This article examines interpatient differences in advanced Hodgkin lymphoma incidence and long‐term survival using data from the SEER database. Socioeconomic status and racial heterogeneities in the U.S. health care system have drawn increasing attention from oncologists and epidemiologists; therefore, whether a patient's sex, age, race, and socioeconomic status affect incidence rate and long‐term survival is a focus.
Keywords: Stage III–IV Hodgkin lymphoma, Relative survival, Period analysis, Incidence
Abstract
Background.
Long‐term survival rates for patients with stage III–IV Hodgkin lymphoma, or advanced Hodgkin lymphoma (aHL), have increased substantially since the 1960s. Because large‐scale research of aHL is rare, we aimed to demonstrate the differences in incidence and survival of aHL according to four patient variables in recent decades, with a focus on the outcomes of treatment of aHL and the advancement of public health care.
Materials and Methods.
Data on aHL cases diagnosed during 1984–2013 were extracted from the Surveillance, Epidemiology, and End Results Program database. Relative survival, Kaplan‐Meier, and Cox proportional hazards regression analyses were performed to identify prognosis indicators for aHL.
Results.
The incidence rates for aHL were 1.1, 0.8, and 1.0 per 100,000 in the first, second, and third decades, respectively, during 1984–2013. The 120‐month relative survival rate improved continuously in each decade from 58.5% to 64.6% to 72.1%. In addition, disparities in the 120‐month relative survival rate between male and female patients and among patients of different races narrowed over time. The difference in long‐term survival rate between the poor (medium and high poverty) and rich (low poverty) groups narrowed across the 3 decades.
Conclusion.
The long‐term survival rate for patients with aHL increased in each decade, whereas survival rate disparities according to sex, race, and socioeconomic status narrowed, except for older patients aged >60 years and the high‐poverty group.
Implications for Practice.
Long‐term survival rates of patients with advanced Hodgkin lymphoma were elaborated in this article. The disparities according to sex, race, and socioeconomic status of survival condition were analyzed and showed the development of the public health care system and modern medicine technology.
Introduction
Hodgkin lymphoma (HL) is a canonical type of lymphoma that is highly predominant in both young and elderly populations. From 1992 to 2013, the incidence rate for HL was only about 2 per 100,000 persons [1], [2]. In 2015, approximately 1,150 patients died of HL in the U.S. [3]. Researchers estimated that 8,260 patients would be diagnosed with HL in 2017 [4]. Although various risk factors may impact prognosis for HL, for instance, patient‐related risk factors that affect outcome of its treatment [5], prognosis is believed to have improved substantially with advances in therapy, such as chemotherapy [6], targeted agents such as rituximab [7], and immunotherapy [8]. Furthermore, the renewed use of diagnostic methods such as computed tomography texture analysis provides prognostic information for HL [9]. These dramatic changes in disease management have transformed HL from an incurable disease to a chronic one [10].
Recent studies demonstrated improvement in the survival of patients with early‐stage HL [11], showing the worse survival of adolescent and young adult HL who were diagnosed at an early stage and resided in low socioeconomic status (SES) neighborhoods [12]. Moreover, inferior overall survival was observed in black and Hispanic patients compared with white patients in previous research [13]. However, studies of stage III–IV HL (also known as advanced HL [aHL]) using large data samples have yet to be published. Therefore, in the present study, we aimed to examine interpatient differences in aHL incidence and long‐term survival using data from the Surveillance, Epidemiology, and End Results Program (SEER) database. In addition, SES and racial heterogeneities in the U.S. health care system have drawn increasing attention from oncologists and epidemiologists. Therefore, we sought to determine whether patients’ sex, age, race, and SES impact incidence rate and long‐term survival for aHL.
Materials and Methods
Data Selection
The data on aHL patients over 3 decades (1984–2013) were extracted from the SEER database, which is one of the most respected tumor registration databases in North America, including data from 18 cancer registries. The SEER*Stat software program (version 8.3.2; National Cancer Institute, Bethesda, MD) was used to collect all data. Data on about 26% of the U.S. population is stored in the SEER database, including number of years of diagnosis since 1973 to 2014, age at diagnosis, disease stage, sex, race, and SES. Patients with all primary aHL pathological patterns and Ann Arbor stage III–IV disease were included. Incidence and survival rates for aHL were obtained from the SEER database.
