This article assesses the expression of the α‐amyloid precursor protein (APP) in a large series of breast carcinomas and the corresponding metastatic lymph nodes.
Keywords: Breast cancer, Nodal metastatsis, Amyloid precursor protein, Immunohistochemistry, Cancer subtype, Patient survival
Abstract
Background.
β‐amyloid precursor protein (APP), a potential target for Alzheimer's disease treatment, has recently been shown to take part in carcinogenesis. Increased APP promotes migration, survival, and proliferation in breast cancer cell lines. We examined the clinical value of APP in breast cancers. A comprehensive examination of clinicopathological features related to APP expression in a large cohort of breast cancers and the corresponding metastatic lymph nodes was performed. APP expression and its prognostic impact in different breast cancer subtypes were examined.
Results.
APP was highly expressed in nonluminal breast cancers and correlated with features associated with nonluminal breast cancers (including higher grade, the presence of necrosis, and higher proliferative index, growth factor receptor, and basal marker expression). Multivariate Cox hazard analysis demonstrated that APP was an independent adverse prognostic factor of disease‐free survival (DFS; hazard ratio [HR], 2.090; p = .013; 95% confidence interval [CI], 1.165–3.748) and breast cancer‐specific survival (BCSS; HR, 2.631; p = .002; 95% CI, 1.408–4.915) in the nonluminal group. The independent prognostic impact was also seen in triple negative breast cancers. Interestingly, a higher expression of APP was found in nodal metastasis compared with primary tumor. Such APP upregulation was correlated with further distal metastasis and poorer outcome (DFS: log‐rank, 12.848; p < .001; BCSS: log‐rank, 13.947; p < .001).
Conclusion.
Our findings provided evidence of oncogenic roles of APP in clinical breast cancers. Patients with positive APP expression, particularly those with APP upregulation in lymph node metastases, may require vigilant monitoring of their disease and more aggressive therapy.
Implications for Practice.
β‐amyloid precursor protein (APP), a potential target for Alzheimer's disease, has recently been implicated in oncogenesis. Here, evidence of its roles in clinical breast cancers is provided. Positive APP expression was found to be an independent prognostic factor in nonluminal cancers, particularly triple negative breast cancers (TNBCs). Interestingly, a higher APP in nodal metastases was associated with distal metastases. TNBCs are heterogeneous and currently have no available target therapy. APP could have therapeutic potential and be used to define the more aggressive cases in TNBCs. Current prognostic analysis is based on primary tumor. The present data suggest that investigation of nodal metastases could provide additional prognostic value.
摘要
背景。β‐淀粉样前体蛋白 (APP) 是阿尔茨海默病的潜在治疗靶点,最近已被证实参与肿瘤生成。APP 的增加促进了乳腺癌细胞系的迁移、存活和增殖。我们对 APP 在乳腺癌治疗中的临床价值进行了研究。我们对一个大型乳腺癌队列和相应的转移淋巴结中与 APP 表达相关的临床病理学特征进行了全面研究。并对 APP 表达及其对不同乳腺癌亚型的预后影响进行了研究。
结果。APP 在非管腔型乳腺癌中具有很高的表达率,并且与引起非管腔型乳腺癌的特征有关(包括等级更高、存在坏死及增殖指数更高,生长因子受体和基础标志物表达)。多变量 Cox 风险分析表明,APP 是无病生存期[DFS;风险比 (HR),2.090;p = 0.013;95% 置信区间 (CI),1.165–3.748]和非管腔型乳腺癌群体特异性生存期(BCSS;HR,2.631;p = 0.002;95% CI,1.408–4.915)的独立不良预后因素。在三阴性乳腺癌中也观察到了独立的预后因素。有趣的是,在淋巴结转移灶中发现了比原发性肿瘤更高的 APP 表达。这种 APP 的上调与进一步的远端转移和预后较差有关(DFS:log‐rank,12.848;p < 0.001;BCSS:log‐rank,13.947;p < 0.001)。
结论。我们的研究结果为 APP 在临床乳腺癌中具有致癌作用提供了证据。APP 阳性表达的患者,特别是淋巴结转移灶中 APP 上调的患者,可能需要谨慎监测病情并接受更积极的治疗。
对临床实践的提示: β‐淀粉样前体蛋白 (APP) 是阿尔茨海默病的潜在靶点,最近被证实参与肿瘤生成。在本研究中,我们为其在临床乳腺癌中发挥作用提供了证据。研究发现,APP 的阳性表达是非管腔型癌症,特别是三阴性乳腺癌 (TNBC) 的独立预后因子。有趣的是,淋巴结转移灶中 APP 的高表达与远端转移有关。TNBC 具有异质性,目前没有可用的靶向疗法。APP 具有治疗的潜力,可用于确定更具侵袭性的 TNBC 病例。目前的预后分析以原发肿瘤为基础。目前的数据表明,淋巴结转移的研究可以提供额外的预后价值。
Introduction
The β‐amyloid precursor protein (APP) is a type I transmembrane protein. Its aberrant processing and mutation can lead to amyloid deposition in the brain, a key pathogenic event in patients with Alzheimer's disease (AD). Despite extensive studies of its role in AD, its normal physiological function remains largely unknown. Based on its protein structure [1], it has been suggested to have various functions. The N‐terminal of APP encompasses a growth factor‐like domain [2], metal binding motifs [3], two heparin sulfate binding sites, and a RERMS sequence with putative growth‐promoting properties [4]. APP has been suggested to act as a cell surface receptor or as a growth factor for cellular adhesion and/or trophic functions. The APP C‐terminal intracellular domain contains a conserved YENPTY motif that enables its interaction with other proteins forming a transcriptional complex in nuclear signaling [5], [6]. In addition, it has been shown to affect several signaling pathways, including the JNK, MAPK, and PI3K/AKT pathways, that regulate cellular functions [7].
