with lung adenocarcinoma (n = 150).
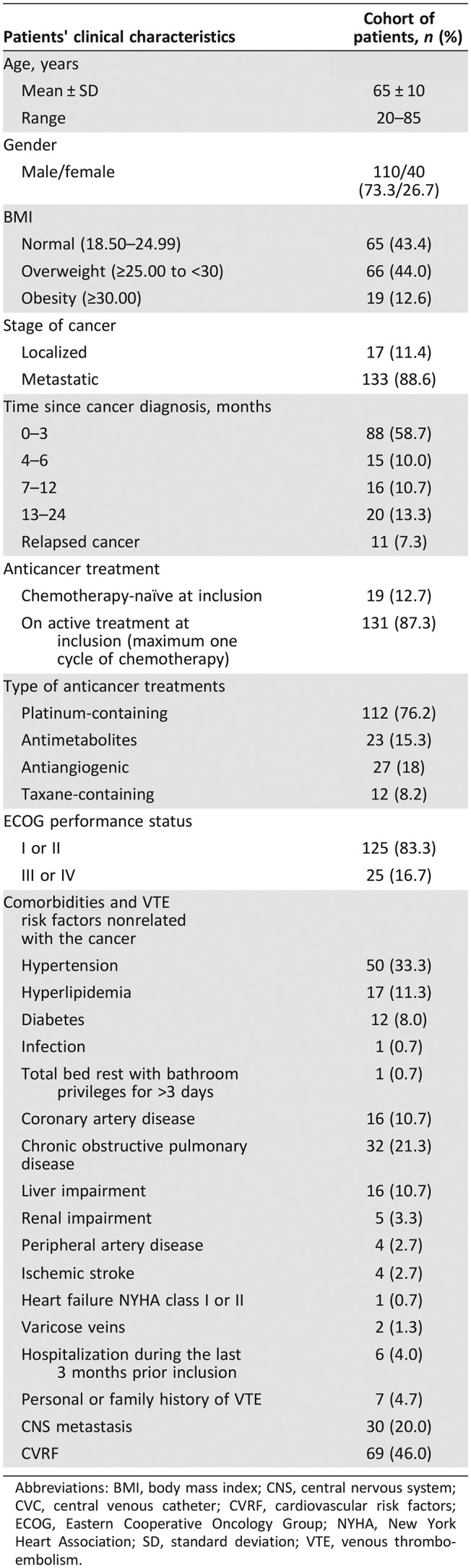
Abbreviations: BMI, body mass index; CNS, central nervous system; CVC, central venous catheter; CVRF, cardiovascular risk factors; ECOG, Eastern Cooperative Oncology Group; NYHA, New York Heart Association; SD, standard deviation; VTE, venous thromboembolism.
Follow‐Up and VTE
During the observation period, a total of 12 patients had VTE (8%). At 3‐months follow‐up, 9 of 12 patients (75%) suffered symptomatic VTE. At 6‐months follow‐up, 3 additional patients out of 12 (25%) had symptomatic VTE. The localization of thrombosis in patients with VTE was as follows: four patients had PE, two patients had proximal DVT, five patients had distal DVT, and one patient had mesenteric vein thrombosis. Mortality rate in the group of patients with VTE was 66.6%. The distribution of patients who died after a VTE episode was as follows: seven at the 3‐months follow‐up and one at the 6‐months follow‐up.
Clinical Predictors for VTE
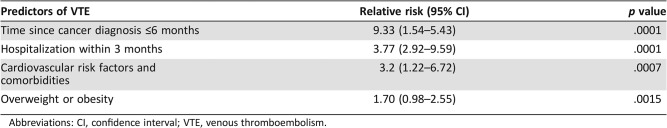
In univariate analysis, the following clinical predictors were found to be significantly associated with the occurrence of symptomatic VTE: time since cancer diagnosis less than 6 months (OR = 9.33, 95% CI, 1.5–5.4; p < .05), hospitalization within 3 months prior to assessment (OR = 3.77, 95% CI, 2.8–9.6; p < .05), presence of cardiovascular risk factors (OR = 3.2, 95% CI, 1.22–6.72; p < .05), and overweight or obesity (OR = 1.70, 95% CI, 0.98–2.55; p < .05; Table 2).