The aHL patient inclusion criteria were based on Lymphoma Subtype Recode/World Health Organization 2008, a primary tumor site and morphology subtype classification scheme for lymphoma. Patients diagnosed as aHL in 1984–2013 with active follow‐up were included, resulting in 7,763 cases for analysis. Cases of HL reported only on a death certificate or diagnosed at autopsy were excluded.
Variable Definitions
Race, sex, SES, and age were the patient variables examined in this study. SES is an economic and sociological measure based on an individual's income, education, and occupation. It is typically broken into three levels: low, medium, and high poverty. In the present study, SES was redefined by changing the low poverty group to the rich group and combining the medium and high poverty groups and calling it the poor group. In addition, the low‐ and medium‐poverty groups were combined and redefined as the majority group, and the high‐poverty group was redefined as the extremely poor group.
Statistical Analysis
The aHL incidence and relative survival rate (RSR) data on the study patients were divided into the 3 decades of the study period and analyzed. In addition, 120‐month RSRs were estimated by fitting a survival rate curve. The survival difference between different patient stratification according to race, sex, age, and SES were analyzed by Kaplan‐Meier analyses. However, when analyzing the patients according to race, data on those who were not white or black were excluded because of heterogeneity in racial composition. All statistical tests were two‐sided, and the significance level was set at .05. All four patient variables were included as covariates in multivariate analysis, which was performed using Cox proportional hazards regression modeling. Hazard ratios and corresponding 95% confidence intervals were calculated for variables and adjusted for all listed covariates of interest. The data were analyzed using the Stata/SE software program (version 12.0; Stata Corp, College Station, TX).
Results
aHL Incidence from 1984 to 2013
We extracted the aHL incidence data over the 3 decades of the study period from the original nine registry sites in the SEER database, resulting in 7,761 cases for use in our study. In the first decade (1984–1993), the aHL incidence rate was highest (1.1 per 100,000). Also, the incidence rate in the second decade (1994–2003: 0.8 per 100,000) was lower than that in the third decade (2004–2013: 1.0 per 100,000; supplemental online Fig. 3; supplemental online Table 1). We found a similar tendency in the HL incidence rate in all patients over the same period. Furthermore, the incidence of stage I–II HL across these 3 decades first increased and then decreased, a totally different trend from that for stage III–IV aHL incidence. The incidence of stage I–II HL was highest in the second decade.
We found similar aHL incidence trends according to age and sex. Specifically, the incidence rate and patient number in the 20‐ to 39‐year age group were the highest among all age groups in all 3 decades. Also, the incidence rate and patient number were higher in male patients (1.4 per 100,000 and 1,543 patients in 1984–1993; 1.1 per 100,000 and 1,379 patients in 1994–2003; and 1.3 per 100,000 and 1,792 patients in 2004–2013) than in female patients (0.8 per 100,000 and 1,017 patients in 1984–1993; 0.6 per 100,000 and 851 patients in 1994–2003; and 0.8 per 100,000 and 1,179 patients in 2004–2013). The aHL incidence rates in the patients 20–39 years old and those older than 60 years were higher than those in the other age groups.
Furthermore, the aHL incidence rate according to SES demonstrated conformation in the rich (1.1 per 100,000 in 1984–1993; 0.9 per 100,000 in 1994–2003; and 1.1 per 100,000 in 2004–2013) and poor groups (1.1 per 100,000 in 1984–1993; 0.8 per 100,000 in 1994–2003; and 1.0 per 100,000 in 2004–2013). The absolute numbers of rich and poor groups were close.
In addition, we found a disparity in aHL incidence rate according to race. In white patients, we saw similar incidence rates over the study period (1.2 per 100,000 in 1984–1993; 0.9 per 100,000 in 1994–2003; and 1.1 per 100,000 in 2004–2013). In black patients, however, the incidence rate was different, as it was higher in the third decade than in the two previous decades (0.9 per 100,000 in 1984–1993 and 1994–2003 and 1.3 per 100,000 in 2004–2013). Additionally, the aHL incidence rates in patients in the other race group (0.4 per 100,000 in 1984–1993; 0.3 per 100,000 in 1994–2003; and 0.5 per 100,000 in 2004–2013) were much lower than those in the white and black groups in each decade.
