This article presents frontline treatments for elderly patients with mantle cell lymphoma who need treatment in three different fitness levels (fit, unfit, or compromised and frail).
Keywords: Mantle cell lymphoma, Upfront treatment, Older patients
Abstract
The natural history of mantle cell lymphoma (MCL) undergoing chemotherapy is a cyclical pattern of remission followed by recurrence of disease due to acquired chemotherapy resistance. The median age of the occurrence of MCL is 65 years, so half of the newly diagnosed MCL patients are considered “elderly.” The tolerance to long‐term chemotherapy in elderly patients is decreased; hence, the response to frontline therapy used is of paramount importance. We hope that our review may guide clinicians in treating such populations in a more personalized and evidence‐based manner.
In the older patients with risk variables, frontline treatment is determined according to different body status of fit, unfit or compromised, and frail. In the fit patients, the pursuit of remission and prolongation of survival might currently justify the use of more intense and toxic therapies. For unfit or compromised older patients, disease control needs to be prioritized, maintaining a balance between the benefits and toxicities of the treatment. For frail patients, tolerance of treatment and minimizing myelotoxicity should be the primary focus. “Chemotherapy‐free” regimens are likely to be considered as the first‐line strategy for this population. On the other hand, in the older MCL population without risk variables, observation or “watch and wait” can prevent overtreatment. Furthermore, more clinical trials and research studies on novel agents and targeted therapies need to be translated into the general population to provide optimal treatment and to guide personalized treatment.
Implications for Practice.
This review emphasizes the importance of frontline therapies for older MCL patients. MCL patients commonly experience a cyclical pattern of remission followed by recurrence of disease due to acquired chemotherapy resistance. As a special population, elderly patients have various comorbidities and decreased organ function, which may reduce the chances of undergoing treatment for recurrent disease. Thus, this older population of patients with MCL should be treated separately and exceptionally. So far, systematic reviews with regard to frontline treatment for older patients with MCL have not been encountered, but the hope is that this review may guide clinicians in treating such populations in a more personalized and evidence‐based manner.
Introduction
Mantle cell lymphoma (MCL) is an incurable subtype of non‐Hodgkin's lymphoma with a median age of occurrence of 65 years [1], [2]. More than half of newly diagnosed MCL patients are thus considered “elderly.” Some scholars consider the threshold of age to be 60 years; on the other hand, some patients aged <70 years with performance status = 0 can tolerate intensive chemotherapy regimens usually reserved for young MCL patients. As a special population, elderly patients have various comorbidities and decreased organ function, which may result in reduced tolerance to chemotherapy. Moreover, social and family factors may lead to more complexities with their management, which could potentially reduce the chances of undergoing treatment for recurrent disease [3], [4], [5].
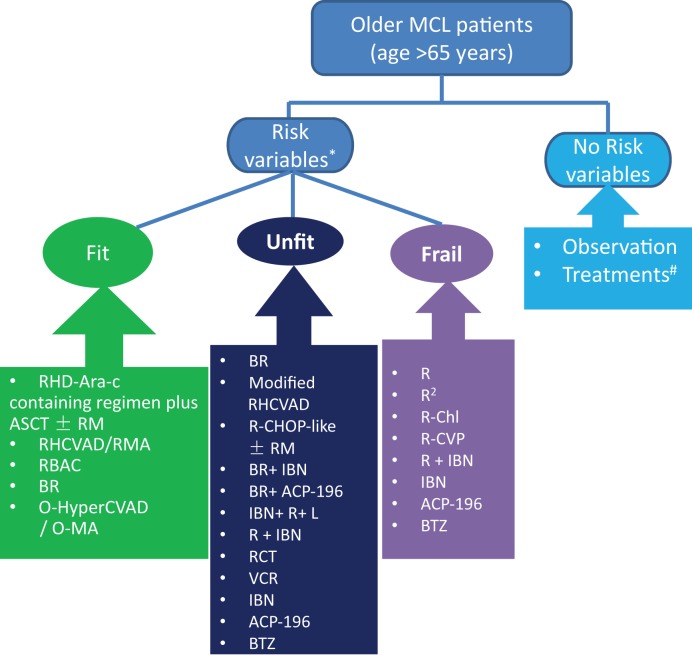
Furthermore, elderly MCL patients with various risk profiles should be treated separately. A personalized treatment for MCL patients is usually based on validated prognostic tools, such as the MIPI‐c (the combination of Mantle Cell Lymphoma International Prognostic Index [MIPI] and the Ki‐67 Proliferative Index), and early predictors of treatment response, such as minimal residual disease analysis, as well as genomic alterations [6] As for prognostic genomic alterations, TP53 was shown to be a significant independent molecular marker predicting dismal outcome for MCL patients [7], [8], [9], [10], and NOTCH1/2 mutations and MYC oncogene have also been associated with an aggressive clinical process [11], [12], [13], [14]. Thus, for elderly MCL patients with adverse prognostic factors, therapy should be offered, while those with good prognostic variables can even be observed due to remarkable treatment‐related toxicity in this population (Fig. 1). We discuss here frontline treatments for elderly MCL patients who need treatment in three different fitness levels—fit, unfit or compromised, and frail. (Fig. 1) Actually, under each fitness level, no randomized controlled trials exist that can be used as evidence that one regimen is better than others. In clinical practice, we still need to evaluate different individual circumstances and the patient's own choice.
Figure 1.
Frontline treatment for older patients with MCL. *Risk variables: intermediate or high MIPI‐c (the combination of the Ki‐67 index and the Mantle Cell Lymphoma International Prognostic Index), positive minimal residual disease, maximum tumor diameter ≥3 cm, abnormal serum LDH and β2–microglobulin levels, B symptoms, adverse mutation like TP53, NOTCH1/2, and MYC, and blastoid/pleomorphic histology. #If localized bulky diseases with risk to end organ are observed, radiotherapy would be given. If disseminated, then observation is the first option.
Abbreviations: ACP‐196, acalabrutinib; ASCT, autologous hematopoietic stem cell transplantation; B, bendamustine; BTZ, bortezomib; Chl, chlorambucil; CVP, cyclophosphamide, vincristine, and prednisone; HCVAD, hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone; HD‐Ara‐c, high‐dose cytorabine; IBN, ibrutinib; MCL, mantle cell lymphoma; O‐HCVAD/O‐MA, ofatumumab hyper‐fractionated cyclophosphamide, doxorubicin, vincristine, and dexamethasone alternating with ofatumumab high‐dose cytarabine and methotrexate; R, rituximab; R2, rituximab plus lenalidomide; RB/RC, rituximab‐bendamustine followed by rituximab‐cytarabine; RCT, rituximab, cladribine, and temsirolimus; RM, rituximab maintenance; VCR, bortezomib, cladribine, and rituximab.
Older MCL Patients with Risk Variables
Frontline Therapy for Elderly Fit MCL Patients (Table 1)
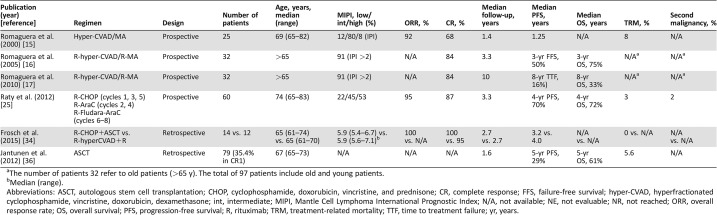
Table 1. Frontline treatment for older fit mantle cell lymphoma patients.
The number of patients 32 refer to old patients (>65 y). The total of 97 patients include old and young patients.
Median (range).
Abbreviations: ASCT, autologous stem cell transplantation; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CR, complete response; FFS, failure‐free survival; hyper‐CVAD, hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone; int, intermediate; MIPI, Mantle Cell Lymphoma International Prognostic Index; N/A, not available; NE, not evaluable; NR, not reached; ORR, overall response rate; OS, overall survival; PFS, progression‐free survival; R, rituximab; TRM, treatment‐related mortality; TTF, time to treatment failure; yr, years.
RHCVAD/MA.
Rituximab plus fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with rituximab plus methotrexate‐cytarabine (HCVAD/MA) with adjustment of the cytarabine (1 g/m2/dose) is an active regimen for elderly patients (>65 years) with untreated MCL [15]. In an early phase II study from MD Anderson Cancer Center, 25 patients, including 6 patients with gastrointestinal (GI) involvement, received HCVAD/MA regimen up to eight cycles, without stem cell transplant (SCT). The overall response rate (ORR) was 92%, and the complete remission (CR) rate was 68%. After a median follow‐up of 17 months, the median failure‐free survival (FFS) was 15 months. Hematologic toxicity was remarkable after four cycles, including 89% of grade 4 neutropenia and 75% of grade 4 thrombocytopenia, but only 5% of the cycles were associated with grade 3 infection. Treatment‐related death occurred in two patients. Subsequently, rituximab (R) was added in HCVAD/MA for elderly MCL patients, and this produced a 3‐year FFS and overall survival (OS) for elderly patients aged >65 years of 50% and 75%, respectively [16]. The principal toxicity in the study was still hematologic, especially significant grade 4 neutropenia and thrombocytopenia. In both young and older patients, 29% of 97 patients did not finish their intended number of cycles because of toxicity. The rate of dose adjustments (decrease in dose) caused by any toxic effect was significantly higher in the elderly patients (>65 years) compared with the younger patients (≤65 years; p = .00001). After 10 years of follow‐up, patients older than 65 years had 8‐year time to treatment failure (TTF) and OS rates of 16% and 33%, respectively, which were significantly less than the TTF and OS rates for patients aged 65 years or younger (46% and 68%, p = .003 and .0007, respectively) [17]. Thus, this intense chemoimmunotherapy without SCT is effective for younger (≤65 years) patients with untreated aggressive MCL but is not recommended for older patients because of significant acute toxicities in this age group [18], [19], [20].