Recent data suggested that APP plays a role in carcinogenesis. Increased APP expression was found in a number of cancers, including pancreatic [8], colon [9], lung [10], prostate [11], thyroid [12], oral squamous [13], and breast [14] cancers. In line with its proposed physiological functions, silencing of APP led to reduced cancer cell growth [9], [11], [15]. Its inhibitory effects on cell growth could be related to cell cycle regulation. APP can modulate the expression of cyclin dependent kinase inhibitor (p27) in breast cancers [15], and its depletion caused cyclin‐C destabilization in lung cancers [10]. It has been reported that APP expression can affect migration in prostate [16] and breast [15] cancer cells. Overexpression of APP increases the expression of epithelial‐mesenchymal transition (EMT)‐related genes in prostate cancers [16]. Of note, APP was functionally linked to AKT activation and the β‐catenin pathway, important signaling processes for promoting EMT in breast cancer [15].
Despite the cellular studies, the clinical significance of APP is not very well understood. The relationship of its expression with poor survival has been demonstrated in oral squamous cell carcinoma [13] and prostate cancer [11]. In the breast, one study has reported that APP was associated with increased risk of recurrence in estrogen receptor‐positive (ER+) cancers, expression of androgen receptor (AR), and higher proliferation index [14]. However, these findings were derived from a small series, which precludes analysis on breast cancer subgroups. Breast cancer is a highly heterogeneous disease with different underlying biology. Hence, the expression and function of APP may differ among different cancer subgroups. Moreover, a comprehensive examination of clinicopathological features related to APP expression and its expression in metastatic cancers is lacking. This study was undertaken to assess the expression of APP, its clinicopathological features, and its prognostic value in a large series of breast carcinomas and the corresponding metastatic lymph nodes. Further analysis was performed in different breast cancer subtypes. The prognostic value of APP expression was analyzed according to REMARK criteria [17].
Materials and Methods
Patient Data
The histologic files of the three involved institutions were searched for breast carcinoma over a period of 4 (2002–2005), 7 (2003–2009), and 2 (2003–2004) years. All the specimens were routinely processed and stained with hematoxylin and eosin (H&E). Diagnosis was confirmed (World Health Organization criteria [18]) and graded (Bloom and Richardson grading [19]). Lymphovascular invasion (LVI), necrosis, phenotypic apocrine features, and extensive in situ components were evaluated as previously [20], [21], [22]. Stromal tumor infiltrating lymphocytes (TILs) were evaluated according to recent guideline [23]. Patients' age, tumor size, lymph node involvement, pathological nodal stage (pN), pathological tumor stage (pT), and outcome data were retrieved from the medical records. Breast cancer‐specific survival (BCSS) was defined as the time interval from the date of initial diagnosis to the date of breast cancer‐related death. Disease‐free survival (DFS) was defined as the duration from the date of initial diagnosis to the first detection of breast cancer‐specific relapse or death. All consecutive cases with excision specimens were included. The study was approved by Joint Chinese University of Hong Kong‐New Territories East Cluster clinical research ethics committee.
Tissue Microarray Construction and Immunohistochemistry
Tissue microarrays (TMAs) containing representative areas from tumor and metastatic node were constructed with duplicated 0.6 mm cores as previously described [24]. One section from each TMA was stained with H&E and reviewed to confirm the presence of representative tumors. Immunohistochemical (IHC) staining was performed on the TMA with the selected antibodies using Ultraview Universal DAB Detection Kit (Ventana, AZ) after deparaffinization, rehydration, and antigen retrieval of the slides. All slides were counterstained with hematoxylin. The TMA slides were assessed for the staining intensity and the actual percentage of stained cells in the nucleus, cytoplasm, or membrane according to different antibodies by two of the authors blinded to the clinical information and the staining results of other markers. APP positive was defined as moderate to intense membranous/cytoplasmic staining in ≥10% of tumor cells. Results for other markers were retrieved from our database and were previously reported. The staining and assessment of all markers involved in the study are shown in supplemental online Table 1.
IHC surrogates for molecular subtype classification were adopted as follows [25]: luminal A (ER+ and progesterone receptor positive [PR+], HER2−, Ki67 <20%, PR ≥20%), luminal B (ER+ and PR+, HER2+, and Ki67 ≥20% and PR <20%), HER2‐overexpressed (HER2‐OE: ER−, PR−, HER2+) and triple negative breast cancers (TNBCs: ER−, PR−, HER2−).
Statistical Analysis
The findings were analyzed using the statistical software SPSS for Windows, version 23 (IBM, Armonk, NY). Chi‐square analysis or Fisher's exact test was used to test for the association of APP expression with categorical variables. The Mann‐Whitney U test was used for analyzing the differences in continuous variables with APP expression. Survival data were evaluated with Kaplan‐Meier analysis. Multivariate analysis with backward Wald model was used. Statistical significance was established at p < .05.