Table 2. Relative risk and 95% confidence intervals of variables, which, according to univariate regression, were significantly associated with the risk of VTE.
Abbreviations: CI, confidence interval; VTE, venous thromboembolism.
The multivariate analysis showed that a time since cancer diagnosis of less than 3 months (OR = 8.13, 95% CI, 1.4–5.1; p < .001) and hospitalization within 3 months prior to assessment (OR = 3.5, 95% CI, 2.3–9.9; p < .001) were the most significant predictors for CAT. Analysis with the Fine and Gray regression model showed that sHR (95% CI) for each predictor for VTE were not sizably affected compared with the OR (95% CI) derived from multivariate analysis.
Biomarkers of Hypercoagulability
Hypercoagulability at the Initiation of Chemotherapy.
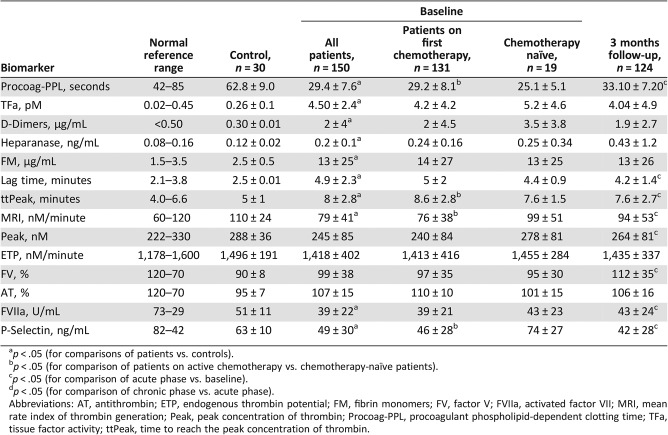
At inclusion, patients showed shorter Procoag‐PPL and significantly higher levels of TFa, D‐Dimers, heparanase, and fibrin monomers compared with controls. Thrombin generation was significantly delayed in patients compared with the controls. The lag‐time and ttPeak were significantly longer and the MRI was significantly reduced in patients as compared with controls. In contrast, the ETP, Peak, FV, and AT were not significantly different in patients compared with controls. The levels of FVIIa and P‐Selectin were significantly lower in patients compared with controls (Table 3).
Table 3. Baseline profile and dynamic changes of the molecular biomarkers for hypercoagulability in patients enrolled in ROADMAP‐CAT study.
p < .05 (for comparisons of patients vs. controls).
p < .05 (for comparison of patients on active chemotherapy vs. chemotherapy‐naïve patients).
p < .05 (for comparison of acute phase vs. baseline).
p < .05 (for comparison of chronic phase vs. acute phase).
Abbreviations: AT, antithrombin; ETP, endogenous thrombin potential; FM, fibrin monomers; FV, factor V; FVIIa, activated factor VII; MRI, mean rate index of thrombin generation; Peak, peak concentration of thrombin; Procoag‐PPL, procoagulant phospholipid‐dependent clotting time; TFa, tissue factor activity; ttPeak, time to reach the peak concentration of thrombin.
Almost all patients had Procoag‐PPL clotting time shorter than the LNL, and in 90% of patients, the levels of TFa and heparanase were above the respective UNL. The levels of D‐Dimers were lower than 1.5 μg/mL in 80 patients (53%) and within the normal range (<0.5 μg/mL) in 28 patients (19%). Levels of FV were inferior to the LNL in 36 patients (24%). When these patients were excluded from the analysis, the lag‐time and the MRI remained significantly different compared with controls.