High‐Dose Cytarabine‐Containing Regimens Except for HCVAD/MA.
In light of favorable outcomes with high‐dose cytarabine (HD‐Ara‐c)‐containing induction chemotherapy in younger MCL patients [21], [22], [23], [24], HD‐Ara‐c‐containing regimens have been used in clinical trials for elderly fit MCL patients. However, randomized prospective controlled studies conducted in this area are scarce. A multicenter, prospective phase 2 study from the Finnish Lymphoma Group added HD‐Ara‐c (cycles 2, 4, and 6–8; the total cumulative dose is 20 g/m2) into induction chemotherapy for 60 cases of MCL (age: 65–83 years). Intermediate‐ and high‐risk untreated MCL patients accounted for 98% and 87% of the patients. Induction therapy included alternating standard dose R‐CHOP and R‐AraC. Thereafter, three cycles (R‐Fludara‐AraC, cycles 6–8) included R‐AraC with two doses of fludarabine, and the last two cycles included standard dose CHOP (cycles 9–10), which resulted in very good response (ORR: 95%, unconfirmed complete remission (CR/Cru): 87%). Additionally, 11 cases of stable disease (SD), partial remission (PR), or unconfirmed complete remission (CRu) obtained further remission after R‐Fludara‐AraC regimen (cycles 6–8). Subsequently, 45 cases were reported to have a negative test for minimal residue disease (MRD) in the bone marrow by flow cytometry. After a median follow‐up of 3.3 years, progression‐free survival (PFS) and OS at 4 years was about 70% each in all patients [25]. This study was characterized by patients with older age, mostly intermediate‐ to high risk, and high intensity of regimens with multiple cycles (up to 10). There were no severe infections during induction, with infections of grade 3 in 12 patients and grade 4 in only 1 patient during the first course (R‐CHOP) of treatment. The treatment‐related mortality (TRM) was low (3%). However, 17% of patients who responded (CR + PR), because of tolerance issues, were not able to receive more than six courses of chemotherapy. In addition, nearly one third of patients needed a reduced dose of chemotherapy. Of note, due to the associated toxicities, the agent fludarabine is no longer used in MCL.
Another regimen containing HD‐Ara‐c is R‐MACLO‐IVAM‐T (Cycle 1 R‐MACLO: rituximab, doxorubicin, cyclophosphamide, vincristine, methotrexate, leucovorin. Cycle 2 R‐IVAM: rituximab, cytarabine with the total cumulative dose of 8 g/m2, etoposide, ifosfamide, and mesna). This study also obtained a favorable survival outcome, with 78% 3‐year PFS in 22 cases of untreated MCL. However, it was also toxic (before mentioning death, you need to mention percentage of grade 3–4 neutropenia, thrombocytopenia, infections, or any other toxicity). Two patients (9%) died: one from sepsis during cycle 1 and another at 38 months while in remission from MCL. However, the total sample size was small and with merely eight cases (37%) aged 60–79 years [26]. Thus, the role of this regimen in older patients needs to be further elucidated.
Autologous Hematopoietic Stem Cell Transplantation.
Autologous hematopoietic stem cell transplantation (ASCT) plays an important role in consolidation in young and fit MCL patients, as shown by numerous phase II studies demonstrating significantly prolonged survival [27], [28], [29], [30]. Thus far, the data with respect to ASCT in older MCL patients are limited primarily due to a small sample size [31], [32], [33], [34], [35]. In the second Nordic MCL trial, patients who received intensive therapy followed by ASCT were mostly younger than 65 years (41 cases older than 60 years) [29]. Very few serial studies reported ASCT as the frontline consolidation therapy in MCL patients older than 65 years.
The European Group for Blood and Marrow Transplantation registry retrospectively analyzed 712 cases of MCL who underwent ASCT between 2000 and 2007. Patients were compared between 79 cases of older than 65 years (patients in CR1 and PR1 at transplantation accounted for 35.4% and 26.6%, respectively) and 633 cases of younger than 65 years. They found that there were no differences in engraftment of neutrophils (12 vs. 12 days) and platelets (13 vs. 13 days), relapse rate (66% vs. 55%), PFS (29% vs. 40%), and OS (61% vs. 67%) at 5 years between both groups. Multivariate analysis showed age ≥65 at transplant was not associated with risk of nonrelapse mortality (NRM). The NRM at 3 months and 5 years after transplantation in patients older than 65 years was not higher than that in patients younger than 65 years. The only factor of importance in NRM was the number of treatment lines (two or more) prior to ASCT. No special toxicity of the patients older than 65 with consolidation SCT was reported in this retrospective study. This study also suggested that ASCT could be performed in selected patients older than 65 years. In addition, these older patients received intensive induction chemotherapy prior to ASCT. Therefore, in order to improve survival for older patient with MCL through transplantation, age is not a major constraint if the decision for ASCT is based on appropriate case selection along with utilization of adequate treatment strategies prior to and after transplantation [36].
In order to understand the efficacy and toxicity of intensive chemotherapy and ASCT for older MCL patients, Abramson Cancer Center retrospectively analyzed 38 cases of intermediate‐ to high‐risk MCL according to the MIPI score and who were older than 60 years (range: 61–74 years) [34] and who had received R‐CHOP or R‐HyperCVAD/MA with or without consolidation ASCT. There were 21 cases (55%) who received consolidation ASCT after R‐CHOP (14 cases, 74%) or after R‐HyperCVAD/MA (7 cases, 37%). All patients achieved CR, including five cases of PR after induction therapy. At a median follow‐up of 2.7 years, 20 patients (57%) developed progressive disease. The median PFS (mPFS) and median OS (mOS) were 3.2 and 6 years, respectively. This study demonstrated a similar PFS between the group that received R‐CHOP followed by ASCT and the group that received R‐HyperCVAD without ASCT as the first‐line therapy for older patients with MCL. Also, the toxicity profile was not significantly different between the two groups. ASCT‐related mortality was not observed. Fifteen patients (71%) experienced infection, and eight patients (38%) experienced mucositis requiring patient‐controlled analgesia or nutritional support. Other adverse events included five patients requiring intensive care and four patients requiring readmission less than 100 days after discharge due to ASCT‐related complications. However, the toxicity was markedly different between R‐HyperCVAD and R‐CHOP for induction. Many more transfusions were required (100% vs. 62%, p = .008) and more dose reductions (33% vs. 6%, p = .08) were observed in the R‐HyperCVAD group than in the R‐CHOP group. All the patients in the R‐CHOP group completed all cycles, whereas only 67% of patients in the HyperCVAD group completed them (p = .01). Of note, compared with the group who only received R‐CHOP chemotherapy, these two intensive therapies could achieve longer PFS for intermediate‐ to high‐risk patients. This needs to be further verified in prospective, randomized, controlled trials with larger sample sizes.
Bendamustine‐Based Regimen plus Low‐Dose Cytarabine.
These intensive regimens mentioned above are effective but with remarkable toxicities. Since the bendamustine plus rituximab (BR) was reported by Rummel et al. [37], the BR regimen has become a standard first‐line therapy for older MCL patients [38], which can be used in elderly fit or unfit MCL patients. We discuss the BR regimen in unfit MCL patients. In elderly fit MCL patients, BR plus low‐dose cytarabine (800–500 mg/m2 × 3 days) was tested.
In a phase II study, R‐BAC (rituximab, bendamustine, and cytarabine 800 mg/m2 × 3d) without maintenance rituximab demonstrated encouraging results in 20 cases of previously untreated fit older (age ≥65 years) patients with MCL [39], with an ORR of 100% and a CR rate of 95%. At a median follow‐up of 2.2 years, the 2‐year PFS and duration of response (DOR) rates were 95% and 100%, respectively. However, hematologic toxicities occurred frequently, with grade 3/4 thrombocytopenia and neutropenia occurring in 70% and 17% of untreated patients, respectively. Grade 3 or 4 infections occurred in five patients, including refractory or relapsed patients. Because of this, another multicenter trial (FIL‐RBAC500) was initiated with a reduced cytarabine dose of 500 mg/m2 [40, 41], which resulted in a substantially reduced hematological toxicity. The proportion of cycles requiring platelet transfusion was reduced from 118 (65%) of 182 cycles of RBAC800 to 89 (29%) of 304 cycles of RBAC500, and the frequencies of severe hemorrhage or infection were low. Efficacy was still excellent, with a CR rate of 91%, negative MRD testing in 51% of tested BM samples, and a 2‐year PFS of 81%. Thus, R‐BAC500 is a safe treatment that can be administered as first‐line therapy to elderly fit patients with MCL.
Elderly fit MCL patients with risk variables need intensive therapy in order to achieve survival benefit. Thus, our recommendation is to use RHD‐Ara‐c containing regimen plus ASCT as frontline therapy in this population.