Results
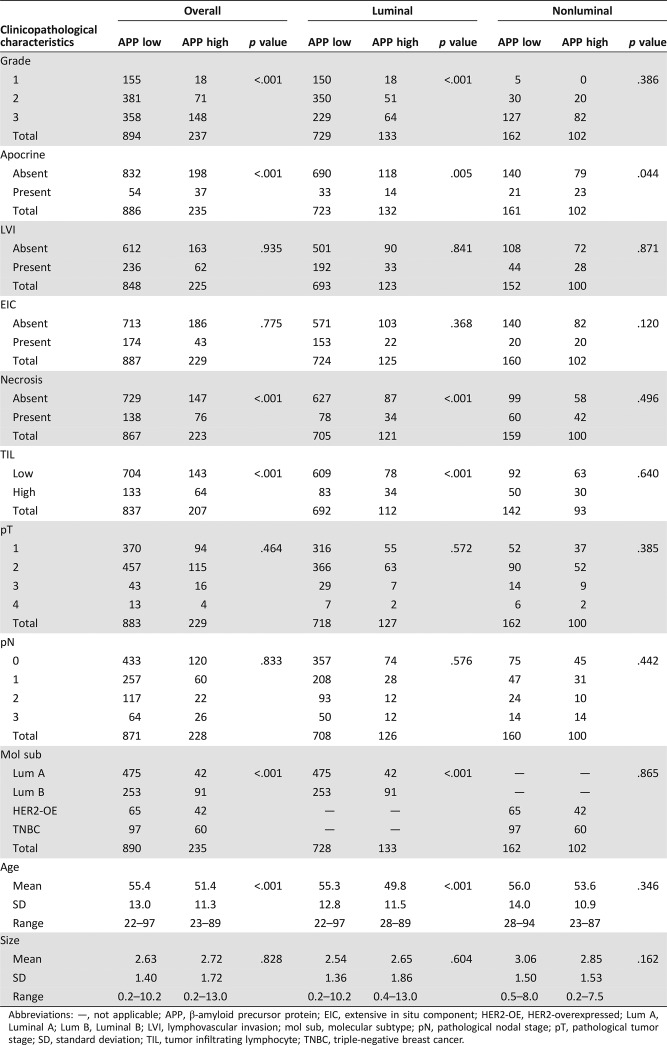
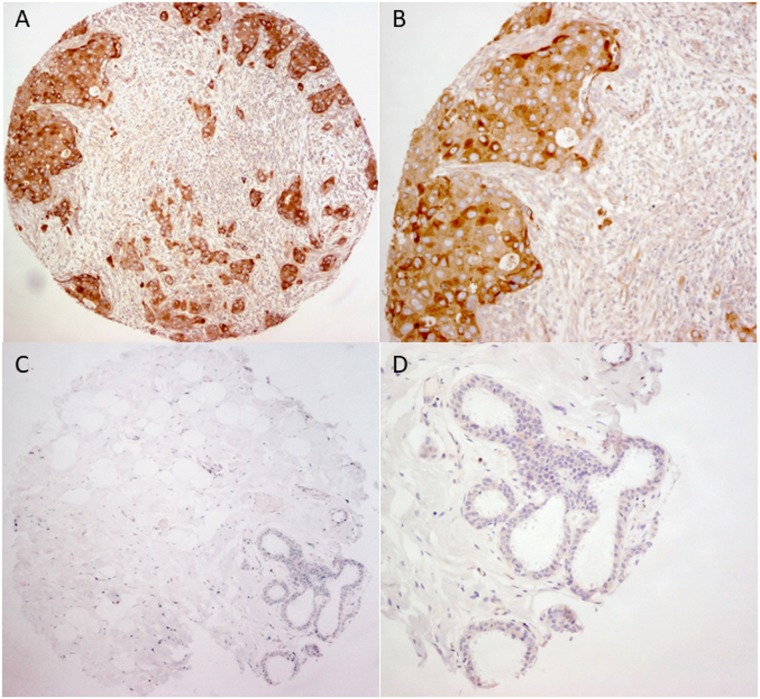
In total, 1,131 primary invasive breast cancers were included in this cohort, and metastatic lymph nodes were present in 302 cases. For the invasive tumors, the mean age of the patients was 54.6 ±12.8 years (range, 22–97 years). The mean tumor size was 2.65 ±1.47 cm (range, 0.2–13.0 cm). One hundred seventy‐three cases (15.3%) were grade 1, 452 cases (40.0%) were grade 2, and 506 cases (44.7%) were grade 3. Details of the clinicopathological features of this cohort are summarized in Table 1. Overall, 237 cases (21.0%) were APP. Representative staining is shown in Figure 1.
Table 1. Clinicopathological association of APP expression in breast cancers.
Abbreviations: —, not applicable; APP, β‐amyloid precursor protein; EIC, extensive in situ component; HER2‐OE, HER2‐overexpressed; Lum A, Luminal A; Lum B, Luminal B; LVI, lymphovascular invasion; mol sub, molecular subtype; pN, pathological nodal stage; pT, pathological tumor stage; SD, standard deviation; TIL, tumor infiltrating lymphocyte; TNBC, triple‐negative breast cancer.