Relative Survival Estimates for 18 SEER Sites from 1984 to 2013
To illustrate the aHL survival trends from 1984 to 2013, we extracted data on 15,635 cases from 18 SEER registry sites with RSR rates of 12, 60, and 120 months in the first, second, and third decades of the study period, respectively. We found that the long‐term survival rate and RSR increased across all 3 decades in all age groups (supplemental online Table 1; Fig. 1A). Also, the 120‐month RSR increased from 61.7% to 66.8% and then to 71.3% across the 3 decades. This trend of increasing RSR was significant. Kaplan‐Meier analysis of survival confirmed improvement in survival times in all age groups over the 3 decades (Fig. 1B). More importantly, the increase in survival rate in the second decade was much larger than that in the third decade. We found a similar trend for the 12‐month RSR (going from 83.8% to 84.8% and then to 86.2%; supplemental online Table 4) and 60‐month RSR (going from 69.3% to 73.1% and then to 77.0%; supplemental online Table 4). Both rates increased over the 3 decades in each age group as well as overall. In addition, increasing range in each age group was larger in the second decade than in the third decade, except for patients >60 years (going from 26.1% to 31.3% and then to 39.2%; Fig. 1; supplemental online Table 4). The 120‐month RSR and long‐term survival rate also increased in both male and female patients over the 3 decades (Table 1).
Figure 1.
Analysis of survival in patients with advanced Hodgkin lymphoma at 18 Surveillance, Epidemiology, and End Results Program sites according to age group (total and 0–19, 20–39, 40–59, and 60+ years) in 1984–1993, 1994–2003, and 2004–2013. (A): Trends in 120‐month relative survival rate. (B): Kaplan‐Meier survival curves.
Table 1. One hundred twenty‐month relative survival rates in Hodgkin lymphoma patients at 18 Surveillance, Epidemiology, and End Results Program sites according to sex, age group, and decade from 1984 to 2013.

p < .0001 compared with the previous decade.
p < .01 compared with the previous decade.
p < .001 compared with the previous decade.
Abbreviation: SEM, standard error of the mean.
In all age groups, in the first and second decades of the study period, the disparity in long‐term survival rate between the two sexes was significant (p < .0001 in 1984–1993 and p = .0002 in 1993–2004). However, in the third decade, this disparity was not significant (p = .9510). Furthermore, in the 20‐ to 39‐year and 40‐ to 59‐year age groups, the long‐term survival rate and RSR in female patients were significantly higher than those in male patients (p < .0001 in the 20‐ to 39‐year and p < .0001 in the 40‐ to 59‐year age group). In the patients 0–19 years old and those older than 60 years, however, the disparity according to sex was not significant. According to our Kaplan‐Meier survival analysis, the gap in long‐term survival between the two sexes narrowed as the survival time increased (Fig. 2). Hazard ratios for aHL patients according to age, sex, race, and SES were greater than 1, which indicated that elder age, male sex, black race, and poor SES were related to decreased survival. Moreover, sex (p < .0001 in 1984–2013) was always significantly related to survival time across the 3 decades of the study period, whereas age (p < .0001 in 1984–2003) was related to long‐term survival over the first 2 decades and SES was related to long‐term survival over the last 2 decades. After stratification of the patients according to age, sex was significantly related to survival in the first and second decades, and SES was related to it in the last 2 decades. Age, sex, and SES were independent predictors of long‐term survival for aHL (Table 2).
Figure 2.
Analysis of survival in male and female patients with Hodgkin lymphoma at 18 Surveillance, Epidemiology, and End Results Program sites according to age group (total and 0–19, 20–39, 40–59, and 60+ years) from 1984 to 2013. (A): One hundred twenty‐month relative survival rates according to age group. (B): Kaplan‐Meier survival curves according to age group. (C): Kaplan‐Meier survival curves according to study decade.