Chemotherapy‐naïve patients, compared with patients on active chemotherapy, showed significantly shorter Procoag‐PPL and ttPeak and significantly increased MRI and P‐Selectin levels. The levels of the other biomarkers were not significantly different between chemotherapy‐naïve patients and those who had received one cycle of chemotherapy (Table 3).
Hypercoagulability at 3‐Months Follow‐Up.
The Procoag‐PPL clotting time significantly increased compared with the baseline but remained significantly shorter than the control group. The Procoag‐PPL was shorter than the LNL for all studied patients. The levels of FV and FVIIa significantly increased compared with the baseline. Lag‐time, ttPeak, and P‐Selectin significantly decreased, whereas Peak and MRI significantly increased as compared with baseline. At 3‐months follow‐up, tTFa, D‐Dimers, AT, ETP, FM, and heparanase did not significantly change as compared with the baseline.
Clinical and Biological Predictors of VTE
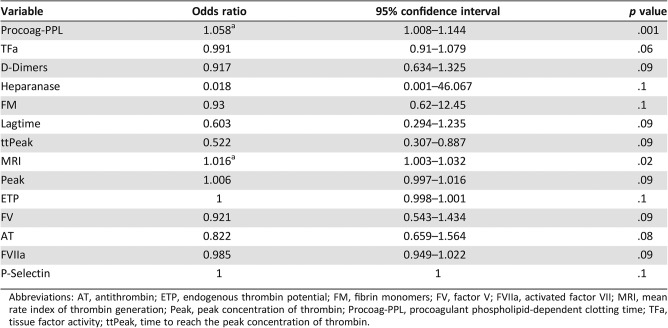
Univariate analysis of the studied biomarkers assessed at inclusion showed that decreased Procoag‐PPL clotting time and decreased MRI were significant and independent risk factors for VTE (Table 4).
Table 4. Relative risk and 95% confidence intervals for VTE risk prediction by the assessment of hypercoagulability biomarkers at the initiation of chemotherapy.
Abbreviations: AT, antithrombin; ETP, endogenous thrombin potential; FM, fibrin monomers; FV, factor V; FVIIa, activated factor VII; MRI, mean rate index of thrombin generation; Peak, peak concentration of thrombin; Procoag‐PPL, procoagulant phospholipid‐dependent clotting time; TFa, tissue factor activity; ttPeak, time to reach the peak concentration of thrombin.
The OR for VTE of the Procoag‐PPL clotting time was 1.058 (95% CI, 1.008–1.144; p = .001) and that of the MRI was 1.016 (95% CI, 1.003–1.032; p = .02; Table 4). In comparison, the univariate analysis of the biomarkers when assessed at the third month of follow‐up did not show any significant association with the risk of VTE.
The multivariate analysis of the studied biomarkers retained the Procoag‐PPL (OR = 1.09, 95% CI, 1.03–1.19, p = .02) and MRI (OR = 1.02, 95% CI, 1.00–1.04, p = .03). When patients were assessed at inclusion, Procoag‐PPL clotting time shorter than 44 seconds and MRI lower than 125 nM/minute were significant predictors of VTE outcome. Multivariate logistic analysis led to the following equation:
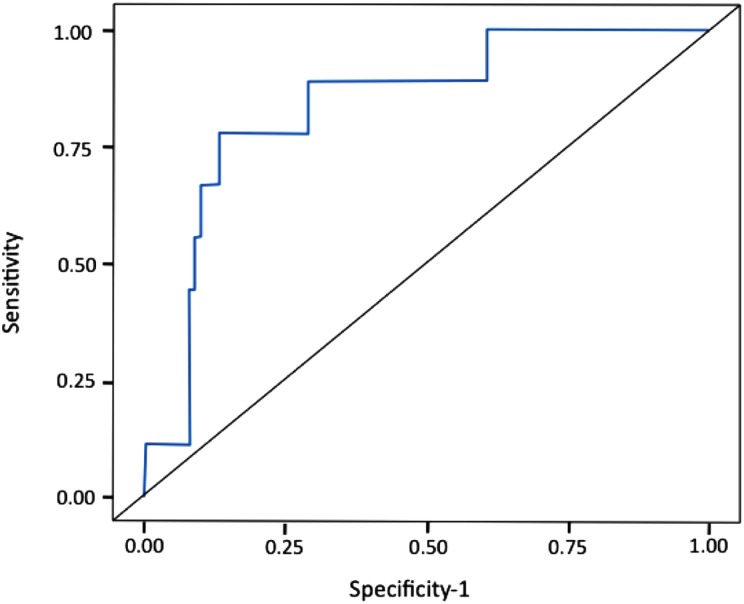