Figure 1.
Representative immunohistochemical staining of β‐amyloid precursor protein in breast cancer and adjacent normal tissue. (A) and (B): Show positive expression of amyloid precusor protein (APP) in tumor at magnification of 100× and 200× respectively. (C) and (D): Show the lower APP expression at the paired adjacent normal tissue at magnification of 100× and 200× respectively.
Correlation with Clinicopathological Features, Biomarkers, and Molecular Subtypes
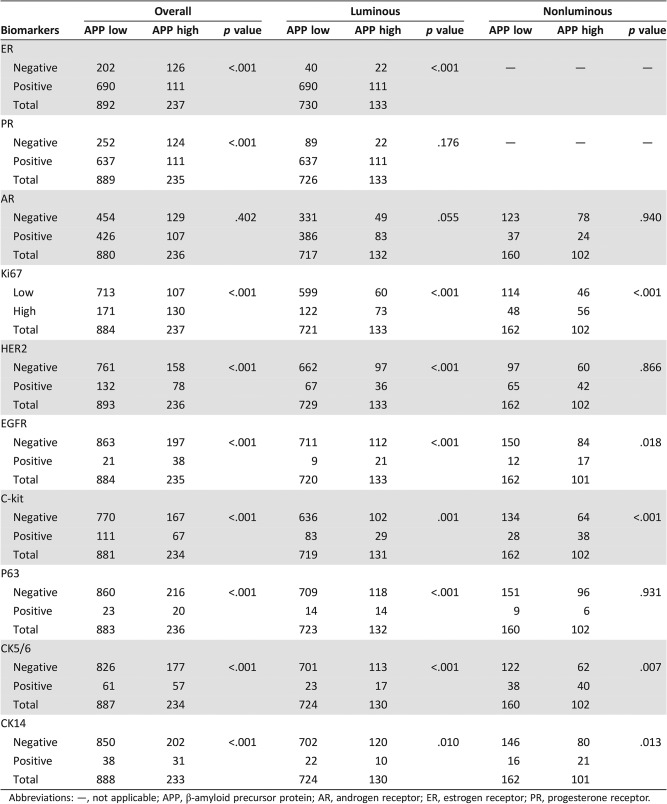
APP expression was positively associated with grade (p < .001), apocrine phenotype (p < .001), necrosis (p < .001), high TIL (p < .001), and younger age (p < .001), but no correlation was found with LVI, pT, pN, and tumor size (Table 1). APP also correlated positively with Ki67, HER2, EGFR, C‐kit, p63, CK5/6 and CK14 (p < .001 for all) but negatively with ER and PR (p < .001 for both; Table 2).
Table 2. Association of APP expression with biomarkers.
Abbreviations: —, not applicable; APP, β‐amyloid precursor protein; AR, androgen receptor; ER, estrogen receptor; PR, progesterone receptor.
Among the 1,125 invasive cancers with complete IHC data for molecular subtyping, 517 (46.0%) were luminal A, 344 (30.6%) were luminal B, 107 (9.5%) were HER2‐OE, and 157 (13.9%) were TNBCs. The expression rate of APP was found to be 8.1% in luminal A subtype, 26.5% in luminal B, 39.3% in HER2‐OE, and 38.2% in TNBCs. A significant differential expression of APP was found among different molecular subtypes (p < .001; Table 1).
Correlation of APP in Luminal and Nonluminal Cases
Two hundred sixty‐four and 862 breast cancers were classified as nonluminal and luminal cancers, respectively. APP expression was found in 38.6% (102/264) and 15.4% (133/862) of nonluminal and luminal cases, respectively. In the nonluminal cases, APP expression was associated with apocrine phenotype (p = .044). In the luminal cases, similar to the overall cohort, APP was associated with grade (p < .001), apocrine phenotype (p = .005), necrosis (p < .001), high TIL (p < .001), and younger age (p < .001); also with luminal B subtype (p < .001; Table 1). For biomarkers, APP was positively associated with Ki67, EGFR, c‐kit, CK5/6, and CK14 in both luminal and nonluminal subgroups (p ≤ .018). Additionally, in luminal cancers, there were significant positive association with HER2 and p63 (p < .001 for both) and negative association with ER expression (p < .001). A marginal association was found with AR in luminal cases (p = .055; Table 2).
Relationship with Patient Outcome
Follow‐up data were available for 1,105 cases. The mean follow‐up duration was 52.7 months (range, 1–210 months). Among them, 179 cases (16.2%) had breast cancer‐specific mortality or relapse. Among these, 131 patients (11.9%) died of breast cancers. Multivariate Cox hazard analysis including age, grade, TIL, LVI, pT stage, pN stage, HER2, ER, PR, Ki67, and APP expression demonstrated that APP was an independent factor of DFS (hazard ratio [HR], 2.090; p = .013; 95% confidence interval [CI], 1.165–3.748) and BCSS (HR, 2.631; p = .002; 95% CI, 1.408–4.915) in the nonluminal group. Further analysis also showed that APP was an independent prognostic factor in TNBC subset among the nonluminal cases (data not shown) but not in luminal cancers (Table 3).
Table 3. Cox regression analysis on DFS and BCSS for APP expression.