Table 2. Summary data for Cox proportional hazards regression analysis of survival in advanced Hodgkin lymphoma patients at 18 Surveillance, Epidemiology, and End Results Program sites from 1984 to 2013.

Abbreviations: 95% CI, 95% confidence interval; SES, socioeconomic status.
aHL Survival in Different Race and SES Groups
We observed that the 120‐month RSR was higher in the white patients than in the black patients. However, this disparity in white and black patients was not significant during the study period except in patients 20–39 years old (75.8% vs. 57.1%, p < .0001 in 1984–1993; 81.8% vs. 71.5%, p < .001 in 1993–2003; and 85.3% vs. 76.7%, p < .01 in 2004–2013). In the third decade, the 120‐month RSR (Table 3) in white patients was significantly higher than that in black patients in the 0‐ to 19‐year group (95.0% vs. 75.0%; p < .0001) and the 40‐ to 59‐year group (74.0% vs. 62.2%; p < .01; Table 3). The trends in 12‐ and 60‐month RSR were similar over time (supplemental online Table 2). The gap in survival rate between the white and black patients was narrowed across all 3 decades, and Kaplan‐Meier survival analysis demonstrated the same survival rate trend (p = .0053 in 1984–1993, p = .7675 in 1994–2003, and p = .09632 in 2004–2013).
Table 3. One hundred twenty‐month relative survival rates in advanced Hodgkin lymphoma patients at 18 Surveillance, Epidemiology, and End Results Program sites according to race, age group, and decade from 1984 to 2013.
p < .0001 compared with the previous decade.
p < .01 compared with the previous decade.
p < .001 compared with the previous decade.
Abbreviation: SEM, standard error of the mean.
The 120‐month RSRs in the different SES groups increased from 1984 to 2013. In addition, the 120‐month RSRs in the rich group were significantly higher than those in the poor group across all 3 decades of the study period (65.0% vs. 58.1%, p < .001 in 1984–1993; 71.4% vs. 63.9%, p < .0001 in 1994–2003; and 75.1% vs. 68.6%, p < .001 in 2004–2013; Table 4). We found similar trends in the 12‐ and 60‐month RSRs (supplemental online Table 3). Moreover, as survival time increased, the differences in RSR and survival time between the rich and poor groups narrowed (Fig. 3).
Table 4. One hundred twenty‐month relative survival rates in advanced Hodgkin lymphoma patients at 18 Surveillance, Epidemiology, and End Results Program sites according to socioeconomic status, age group, and decade from 1984 to 2013.
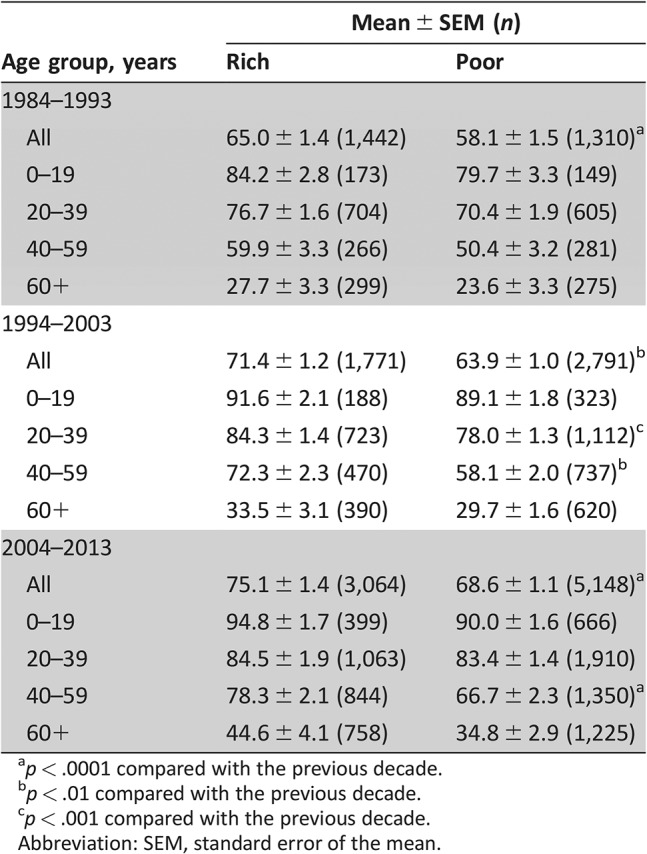
p < .0001 compared with the previous decade.
p < .01 compared with the previous decade.
p < .001 compared with the previous decade.