Accordingly, a score was formulated where the dependent variable is the VTE risk and the predictors are binary: 1 (if Procoag‐PPL <44 seconds and MRI <125 nM/minute) or 0 (ifProcoag‐PPL >44 seconds or MRI >125 nM/minute) and patients were stratified at high‐ or intermediate/low‐risk group (Fig. 1). All patients with VTE had Procoag‐PPL <44 seconds. The MRI <125 was found in 10 of 12 VTE patients. The rate of VTE was 3.4% in the intermediate/low‐risk group and 12.2% in high‐risk group. The AUC of the ROC analysis was 0.77 (Fig. 1). The sensitivity and the specificity of thescore was 88% and 52%, respectively. According to the Hosmer‐Lemeshow test, a value of p = .23 showed that the model was well calibrated.
Figure 1.
Receiver operating characteristic analysis of the experimental model for venous thromboembolism prediction including procoagulant phospholipid‐dependent clotting time and mean rate index (area under the curve = 0.77).
VTE Risk Assessment with the COMPASS‐CAT Score
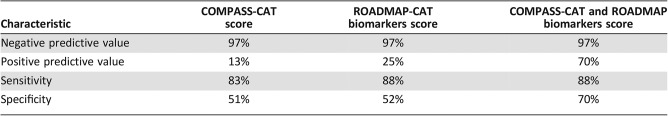
Application of the COMPASS‐CAT RAM resulted in accurate stratification of patients at high and intermediate/low risk for VTE (Table 5). The incorporation of the Procoag‐PPL and MRI into the COMPASS score significantly improved its specificity (Table 5).
Table 5. Improvement of the qualitative characteristics of the clinical COMPASS‐CAT score by the addition of procoagulant phospholipid‐dependent clotting time Procoag‐PPL clotting time and MRI of thrombin generation.
Discussion
The prospective ROADMAP‐CAT study conducted in ambulatory patients with lung adenocarcinoma aimed to identify the most clinically relevant biomarkers of hypercoagulability for the evaluation of VTE risk. In the studied cohort of patients, the annual cumulative incidence of symptomatic VTE was 8%, most VTE episodes occurred early after cancer diagnosis, and VTE was associated with high mortality, being in accordance with previously published studies [2], [3], [5], [6], [7]. The prospective design of the study showed that the Procoag‐PPL clotting time and the MRI of the propagation phase of thrombin generation are mandatory biomarkers for the classification of patients into high or intermediate/low risk for VTE. Indeed, shortened Procoag‐PPL clotting time (<44 seconds) and decreased MRI of thrombin generation (<125 nM/minute) were independently associated with VTE risk. The design of the study allowed to define that measurement of Procoag‐PPL and MRI should be performed before or within 1 month after the administration of the first chemotherapy cycle. This is based on the fact that most VTE episodes occurred within the first 3 months from inclusion. The studied biomarkers assessed at the end of the third cycle of chemotherapy were not related with VTE risk (data not shown).