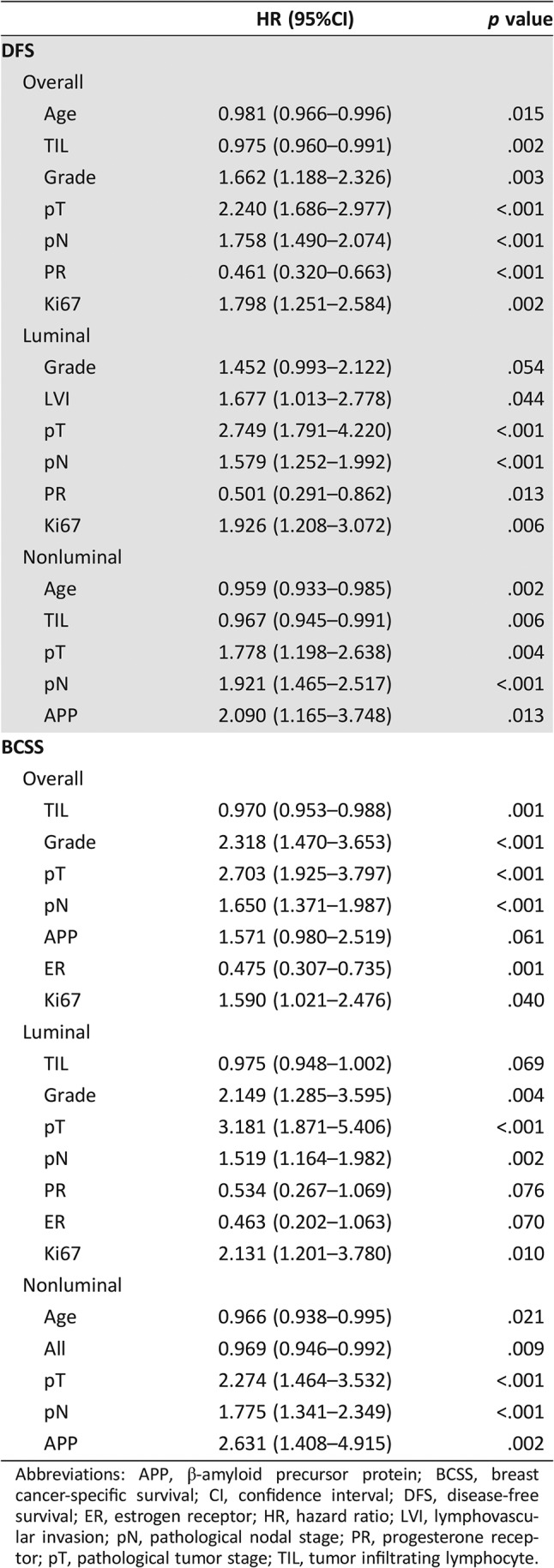
Abbreviations: APP, β‐amyloid precursor protein; BCSS, breast cancer‐specific survival; CI, confidence interval; DFS, disease‐free survival; ER, estrogen receptor; HR, hazard ratio; LVI, lymphovascular invasion; pN, pathological nodal stage; PR, progesterone receptor; pT, pathological tumor stage; TIL, tumor infiltrating lymphocyte.
Expression in Nodal Metastases and Corresponding Primary Tumors
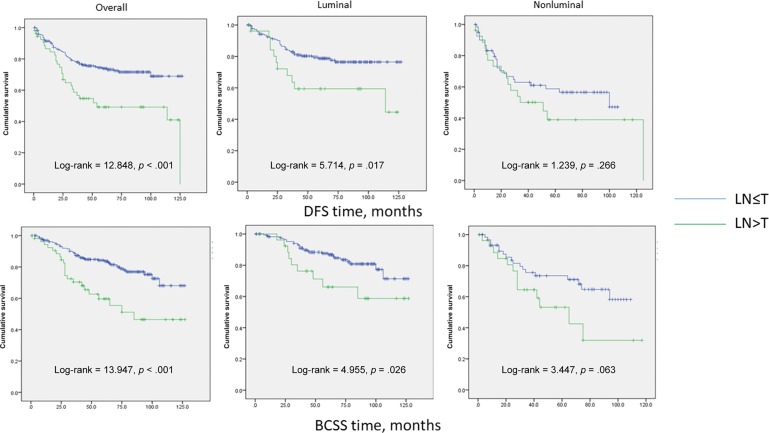
Three hundred two breast carcinomas and matched nodal metastases were analyzed. APP was expressed in 19.9% (60/302 cases) of primary breast cancers and 25.5% (77/302 cases) of metastatic lymph nodes (LNs). APP status in nodal metastases and primary tumor strongly correlated with each other (p < .001). Nevertheless, there was a significant upregulation of APP expression in metastatic LNs (p = .001). Fifty‐five cases showed a higher percentage of APP‐stained cells in LNs than in the primary tumor, whereas 215 cases did not show any change in APP expression between the pairs and 32 cases showed a diminution of APP staining in metastatic LNs. The upregulation of APP expression in LNs was associated with higher grade (p = .010), higher pN stage (p = .011), and distant metastases (p = .003). There was also a differential upregulation of APP in LN among the molecular subtypes (with the highest rate in TNBC, 34%; p < .001; Table 4). Interestingly, patients with APP upregulation in LN showed significantly poorer DFS (log‐rank, 12.848; p < .001) and BCSS (log‐rank, 13.947; p < .001). The significant correlation of APP upregulation in LNs with patients' outcome was also observed within the luminal subgroups (DFS: log‐rank, 5.714; p = .017; BCSS: log‐rank, 4.955; p = .026). A trend of poor outcome was also noted for the nonluminal cancers (DFS: log‐rank, 1.239; p = .266; BCSS: log‐rank, 3.447; p = .063; Fig. 2).