Abbreviation: SEM, standard error of the mean.
Figure 3.
Analysis of survival in Hodgkin lymphoma patients at 18 Surveillance, Epidemiology, and End Results Program sites according to race and SES from 1984 to 2013. (A): 12‐, 60‐, and 120‐month relative survival rates (RSRs) according to race. (B): 12‐, 60‐, and 120‐month RSRs according to SES. (C): Kaplan‐Meier survival curves according to race and study decade. (D): Kaplan‐Meier survival curves according to SES and study decade.
Abbreviation: SES, socioeconomic status.
Most of the black patients (76.4%) were in the poor group, whereas only half of the white patients were in the poor group (56.6%). In addition, over time, the percentage of white patients in the rich group decreased (from 56.5% in 1984–1993 to 42.2% in 1994–2003 to 39.2% in 2004–2013), but the distribution of black patients in this group changed little over the same period. This difference in racial makeup between the rich and poor group may explain the narrowing gaps in RSR and survival time among the two SES groups (Fig. 1).
Discussion
We observed that the incidence rate for aHL fluctuated over the 3 decades of the study period. In addition, the long‐term survival rate for aHL increased over the same period.
The trend in incidence of aHL was the same as that of HL at all stages but was different from that of stage I–II HL (supplemental online Fig. 2). Because the incidence rate for aHL decreased in the second decade of the study period, the incidence rate for HL at all stages also decreased. Furthermore, the incidence rate for stage I–II HL in the same period increased, demonstrating an increasing number of patients diagnosed with HL at an early stage (stage I–II), which can prevent transformation of HL into aHL in some patients. The diagnosis of HL at an early stage was attributed to advances in the U.S. public health care system.
The increasing long‐term survival rate for aHL across the 3 decades of the study period in all age groups demonstrated improvement in the treatment of HL. Moreover, the long‐term prognosis for aHL was relatively good, with a 120‐month RSR of 68.2% for the entire study period. Also, the 120‐month RSR increased across the 3 decades, going from 61.7% in the first decade to 66.8% in the second decade to 71.3% in the third decade. The Kaplan‐Meier survival analysis demonstrated the same trend. The rapid development of modern medicine (e.g., the discovery of the prognostic significance of expression of the B‐cell marker CD20 [14] as well as the approval of rituximab [15], [16] in 1997) may explain the substantial increase in long‐term survival rates and 120‐month RSR over these 3 decades. The survival improvement also may be attributed to early diagnosis, accurate assessment of the aHL stage, and early treatment of aHL complications [17]. However, in patients older than 60 years, significant disparity among 3 decades could be observed. A recent study showed that the bleomycin lung toxicity (BLT)‐related mortality rate in patients >60 years of age was 30 times higher than that in patients <60 years [18], [19], [20]. With the enrichment of clinical research, clinicians have become more cautious in using drugs, especially bleomycin, in older HL patients, which might further improve the survival rate of HL patients >60 years of age.
The incidence of aHL in male patients was higher than that in female patients across the 3 decades of the study period. Furthermore, female patients had higher long‐term survival rates than did male patients, especially in the 20‐ to 39‐year and 40‐ to 59‐year age groups. The effect on aHL of estrogen may be the main reason for this difference. The protection of estrogen [21] can help female patients survive aHL. Also, a study demonstrated that estrogens, via estrogen receptor β, inhibit proliferation and promote apoptosis of HL cells, producing significant effects on immune functions and lymphoid malignancies [22]. Of note, the gap in long‐term survival between the male and female patients over the 3 decades of the study period narrowed, with no obvious disparity between them in the third decade. This narrowing gap may be attributed to developments in modern medicine. Kaplan‐Meier analysis also illustrated this trend. The age‐dependent survival superiority and reduced incidence of aHL in the female patients indicated the protective role of estrogen in aHL cases.
