Abstract
Lessons Learned.
The combination of the antiangiogenic agent ziv‐aflibercept and the heat shock protein 90 inhibitor ganetespib was associated with several serious and unexpected adverse events and was not tolerable on the dosing schedule tested.
Studies such as these emphasize the importance of considering overlapping toxicities when designing novel treatment combination regimens.
Background.
Although inhibition of angiogenesis is an effective strategy for cancer treatment, acquired resistance to antiangiogenic therapy is common. Heat shock protein 90 (Hsp90) is a molecular chaperone that regulates various oncogenic signaling pathways involved in acquired resistance and has been shown to play a role in angiogenesis. Combining an antiangiogenic agent with an Hsp90 inhibitor has therefore been proposed as a strategy for preventing resistance and improving antitumor activity. We conducted a single‐arm phase I study evaluating the combination of ziv‐aflibercept, an antiangiogenic drug, with the Hsp90 inhibitor ganetespib.
Methods.
Adult patients were eligible if they had recurrent or metastatic gastrointestinal carcinomas, nonsquamous non‐small cell lung carcinomas, urothelial carcinomas, or sarcomas that had progressed after at least one line of standard therapy. Ziv‐aflibercept was administered intravenously on days 1 and 15, and ganetespib was administered intravenously on days 1, 8, and 15, of each 28‐day cycle.
Results.
Five patients were treated with the combination. Although three patients achieved stable disease, study treatment was associated with several serious and unexpected adverse events.
Conclusion.
The dose escalation phase of this study was not completed, but the limited data obtained suggest that this combination may be too toxic when administered on this dosing schedule.
Abstract
经验获取
• 抗血管生成剂阿柏西普与热休克蛋白90抑制剂 ganetespib 联合使用与一些严重及意外的不良事件有关,并且试验给药计划不耐受。
• 这些研究都强调了在设计新型治疗组合方案时考虑交叉毒性的重要性。
摘要
背景。虽然抑制血管生成是治疗癌症的有效策略,但血管生成治疗经常会造成获得性耐药。热休克蛋白 90(Hsp90) 是一种分子伴侣,可调节多种涉及获得性耐药的致癌信号通路,已被证明在血管生成中发挥重要作用。因此,抗血管生成剂与 Hsp90 抑制剂联合使用,被提出为一种预防耐药性和提高抗肿瘤活性的策略。我们开展了一项单组I期研究,评估抗血管生成药物阿柏西普与 Hsp90 抑制剂 ganetespib 联合使用的疗效。
方法. 符合条件的成年患者需患有复发或转移性胃肠道肿瘤、非鳞状非小细胞肺癌、尿路上皮癌或肉瘤经过至少一次标准治疗后出现进展。第1天和第15天静脉注射阿柏西普,第1天、第8天和第15天静脉注射 ganetespib,每28天为一疗程。
结果。 5例患者接受了联合治疗。虽然有3例患者病情稳定,但研究治疗与数种严重及意外的不良事件有关。
结论。本研究剂量递增阶段尚未完成,但所获得的有限数据表明,此剂量计划中的组合可能毒性过大。
Discussion
The combination of antiangiogenic agents and Hsp90 inhibitors is theoretically a promising strategy for targeting compensatory oncogenic pathways. Antitumor efficacy can be achieved with therapies that block blood vessel formation, and the addition of an Hsp90 inhibitor to these agents could enhance antiangiogenic effects while also blocking oncogenic pathways that contribute to resistance. Based on this rationale, we conducted a phase I dose escalation trial (NCT02192541) testing the combination of ganetespib, an Hsp90 inhibitor, and ziv‐aflibercept (Zaltrap; sanofi‐aventis, Bridgewater, NJ; also known as vascular endothelial growth factor [VEGF]‐Trap), a fusion protein that binds and traps VEGF‐A, VEGF‐B, and placental growth factor. Adult patients were eligible if they had recurrent or metastatic gastrointestinal carcinomas, non‐small cell lung carcinomas (NSCLC), urothelial carcinomas, or sarcomas with disease progression following all treatments known to prolong survival.
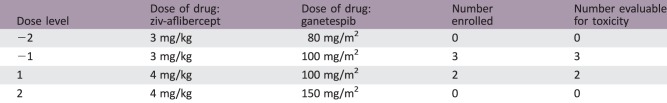
Five patients with solid tumors (three colon adenocarcinomas, one small bowel adenocarcinoma, and one rectal adenocarcinoma) were enrolled in the study. Patients received ganetespib on days 1, 8, and 15, and ziv‐aflibercept on days 1 and 15, of each 28‐day cycle. The starting dose level (DL 1) was ganetespib at 100 mg/m2 intravenously (IV) and ziv‐aflibercept at 4 mg/kg IV; after the second patient, the dose was de‐escalated from DL 1 to DL −1 (ganetespib 100 mg/m2 IV and ziv‐aflibercept 3 mg/kg IV).