Frontline Therapy for “Unfit or Compromised” Older MCL Patients
For the “unfit or compromised” patients, the aim of treatment is to get the disease under adequate control, because the pursuit of remission with intense toxic therapy could induce damage from side effects due to the comorbidities and decreased organ function. Therefore, regimens like bendamustine‐based therapy, modified R‐HCVAD, and RCHOP are proposed to these unfit older patients with MCL.
For the “unfit or compromised” patients, the aim of treatment is to get the disease under adequate control, because the pursuit of remission with intense toxic therapy could induce damage from side effects due to the comorbidities and decreased organ function. Therefore, regimens like bendamustine‐based therapy, modified R‐HCVAD, and RCHOP are proposed to these unfit older patients with MCL.
Bendamustine‐Based Therapy (Table 2)
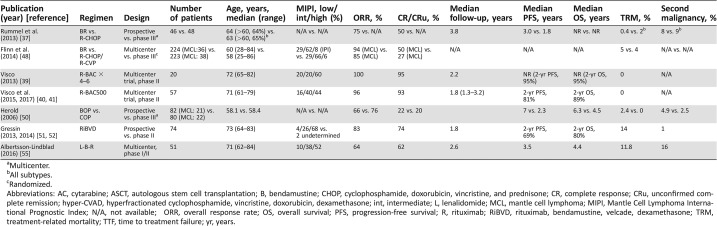
Table 2. Bendamustine‐based frontline treatment for older fit or unfit mantle cell lymphoma patients.
Multicenter.
All subtypes.
Randomized.
Abbreviations: AC, cytarabine; ASCT, autologous stem cell transplantation; B, bendamustine; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CR, complete response; CRu, unconfirmed complete remission; hyper‐CVAD, hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone; int, intermediate; L, lenalidomide; MCL, mantle cell lymphoma; MIPI, Mantle Cell Lymphoma International Prognostic Index; N/A, not available; ORR, overall response rate; OS, overall survival; PFS, progression‐free survival; R, rituximab; RiBVD, rituximab, bendamustine, velcade, dexamethasone; TRM, treatment‐related mortality; TTF, time to treatment failure; yr, years.
Bendamustine, as a single agent or in combination with rituximab, has been a very effective and well‐tolerated treatment for relapsed or refractory MCL patients [42], [43], [44], [45], [46], [47]. This prompted its use in frontline combination therapy for elderly MCL patients. The BR regimen was studied in the StiL trial, which was an open‐label, randomized, controlled phase III trial among 81 centers in Germany between 2003 and 2008. In this trial, patients were randomized to either BR or R‐CHOP for a maximum of six courses. Of 514 patients entered in the study, 94 were MCL (ages: 64.5–74, median: 70) and 420 were cases of other types of indolent lymphoma (279 follicular lymphoma, 67 marginal zone lymphoma, 41 lymphoplasmacytic lymphoma, 21 small lymphocytic lymphoma, 12 low‐grade, unclassifiable lymphoma). The ORR did not differ between the two treatment arms (242 [93%] of 261 patients in the BR group vs. 231 [91%] of 253 in the R‐CHOP group); however, the CR rate was significantly increased in patients in the BR group (104 [40%] vs. 76 [30%]; p = .021). At median follow‐up of 45 months, median PFS was significantly longer in the BR group than in the R‐CHOP group for MCL patients (35.4 [28.8–54.9] vs. 22.1 [15.1–33.8]; p = .0044), whereas OS did not differ between the treatment groups and median OS was not reached in either group for all subtypes of lymphoma [37]. Fewer adverse events were observed in the BR group than in the R‐CHOP group. There were significantly fewer hematological toxic effects in patients in the BR group than in the R‐CHOP group, with less grade 3–4 leucocytopenia (p < .0001) and neutropenia (p < .0001). Infections were significantly less frequent in patients in the BR group than in the R‐CHOP group. Severe infectious complications like sepsis with a fatal outcome were also less frequent in the BR group than in the R‐CHOP group (one patient vs. five patients). Twenty secondary malignancies were observed in the BR group (261 assessed) compared with 23 in the R‐CHOP group (253 assessed), with one hematological malignancy in each group (one case of myelodysplasticsyndrome (MDS) in the BR group and one of acute myelocytic leukemia (AML) in the R‐CHOP group). This study suggested that the BR regimen had higher CR rates and longer PFS as frontline therapy for untreated older MCL patients compared with the R‐CHOP regimen. Since then, the BR regimen has gradually become the standard therapy for newly diagnosed older MCL patients. However, the CR rate (40%) of the BR regimen is still low.
In order to verify the efficacy and toxicity of the BR regimen, the randomized, noninferiority, global, phase III BRIGHT trial compared BR (n =213 [n = 36 MCL]) with R‐CHOP/R‐CVP (n = 206 [n = 38 MCL]) in indolent NHL (iNHL) and MCL, suggesting that BR is noninferior to R‐CHOP/R‐CVP with regard to clinical response [48]. In MCL patients, the CR rate in the BR arm was superior to that in standard therapy (CR rate ratio: 1.95; p = .018; 22 patients received R‐CHOP and 11 R‐CVP). After a median follow‐up of about 65 months, the updated BRIGHT study has confirmed that PFS, event‐free survival, and DOR were significantly better for BR, and OS was not yet statistically different between BR and R‐CHOP/R‐CVP [49]. The hazard ratio (95% confidence interval) for PFS was 0.70 (p = .0582) in iNHL and PFS 0.40 (p = .0035) in MCL comparing BR versus R‐CHOP/R‐CVP, which indicated MCL patients benefit most from BR. Adverse events including vomiting and drug‐hypersensitivity reactions were markedly higher in BR group (p < .05), and peripheral neuropathy/paresthesia and alopecia were significantly higher in the standard‐therapy regimens group (p < .05). The incidence of infections was not significantly different across treatment groups. Grade 3/4 leucocytopenia were more common in the BR group, and grade 3/4 neutropenia were more common in the standard chemotherapy group, most notably R‐CHOP. No significant difference of death incidence was observed between the BR group and the standard‐therapy group (12 deaths, 5% vs. 9 deaths, 4%). These data indicate BR is noninferior to standard therapy with acceptable safety profile. However, this BRIGHT trial included not only old patients but also young patients (age: 25–86 years).
An early randomized phase III study from Germany reported that the combination of bendamustine, vincristine, and prednisone (BOP) had similar CR rates to those of the cyclophosphamide, vincristine, prednisone (COP) regimen (22% vs. 20%), but the projected 5‐year survival rate of the BOP group was higher than that of the COP group (61% vs. 46%). The most common adverse event requiring dosage reduction was leucopenia, and the two groups had a similar frequency of leucopenia (63% of COP cycles vs. 55% of BOP cycles). Except for old patients with MCL, the 164 untreated cases (18–75 years of age) included young MCL patients and other B cell lymphoma (follicular lymphoma and plasmacytic lymphoma); however, the study still demonstrated the efficacy of the BOP regimen for old MCL patients, with a 5‐year survival of 43% [50].
A study based on the combination of bendamustine and bortezomib was conducted by the LYSA group, bortezomib being the first drug approved for use in the treatment of relapsed MCL. In this prospective phase II trial “Lymphome Du Manteau 2010 SA,” the RiBVD regimen (rituximab IV, bendamustine IV, velcade 1.3 mg/m2 subcutaneously on days 1, 4, 8, and 11, dexamethasone IV) was applied in 74 cases of previously untreated elderly MCL (>65 years or not eligible for ASCT). Patients with intermediate‐ and high‐risk MIPI scores accounted for 94% of the total cases. After four courses, the ORR and CR/CRu rates were 86% and 57%, respectively. Treatment with an additional two courses raised the CR/CRu rate by 17%. Eighty‐six percent of patients achieved MRD negativity in the peripheral blood after six courses, and this was significantly correlated with PFS and OS. At a median follow‐up of 1.8 years, 2‐year PFS and OS were 69% and 80%, respectively. Thus, this regimen was shown to be very effective as the first‐line chemotherapy for older MCL patients but with considerably high toxicities [51], [52]. The adverse events included grade 3 or 4 neutropenia (21%), grade 3 or 4 thrombocytopenia (15%), grade 3 or 4 anemia (2%), and four (6%) toxic deaths (one pneumonia, two cardiac arrest, and one following progressive multifocal leukoencephalopathy).
One of the newer agents approved in MCL is lenalidomide [53], [54]. The Nordic Lymphoma Group evaluated in a phase I + II trial (NLG‐MCL4 trial) the combination of L‐R‐B (lenalidomide‐rituximab‐bendamustine) for frontline treatment of elderly MCL patients [55]. The CR/CRu rate achieved after an induction phase was 64%. This result was higher than the 50% CR rate in the MCL subgroup of the R‐B arm in the BRIGHT trial, although the BRIGHT trial included positron emission tomography (PET) as part of the response evaluation, and it is difficult to compare separate clinical trial results [48]. However, the CR rate of L‐R‐B was inferior to that of 74% achieved after six cycles of R‐B plus bortezomib (RiBVD) in untreated patients with similar patient characteristics, as well as to the CR rate of 93%–95%, observed with R‐B in combination with cytarabine (R‐BAC) in the subgroup of untreated MCL patients after 4–6 cycles [39], [40], [52]. Of note, the L‐R‐B regimen in this study was associated with an unfavorable safety profile including a high rate of infections (42%) and of second malignancies (16%). The hematologic adverse events included grade 3–4 neutropenia (38%) and thrombocytopenia (10%). Six cases (11.8%) of TRM were observed, including three due to infection and two due to secondary malignancies (lung cancer and chronicmyelomonocyticleukemia (CMML)). One patient with progressive disease died without a report of the cause of death. Nine secondary malignancies were found in eight patients (16%) during follow‐up.
