Abstract
Lessons Learned.
RAS‐ or BRAF‐mutated metastatic colorectal cancers (mCRCs) progressing after first‐line treatment have a poor prognosis.
European and U.S. guidelines include the multikinase inhibitor regorafenib as a standard option for second‐line therapy and beyond, based on the results of the randomized phase III CORRECT trial demonstrating improvement in survival.
Although stopped prematurely for failing to accrue, the PREVIUM trial, the first prospective interventional study exploring regorafenib as second‐line treatment for patients with mCRC bearing RAS or BRAF mutations, failed to demonstrate clinical activity in the population analyzed.
Background.
Patients with RAS‐ or BRAF‐mutated (mut) metastatic colorectal cancer (mCRC) progressing on first‐line bevacizumab plus 5‐FU/irinotecan/oxaliplatin (FOLFOXIRI) have a poor prognosis. We aimed to assess the efficacy and safety of regorafenib in this population.
Methods.
Regorafenib was administered daily for 3 weeks of each 4‐week cycle until disease progression or other reason. The primary endpoint was 6‐month progression‐free survival (PFS).
Results.
KRAS, NRAS, or BRAF was mutated in mCRC samples in 60%, 20%, and 13% of patients, respectively. Median time from initial diagnosis of metastases to the start of regorafenib and treatment duration was 13.8 months and 7 weeks, respectively. Reasons for discontinuation included disease progression (80%), investigator decision (13%), and adverse events (AEs; 7%). Seven patients (47%) required dose reduction, mostly for asthenia (43%). The most common regorafenib‐related grade 3 AEs were asthenia (33%), dysphonia (13%), and hypertension (13%) (Table 1). There were no grade 4 toxicities. No patient was progression‐free at 6 months. Median PFS, time to progression (TTP), and overall survival (OS) were 2.2, 2.0, and 3.3 months, respectively.
Conclusion.
Although stopped prematurely for failing to accrue, in the population analyzed, regorafenib failed to demonstrate clinical activity in KRAS‐ or BRAF‐mutated mCRC with progression following first‐line with FOLFOXIRI plus bevacizumab, although tolerability was acceptable. Our trial suggests that exploring regorafenib efficacy in an earlier line of therapy should not be undertaken without better population refinement.
Abstract
经验获取
• 一线治疗后进展的 RAS 或 BRAF 基因突变转移性结直肠癌 (mCRC) 预后不良。
• 欧洲和美国的指南将多激酶抑制剂瑞格非尼作为二线治疗及后线治疗的标准选择,是根据随机III期CORRECT试验证实生存期改善的结果。
• 虽然因招募失败而提早结束,但PREVIUM 试验是首个前瞻性干预研究探索瑞格非尼作为二线治疗 RAS 或 BRAF 基因突变的 mCRC 患者,未能对分析人群取得临床活性。
摘要
背景。贝伐珠单抗 + 5‐FU/伊立替康/奥沙利铂 (FOLFOXIRI) 一线治疗后病情进展的 RAS 或 BRAF 基因突变 (mut) 转移性结直肠癌 (mCRC) 患者预后差。我们旨在评估瑞格非尼对该人群的有效性和安全性。
方法。瑞格非尼每天一次,连续给药 3 周,每 4 周为一个周期,直到患者病情出现进展或存在其他原因。主要终点是 6 个月无进展生存期 (PFS)。
结果。分别有 60%、20% 和 13% 的患者的 mCRC 样本出现 KRAS、NRAS 或 BRAF 基因突变。从转移的初始诊断到开始使用瑞格非尼和持续治疗的中位时间分别为 13.8 个月和 7 周。停药的原因包括病情进展 (80%)、研究者决定 (13%) 和不良事件 (AE;7%)。七名患者 (47%) 需要减少剂量,主要是因为身体虚弱 (43%)。最常见的瑞格非尼相关的 3 级 AE 是身体虚弱 (33%)、发声困难 (13%) 和高血压 (13%)(表 1)。未出现 4 级毒性。没有患者在 6 个月时病情无进展。中位PFS、至进展时间 (TTP) 和总生存期 (OS) 分别为 2.2、2.0 和 3.3 个月。
结论。虽然在招募失败的情况下提早结束,但在所分析的人群中,未证实瑞格非尼对 FOLFOXIRI + 贝伐珠单抗一线治疗后病情进展的 KRAS 或 BRAF 基因突变 mCRC 患者具有临床活性,尽管耐受性尚可。我们的试验表明,如果没有进行更好的人群细化,则不应在较早期的治疗中探索瑞格非尼的有效性。
Discussion
In relapsed wild‐type‐RAS mCRC, the current standard treatment is chemotherapy plus an anti‐epidermal growth factor receptor (EGFR) antibody. For patients with mut‐KRAS mCRC whose disease progresses following a first‐line combination of FOLFOXIRI/bevacizumab, the choice of optimal systemic therapy has not yet been established. In this scenario, switching to a targeted agent such as a multikinase inhibitor is a valid and recommended approach, included in current European Society for Medical Oncology and National Comprehensive Cancer Network guidelines.