Table 4. Association of APP upregulation in nodal metastasis with clinicopathological features.
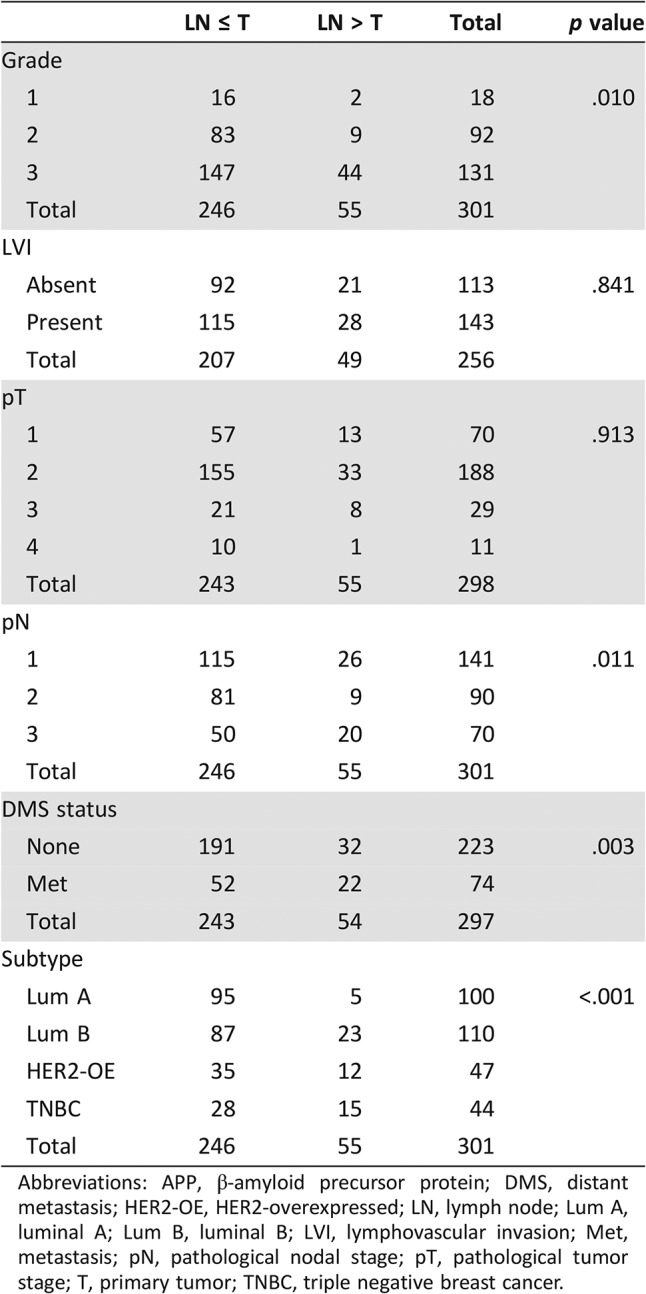
Abbreviations: APP, β‐amyloid precursor protein; DMS, distant metastasis; HER2‐OE, HER2‐overexpressed; LN, lymph node; Lum A, luminal A; Lum B, luminal B; LVI, lymphovascular invasion; Met, metastasis; pN, pathological nodal stage; pT, pathological tumor stage; T, primary tumor; TNBC, triple negative breast cancer.
Figure 2.
Kaplan‐Meier analysis of DFS and BCSS for patients with upregulation of APP expression in LNs compared with the primary tumor.
Abbreviations: BCSS, breast cancer‐specific survival; DFS, disease‐free survival; LN, lymph node; T, primary tumor.
Discussion
Several studies have demonstrated positive expression of APP in a number of cancers, including breast cancers [14]. However, those studies were limited by small sample sizes. Moreover, the clinicopathological associations and clinical value of APP expression have not been thoroughly investigated. We performed a comprehensive analysis of APP expression in a large series of clinical breast cancers and provided evidence on its oncogenic role. Similar to the result from an earlier study, we found an association of APP with Ki67 and poor outcome [14]. With a larger cohort, we furnished further information regarding the less frequent subtypes. A high APP expression was found in nonluminal breast cancers, including both HER2‐OE (39.3%) and TNBC (38.2%), compared with 8% in luminal A cancers. Concordantly, APP was associated with the related biomarker profile (high Ki67, basal markers, and growth hormone receptor expression) and clinicopathological features (patients' young age, high grade, necrosis, apocrine, and TIL) in this breast cancer cohort.
TNBC is a heterogeneous group of breast cancer, and there are many variants even among high‐grade TNBCs. To date, the clinical management of all high‐grade TNBC is similar if not the same. In the current study, it was found that APP was an independent poor prognostic factor in nonluminal cases, particularly the TNBC subset. Thus, APP may be useful in triaging subsets of patients with TNBC with more aggressive cancers, for which the classical pathological variables, such as tumor grade, nodal status, and tumor size, may not be useful [26]. Our findings suggested that APP could be used to refine TNBC classification.
