Programmed cell death‐1 (PD‐1) signaling is a new target of anti‐tumor therapy that involves immune reactivation; however, its antitumor effect on subsequent chemotherapy is unclear. We encountered several cases of unexpected antitumor response among patients who underwent palliative chemotherapy in the follow‐up study of our phase II nivolumab trial. Two representative cases are described here.
Abstract
Platinum‐resistant recurrent ovarian cancer is generally refractory to chemotherapy. Programmed cell death‐1 (PD‐1) signaling is a new target for antitumor therapy. The anti‐PD‐1 antibody nivolumab had a 10% durable complete response rate in our phase II clinical trial. However, how nivolumab affects sensitivity to subsequent chemotherapy remains unclear. We encountered several cases of unexpected antitumor response among patients who underwent palliative chemotherapy in the follow‐up study of our phase II nivolumab trial (UMIN000005714). Several agents had an unexpected antitumor response in patients who were resistant or refractory to standard chemotherapeutic agents. In one patient, both pegylated liposomal doxorubicin (PLD) and nedaplatin (CDGP) resulted in partial response. In another patient, PLD and CDGP resulted in partial response and stable disease, respectively. These two patients remained alive on the cutoff date. These two cases raise the possibility that nivolumab might improve sensitivity to adequate chemotherapy for ovarian cancer.
Introduction
The prognosis of platinum‐resistant recurrent ovarian cancer is extremely poor. A new treatment strategy is urgently required [1]. Programmed cell death‐1 (PD‐1) signaling is a new target of antitumor therapy that involves immune reactivation [2]; however, its antitumor effect on subsequent chemotherapy remains unclear.
Nivolumab resulted in a complete response in 2 patients, a partial response (PR) in 1 patient, stable disease (SD) in 6 patients, and progressive disease (PD) in 10 patients, whereas 1 patient was not evaluated (15% response rate and 45% disease control rate) in a phase II clinical trial at our hospital [3]. In a follow‐up study of this trial, we encountered several cases of an unexpected antitumor response to palliative chemotherapy (supplemental online Fig. 1, supplemental online Table 1) and describe two representative cases here.
Representative Cases
Case 1
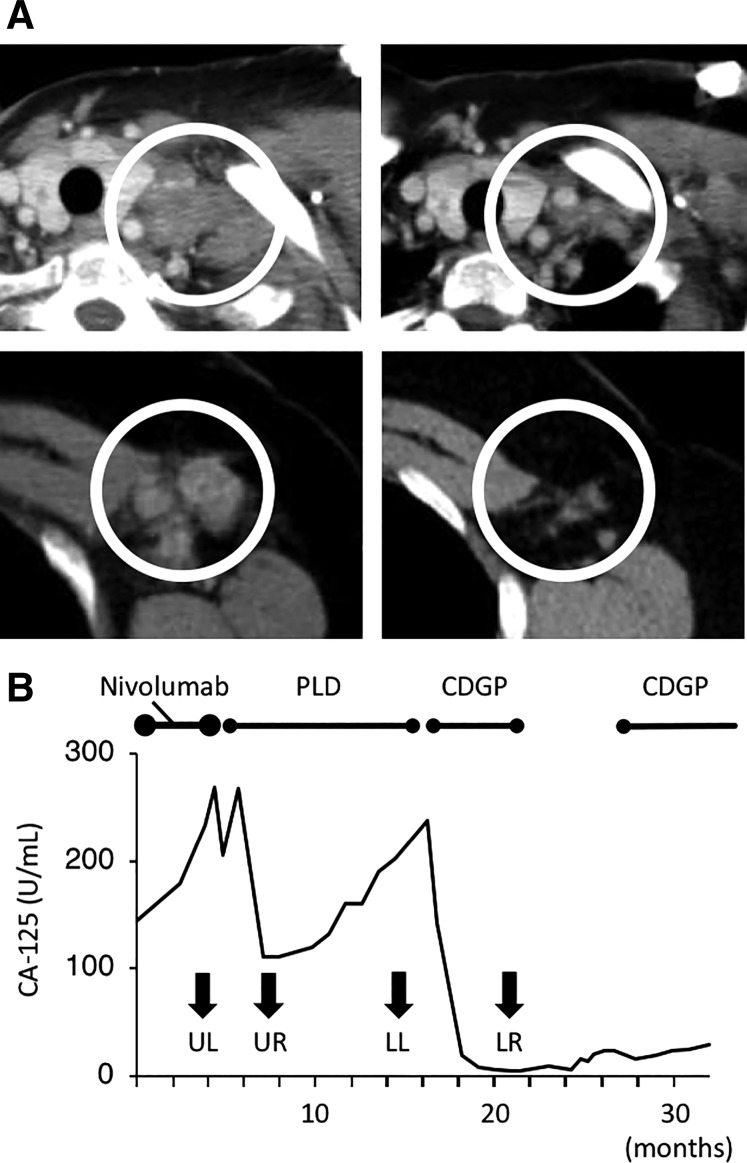