The clinical features of the studied cohort of patients respond to the principal generalizability criteria for risk assessment tools [29], [30]. Moreover, the studied patients had similar characteristics, particularly regarding the status of chemotherapy, to the derivation cohort of the COMPASS‐CAT RAM [18]. The principal characteristics of the derivation cohort of patients were (a) metastatic lung adenocarcinoma, (b) cancer diagnosis within 6 months prior to assessment, (c) good performance status, and (d) administration of a maximum of one cycle of the recommended chemotherapeutic regimen. The derivation cohort is representative of patients with several other characteristics that frequently appear in patients with lung adenocarcinoma, such as poor performance status, interval since cancer diagnosis longer than 6 months, various types of chemotherapy, etc. Following the validation study, the ROADMAP‐CAT score will be applicable to any patient with lung adenocarcinoma. The association of the biological ROADMAP‐CAT score with the COMPASS‐CAT score in patients with lung adenocarcinoma is expected to increase the positive predictive value for the identification of patients at VTE risk. However, caution is advised before extrapolating the results of the present study into unselected patient populations with lung cancer types other than adenocarcinoma.
The ROADMAP‐CAT study also identified the most relevant clinical predictors of VTE in outpatients with lung adenocarcinoma on chemotherapy. These are hospitalization within the last 3 months prior to assessment, presence of cardiovascular risk factors and/or cardiovascular comorbidities, overweight or obesity, personal history of thrombosis, and a time since cancer diagnosis less than 6 months from the assessment. The clinical predictors of VTE identified in the ROADMAP‐CAT study are the same as those identified in a large prospective study performed in outpatients with solid tumors, including lung cancer, which resulted in the derivation of the COMPASS‐CAT RAM [18].
The concept that the assessment of hypercoagulability biomarkers could improve the accuracy of clinical RAMs to stratify ambulatory patients at VTE risk has been tested in previous studies [31]. The Vienna prediction score for patients with various solid and hematologic malignancies improved the performance of the Khorana RAM by incorporating soluble P‐Selectin and D‐Dimers [31]. In addition, biomarkers of coagulation or fibrinolysis activation, as reflected by high levels of D‐dimers, are independently associated with an unfavorable prognosis in patients with solid tumors, which is, however, not necessarily associated with an increased risk of VTE [32]. The ROADMAP‐CAT study showed that D‐Dimers and P‐Selectin were not mandatory for VTE risk, and this is not in the same line with the findings of the Vienna Cancer and Thrombosis Study (CATS) [31]. However, some substantial differences in the design of the two studies could explain this discrepancy. The ROADMAP‐CAT study enrolled patients with the same type of cancer, whereas the Vienna CATS cohort included a large variety of malignancies with different potential for blood coagulation activation and also different risk of CAT. This difference between the two studies strongly suggests that the value of hypercoagulability biomarkers for identification of VTE risk in cancer patients needs to be assessed in trials dedicated to a given histological type. Applying the COMPASS‐CAT RAM to the ROADMAP‐CAT cohort accurately stratified patients into high and intermediate/low risk for VTE. Despite the weak, although significant, association of Procoag‐PPL clotting time and MRI with VTE events, the introduction of these tests into the COMPASS‐CAT clinical RAM significantly improved its positive predictive value (Table 5).
The feasibility of the integral clinicobiological analysis of VTE risk in ambulatory cancer patients is a major advantage of this risk assessment strategy because both biomarkers selected by the ROADMAP‐CAT study are commercially available, easy to perform, and do not require a specialized laboratory infrastructure.
The sample size and the monocentric design are the limitations of the ROADMAP‐CAT study. The number of patients and events provides sufficient statistical power to evaluate the predictive value of the studied biomarkers; however, it did not allow internal validation of the model. Although the recommended treatments were applied, the monocentric design of the study did not allow evaluation of any potential influence of other therapeutic practices or supportive treatments on the predictive capacity of the studied biomarkers. In contrast, the prospective evaluation of hypercoagulability biomarkers throughout the course of chemotherapy and the homogeneity of the patient population, comprising only ambulatory patients with lung adenocarcinoma, are the major strengths of the ROADMAP‐CAT study.