It appears that some of our findings did not concur with the earlier data [14]. The differences could be due to variations in methodology and cohort characteristics. A positive association of APP with AR was observed previously [14] but not here. The previous study had predominantly ER+ cases and used a different AR cutoff. In the current series, there was a marginal association between APP and AR expression in our luminal cancers, and when the data were analyzed as continuous variables, a significant correlation between AR and APP in luminal cancer was noted. The other difference was the risk of recurrence in ER+ cancers. The discrepancy may be attributed to the shorter follow‐up time of our cohort (mean follow‐up of 52.7 months compared with 100 months in the previous report [14]). Recurrence of luminal cancers could occur beyond 10 years [27]. We did observe an association of APP in luminal cancers with a poor prognostic profile, namely higher tumor grade, younger age, expression of Ki67, basal markers, and HER2, as well as the luminal B subtype. An extended follow‐up analysis may be able to capture fully the recurrent events in the current series.
Another interesting finding was a higher APP expression in nodal metastases compared with the primary tumor. One could speculate that APP expression may be acquired during disease progression. The enhanced expression in nodal metastases was associated with higher pN stage and conferred higher chance of distant metastases, thus poorer survival. Localized breast cancer has a higher chance of curative outcome, whereas metastatic disease carries a dismal prognosis and is responsible for the majority of cancer‐related mortality. For effective disease control, there is a major need for understanding the molecular mechanisms driving tumor metastasis. Our data provided clinical evidence showing the role of APP in cancer metastases. The precise mechanisms by which APP mediate migration in cancers remain to be investigated. The large extracellular domain of APP is capable of binding to several extracellular matrix proteins that mediate cell‐cell and cell‐matrix adhesion. In neuronal progenitor cells, it binds to extracellular matrices, such as laminin, to promote cell migration and neuronal growth cone adhesion [28], [29]. Moreover, APP is functionally linked with AKT activation and GSK‐3β/β‐catenin pathways, which are closely engaged in cell migration and the EMT process [15], [30], [31]. APP expression at nodal metastatic foci may also be applied in predicting the prognosis of patients. A common route of metastasis of breast cancer is through axillary LNs. APP in cancer cells residing in LNs may confer capacity for further distant organ invasion. Biomarkers are analyzed mainly at the primary tumor, and patient management is based on those results. In view of the significance of tumor heterogeneity being increasingly acknowledged, the spatial differences of the tumors within the same patient may affect treatment responses and the behavior of the cancer [32]. Analysis of the primary tumor alone may not be sufficiently informative, and investigation in nodal metastases could provide additional prognostic value.
Conclusion
APP was associated with nonluminal breast cancers and features associated with those cancers. It could be a novel prognostic marker, particularly in TNBC. A higher expression of APP was found in nodal metastasis compared with primary tumor. Such APP upregulation was correlated with further distal metastasis and poorer outcome. Our findings provide evidence of the oncogenic roles of APP in clinical breast cancers. Patients with positive APP expression, particularly those with upregulation in LNs, may require closer monitoring of their disease and more aggressive therapy.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
This study was supported by the Health and Medical Research Fund of Hong Kong (02130746).
Author Contributions
Conception/design: Gary M.K. Tse
Provision of study material or patients: Siu‐Ki Chan, Sai‐Yin Cheung, Gary M.K. Tse
Collection and/or assembly of data: Michelle A. Lee, Yun‐Bi Ni, Wai‐Wa Chan
Data analysis and interpretation: Julia Y.S. Tsang
Manuscript writing: Julia Y.S. Tsang, Gary M.K. Tse
Final approval of manuscript: Julia Y.S. Tsang, Michelle A. Lee, Yun‐Bi Ni, Siu‐Ki Chan, Sai‐Yin Cheung, Wai‐Wa Chan, Kwok‐Fai Lau, Gary M.K. Tse
Disclosures
The authors indicated no financial relationships.