A 65‐year‐old woman developed recurrence of serous ovarian carcinoma and multiple lymph node metastases (supplemental online Table 1, Patient 13). She initially underwent exploratory laparotomy, followed by interval debulking surgery and adjuvant chemotherapy. She experienced disease recurrence several times and received paclitaxel (PAC) and carboplatin (CBDCA), nedaplatin (CDGP), irinotecan (CPT‐11), and weekly PAC plus CBDCA therapy, and surgical treatment. She joined our clinical trial and received nivolumab. After two cycles, she developed PD, and nivolumab was discontinued. After the trial, she was treated with pegylated liposomal doxorubicin (PLD). The overall response was PR (supplemental online Table 2‐1). The representative lesion is described in Figure 1A. The short axis of this lymph node decreased by 65% after three cycles of PLD. The patient received 12 cycles of PLD, which eventually resulted in PD. CDGP was administered (supplemental online Table 2‐2). The short axis of the left axillary lymph node shrank by 54% with six cycles of CDGP (Fig. 1A). After 2 months, metastasis was identified through biopsy of a cervical lymph node. She received six cycles of weekly gemcitabine (GEM); however, she developed PD. Subsequently, CDGP was administered again, with PR. Serum carbohydrate antigen 125 (CA‐125) levels after the trial are shown in Figure 1B. She remained alive on the cutoff date.
Figure 1.
Antitumor effect of palliative chemotherapy after the nivolumab trial in Case 1 (Patient 13). (A): Computed tomography images. (Upper left): A left subclavicular lymph node before PLD therapy. (Upper right): The same lymph node after three cycles of PLD. (Lower left): A left axillary lymph node before CDGP therapy. (Lower right): The same node after six cycles of CDGP. PLD was used after the patient developed progressive disease (PD) with nivolumab. With PLD treatment, the short axis of the lymph node decreased from 22.5 mm to 7.9 mm (−65%). CDGP was administered after the patient developed PD after 12 cycles of PLD. The short axis of the lymph node decreased from 14.9 mm to 6.9 mm (−54%). The white circles in each image indicate enlarged lymph nodes. (B): Serum CA‐125 levels. Serum CA‐125 levels decreased after PLD and CDGP. Both agents resulted in a partial response. Arrows indicate CA‐125 levels at the time when images in (A) were obtained. CDGP was administered again as palliative chemotherapy after a 6‐month chemotherapy‐free period because it had been quite effective.
Abbreviations: CA‐125, carbohydrate antigen 125; CDGP, nedaplatin; LL, Figure 1A lower left; LR, Figure 1A lower right; PLD, pegylated liposomal doxorubicin; UL, Figure 1A upper left; UR, Figure 1A upper right.