Conclusion
The prospective ROADMAP‐CAT study demonstrates the presence of pronounced blood hypercoagulability in ambulatory patients with lung adenocarcinoma, characterized by decreased Procoag‐PPL clotting time, enhanced endothelial cell activation, increased degradation of fibrin, and exhausted thrombin generation. Among a large number of biomarkers of hypercoagulability, only the Procoag‐PPL clotting time and the MRI of thrombin generation were found to be independently associated with the risk of VTE. The measurement of these biomarkers before or within 1 month after administration of the first cycle and their incorporation into the COMPASS‐CAT RAM significantly improved the capacity of this RAM to stratify patients into high or intermediate/low VTE risk groups. The evaluation of these biomarkers is feasible in most hospitals and should be taken into consideration when designing phase III clinical trials that evaluate the efficacy and safety of pharmacological thromboprophylaxis in outpatients with lung adenocarcinoma.
Acknowledgments
We acknowledge Dr. Costas Sitaras, from Leo Pharma Greece, for precious support of the ROADMAP project. We thank Hayat Mokrani and Matthieu Grusse for their skillful technical assistance.
Footnotes
For Further Reading: Grigoris T. Gerotziafas, Ali Taher, Hikmat Abdel‐Razeq et al. A Predictive Score for Thrombosis Associated with Breast, Colorectal, Lung, or Ovarian Cancer: The Prospective COMPASS‐Cancer‐Associated Thrombosis Study. The Oncologist 2017;22:1222–1231.
Implications for Practice: The Prospective Comparison of Methods for thromboembolic risk assessment with clinical Perceptions and AwareneSS in real life patients‐Cancer Associated Thrombosis (COMPASS‐CAT) study provides a new risk assessment model (RAM) for venous thromboembolism (VTE) applicable in outpatients with breast, colorectal, lung or ovarian cancer. The COMPASS‐CAT RAM is robust, applicable during chemotherapy and determines the need for VTE prevention by including reliable and easily collected VTE predictors associated with cancer status, its treatment as well as with patients' characteristics and comorbidities. An independent external validation of the RAM is indicated before its use in clinical practice.
Author Contributions
Conception/design: Konstantinos Syrigos, Dimitra Grapsa, Rabiatou Sangare, Ilias Evmorfiadis, Annette K. Larsen, Patrick Van Dreden, Paraskevi Boura, Andriani Charpidou, Elias Kotteas, Theodoros N. Sergentanis, Ismail Elalamy, Anna Falanga, Grigoris T. Gerotziafas
Provision of study material or patients: Konstantinos Syrigos, Dimitra Grapsa, Rabiatou Sangare, Ilias Evmorfiadis, Annette K. Larsen, Patrick Van Dreden, Paraskevi Boura, Andriani Charpidou, Elias Kotteas, Theodoros N. Sergentanis, Ismail Elalamy, Anna Falanga, Grigoris T. Gerotziafas
Collection and/or assembly of data: Konstantinos Syrigos, Dimitra Grapsa, Rabiatou Sangare, Ilias Evmorfiadis, Annette K. Larsen, Patrick Van Dreden, Paraskevi Boura, Andriani Charpidou, Elias Kotteas, Theodoros N. Sergentanis, Ismail Elalamy, Anna Falanga, Grigoris T. Gerotziafas
Data analysis and interpretation: Konstantinos Syrigos, Dimitra Grapsa, Rabiatou Sangare, Ilias Evmorfiadis, Annette K. Larsen, Patrick Van Dreden, Paraskevi Boura, Andriani Charpidou, Elias Kotteas, Theodoros N. Sergentanis, Ismail Elalamy, Anna Falanga, Grigoris T. Gerotziafas
Manuscript writing: Konstantinos Syrigos, Dimitra Grapsa, Rabiatou Sangare, Ilias Evmorfiadis, Annette K. Larsen, Patrick Van Dreden, Paraskevi Boura, Andriani Charpidou, Elias Kotteas, Theodoros N. Sergentanis, Ismail Elalamy, Anna Falanga, Grigoris T. Gerotziafas