References
- 1.Liu X, Yu X, Zack DJ et al. TiGER: A database for tissue‐specific gene expression and regulation. BMC Bioinformatics 2008;9:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossjohn J, Cappai R, Feil SC et al. Crystal structure of the N‐terminal, growth factor‐like domain of Alzheimer amyloid precursor protein. Nat Struct Biol 1999;6:327–331. [DOI] [PubMed] [Google Scholar]
- 3.Kong GK, Adams JJ, Harris HH et al. Structural studies of the Alzheimer's amyloid precursor protein copper‐binding domain reveal how it binds copper ions. J Mol Biol 2007;367:148–161. [DOI] [PubMed] [Google Scholar]
- 4.Ninomiya H, Roch JM, Sundsmo MP et al. Amino acid sequence RERMS represents the active domain of amyloid beta/A4 protein precursor that promotes fibroblast growth. J Cell Biol 1993;121:879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lau KF, Chan WM, Perkinton MS et al. Dexras1 interacts with FE65 to regulate FE65‐amyloid precursor protein‐dependent transcription. J Biol Chem 2008;283:34728–34737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Rotz RC, Kohli BM, Bosset J et al. The APP intracellular domain forms nuclear multiprotein complexes and regulates the transcription of its own precursor. J Cell Sci 2004;117:4435–4448. [DOI] [PubMed] [Google Scholar]
- 7.Deyts C, Thinakaran G, Parent AT. APP receptor? To be or not to be. Trends Pharmacol Sci 2016;37:390–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansel DE, Rahman A, Wehner S et al. Increased expression and processing of the Alzheimer amyloid precursor protein in pancreatic cancer may influence cellular proliferation. Cancer Res 2003;63:7032–7037. [PubMed] [Google Scholar]
- 9.Meng JY, Kataoka H, Itoh H et al. Amyloid beta protein precursor is involved in the growth of human colon carcinoma cell in vitro and in vivo. Int J Cancer 2001;92:31–39. [PubMed] [Google Scholar]
- 10.Sobol A, Galluzzo P, Liang S et al. Amyloid precursor protein (APP) affects global protein synthesis in dividing human cells. J Cell Physiol 2015;230:1064–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takayama K, Tsutsumi S, Suzuki T et al. Amyloid precursor protein is a primary androgen target gene that promotes prostate cancer growth. Cancer Res 2009;69:137–142. [DOI] [PubMed] [Google Scholar]
- 12.Krause K, Karger S, Sheu SY et al. Evidence for a role of the amyloid precursor protein in thyroid carcinogenesis. J Endocrinol 2008;198:291–299. [DOI] [PubMed] [Google Scholar]
- 13.Ko SY, Lin SC, Chang KW et al. Increased expression of amyloid precursor protein in oral squamous cell carcinoma. Int J Cancer 2004;111:727–732. [DOI] [PubMed] [Google Scholar]
- 14.Takagi K, Ito S, Miyazaki T et al. Amyloid precursor protein in human breast cancer: An androgen‐induced gene associated with cell proliferation. Cancer Sci 2013;104:1532–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim S, Yoo BK, Kim HS et al. Amyloid‐beta precursor protein promotes cell proliferation and motility of advanced breast cancer. BMC Cancer 2014;14:928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyazaki T, Ikeda K, Horie‐Inoue K et al. Amyloid precursor protein regulates migration and metalloproteinase gene expression in prostate cancer cells. Biochem Biophys Res Commun 2014;452:828–833. [DOI] [PubMed] [Google Scholar]
- 17.McShane LM, Altman DG, Sauerbrei W et al. REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat 2006;100:229–235. [DOI] [PubMed] [Google Scholar]
- 18.Lakhani SR, Ellis IO, Schnitee SJ. et al., eds. World Health Organisation Classification of Tumors of the Breast. 4th ed Lyon: IARC Press; 2012. [Google Scholar]
- 19.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long‐term follow‐up. Histopathology 1991;19:403–410. [DOI] [PubMed] [Google Scholar]
- 20.Ni YB, Tsang JY, Chan SK et al. A novel morphologic‐molecular recurrence predictive model refines traditional prognostic tools for invasive breast carcinoma. Ann Surg Oncol 2014;21:2928–2933. [DOI] [PubMed] [Google Scholar]
- 21.Mujtaba SS, Ni YB, Tsang JY et al. Fibrotic focus in breast carcinomas: Relationship with prognostic parameters and biomarkers. Ann Surg Oncol 2013;20:2842–2849. [DOI] [PubMed] [Google Scholar]
- 22.Tsang JY, Ni YB, Chan SK et al. CX3CL1 expression is associated with poor outcome in breast cancer patients. Breast Cancer Res Treat 2013;140:495–504. [DOI] [PubMed] [Google Scholar]
- 23.Salgado R, Denkert C, Demaria S et al. The evaluation of tumor‐infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann Oncol 2015;26:259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsang JYS, Chan KW, Ni YB et al. Expression and clinical significance of herpes virus entry mediator (HVEM) in breast cancer. Ann Surg Oncol 2017;24:4042–4050. [DOI] [PubMed] [Google Scholar]
- 25.Goldhirsch A, Winer EP, Coates AS et al. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 2013;24:2206–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brouckaert O, Wildiers H, Floris G et al. Update on triple‐negative breast cancer: Prognosis and management strategies. Int J Womens Health 2012;4:511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishimura R, Osako T, Nishiyama Y et al. Evaluation of factors related to late recurrence‐‐later than 10 years after the initial treatment‐‐in primary breast cancer. Oncology 2013;85:100–110. [DOI] [PubMed] [Google Scholar]
- 28.Young‐Pearse TL, Bai J, Chang R et al. A critical function for beta‐amyloid precursor protein in neuronal migration revealed by in utero RNA interference. J Neurosci 2007;27:14459–14469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sosa LJ, Bergman J, Estrada‐Bernal A et al. Amyloid precursor protein is an autonomous growth cone adhesion molecule engaged in contact guidance. PLoS One 2013;8:e64521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larue L, Bellacosa A. Epithelial‐mesenchymal transition in development and cancer: Role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene 2005;24:7443–7454. [DOI] [PubMed] [Google Scholar]
- 31.Gilles C, Polette M, Mestdagt M et al. Transactivation of vimentin by beta‐catenin in human breast cancer cells. Cancer Res 2003;63:2658–2664. [PubMed] [Google Scholar]
- 32.Fisher R, Pusztai L, Swanton C. Cancer heterogeneity: Implications for targeted therapeutics. Br J Cancer 2013;108:479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]