Modified R‐HCVAD plus Maintenance R.
The Wisconsin Oncology Network study recently published on a nonintensive regimen incorporating bortezomib into a previously reported modified R‐HyperCVAD regimen VcR‐CVAD for the treatment of 30 cases (median age: 61, range: 48–74) of previously untreated MCL (60% intermediate‐/high‐risk MIPI) [56] The VcR‐CVAD regimen is built upon a modified R‐hyperCVAD chemotherapy with maintenance rituximab, by the incorporation of bortezomib (VcR‐CVAD). The VcR‐CVAD regimen achieved higher ORR (90% vs. 77%) and CR rate (77% vs. 64%) than modified R‐hyperCVAD. The active anti‐MCL component of this regimen was due to the suppression of the crucial NF‐kappa B pathway of MCL by bortezomib [57], [58], along with the synergistic effect of bortezomib with R and cyclophosphamide [59], and the shorter cycle interval (21 days vs. 28 days) [56], [60]. After a median follow‐up of 3.5 years, the 3‐year PFS and OS were 63% and 86%, respectively. There were no significant differences noted in PFS or OS between patients <60 and patients ≥60 years of age. Thus, by adding bortezomib for elderly patients with previously untreated MCL, VcR‐CVAD showed an approximately 10% improvement in 3‐year PFS and OS [16], which were also comparable to the outcomes with other more intensive regimens [61]. Major toxicities from VcR‐CVAD included myelosuppression and peripheral neuropathy (PPN). Grade 3–4 neutropenia and thrombocytopenia were observed in 20% and 23% of patients, respectively. Nine patients experienced grade 3–4 PPN. The addition of bortezomib seemed not to worsen the myelosuppression but did increase the risk of peripheral neuropathy, requiring dose adjustments in bortezomib and vincristine. However, patients younger than 65 years accounted for the majority in these studies. Hence, it is still difficult to compare between this regimen and regimens that have patients uniformly older than 65 years. This study provided the basis for combination chemotherapy with bortezomib in a clinical practice setting [56], which was further confirmed in the multicenter setting by the Eastern Cooperative Oncology Group E1405 study, in which VcR‐CVAD followed by maintenance rituximab was used in newly diagnosed MCL (median age: 62 years, range: 40–76 years) [62].
Subsequently, a pivotal LYM‐3002 study phase III trial compared R‐CHOP with VR‐CAP (vincristine in R‐CHOP regimen is replaced by bortezomib) in previously untreated MCL patients. The two groups had similar sample sizes (n = 243–244) and age distribution (median: 65–66, ≥60 years accounting for 73% of the population). The CR rate of VR‐CAP (53%) was significantly higher than that of R‐CHOP (42%). At a median follow‐up of 3.3 years, the median duration time of CR of VR‐CAP group was significantly longer (1.5 years vs. 3.5 years). The mPFS was also improved by 59% (1.2 years vs. 2.1 years, p < .001), but there were no significant differences in mOS among the two groups. In this study, most patients were older, and hematologic toxicity was common. As compared with the VR‐CAP group, lower rates of grade 3–4 neutropenia (67% vs. 85%) and thrombocytopenia (6% vs. 57%) were observed in the R‐CHOP group. Drug‐related death, such as infection, cardiac failure, etc., was observed in 14 patients (6%) in the R‐CHOP group and 11 patients (5%) in the VR‐CAP group. Low‐ and intermediate‐risk patients benefited most from this regimen [63]. Therefore, this study led to extended U.S. Food and Drug Administration approval for bortezomib in previously untreated MCL in October 2014 [64]. Later, a LYM‐3002 clinical trial compared R‐CHOP with VR‐CAP in newly diagnosed MCL patients unsuitable for SCT, which showed that the VR‐CAP regimen almost doubled PFS relative to R‐CHOP (24.7 vs. 14.4 months; hazard ratio = 0.63, p < .001). VR‐CAP was also a cost‐effective treatment [65].
Another bortezomib‐containing combination therapy comes from GOELAMS in France, which predominantly focuses on patients with high‐risk MIPI score. This prospective, multi‐institutional phase II study utilized the RiPADC regimen (bortezomib in combination with doxorubicin, dexamethasone, chlorambucil, and rituximab) without maintenance therapy for newly diagnosed older (age >65 years) MCL patients [66]. MIPI high‐risk‐score patients accounted for 70% of the participants in the study (n = 39). Patients who achieved PR or CR after four courses could receive two more courses, accounting for a total of six courses. The ORR and CR was 74% and 59%, respectively. At a median follow‐up of 2.3 years, the mPFS was 26 months and mOS was not reached. Treatment‐related grade 3 and 4 adverse events predominantly comprised myelosuppression (neutropenia for 8% of patients and thrombocytopenia for 11%), infection, lung toxicity, and peripheral neuropathy. TRM was 5%, which is high for this age population.
R‐CHOP–Like Therapy (Table 3)
Table 3. Doxorubicin‐based frontline treatment for older unfit mantle cell lymphoma patients.
Abbreviations: αIFN, α interferon; ASCT, autologous stem cell transplantation; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CR, complete response; hyper‐CVAD, hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone; int, intermediate; MIPI, Mantle cell lymphoma International Prognostic Index; N/A, not available; NE, not evaluable; NR, not reached; ORR, overall response rate; OS, overall survival; PFS, progression‐free survival; R, rituximab; RiPAD+C, rituximab, bortezomib, doxorubicin, dexamethasone and chlorambucil; TRM, treatment‐related mortality; TTF, time to treatment failure; VR‐CAP, cyclophosphamide, doxorubicin, bortezomib, prednisone; yr, years.
The European MCL network conducted a large, prospective, phase III, randomized controlled trial that compared R‐CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) with R‐FC (rituximab, fludarabine and cyclophosphamide) in 457 evaluable patients older than 60 years. Patients who showed response underwent a second randomization to maintenance therapy with rituximab (R) or interferon alfa [2]. The CR rate was similar between the R‐CHOP and R‐FC regimen (40% vs. 34%), but the ORR was lower after R‐FC (78% vs. 87%; p = .0508). Specifically, progression of disease was more frequent during treatment with R‐FC (14% vs. 5%), and the mOS was significantly inferior after R‐FC (40 vs. 64 months; p = .0072). In addition, more patients in R‐FC group relapsed. Fludarabine in the R‐FC arm was thought to have contributed to the remarkable toxicities in older patients, which included more frequent, long‐lasting hematologic grade 3–4 toxicity and the immunosuppression of T cell functions and more life‐threatening infections.
In an unplanned statistical analysis of this study, among patients who had a response to R‐CHOP, maintenance therapy with R significantly improved OS. Subsequently, a study from Japan demonstrated R maintenance therapy is effective as well and prolonged OS for patients 60 years of age and older with newly diagnosed MCL [67]. Only grade 1 or 2 infections were observed during long‐term R maintenance after R‐CHOP. Therefore, R‐CHOP induction followed by maintenance therapy with R can be recommended for older patients with newly diagnosed MCL.
So far, the longest mOS achieved for older patients aged >65 years is 5.7 years, which was reported by a multicenter phase II trial in which R‐CHOP was given for four cycles followed by radioimmunotherapy (RIT; 90Y–ibritumomab tiuxetanin) to 21 cases of MCL patients aged >65 years. The ORR and CR rate was found to be 76% and 48%, respectively. Response rates did not differ by age, which were 86% (30 of 35) for patients 65 years old compared with 76% (16 of 21) for patients older than age 65 [68]. Although the number of patients in the trial is small, these data correlated with the known sensitivity of MCL to radiotherapy. In addition, this combination has no unexpected short‐ or long‐term toxicities. Of the 51 patients who received RIT, 46 patients had a grade 2 or greater reduction in neutrophils, hemoglobin, or platelets. After 10‐year follow‐up, all second malignancies included therapy‐related myeloid neoplasia (n = 1), non‐small cell lung cancer (n = 2), bladder cancer (n = 1), ampullary adenocarcinoma (n = 1), and resected localized nonmelanoma skin cancers (n = 2). Long‐term follow‐up of this cohort of patients treated with a brief <4‐month therapeutic regimen consisting of R‐CHOP for four cycles followed by 90Y‐RIT demonstrated this combination is an effective regimen for the initial treatment of MCL [69]. However, in this new drug era, R‐CHOP followed by RIT may not be of high priority in clinical practice. Furthermore, this combination is currently not a recommended regimen as part of the National Comprehensive Cancer Network Guidelines for B‐cell Lymphomas (version 5.2017, September 26, 2017) for the frontline treatment of MCL. In our opinion, as compared with RCHOP, BR is more effective and has less toxicity. Thus, we favor BR over the combination of bendamustine with other myelotoxic regimens such as immunoradiotherapy in elderly MCL patients.
For unfit or compromised elderly MCL patients, a comprehensive risk‐benefit assessment is needed to find a balance between the benefit and the toxicities of the treatment in order to decide the best available treatment. Thus, our recommendation is to use the BR regimen as frontline therapy in this population.