Final approval of manuscript: Konstantinos Syrigos, Dimitra Grapsa, Rabiatou Sangare, Ilias Evmorfiadis, Annette K. Larsen, Patrick Van Dreden, Paraskevi Boura, Andriani Charpidou, Elias Kotteas, Theodoros N. Sergentanis, Ismail Elalamy, Anna Falanga, Grigoris T. Gerotziafas
Disclosures
Konstantinos Syrigos: Leo Pharma (H); Ismail Elalamy: Sanofi, Leo Pharma, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb (C/A, H); Anna Falanga: Bayer, Rovi, Boehringer Ingelheim (C/A, H); Grigoris T. Gerotziafas: Sanofi (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Ay C, Unal UK. Epidemiology and risk factors for venous thromboembolism in lung cancer. Curr Opin Oncol 2016;28:145–149. [DOI] [PubMed] [Google Scholar]
- 2.Connolly GC, Dalal M, Lin J et al. Incidence and predictors of venous thromboembolism (VTE) among ambulatory patients with lung cancer. Lung Cancer 2012;78:253–258. [DOI] [PubMed] [Google Scholar]
- 3.Ay C, Pabinger I, Cohen AT. Cancer‐associated venous thromboembolism: Burden, mechanisms and management. Thromb Haemost 2017;117:219–230. [DOI] [PubMed] [Google Scholar]
- 4.Lyman GH, Khorana AA, Falanga A. Thrombosis and cancer: Emerging data for the practicing oncologist. Am Soc Clin Oncol Educ Book 10.1200/EdBook_AM. 201333e337. [DOI] [PubMed] [Google Scholar]
- 5.Chew HK, Davies AM, Wun T et al. The incidence of venous thromboembolism among patients with primary lung cancer. JThromb Haemost 2008;6:601–608. [DOI] [PubMed] [Google Scholar]
- 6.Tesselaar ME, Osanto S. Risk of venous thromboembolism in lung cancer. Curr Opin Pulm Med 2007;13:362–367. [DOI] [PubMed] [Google Scholar]
- 7.Alexander M, Kirsa S, Wolfe R et al. Thromboembolism in lung cancer ‐ An area of urgent unmet need. Lung Cancer 2014;84:275–280. [DOI] [PubMed] [Google Scholar]
- 8.Kahn SR, Lim W, Dunn AS et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest 2012;141(suppl 2):e195S–e226S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicolaides AN, Fareed J, Kakkar AK et al. Prevention and treatment of venous thromboembolism–International Consensus Statement. Int Angiol 2013;32:111–260. [PubMed] [Google Scholar]
- 10.Debourdeau P, Farge D, Beckers M et al. International clinical practice guidelines for the treatment and prophylaxis of thrombosis associated with central venous catheters in patients with cancer. JThromb Haemost 2013;11:71–80. [DOI] [PubMed] [Google Scholar]
- 11.Di Nisio M, Porreca E, Otten HM et al. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst Rev 2014;CD008500. [DOI] [PubMed] [Google Scholar]
- 12.Lyman GH, Bohlke K, Khorana AA et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology Clinical Practice Guideline Update 2014. JClin Oncol 2015;33:654–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khorana AA, Kuderer NM, Culakova E et al. Development and validation of a predictive model for chemotherapy‐associated thrombosis. Blood 2008;111:4902–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agnelli G, George DJ, Kakkar AK et al. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med 2012;366:601–609. [DOI] [PubMed] [Google Scholar]
- 15.George D, Agnelli G, Fisher W et al. Venous thromboembolism (VTE) prevention with semuloparin in cancer patients initiating chemotherapy: Benefit‐risk assessment by VTE risk in SAVE‐ONCO. American Society of Hematology 53rd meeting; 2011. Available at http://www.bloodjournal.org/content/118/21/206. Accessed March 7, 2018.