Case 2
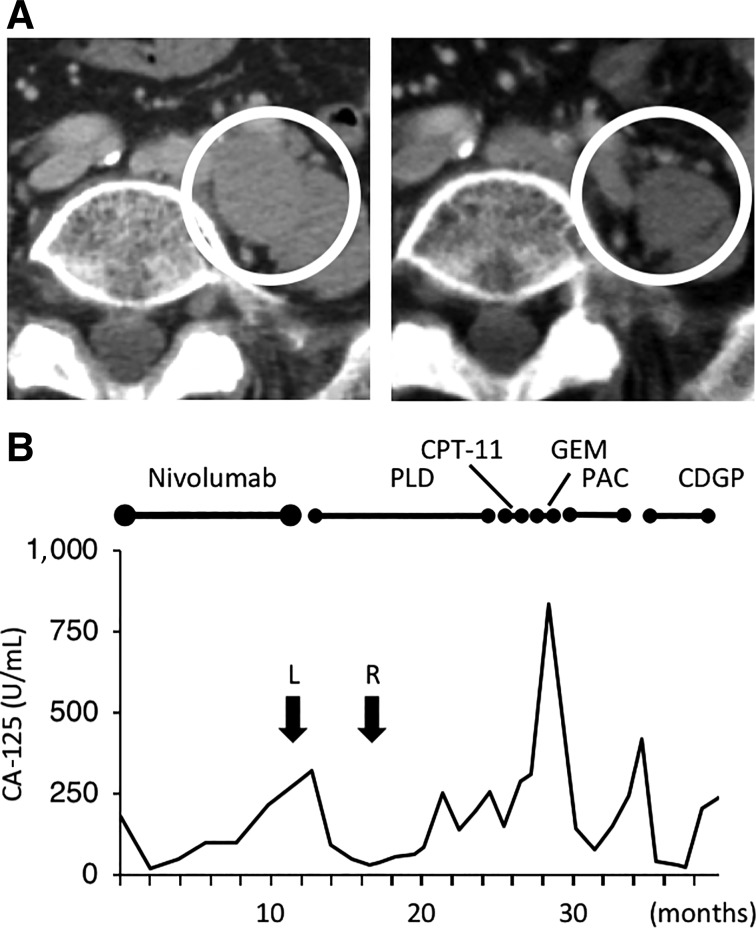
A 69‐year‐old woman received six cycles of nivolumab, which resulted in PD (supplemental online Table 1, Patient 4). She had been diagnosed with ovarian cancer with peritoneal dissemination and multiple lymph nodes metastases 3 years before nivolumab treatment. She underwent interval debulking surgery, followed by adjuvant PAC plus CBDCA chemotherapy. Seven months later, she experienced recurrence and underwent two chemotherapy regimens (PAC plus CBDCA and PAC plus cisplatin, respectively); however, she ultimately developed PD. Following participation in the nivolumab trial, the patient received six cycles of PLD and achieved a PR (supplemental online Table 3). The representative lesion is described in Figure 2A. However, after 10 cycles of PLD, exacerbation of lymph node metastasis was observed. CPT‐11, GEM, and weekly PAC plus bevacizumab were used sequentially, all resulting in PD. Next, four cycles of CDGP were administered. Although the patient experienced SD, chemotherapy was stopped because of pancytopenia. Serum CA‐125 levels during treatment are shown in Figure 2B. She remained alive on the cutoff date.
Figure 2.
Antitumor effect on palliative chemotherapy after the nivolumab trial in Case 2 (Patient 4). (A): Computed tomography images. (Left): Pelvic lymph nodes before PLD therapy. PLD was used after the patient developed progressive disease with nivolumab. (Right): The same lymph nodes after four cycles of PLD. With PLD treatment, the short diameter of the largest node decreased from 22.0 mm to 5.6 mm (−75%). The white circles in each picture indicate enlarged lymph nodes. (B): Serum CA‐125 levels. Serum CA‐125 levels decreased after the administration of PLD, PAC, and CDGP. PLD resulted in a partial response, CDGP resulted in stable disease, and PAC resulted in progressive disease. Arrows indicate CA‐125 levels at the time when images in Figure 2A were obtained.
Abbreviations: CA‐125, carbohydrate antigen 125; CDGP, nedaplatin; CPT‐11, irinotecan; GEM, gemcitabine; L, Figure 2A left; PAC, paclitaxel; PLD, pegylated liposomal doxorubicin; R, Figure 2A right.
Discussion
Platinum‐resistant recurrent ovarian cancer is generally refractory to chemotherapy. Although the sample size was small, we found that PLD and CDGP had an unexpected antitumor effect (supplemental online Table 1), in contrast to the reported low efficacy of these drugs for platinum‐resistant ovarian cancer [4], [5]. In addition, overall survival in patients who received either PLD or CDGP subsequent to nivolumab might be longer than that in patients who received other agents, although the difference was not statistically significant; this is possibly because of the limited number of patients (supplemental online Fig. 2). These data suggest that PLD and CDGP might be effective after nivolumab treatment.