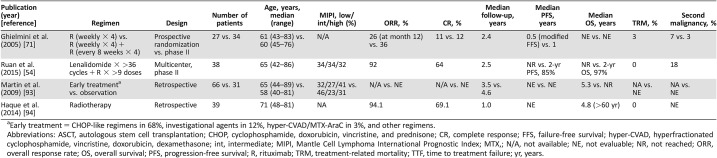
Frontline Therapy for “Frail” Older MCL Patients (Table 4)
Table 4. Frontline treatment for older frail mantle cell lymphoma patients or older patients without risk variables.
Early treatment = CHOP‐like regimens in 68%, investigational agents in 12%, hyper‐CVAD/MTX‐AraC in 3%, and other regimens.
Abbreviations: ASCT, autologous stem cell transplantation; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CR, complete response; FFS, failure‐free survival; hyper‐CVAD, hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone; int, intermediate; MIPI, Mantle Cell Lymphoma International Prognostic Index; MTX,; N/A, not available; NE, not evaluable; NR, not reached; ORR, overall response rate; OS, overall survival; PFS, progression‐free survival; R, rituximab; TRM, treatment‐related mortality; TTF, time to treatment failure; yr, years.
For “frail” MCL patients, improvement in quality of life and palliative treatment should take precedence over prolonged survival. Thus, a mild immune‐chemotherapy regimen is suitable. A study from the Swiss Group for Clinical Cancer Research showed the monotherapy with R is active in older previously untreated MCL patients [71]. This study included 104 cases of MCL aged from 41 to 83 years (median age: 65 years). After the induction phase of monotherapy R, patients who achieved SD, PR, and CR were then randomized to two arms. Patients in arm A did not receive any treatment, whereas patients in arm B received rituximab 375 mg/m2 every other month for an additional four doses. After the induction phase, 34 cases of previous untreated MCL showed ORR and CR rates of 27% and 3%, respectively. At a median follow‐up of 2.4 years, the ORR and the duration time of response in the two arms were similar. Also, there was no significant difference in median survival time between the two arms (0.5 year vs. 1 year, p = .1). Due to mild overall toxicity with about 12% of grade 3–4 hematologic toxicities, monotherapy with R is suitable for the treatment of frail older patients with MCL.
Lenalidomide plus rituximab (R2) is another potential first‐line therapy for “unfit or compromised” older MCL patients. A multicenter phase II study from Weill Cornell Medical College designed the R2 regimen as the first‐line therapy for 38 cases of MCL (median age: 65 years, range: 42–86 years). The trial included induction and maintenance phases. During the induction phase, lenalidomide was given at a dose of 20 mg daily on days 1–21 of every 28‐day cycle for 12 cycles; If there were no evident side effects at the first course, the dose of lenalidomide was increased to 25 mg daily. Then it was reduced to 15 mg daily at the maintenance phase. Rituximab was given once a week in the first 4 weeks and then once every other cycle for at least 36 cycles or until the disease progressed. This trial demonstrated a very good ORR (92%) and CR rate (64%), with improvement in quality of life and with tolerable hematologic and nonhematologic (mainly rash) toxicity. At a median follow‐up of 2.5 years, the 2‐year PFS and OS was 85% and 97%, respectively [54]; although this was not a randomized trial, the ORR and PFS were similar to historical controls.
Other oral chemotherapy such as chlorambucil [72], [73], prednisone, cyclophosphamide [74], [75], [76], [77], or the oral combination of these drugs can be used as frontline therapy for “frail” older MCL patients [78], [79], [80]. The tolerance to oral single‐agent chlorambucil [73] and combination of chlorambucil with R was good in published studies [81], [82]. None of the patients developed granulocytopenia or significant nausea and vomiting after the treatment of single‐agent chlorambucil with a mean total dose of 600 mg [73]. The most important toxicity for the combination of chlorambucil with R was hematological, with grade 3–4 neutropenia and thrombopenia in five (36%) and three (21%) patients, respectively [82]. In this study, patients received a median of six cycles (range: 2–11) of chlorambucil and four cycles (range: 2–12) of rituximab [82]. However, the sample size of the trial was not adequately large, with only two cases of previously untreated MCL being reported [82]. Also, frail old patients with MCL can be treated with the R‐CVP regimen, in which anthracycline‐like drugs are removed from the R‐CHOP regimen due to cardiac toxicity.
In addition, with the development of targeted, less‐toxic therapies for lymphoma such as ibrutinib [83], acalabrutinib [84], [85], and venetoclax [86], among others currently on clinical trials [87], [88], [89], [90], [91], [92], “unfit” and “frail” elderly MCL patients stand to benefit more from their use in the frontline setting.
For frail elderly MCL patients, the primary focus should be on tolerance of treatment and minimizing myelotoxicity. Thus, we recommend use of the “chemo‐free” regimen R plus ibrutinib or R2 as frontline therapy in this population.
For frail elderly MCL patients, the primary focus should be on tolerance of treatment and minimizing myelotoxicity. Thus, we recommend use of the “chemo‐free” regimen R plus ibrutinib or R2 as frontline therapy in this population.
Older MCL Patients Without Risk Variables (Table 4)
For the older MCL population without adverse prognostic variables, observation or “watch and wait” could be proposed. In a retrospective analysis of 97 patients with MCL, 31 patients (32%) were observed for more than 3 months before initial systemic therapy. The observation group had a median age of 58 years (range, 40–81 years). Compared with the treatment group, better performance status and lower‐risk standard International Prognostic Index scores were more commonly present in those undergoing observation. After a median follow‐up of 55 months, the survival of the observation group was significantly superior to that of the early treatment group (not reached vs. 64 months, p = .004) [93]. Thus, in older MCL patients with low‐risk variables, observation or “watch and wait” can be an acceptable first management choice.
However, if localized bulky diseases with risk to end organ are observed, radiotherapy would be given (Fig. 1). Accumulating evidence demonstrated that MCL is a radio‐sensitive disease with excellent responses to relatively low radiation doses [94].
We recommend observation of patients fulfilling all the following: Ki‐67 less than 30%, maximum tumor diameter less than 3 cm, normal serum LDH and β 2 –microglobulin levels, lack of B symptoms, low MIPI score, without C‐myc, TP53 and NOTCH1/2 mutations, and nonblastoid/pleomorphic histology [95].
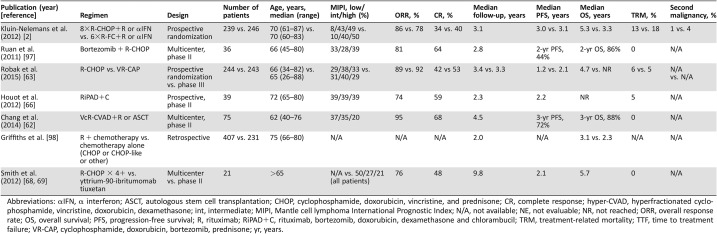
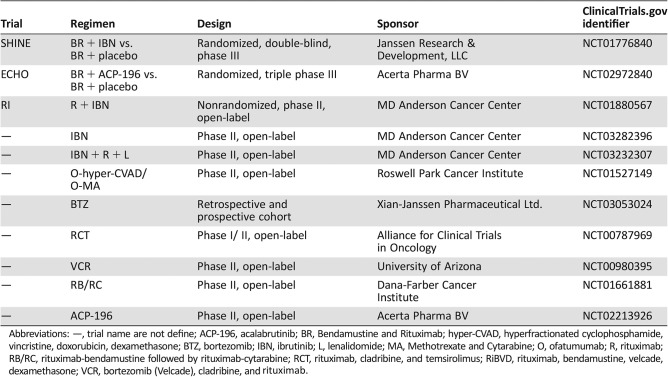
Ongoing Trials on Frontline Therapy for Older MCL Patients (Table 5)
Table 5. Ongoing trials using novel agents alone or in combination as frontline therapy for older MCL patients.
Abbreviations: —, trial name are not define; ACP‐196, acalabrutinib; BR, Bendamustine and Rituximab; hyper‐CVAD, hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone; BTZ, bortezomib; IBN, ibrutinib; L, lenalidomide; MA, Methotrexate and Cytarabine; O, ofatumumab; R, rituximab; RB/RC, rituximab‐bendamustine followed by rituximab‐cytarabine; RCT, rituximab, cladribine, and temsirolimus; RiBVD, rituximab, bendamustine, velcade, dexamethasone; VCR, bortezomib (Velcade), cladribine, and rituximab.
In order to increase the CR rate and prolong survival, additional drugs are being tested in combination with the BR regimen. The international, double‐blind, randomized, controlled phase III trial SHINE enrolled patients 65 years or older with newly diagnosed MCL (No. 2013‐0056) and treated them with six cycles of a standard regimen of bendamustine and rituximab plus either ibrutinib or placebo. The objective of the trial was to prolong PFS and, potentially, OS. If the primary objective is achieved, this could become the new standard for elderly MCL patients around the world. The RI (rituximab plus ibrutinib) trial has achieved high ORR (100%) and CR (90%) in young newly diagnosed MCL patients [96]. However, it is still not clear in older patients. If this RI trial can bring favorable outcome, this combination will become another “chemo‐free” option for newly diagnosed older MCL patients.