- 16.Mansfield A, Tafur AJ, Wang CE et al. Predictors of active cancer thromboembolic outcomes: Validation of the Khorana score among patients with lung cancer. JThromb Haemost 2016;14:1773–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandala M, Falanga A, Roila F et al. Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol 2011;22(suppl 6):vi85–vi92. [DOI] [PubMed] [Google Scholar]
- 18.Gerotziafas GT, Taher A, Abdel‐Razeq H et al. A predictive score for thrombosis associated with breast, colorectal, lung, or ovarian cancer. The prospective COMPASS‐Cancer‐Associated Thrombosis study. The Oncologist 2017;22:1222–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verso M, Agnelli G, Barni S et al. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: The Protecht score. Intern Emerg Med 2012;7:291–292. [DOI] [PubMed] [Google Scholar]
- 20.Ay C, Dunkler D, Marosi C et al. Prediction of venous thromboembolism in cancer patients. Blood 2010;116:5377–5382. [DOI] [PubMed] [Google Scholar]
- 21.Alexander M, Burbury K. A systematic review of biomarkers for the prediction of thromboembolism in lung cancer ‐ Results, practical issues and proposed strategies for future risk prediction models. Thromb Res 2016;148:63–69. [DOI] [PubMed] [Google Scholar]
- 22.Alikhan R, Cohen AT, Combe S et al. Risk factors for venous thromboembolism in hospitalized patients with acute medical illness: Analysis of the MEDENOX Study. Arch Intern Med 2004;164:963–968. [DOI] [PubMed] [Google Scholar]
- 23.Gerotziafas GT, Depasse F, Busson J et al. Towards a standardization of thrombin generation assessment: The influence of tissue factor, platelets and phospholipids concentration on the normal values of Thrombogram‐Thrombinoscope assay. Thromb J 2005;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spronk HM, Dielis AW, De Smedt E et al. Assessment of thrombin generation II: Validation of the Calibrated Automated Thrombogram in platelet‐poor plasma in a clinical laboratory. Thromb Haemost 2008;100:362–364. [PubMed] [Google Scholar]
- 25.Van Dreden P, Rousseau A, Savoure A et al. Plasma thrombomodulin activity, tissue factor activity and high levels of circulating procoagulant phospholipid as prognostic factors for acute myocardial infarction. Blood Coagul Fibrinolysis 2009;20:635–641. [DOI] [PubMed] [Google Scholar]
- 26.Schneider P, Van Dreden P, Rousseau A et al. Increased levels of tissue factor activity and procoagulant phospholipids during treatment of children with acute lymphoblastic leukaemia. Br J Haematol 2010;148:582–592. [DOI] [PubMed] [Google Scholar]
- 27.Peduzzi P, Concato J, Kemper E et al. A simulation study of the number of events per variable in logistic regression analysis. JClin Epidemiol 1996;49:1373–1379. [DOI] [PubMed] [Google Scholar]
- 28.Wasson JH, Sox HC, Neff RK et al. Clinical prediction rules: Applications and methodological standards. N Engl J Med 1985;313:793–799. [DOI] [PubMed] [Google Scholar]
- 29.Hendriksen JM, Geersing GJ, Moons KG et al. Diagnostic and prognostic prediction models. JThromb Haemost 2013;11(suppl 1):129–141. [DOI] [PubMed] [Google Scholar]
- 30.Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361–387. [DOI] [PubMed] [Google Scholar]
- 31.Ay C, Dunkler D, Simanek R et al. Prediction of venous thromboembolism in patients with cancer by measuring thrombin generation: Results from the Vienna Cancer and Thrombosis Study. JClin Oncol 2011;29:2099–2103. [DOI] [PubMed] [Google Scholar]
- 32.Ay C, Dunkler D, Pirker R et al. High D‐dimer levels are associated with poor prognosis in cancer patients. Haematologica 2012;97:1158–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]