In the first decade of the study period, the aHL incidence rate in white patients was higher than that in black patients, but this disparity became insignificant in the second decade, and the findings reversed in the third decade, when the rate was higher in black patients than in white ones. Additionally, the aHL incidence rate in the other race group was always much lower than that in the black and white groups. Genetic differences in the other race group and black and white individuals may be the main reason for the incidence difference in these three groups. In addition, the distribution proportion of black patients in the poor group increased across the 3 decades. Furthermore, the disparity in survival rates between the black and white patients was obvious in the first decade. Specifically, white patients had higher 12‐, 60‐, and 120‐month RSRs than did black patients. However, as described above, the disparity in survival rates between these two groups was unobvious in the last 2 decades. Also, Kaplan‐Meier survival analysis demonstrated no significant differences in survival rate between black and white patients except in the first decade. This may be attributed to developments in public health care and increasing access to medicine and other treatments for the black population. Moreover, the increasing incidence rate for stage I–II HL in black patients across the 3 decades reflected enhancement of health consciousness in black patients. HL can be diagnosed at an early stage in black patients more often than in the past, which can improve the effect of therapy for and long‐term survival of this disease. In addition, improvements in treatment played an important role in narrowing the survival gap between the two races. Finally, developments in public health care may have helped increase the diagnosis of stage I–II HL in black patients, explaining the increasing incidence of HL in the black group in our study across the 3 decades.
With respect to SES, we found no significant differences in aHL incidence rate between the rich and poor groups over the 3‐decade study period. However, the rich group had a higher long‐term survival rate than the poor group. Kaplan‐Meier survival analysis also demonstrated a significant disparity in survival rate between the two groups. However, the gap in survival rate between the rich and poor groups narrowed over the 3 decades. This is further evidence of the positive effects of advances in public health care, providing those in the poor group with more medical resources like those available to patients in the rich group, resulting in longer lives. However, in comparing the majority and extremely poor groups, we found that the survival disparity increased across the 3 decades, showing that more should be done in the area of public health care (supplemental online Fig. 4).
Conclusion
This study demonstrated improvement of survival rates in aHL patients over time [23]. It also updates incidence and survival information for aHL cases. We extracted and analyzed information on the aHL incidence and survival rates and their trends for 1984–2013 from the SEER database. Our study was limited by under‐registration, misclassification, and variations in the distribution of patients according to SEER. More importantly, we found that disparities in aHL incidence and survival among patients according to sex, race, and SES narrowed over time. This finding confirms that public health care in the U.S. has improved. Developments in public health care can not only reduce differences between patients in different race and SES groups but also improve the long‐term survival rate for HL via diagnosis of it at an early stage [24], reducing the secondary solid cancer risk in HL patents [25]. As researchers have shown, the major risk factors for HL are unclear. These factors may include genetic factors, Epstein‐Barr virus infection [26], and exposure to some chemicals. Also, researchers have shown that rapid fetal growth and a history of HL in siblings and parents were associated with an increased risk of HL at an early age (0–37 years) [27]. The advances in public health care may reduce the rate of Epstein‐Barr virus infection, which is indirectly connected to HL [28]. In addition, as some studies showed, Epstein‐Barr virus (EBV) infection may decrease survival rates in HL patients, but at the same time, the virus can be used to defeat these EBV‐related lymphomas [29]. For example, by improving the understanding of the role this virus plays in the development of these lymphomas, we can develop drugs that target Epstein‐Barr viral antigens [30]. Moreover, the incidence of HL in patients who receive antiretroviral therapy [31] after human immunodeficiency virus is 5‐fold to 15‐fold higher than that in the general population [32]. Further studies demonstrating these solutions should be performed. In general, treatment of HL and U.S. public health care improved continually over the 3 decades of the study period, resulting in improvement of survival rates and narrowing of the differences in long‐term survival rates among patients of different races and in different SES groups [33]. Furthermore, in the present study, we showed the protective effect of estrogen by detecting differences in the incidence of and survival rates for aHL between male and female patients as well as in patients in different age groups. However, the protective effect of estrogen remains to be confirmed.