Conclusion
The frontline treatment for older MCL patients is of paramount importance, in that older patients are a population with specific characteristics. The treatment goals for older patients with MCL are markedly different from those of young MCL patients (<65 years). In the fit older population with risk variables, the pursuit of remission and prolongation of survival might currently justify the use of more intense and toxic therapies. Our first choice of frontline therapy for this population is RHD‐Ara‐c‐containing regimen plus ASCT. For unfit or compromised older patients, a balance between the benefits and toxicities of the treatment should be considered. Our first choice of frontline therapy for this population is BR regimen. For frail patients, tolerance of treatment and minimizing myelotoxicity should be the primary focus. We recommend “chemotherapy‐free” regimens, such as R plus ibrutinib or R2, as the first choice for this population. On the other hand, in the older MCL population without risk variables, observation or “watch and wait” can prevent overtreatment. Furthermore, more clinical trials and research studies on novel agents and targeted therapies need to be translated into the general population in order to provide optimal treatment and to guide personalized treatment.
Acknowledgments
This work was supported by the generous philanthropic contributions to The University of Texas MD Anderson Moon Shots Program. It was also supported by Zhejiang Provincial Natural Science Foundation of China (LY13H080003) to Haige Ye.
Contributed equally
Author Contributions
Conception/design: Michael L. Wang
Provision of study material or patients: Haige Ye, Aakash Desai
Collection and/or assembly of data: Haige Ye, Aakash Desai, Dongfeng Zeng
Data analysis and interpretation: Haige Ye
Manuscript writing: Haige Ye, Aakash Desai
Final approval of manuscript: Haige Ye, Aakash Desai, Dongfeng Zeng, Jorge Romaguera, Michael L. Wang
Disclosures
The authors indicated no financial relationships.
References
- 1. Dreyling M, Hiddemann W, European MCL Network. Current treatment standards and emerging strategies in mantle cell lymphoma. Hematology Am Soc Hematol Educ Program 2009:542–551. [DOI] [PubMed] [Google Scholar]
- 2. Kluin‐Nelemans HC, Hoster E, Hermine O et al. Treatment of older patients with mantle‐cell lymphoma. N Engl J Med 2012;367:520–531. [DOI] [PubMed] [Google Scholar]
- 3. Repetto L, Fratino L, Audisio RA et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: An Italian Group for Geriatric Oncology study. J Clin Oncol 2002;20:494–502. [DOI] [PubMed] [Google Scholar]
- 4. Tucci A, Ferrari S, Bottelli C et al. A comprehensive geriatric assessment is more effective than clinical judgment to identify elderly diffuse large cell lymphoma patients who benefit from aggressive therapy. Cancer 2009;115:4547–4553. [DOI] [PubMed] [Google Scholar]
- 5. Balducci L. New paradigms for treating elderly patients with cancer: The comprehensive geriatric assessment and guidelines for supportive care. J Support Oncol 2003;1:30–37. [PubMed] [Google Scholar]
- 6. Dreyling M, Ferrero S, European Mantle Cell Lymphoma Network. The role of targeted treatment in mantle cell lymphoma: Is transplant dead or alive? Haematologica 2016;101:104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Halldorsdottir AM, Lundin A, Murray F et al. Impact of TP53 mutation and 17p deletion in mantle cell lymphoma. Leukemia 2011;25:1904–1908. [DOI] [PubMed] [Google Scholar]
- 8. Slotta‐Huspenina J, Koch I, de Leval L et al. The impact of cyclin D1 mRNA isoforms, morphology and p53 in mantle cell lymphoma: P53 alterations and blastoid morphology are strong predictors of a high proliferation index. Haematologica 2012;97:1422–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greiner TC, Moynihan MJ, Chan WC et al. P53 mutations in mantle cell lymphoma are associated with variant cytology and predict a poor prognosis. Blood 1996;87:4302–4310. [PubMed] [Google Scholar]
- 10. Hernandez L, Fest T, Cazorla M et al. P53 gene mutations and protein overexpression are associated with aggressive variants of mantle cell lymphomas. Blood 1996;87:3351–3359. [PubMed] [Google Scholar]
- 11. Kridel R, Meissner B, Rogic S et al. Whole transcriptome sequencing reveals recurrent NOTCH1 mutations in mantle cell lymphoma. Blood 2012;119:1963–1971. [DOI] [PubMed] [Google Scholar]
- 12. Bea S, Valdes‐Mas R, Navarro A et al. Landscape of somatic mutations and clonal evolution in mantle cell lymphoma. Proc Natl Acad Sci U S A 2013;110:18250–18255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin CY, Loven J, Rahl PB et al. Transcriptional amplification in tumor cells with elevated c‐MYC. Cell 2012;151:56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klapproth K, Wirth T. Advances in the understanding of MYC‐induced lymphomagenesis. Br J Haematol 2010;149:484–497. [DOI] [PubMed] [Google Scholar]
- 15. Romaguera JE, Khouri IF, Kantarjian HM et al. Untreated aggressive mantle cell lymphoma: Results with intensive chemotherapy without stem cell transplant in elderly patients. Leuk Lymphoma 2000;39:77–85. [DOI] [PubMed] [Google Scholar]
- 16. Romaguera JE, Fayad L, Rodriguez MA et al. High rate of durable remissions after treatment of newly diagnosed aggressive mantle‐cell lymphoma with rituximab plus hyper‐CVAD alternating with rituximab plus high‐dose methotrexate and cytarabine. J Clin Oncol 2005;23:7013–7023. [DOI] [PubMed] [Google Scholar]
- 17. Romaguera JE, Fayad LE, Feng L et al. Ten‐year follow‐up after intense chemoimmunotherapy with rituximab‐hyperCVAD alternating with rituximab‐high dose methotrexate/cytarabine (R‐MA) and without stem cell transplantation in patients with untreated aggressive mantle cell lymphoma. Br J Haematol 2010;150:200–208. [DOI] [PubMed] [Google Scholar]
- 18. Bernstein SH, Epner E, Unger JM et al. A phase II multicenter trial of hyperCVAD MTX/Ara‐C and rituximab in patients with previously untreated mantle cell lymphoma; SWOG 0213. Ann Oncol 2013;24:1587–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chihara D, Cheah CY, Westin JR et al. Rituximab plus hyper‐CVAD alternating with MTX/Ara‐C in patients with newly diagnosed mantle cell lymphoma: 15‐year follow‐up of a phase II study from the MD Anderson Cancer Center. Br J Haematol 2016;172:80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Merli F, Luminari S, Ilariucci F et al. Rituximab plus HyperCVAD alternating with high dose cytarabine and methotrexate for the initial treatment of patients with mantle cell lymphoma, a multicentre trial from Gruppo Italiano Studio Linfomi. Br J Haematol 2012;156:346–353. [DOI] [PubMed] [Google Scholar]
- 21. Hermine O, Hoster E, Walewski J et al. Alternating courses of 3x CHOP and 3x DHAP plus rituximab followed by a high dose ARA‐C containing myeloablative regimen and autologous stem cell transplantation (ASCT) increases overall survival when compared to 6 courses of CHOP plus rituximab followed by myeloablative radiochemotherapy and ASCT in mantle cell lymphoma: Final analysis of the MCL younger trial of the European Mantle Cell Lymphoma Network (MCL net). Blood 2012;120:151a. [Google Scholar]
- 22. Gouill SL, Thieblemont C, Oberic L et al. Rituximab maintenance versus wait and watch after four courses of R‐DHAP followed by autologous stem cell transplantation in previously untreated young patients with mantle cell lymphoma: First interim analysis of the phase III prospective Lyma trial, a Lysa study. Blood 2014:124146a. [Google Scholar]
- 23. Delarue R, Haioun C, Ribrag V et al. CHOP and DHAP plus rituximab followed by autologous stem cell transplantation in mantle cell lymphoma: A phase 2 study from the Groupe d'Etude des Lymphomes de l'Adulte. Blood 2013;121:48–53. [DOI] [PubMed] [Google Scholar]
- 24. van 't Veer MB, de Jong D, MacKenzie M et al. High‐dose Ara‐C and beam with autograft rescue in R‐CHOP responsive mantle cell lymphoma patients. Br J Haematol 2009;144:524–530. [DOI] [PubMed] [Google Scholar]
- 25. Raty R, Honkanen T, Jantunen E et al. Prolonged immunochemotherapy with rituximab, cytarabine and fludarabine added to cyclophosphamide, doxorubicin, vincristine and prednisolone and followed by rituximab maintenance in untreated elderly patients with mantle cell lymphoma: A prospective study by the Finnish Lymphoma Group. Leuk Lymphoma 2012;53:1920–1928. [DOI] [PubMed] [Google Scholar]
- 26. Lossos IS, Hosein PJ, Morgensztern D et al. High rate and prolonged duration of complete remissions induced by rituximab, methotrexate, doxorubicin, cyclophosphamide, vincristine, ifosfamide, etoposide, cytarabine, and thalidomide (R‐MACLO‐IVAM‐T), a modification of the National Cancer Institute 89‐C‐41 regimen, in patients with newly diagnosed mantle cell lymphoma. Leuk Lymphoma 2010;51:406–414. [DOI] [PubMed] [Google Scholar]
- 27. Vigouroux S, Gaillard F, Moreau P et al. High‐dose therapy with autologous stem cell transplantation in first response in mantle cell lymphoma. Haematologica 2005;90:1580–1582. [PubMed] [Google Scholar]