To our knowledge, this is the first prospective interventional study exploring regorafenib as second‐line treatment for a mCRC population pretreated with the triplet FOLFOXIRI/bevacizumab, specifically enrolling patients with mut‐KRAS or BRAF mCRC. Although enrollment was closed prematurely due to poor accrual, and definitive statements regarding the efficacy of regorafenib cannot be made, the study highlights several important lessons. The disappointing disease control rate compared with the pivotal phase III trial might be explained by the fact that patients included in our trial had some clinical and molecular high‐risk and poor prognosis features. Noticeably, the median TTP on their first‐line therapy was <14 months. Likewise, circulating tumor cell (CTC) counts were obtained. CTC counts have been validated as a prognostic marker in multiple clinical studies. In our study, 87% of patients had CTC counts >3 per 7.5 mL before their first‐line treatment, this being considered a poor prognostic feature.
Lastly, our data on this selected population would suggest that KRAS‐ or BRAF‐mutated mCRC is associated with inferior PFS and OS, reflecting that an interaction between baseline mutational status and treatment effect could not be excluded. Indeed, frequently debated is the influence of the mutated‐EGFR status of patients included in regorafenib studies, particularly patients with tumors bearing mutations in RAS, which tend to be even less responsive to chemotherapy than tumors in a population with wild‐type disease. Furthermore, mut‐RAS mCRC exhibits a markedly higher cumulative incidence of lung, bone, and brain metastasis. In our study, 12 patients (80%) had a RAS mutation.
Our study results, in line with other similar trials, do not provide a signal that regorafenib would lead to improved clinical benefit for high‐risk mCRC patients. As vascular endothelial growth factor (VEGF) axis inhibition underlies regorafenib activity, antiangiogenic properties and inflammation‐related factors could play a role in modulating this lack of activity, as an inflammation‐mediated cross‐talk between endothelial cells and immune system effectors has been hypothesized to play a critical role in antiangiogenic therapy resistance. This systemic inflammatory status in advanced disease could reflect changes in tumor inflammatory microenvironment that would activate VEGF‐independent angiogenesis.
With regard to its tolerability, regorafenib appears to be safe according to the observed toxicity profile. The frequency and severity of AEs was thus consistent with the known safety profile of regorafenib and other multikinase inhibitors.
Trial Information
- Disease
Colorectal cancer
- Stage of Disease/Treatment
Metastatic/advanced
- Prior Therapy
1 prior regimen
- Type of Study – 1
Phase II
- Type of Study – 2
Single arm
- Primary Endpoint
Progression‐free survival
- Secondary Endpoint
Overall survival
- Additional Details of Endpoints or Study Design
- Primary endpoint: 6‐month PFS rate as per modified RECIST criteria. A sample size of 53 evaluable patients was necessary assuming a minimum of 30% patients achieving a PFS at 6 months, rejecting the null hypothesis of <15% PFS at 6 months, with an alpha error of 0.05 and statistical power of 80%.
- Evidence‐based medicine must be based on the best evidence, which may include negative studies. This research article, although stopped prematurely for failing to accrue, clearly documented a lack of efficacy of a targeted therapeutic agent in RAS‐ or BRAF‐mutated mCRC progressing following first‐line with FOLFOXIRI/bevacizumab.
- Investigator's Analysis
- Level of activity did not meet planned endpoint
Drug Information
- Drug 1
- Generic/Working Name
New drug
- Trade Name
Stivarga
- Company Name
Bayer AG
- Drug Type
Small molecule
- Drug Class
Angiogenesis
- Dose
160 mg per flat dose
- Route
p.o.
- Schedule of Administration
Patients received regorafenib 160 mg once daily, for the first 21 days of each 28‐day cycle until disease progression, unacceptable toxicity, withdrawal of the patient from the study, or death.
Patient Characteristics
- Number of Patients, Male
13
- Number of Patients, Female
2
- Stage
Metastatic stage IV: 15 patients
- Age
Median (range): 68
- Number of Prior Systemic Therapies
Median (range): 1
- Performance Status: ECOG
-
0 — 5
1 — 10
2 — 0
3 — 0
Unknown — 0
- Other
-
9 patients: mut‐KRAS
3 patients: mut‐NRAS
2 patients: mut‐BRAF
1 patient Not available
Adverse Events
Regorafenib‐related grade 3 adverse events: asthenia (33%), dysphonia (13%), and hypertension (13%).