By analyzing the differences in aHL survival in patients across 3 decades, we showed the positive effects of significant advances in U.S. public health care. We also showed that adequate health care can overcome disparities in the susceptibility to aHL due to genetic differences according to race, which may predict future trends in the incidence of and long‐term prognosis for aHL.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
This research was supported by the National Natural Science Foundation of China (81500030) and the Natural Science Foundation of Guangdong Province (2016A030313272, 2016A030313277, 2017A030313573).
Contributed equally
Author Contributions
Conception/design: Yan Yan, Shuncong Wang, Haiqing Ma
Collection and/or assembly of data: Yushi Li, Huanhuan Sun, Haiqing Ma
Data analysis and interpretation: Yushi Li, Tiantian Sun
Manuscript writing: Yushi Li, Huanhuan Sun, Haiqing Ma
Final approval of manuscript: Yushi Li, Huanhuan Sun, Yan Yan, Tiantian Sun, Shuncong Wang, Haiqing Ma
Disclosures
The authors indicated no financial relationships.
References
- 1.National Cancer Institute, SEER Stat, Vol. 2016. https://seer.cancer.gov/statfacts/html/hodg.html
- 2. Evens AM, Hutchings M, Diehl V. Treatment of Hodgkin lymphoma: The past, present, and future. Nat Clin Pract Oncol 2008;5:543–556. [DOI] [PubMed] [Google Scholar]
- 3. Hoppe RT, Advani RH, Ai WZ. Hodgkin lymphoma, version 2.2015. J Natl Compr Canc Netw 2015;13:554–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 5. Connors JM. Risk assessment in the management of newly diagnosed classical Hodgkin lymphoma. Blood 2015;125:1693–1702. [DOI] [PubMed] [Google Scholar]
- 6. Nicholson WM, Beard ME, Crowther D et al. Combination chemotherapy in generalized Hodgkin's disease. Br Med J 1970;3:7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Molin D, Linderoth J, Wahlin BE. Nodular lymphocyte predominant Hodgkin lymphoma in Sweden between 2000 and 2014: An analysis of the Swedish Lymphoma Registry. Br J Haematol 2017;177:449–456. [DOI] [PubMed] [Google Scholar]
- 8. Lulla P, Heslop HE. Checkpoint inhibition and cellular immunotherapy in lymphoma. Hematology Am Soc Hematol Educ Program 2016;2016:390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ganeshan B, Miles KA, Babikir S et al. CT‐based texture analysis potentially provides prognostic information complementary to interim FDG‐PET for patients with Hodgkin's and aggressive non‐Hodgkin's lymphomas. Eur Radiol 2017;27:1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bessell EM, Bouliotis G, Armstrong S et al. Long‐term survival after treatment for Hodgkin's disease (1973–2002): Improved survival with successive 10‐year cohorts. Br J Cancer 2012;107:531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Engert A, Raemaekers J. Treatment of early‐stage Hodgkin lymphoma. Semin Hematol 2016;53:165–170. [DOI] [PubMed] [Google Scholar]
- 12. Keegan TH, DeRouen MC, Parsons HM et al. Impact of treatment and insurance on socioeconomic disparities in survival after adolescent and young adult Hodgkin lymphoma: A population‐based study. Cancer Epidemiol Biomarkers Prev 2016;25:264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Evens AM, Antillon M, Aschebrook‐Kilfoy B et al. Racial disparities in Hodgkin's lymphoma: A comprehensive population‐based analysis. Ann Oncol 2012;23:2128–2137. [DOI] [PubMed] [Google Scholar]
- 14. Fu XH, Wang SS, Huang Y et al. Prognostic significance of CD20 expression in Hodgkin and Reed‐Sternberg cells of classical Hodgkin's lymphoma [in Chinese]. Ai Zheng 2008;27:1197–1203. [PubMed] [Google Scholar]
- 15. Maeda LS, Advani RH. The emerging role for rituximab in the treatment of nodular lymphocyte predominant Hodgkin lymphoma. Curr Opin Oncol 2009;21:397–400. [DOI] [PubMed] [Google Scholar]