- 28. Dreger P, Rieger M, Seyfarth B et al. Rituximab‐augmented myeloablation for first‐line autologous stem cell transplantation for mantle cell lymphoma: Effects on molecular response and clinical outcome. Haematologica 2007;92:42–49. [DOI] [PubMed] [Google Scholar]
- 29. Geisler CH, Kolstad A, Laurell A et al. Long‐term progression‐free survival of mantle cell lymphoma after intensive front‐line immunochemotherapy with in vivo‐purged stem cell rescue: A nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood 2008;112:2687–2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Robinson S, Dreger P, Caballero D et al. The EBMT/EMCL consensus project on the role of autologous and allogeneic stem cell transplantation in mantle cell lymphoma. Leukemia 2015;29:464–473. [DOI] [PubMed] [Google Scholar]
- 31. Buadi FK, Micallef IN, Ansell SM et al. Autologous hematopoietic stem cell transplantation for older patients with relapsed non‐Hodgkin's lymphoma. Bone Marrow Transplant 2006;37:1017–1022. [DOI] [PubMed] [Google Scholar]
- 32. Gopal AK, Rajendran JG, Gooley TA et al. High‐dose [131i]tositumomab (anti‐CD20) radioimmunotherapy and autologous hematopoietic stem‐cell transplantation for adults > or = 60 years old with relapsed or refractory B‐cell lymphoma. J Clin Oncol 2007;25:1396–1402. [DOI] [PubMed] [Google Scholar]
- 33. Gopal AK, Gooley TA, Golden JB et al. Efficacy of high‐dose therapy and autologous hematopoietic stem cell transplantation for non‐hodgkin's lymphoma in adults 60 years of age and older. Bone Marrow Transplant 2001;27:593–599. [DOI] [PubMed] [Google Scholar]
- 34. Frosch Z, Luskin MR, Landsburg DJ, et al. R‐CHOP or R‐HyperCVAD with or without autologous stem cell transplantation for older patients with mantle cell lymphoma. Clin Lymphoma Myeloma Leuk 2015;15:92–97. [DOI] [PubMed] [Google Scholar]
- 35. de la Rubia J, Saavedra S, Sanz GF et al. Transplant‐related mortality in patients older than 60 years undergoing autologous hematopoietic stem cell transplantation. Bone Marrow Transplant 2001;27:21–25. [DOI] [PubMed] [Google Scholar]
- 36. Jantunen E, Canals C, Attal M et al. Autologous stem‐cell transplantation in patients with mantle cell lymphoma beyond 65 years of age: A study from the European Group for Blood and Marrow Transplantation (EBMT). Ann Oncol 2012;23:166–171. [DOI] [PubMed] [Google Scholar]
- 37. Rummel MJ, Niederle N, Maschmeyer G et al. Bendamustine plus rituximab versus CHOP plus rituximab as first‐line treatment for patients with indolent and mantle‐cell lymphomas: An open‐label, multicentre, randomised, phase 3 non‐inferiority trial. Lancet 2013;381:1203–1210. [DOI] [PubMed] [Google Scholar]
- 38. Lipsky A, Martin P. Bendamustine‐rituximab in mantle cell lymphoma. Lancet Haematol 2017;4:e2–e3. [DOI] [PubMed] [Google Scholar]
- 39. Visco C, Finotto S, Zambello R et al. Combination of rituximab, bendamustine, and cytarabine for patients with mantle‐cell non‐hodgkin lymphoma ineligible for intensive regimens or autologous transplantation. J Clin Oncol 2013;31:1442–1449. [DOI] [PubMed] [Google Scholar]
- 40.Visco.C CA S, Franceschetti C, Patti S, et al. Rituximab, bendamustine and cytarabine (RBAC500) as induction therapy in elderly patients with mantle cell lymphoma: A phase 2 study from the Fondazione Italiana Linfomi. Hematol Oncol 2015;33:100–180. [Google Scholar]
- 41. Visco C CA S, Franceschetti C, Patti S et al. Rituximab, bendamustine and cytarabine (rbac500) as induction therapy in elderly patients with mantle cell lymphoma: A phase 2 study from the fondazione italiana linfomi. Hematological oncology 2015;33:100–180. [Google Scholar]
- 42. Cheson BD, Rummel MJ. Bendamustine: Rebirth of an old drug. J Clin Oncol 2009;27:1492–1501. [DOI] [PubMed] [Google Scholar]
- 43. Rummel MJ, Al‐Batran SE, Kim SZ et al. Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low‐grade non‐Hodgkin's lymphoma. J Clin Oncol 2005;23:3383–3389. [DOI] [PubMed] [Google Scholar]
- 44. Robinson KS, Williams ME, van der Jagt RH et al. Phase II multicenter study of bendamustine plus rituximab in patients with relapsed indolent B‐cell and mantle cell non‐Hodgkin's lymphoma. J Clin Oncol 2008;26:4473–4479. [DOI] [PubMed] [Google Scholar]
- 45. Kahl BS, Bartlett NL, Leonard JP et al. Bendamustine is effective therapy in patients with rituximab‐refractory, indolent B‐cell non‐Hodgkin lymphoma: Results from a multicenter study. Cancer 2010;116:106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rummel M, Kaiser U, Balser C et al. Bendamustine plus rituximab versus fludarabine plus rituximab for patients with relapsed indolent and mantle‐cell lymphomas: A multicentre, randomised, open‐label, non‐inferiority phase 3 trial. Lancet Oncol 2016;17:57–66. [DOI] [PubMed] [Google Scholar]
- 47. Matsumoto K, Takayama N, Aisa Y et al. A phase II study of bendamustine plus rituximab in Japanese patients with relapsed or refractory indolent B‐cell non‐Hodgkin lymphoma and mantle cell lymphoma previously treated with rituximab: BRB study. Int J Hematol 2015;101:554–562. [DOI] [PubMed] [Google Scholar]
- 48. Flinn IW, van der Jagt R, Kahl BS et al. Randomized trial of bendamustine‐rituximab or R‐CHOP/R‐CVP in first‐line treatment of indolent NHL or MCL: The BRIGHT study. Blood 2014;123:2944–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Flinn I, van der Jagt R, Chang JE et al. First‐line treatment of iNHL or MCL patients with BR or R‐CHOP/R‐CVP: Results of the BRIGHT 5‐year follow‐up study. J Clin Oncol 2017;35(suppl 15):7500a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Herold M, Schulze A, Niederwieser D et al. Bendamustine, vincristine and prednisone (BOP) versus cyclophosphamide, vincristine and prednisone (COP) in advanced indolent non‐Hodgkin's lymphoma and mantle cell lymphoma: Results of a randomised phase III trial (OSHO# 19). J Cancer Res Clin Oncol 2006;132:105–112. [DOI] [PubMed] [Google Scholar]
- 51. Gressin R, Callanan M, Daguindau N et al. The RIBVD regimen (rituximab IV, bendamustine IV, velcade SC, dexamethasone IV) offers a high complete response rate in elderly patients with untreated mantle cell lymphoma. Preliminary results of the Lysa trial “Lymphome Du Manteau 2010 SA”. Blood 2013;122:370a. [Google Scholar]
- 52. Gressin R, Callanan M, Daguindau N et al. Frontline therapy with the RIBVD regimen elicits high clinical and molecular response rates and long PFS in elderly patients mantle cell lymphoma (MCL); final results of a prospective phase II trial by the Lysa group. Blood 2014;124:148. [Google Scholar]
- 53. Gunnellini M, Falchi L. Therapeutic activity of lenalidomide in mantle cell lymphoma and indolent non‐Hodgkin's lymphomas. Adv Hematol 2012;2012:523842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ruan J, Martin P, Shah B et al. Lenalidomide plus rituximab as initial treatment for mantle‐cell lymphoma. N Engl J Med 2015;373:1835–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Albertsson‐Lindblad A, Kolstad A, Laurell A et al. Lenalidomide‐bendamustine‐rituximab in untreated mantle cell lymphoma > 65 years, the Nordic Lymphoma Group phase I+II trial NLG‐MCl4. Blood 2016;128:1814–1820. [DOI] [PubMed] [Google Scholar]
- 56. Chang JE, Peterson C, Choi S et al. VcR‐CVAD induction chemotherapy followed by maintenance rituximab in mantle cell lymphoma: A Wisconsin Oncology Network Study. Br J Haematol 2011;155:190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pham LV, Tamayo AT, Yoshimura LC et al. Inhibition of constitutive NF‐kappa B activation in mantle cell lymphoma B cells leads to induction of cell cycle arrest and apoptosis. J Immunol 2003;171:88–95. [DOI] [PubMed] [Google Scholar]
- 58. Lv H, Li Y, Du H et al. The synthetic compound norcantharidin induced apoptosis in mantle cell lymphoma in vivo and in vitro through the PI3K‐Akt‐NF‐kappa B signaling pathway. Evid Based Complement Alternat Med 2013;2013:461487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang M, Han XH, Zhang L et al. Bortezomib is synergistic with rituximab and cyclophosphamide in inducing apoptosis of mantle cell lymphoma cells in vitro and in vivo. Leukemia 2008;22:179–185. [DOI] [PubMed] [Google Scholar]
- 60. Kahl BS, Longo WL, Eickhoff JC et al. Maintenance rituximab following induction chemoimmunotherapy may prolong progression‐free survival in mantle cell lymphoma: A pilot study from the Wisconsin Oncology Network. Ann Oncol 2006;17:1418–1423. [DOI] [PubMed] [Google Scholar]
- 61. Damon LE, Johnson JL, Niedzwiecki D et al. Immunochemotherapy and autologous stem‐cell transplantation for untreated patients with mantle‐cell lymphoma: CALGB 59909. J Clin Oncol 2009;27:6101–6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chang JE, Li H, Smith MR et al. Phase 2 study of VcR‐CVAD with maintenance rituximab for untreated mantle cell lymphoma: An Eastern Cooperative Oncology Group study (e1405). Blood 2014;123:1665–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Robak T, Huang H, Jin J et al. Bortezomib‐based therapy for newly diagnosed mantle‐cell lymphoma. N Engl J Med 2015;372:944–953. [DOI] [PubMed] [Google Scholar]
- 64.Velcade (bortezomib) for injection prescribing information. Available at http://www.velcade.com/files/pdfs/velcade_prescribing_information.pdf. Accessed May 13, 2003.