The interaction between the tumor microenvironment and chemotherapy is an interesting issue [2]. Tumors are complex tissues containing multiple cell types, such as cancer‐associated structural cells, vascular cells, fibroblasts, and immune cells, and some of these cell types promote tumor progression and metastasis [6], [7]. A recent study in ovarian cancer suggested that immunotherapy might modulate tumor sensitivity to chemotherapy; immunotherapy can abolish cancer‐associated fibroblasts that mediate chemoresistance via interferon γ/signal transducers and activators of transcription (STAT) signaling, which induces cystine and glutathione metabolism [8]. These findings raise the possibility that immunotherapy might modulate the tumor microenvironment and improve sensitivity to subsequent chemotherapy. In fact, concurrent nivolumab and platinum‐based doublet chemotherapy were more effective than chemotherapy alone in advanced lung cancer [9]. However, the effect of sequential chemotherapy and anti‐PD‐1 antibodies has not been investigated in other solid tumors. This study suggests that nivolumab might improve chemosensitivity to subsequent chemotherapy in ovarian cancer, even when nivolumab itself resulted in PD.
The number of patients in this study was very limited. Thus, we cannot conclude that PLD and CDGP are actually effective after nivolumab. A phase III clinical trial of nivolumab in patients with advanced or recurrent ovarian cancer (JapicCTI‐153004) has started in Japan. A follow‐up study of patients from that trial may be used to evaluate our hypothesis.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
We thank the doctors from the following institutions for supporting the follow‐up study: Japanese Red Cross Society Kyoto Daiichi Hospital, Dr. Morio Sawada; Kansai Rosai Hospital, Dr. Kimihiko Ito; Kitano Hospital, Dr. Makoto Sono; Kobe City Medical Center General Hospital, Dr. Shinya Yoshioka; Kyoto Second Red Cross Hospital, Dr. Hiroyuki Fujita; Kyoto Medical Center, Dr. Masahito Takakura; Mitsubishi Kyoto Hospital, Dr. Masumi Sunada; Nagahama Red Cross Hospital, Dr. Hiromi Kawaguchi; Osaka Hospital, Dr. Aya Fukuda; Osaka Red Cross Hospital, Dr. Takafumi Nonogaki; Osaka University Hospital, Dr. Yutaka Ueda; Shizuoka Cancer Center, Dr. Yasuyuki Hirashima; Shiga University Hospital, Dr. Tsukuru Amano. Nivolumab and safety data on nivolumab were kindly supplied by Ono Pharmaceuticals Company (Japan) and Bristol‐Myers Squibb (U.S.) in our physician‐initiated clinical trial (UMIN000005714).
Disclosures
The authors indicated no financial relationships.
References
- 1.Naumann RW and Coleman RL. Management strategies for recurrent platinum‐resistant ovarian cancer. Drugs 2011;71:1397–1412. [DOI] [PubMed] [Google Scholar]
- 2.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011;480:480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamanishi J, Mandai M, Ikeda T et al. Safety and antitumor activity of anti‐PD‐1 antibody, nivolumab, in patients with platinum‐resistant ovarian cancer. J Clin Oncol 2015;33:4015–4022. [DOI] [PubMed] [Google Scholar]
- 4.Goto T, Takano M, Ohishi R et al. Single nedaplatin treatment as salvage chemotherapy for platinum/taxane‐resistant/refractory epithelial ovarian, tubal and peritoneal cancers. J Obstet Gynaecol Res 2010;36:764–768. [DOI] [PubMed] [Google Scholar]
- 5.Mutch DG, Orlando M, Goss T et al. Randomized phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in patients with platinum‐resistant ovarian cancer. J Clin Oncol 2007;25:2811–2818. [DOI] [PubMed] [Google Scholar]
- 6.Hwang RF, Moore T, Arumugam T et al. Cancer‐associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res 2008;68:918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orimo A, Gupta PB, Sgroi DC et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF‐1/CXCL12 secretion. Cell 2005;121:335–348. [DOI] [PubMed] [Google Scholar]
- 8.Wang W, Kryczek I, Dostál L et al. Effector T cells abrogate stroma‐mediated chemoresistance in ovarian cancer. Cell 2016;165:1092–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rizvi NA, Hellmann MD, Brahmer JR et al. Nivolumab in combination with platinum‐based doublet chemotherapy for first‐line treatment of advanced non‐small‐cell lung cancer. J Clin Oncol 2016;34:2969–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]