- 16. Oki Y, Younes A. Does rituximab have a place in treating classic Hodgkin lymphoma? Curr Hematol Malig Rep 2010;5:135–139. [DOI] [PubMed] [Google Scholar]
- 17. Ansell SM. Hodgkin lymphoma: 2016 update on diagnosis, risk‐stratification, and management. Am J Hematol 2016;91:434–442. [DOI] [PubMed] [Google Scholar]
- 18. Evens AM, Helenowski I, Ramsdale E et al. A retrospective multicenter analysis of elderly Hodgkin lymphoma: Outcomes and prognostic factors in the modern era. Blood 2012;119:692–695. [DOI] [PubMed] [Google Scholar]
- 19. Evens AM, Hong F, Gordon LI et al. The efficacy and tolerability of adriamycin, bleomycin, vinblastine, dacarbazine and Stanford V in older Hodgkin lymphoma patients: A comprehensive analysis from the North American intergroup trial E2496. Br J Haematol 2013;161:76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boll B, Goergen H, Behringer K et al. Bleomycin in older early‐stage favorable Hodgkin lymphoma patients: Analysis of the German Hodgkin Study Group (GHSG) HD10 and HD13 trials. Blood 2016;127:2189–2192. [DOI] [PubMed] [Google Scholar]
- 21. Pierdominici M, Maselli A, Locatelli SL et al. Estrogen receptor β ligation inhibits Hodgkin lymphoma growth by inducing autophagy. Oncotarget 2017;8:8522–8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yakimchuk K, Jondal M, Okret S. Estrogen receptor α and β in the normal immune system and in lymphoid malignancies. Mol Cell Endocrinol 2013;375:121–129. [DOI] [PubMed] [Google Scholar]
- 23. Koshy M, Fairchild A, Son CH et al. Improved survival time trends in Hodgkin's lymphoma. Cancer Med 2016;5:997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Noordijk EM, Carde P, Dupouy N et al. Combined‐modality therapy for clinical stage I or II Hodgkin's lymphoma: Long‐term results of the European Organisation for Research and Treatment of Cancer H7 randomized controlled trials. J Clin Oncol 2006;24:3128–3135. [DOI] [PubMed] [Google Scholar]
- 25. Hodgson DC, Gilbert ES, Dores GM et al. Long‐term solid cancer risk among 5‐year survivors of Hodgkin's lymphoma. J Clin Oncol 2007;25:1489–1497. [DOI] [PubMed] [Google Scholar]
- 26. Gottschalk S, Ng CY, Perez M et al. An Epstein‐Barr virus deletion mutant associated with fatal lymphoproliferative disease unresponsive to therapy with virus‐specific CTLs. Blood 2001;97:835–843. [DOI] [PubMed] [Google Scholar]
- 27. Crump C, Sundquist K, Sieh W et al. Perinatal and family risk factors for Hodgkin lymphoma in childhood through young adulthood. Am J Epidemiol 2012;176:1147–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Glaser SL, Lin RJ, Stewart SL et al. Epstein‐Barr virus‐associated Hodgkin's disease: Epidemiologic characteristics in international data. Int J Cancer 1997;70:375–382. [DOI] [PubMed] [Google Scholar]
- 29. Kanakry JA, Ambinder RF. EBV‐related lymphomas: New approaches to treatment. Curr Treat Options Oncol 2013;14:224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roschewski M, Wilson WH. EBV‐associated lymphomas in adults. Best Pract Res Clin Haematol 2012;25:75–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee JY, Dhakal I, Casper C et al. Risk of cancer among commercially insured HIV‐infected adults on antiretroviral therapy. J Cancer Epidemiol 2016;2016:2138259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goedert JJ, Bower M. Impact of highly effective antiretroviral therapy on the risk for Hodgkin lymphoma among people with human immunodeficiency virus infection. Curr Opin Oncol 2012;24:531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Canellos GP, Rosenberg SA, Friedberg JW et al. Treatment of Hodgkin lymphoma: A 50‐year perspective. J Clin Oncol 2014;32:163–168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