Abbreviation: NC/NA, no change from baseline/no adverse event.
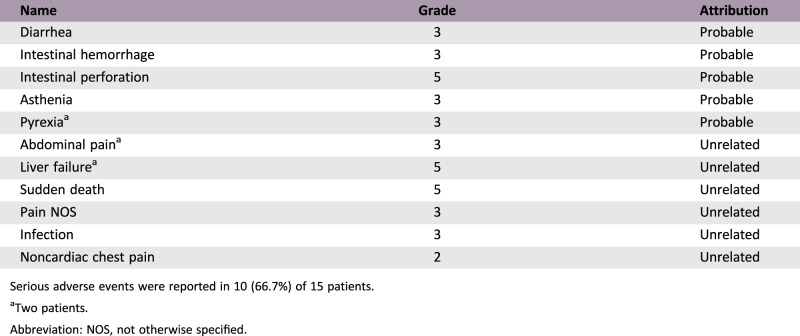
Serious Adverse Events
Serious adverse events were reported in 10 (66.7%) of 15 patients.
Two patients.
Abbreviation: NOS, not otherwise specified.
Assessment, Analysis, and Discussion
- Completion
Study terminated before completion
- Terminated Reason
Did not fully accrue
Investigator's Assessment
- Level of activity did not meet planned endpoint
For patients with metastatic colorectal cancer (mCRC), combination chemotherapy schedules with oxaliplatin or irinotecan‐based doublets or the 5‐FU/irinotecan/oxaliplatin (FOLFOXIRI) triple regimen plus a molecularly targeted compound in the first‐line setting have been established as the standard of care on the basis of reported clinical efficacy and safety profile. Namely, in the TRIBE trial, FOLFOXIRI plus bevacizumab showed an improved progression‐free survival (PFS) and overall survival (OS) when compared with FOLFIRI plus bevacizumab [1]. In the PREVIUM study, the selection of the FOLFOXIRI/bevacizumab triplet in the first‐line treatment was considered the best approach due to its potential to maximize the survival benefit and based on the age, PS, and the clinical and molecular high‐risk profile of our population.
However, if disease progression occurs shortly or while on FOLFOXIRI plus bevacizumab, other therapeutic options with a different mechanism of action should be considered. Whereas for relapsed wild‐type‐KRAS mCRC the current standard treatment is chemotherapy plus an anti‐epidermal growth factor receptor antibody, for patients whose tumors bear mutated (mut)‐KRAS and/or mut‐BRAF and whose disease progresses on a first‐line combination of FOLFOXIRI/bevacizumab, the choice of optimal systemic therapy has not yet been established. Objective responses and delayed tumor progression have been reported when second‐line treatments containing the same cytotoxic agents have been used. However, for the patients with early relapse as well as patients with toxicities of previous exposure to a fluropyrimidine‐based combination regimen, this approach is not feasible [2], [3]. In this scenario, switching to a targeted agent such as a multikinase inhibitor is a valid and recommended approach in the current European Society for Medical Oncology and National Comprehensive Cancer Network guidelines. Regorafenib, a U.S. Food and Drug Administration‐ and European Medicines Agency‐approved biological agent in previously treated mCRC, targets a broad range of angiogenic, stromal, and oncogenic kinases including BRAF, which is approved for the treatment of mCRC, based on the results of the randomized phase III CORRECT trial demonstrating improvement in survival [4].
To our knowledge, this is the first prospective interventional study exploring regorafenib as second‐line treatment for an mCRC population pretreated with the triplet FOLFOXIRI/bevacizumab, specifically enrolling mut‐KRAS or BRAF patients. However, activity did not reach the level seen in later lines of therapy in patients enrolled on the CORRECT pivotal trial [4]. In this study, OS was 6.4 months (95% confidence interval [CI]: 5.8–7.3) in the regorafenib group and 5.0 months (95% CI: 4.4–5.8) in the placebo group. Median progression‐free survival was 2.0 months (95% CI: 1.9–2.3) and 1.7 months (95% CI: 1.7–1.8), respectively. Our data in the study reported here, in second‐line, revealed PFS and OS at 2.2 and 3.3 months, respectively.