- 65. Van Keep MM, Gairy K, Seshagiri D et al. Cost effectiveness of bortezomib, rituximab, cyclophosphamide, doxorubicin and prednisone for the first‐line treatment of mantle cell lymphoma not eligible for stem cell transplantation: A Scottish perspective. Value Health 2015;18:A453. [Google Scholar]
- 66. Houot R, Le Gouill S, Ojeda Uribe M et al. Combination of rituximab, bortezomib, doxorubicin, dexamethasone and chlorambucil (RIPAD+C) as first‐line therapy for elderly mantle cell lymphoma patients: Results of a phase II trial from the GOELAMS. Ann Oncol 2012;23:1555–1561. [DOI] [PubMed] [Google Scholar]
- 67. Inoue N, Nishimura N, Takahashi A et al. Rituximab maintenance therapy is an effective therapy in over‐sixties with mantle cell lymphoma. Blood 2015;126:5081. [Google Scholar]
- 68. Smith MR, Li H, Gordon L et al. Phase II study of rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone immunochemotherapy followed by yttrium‐90‐ibritumomab tiuxetan in untreated mantle‐cell lymphoma: Eastern Cooperative Oncology Group study e1499. J Clin Oncol 2012;30:3119–3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Smith MR, Hong F, Li H, et al. Mantle cell lymphoma initial therapy with abbreviated r-chop followed by (90)y-ibritumomab tiuxetan: 10-year follow-up of the phase 2 ecog-acrin study e1499. Leukemia 2017;31:517–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ghosh DB, Karki BB. Solid‐liquid density and spin crossovers in (Mg, Fe)O system at deep mantle conditions. Sci Rep 2016;6:37269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ghielmini M, Schmitz SF, Cogliatti S et al. Effect of single‐agent rituximab given at the standard schedule or as prolonged treatment in patients with mantle cell lymphoma: A study of the Swiss Group for Clinical Cancer Research (SAKK). J Clin Oncol 2005;23:705–711. [DOI] [PubMed] [Google Scholar]
- 72. Rospond‐Kubiak I, Kociecki J, Stopa M. Primary uveal lymphoma effectively managed with oral chlorambucil: A case report. J Med Case Rep 2013;7:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ben Simon GJ, Cheung N, McKelvie P et al. Oral chlorambucil for extranodal, marginal zone, B‐cell lymphoma of mucosa‐associated lymphoid tissue of the orbit. Ophthalmology 2006;113:1209–1213. [DOI] [PubMed] [Google Scholar]
- 74. Warry E, Hansen RJ, Gustafson DL et al. Pharmacokinetics of cyclophosphamide after oral and intravenous administration to dogs with lymphoma. J Vet Intern Med 2011;25:903–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Smith SM, Johnson J, Cheson BD et al. Recombinant interferon‐alpha2b added to oral cyclophosphamide either as induction or maintenance in treatment‐naive follicular lymphoma: Final analysis of CALGB 8691. Leuk Lymphoma 2009;50:1606–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lepicard A, Lamarque D, Levy M, et al. Duodenal mucosa‐associated lymphoid tissue lymphoma: Treatment with oral cyclophosphamide. Am J Gastroenterol 2000;95:536–539. [DOI] [PubMed] [Google Scholar]
- 77. Benboubker L, Linassier C, Delain M et al. Mediastinal large‐cell lymphoma with sclerosis refractory to conventional chemotherapy can respond after daily oral cyclophosphamide. Leuk Lymphoma 1998;29:199–203. [DOI] [PubMed] [Google Scholar]
- 78. Coleman M, Martin P, Ruan J et al. Prednisone, etoposide, procarbazine, and cyclophosphamide (PEP‐C) oral combination chemotherapy regimen for recurring/refractory lymphoma: Low‐dose metronomic, multidrug therapy. Cancer 2008;112:2228–2232. [DOI] [PubMed] [Google Scholar]
- 79. Tucci A, Cerqui E, Ungari M et al. Continuous oral cyclophosphamide and prednisolone as a valuable treatment option for peripheral T cell lymphoma. Br J Haematol 2011;152:113–116. [DOI] [PubMed] [Google Scholar]
- 80. Goss P, Burkes R, Rudinskas L et al. A phase II trial of prednisone, oral etoposide, and novantrone (PEN) as initial treatment of non‐Hodgkin's lymphoma in elderly patients. Leuk Lymphoma 1995;18:145–152. [DOI] [PubMed] [Google Scholar]
- 81. Ghielmini M, Zucca E. How I treat mantle cell lymphoma. Blood 2009;114:1469–1476. [DOI] [PubMed] [Google Scholar]
- 82. Bauwens D, Maerevoet M, Michaux L et al. Activity and safety of combined rituximab with chlorambucil in patients with mantle cell lymphoma. Br J Haematol 2005;131:338–340. [DOI] [PubMed] [Google Scholar]
- 83. Maffei R, Fiorcari S, Martinelli S et al. Targeting neoplastic B cells and harnessing microenvironment: The “double face” of ibrutinib and idelalisib. J Hematol Oncol 2015;8:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wu J, Zhang M, Liu D. Acalabrutinib (ACP‐196): A selective second‐generation BTK inhibitor. J Hematol Oncol 2016;9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wu J, Liu C, Tsui ST et al. Second‐generation inhibitors of bruton tyrosine kinase. J Hematol Oncol 2016;9:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. King AC, Peterson TJ, Horvat TZ et al. Venetoclax: A first‐in‐class oral BCL‐2 inhibitor for the management of lymphoid malignancies. Ann Pharmacother 2017;51:410–416. [DOI] [PubMed] [Google Scholar]
- 87. Kato H, Kinoshita T. Molecular target therapy for non‐Hodgkin lymphoma [in Japanese]. Nihon Rinsho 2014;72:1104–1112. [PubMed] [Google Scholar]
- 88. Contag CH, Sikorski R, Negrin RS et al. Definition of an enhanced immune cell therapy in mice that can target stem‐like lymphoma cells. Cancer Res 2010;70:9837–9845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Suzuki R. Molecular target therapy for malignant lymphoma [in Japanese]. Rinsho Ketsueki 2008;49:18–29. [PubMed] [Google Scholar]
- 90. Rankin CT, Veri MC, Gorlatov S et al. Cd32b, the human inhibitory Fc‐gamma receptor IIB, as a target for monoclonal antibody therapy of B‐cell lymphoma. Blood 2006;108:2384–2391. [DOI] [PubMed] [Google Scholar]
- 91. Tobinai K. Rituximab and other emerging antibodies as molecular target‐based therapy of lymphoma. Int J Clin Oncol 2003;8:212–223. [DOI] [PubMed] [Google Scholar]
- 92. Wang Z, Wu Z, Liu Y et al. New development in CAR‐T cell therapy. J Hematol Oncol 2017;10:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Martin P, Chadburn A, Christos P et al. Outcome of deferred initial therapy in mantle‐cell lymphoma. J Clin Oncol 2009;27:1209–1213. [DOI] [PubMed] [Google Scholar]
- 94. Haque W, Voong KR, Shihadeh F et al. Radiation therapy is an effective modality in the treatment of mantle cell lymphoma, even in heavily pretreated patients. Clin Lymphoma Myeloma Leuk 2014;14:474–479. [DOI] [PubMed] [Google Scholar]
- 95. Cheah CY, Seymour JF, Wang ML. Mantle cell lymphoma. J Clin Oncol 2016;34:1256–1269. [DOI] [PubMed] [Google Scholar]
- 96. Wang M, Lee HJ, Thirumurthi S et al. Chemotherapy‐free induction with ibrutinib‐rituximab followed by shortened cycles of chemo‐immunotherapy consolidation in young, newly diagnosed mantle cell lymphoma patients: A phase II clinical trial. Blood 2016;128:147. 27418621 [Google Scholar]
- 97. Ruan J, Martin P, Furman RR et al. Bortezomib plus CHOP‐rituximab for previously untreated diffuse large B‐cell lymphoma and mantle cell lymphoma. J Clin Oncol 2011;29:690–697. [DOI] [PubMed] [Google Scholar]
- 98. Griffiths R, Mikhael J, Gleeson M et al. Addition of rituximab to chemotherapy alone as first‐line therapy improves overall survival in elderly patients with mantle cell lymphoma. Blood 2011;118:4808–4816. [DOI] [PMC free article] [PubMed] [Google Scholar]