This disappointing disease control rate compared with the pivotal phase III trial might be explained by the fact that patients included in our trial had some clinical and molecular high‐risk and poor prognosis features. Patients harboring tumors bearing mutations in RAS tend to be even less responsive to chemotherapy than a population with wild‐type disease. Furthermore, mut‐RAS mCRC exhibits a markedly higher cumulative incidence of lung, bone, and brain metastasis [5], [6]. In our study, 12 patients (80%) had an RAS mutation. In addition, 13 of 15 patients included had synchronous metastatic liver and/or lung stage IV disease at initial first diagnosis, and had disease progression <1 month after the end of treatment or while on the oxaliplatin‐containing first‐line therapy. Worse prognostic variables, defined as having a poor Eastern Cooperative Oncology Group performance score, short time from initial diagnosis of metastases to the start of regorafenib, low initial regorafenib dose, >3 metastatic sites, liver metastases, and mut‐KRAS profile, independently and negatively affected survival in patients on regorafenib enrolled in the real‐life REBECCA trial [7]. Noticeably, and based on these factors, a high‐risk prognostic group with a median survival of 2.5 months was identified. Furthermore, they reproduced this predictive pattern in the FAS‐CORRECT population, identifying a particularly poor subpopulation with a median OS of 3.4 months. Likewise, in our study, 87% of patients had detectable circulating tumor cells >3 per 7.5 mL before their first‐line treatment, this being considered a poor prognostic feature [8], [9], [10].
Regarding tolerability, the frequency and severity of adverse events were consistent with the known safety profile of regorafenib and other multikinase inhibitors, and in the expected range regarding the morbidity of patients previously treated with a fluropyrimidine‐based combination plus bevacizumab.
In summary, although stopped prematurely for failing to accrue, on the population analyzed, regorafenib failed to demonstrate clinical activity in patients with very poor prognosis mCRC that progressed following first‐line therapy with the triplet FOLFOXIRI plus bevacizumab, although tolerability was acceptable. Median PFS was consistent with what was reported previously in the pivotal phase III CORRECT trial. Several biomarkers have been implemented to define the best therapeutic strategy in mCRC. Unfortunately, none can be related to regorafenib efficacy. Our trial suggests that exploring regorafenib efficacy in earlier line should not be undertaken without better population refinement.
Acknowledgments
We thank the patients, their families, and the study investigators.
Footnotes
ClinicalTrials.gov Identifier: NCT02175654
Sponsor(s): Spanish Cooperative Group for the Treatment of Digestive Tumors (TTD)
Principal Investigator: Pilar Garcia‐Alfonso
IRB Approved: Yes
Disclosures
Manuel Benavides: Bayer (C/A, H); Fernando Rivera: Amgen (C/A, H, RF), Bayer (C/A, H, RF), Roche (C/A, H, RF); Amelia Lopez‐Ladron: Roche (C/A); Rafael Lopez: Roche (RF); Enrique Aranda: Amgen (C/A), Bayer (C/A), Celgene (C/A), Merck (C/A), Roche (C/A), Sanofi (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Loupakis F, Cremolini C, Masi G et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 2014;371:1609–1618. [DOI] [PubMed] [Google Scholar]
- 2.Fornaro L, Vasile E, Masi G et al. Outcome of second‐line treatment after first‐line chemotherapy with the GONO FOLFOXIRI regimen. Clin Colorectal Cancer 2012;11:71–76. [DOI] [PubMed] [Google Scholar]
- 3.Rossini D, Moretto R, Cremolini C et al. Treatments (tx) after progression to first‐line FOLFOXIRI plus bevacizumab (bev) in metastatic colorectal cancer (mCRC) patients (pts): A pooled analysis of TRIBE and MOMA studies by GONO group. J Clin Oncol 2017;35(suppl 15):3542a. [Google Scholar]
- 4.Grothey A, Van Cutsem E, Sobrero A et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo‐controlled, phase 3 trial. Lancet 2013;381:303–312. [DOI] [PubMed] [Google Scholar]
- 5.Modest DP, Ricard I, Heinemann V et al. Outcome according to KRAS‐, NRAS‐ and BRAF‐mutation as well as KRAS mutation variants: Pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Ann Oncol 2016;27:1746–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yaeger R, Cowell E, Chou JF et al. RAS mutations affect pattern of metastatic spread and increase propensity for brain metastasis in colorectal cancer. Cancer 2015;121:1195–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adenis A, de la Fouchardiere C, Paule B et al. Survival, safety, and prognostic factors for outcome with Regorafenib in patients with metastatic colorectal cancer refractory to standard therapies: Results from a multicenter study (REBECCA) nested within a compassionate use program. BMC Cancer 2016;16:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang X, Gao P, Song Y et al. Relationship between circulating tumor cells and tumor response in colorectal cancer patients treated with chemotherapy: A meta‐analysis. BMC Cancer 2014;14:976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen SJ, Punt CJ, Iannotti N et al. Relationship of circulating tumor cells to tumor response, progression‐free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:3213–3221. [DOI] [PubMed] [Google Scholar]
- 10.Tabernero J, Lenz HJ, Siena S et al. Analysis of circulating DNA and protein biomarkers to predict the clinical activity of regorafenib and assess prognosis in patients with metastatic colorectal cancer: A retrospective, exploratory analysis of the CORRECT trial. Lancet Oncol 2015;16:937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]