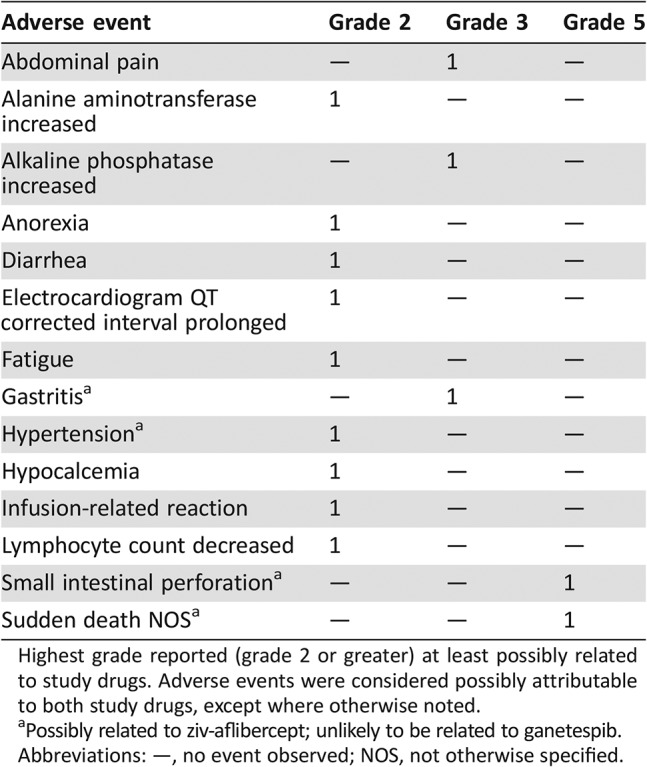
Although three of four evaluable patients on study exhibited stable disease, patients experienced multiple adverse events (AEs) ranging from grade 2 to grade 5, with gastrointestinal toxicities being the most common (Table 1). Four of five patients treated with the combination experienced at least one grade 2 AE at least possibly related to the study drugs. Grade 3 events included abdominal pain, gastritis, and an increase in alkaline phosphatase. There were two deaths on trial from events deemed possibly related to study treatment: one from grade 5 perforation of the small bowel and one sudden death not otherwise specified, potentially due to a gastrointestinal hemorrhage. In addition, one patient in the study died from grade 5 rectal perforation, but this event was attributed to surgical complications and not study drug administration. The trial was closed before the maximum tolerated dose (MTD) was reached.
Table 1. Adverse events by patient.
Highest grade reported (grade 2 or greater) at least possibly related to study drugs. Adverse events were considered possibly attributable to both study drugs, except where otherwise noted.
Possibly related to ziv‐aflibercept; unlikely to be related to ganetespib.
Abbreviations: —, no event observed; NOS, not otherwise specified.
The escalation phase of this study was not completed, but based on the limited data obtained, the combination of ganetespib and ziv‐aflibercept may be too toxic for clinical use. Ziv‐aflibercept is known to be associated with gastrointestinal perforation; concurrent administration with ganetespib, a drug that carries its own risk of gastrointestinal AEs, does not appear to be tolerable. It remains to be determined whether similar toxicities would be observed in patients treated with combinations of different VEGF and Hsp90 inhibitors. These results emphasize the need for caution when conducting studies of novel drug combinations.
Trial Information
- Disease
Bladder cancer
- Disease
Colorectal cancer
- Disease
Lung cancer—NSCLC
- Disease
Sarcomas—Adult
- Stage of Disease/Treatment
Metastatic/advanced
- Prior Therapy
No designated number of regimens
- Type of Study ‐ 1
Phase I
- Type of Study ‐ 2
3 + 3
- Primary Endpoint
Safety
- Primary Endpoint
Tolerability
- Primary Endpoint
Maximum tolerated dose
- Secondary Endpoint
Pharmacodynamic
- Additional Details of Endpoints or Study Design
- Eligibility Criteria
- Patients 18 years or older were eligible if they had histologically confirmed recurrent or metastatic gastrointestinal carcinomas, non‐small cell lung carcinomas, urothelial carcinomas, or sarcomas with disease progression following all treatments known to prolong survival, unless a given treatment was contraindicated. Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status ≤2; a life expectancy of >3 months; and normal organ and marrow function as defined by an absolute neutrophil count ≥1,500/μL, platelets ≥100,000/μL, total bilirubin ≤1.5 × institutional upper limit of normal (ULN), aspartate transaminase and alanine transaminase ≤3 × ULN, creatinine ≤1.2 × ULN (or creatinine clearance ≥60 mL/minute/1.73 m2 for patients with creatinine levels above institutional normal), urine protein/creatinine <1 mg/mg, and international normalized ratio (INR) <1.5.
- Patients were required to have optimally controlled blood pressure (defined as blood pressure below 140/90 mm Hg) prior to enrollment and to have cardiac function within institutional normal limits on echocardiogram.
- Patients were excluded from the study if they had undergone chemotherapy or radiotherapy within 3 weeks (6 weeks for nitrosoureas or mitomycin C) prior to entering the study, had active brain metastases or carcinomatous meningitis, uncontrolled intercurrent illness including ongoing or active untreated infection, serious cardiac illness, or medical conditions, or had undergone major surgery within 4 weeks prior to enrollment. Human immunodeficiency virus‐positive patients on combination antiretroviral therapy were considered ineligible because of the potential for pharmacokinetic interactions with ganetespib and ziv‐aflibercept. Patients were advised to avoid concomitant drugs that may cause QTc prolongation. Pregnant women and women who were breastfeeding were excluded.
- Study Design
- This was an open‐label, single‐arm, phase I trial evaluating the combination of ganetespib and ziv‐aflibercept in patients with refractory gastrointestinal carcinomas, nonsquamous non‐small cell lung carcinomas, urothelial carcinomas, and sarcomas. Ganetespib and ziv‐aflibercept were supplied by the National Cancer Institute's Division of Cancer Treatment and Diagnosis under Collaborative Research Agreements with Sanofi and Synta Pharmaceuticals (now Madrigal Pharmaceuticals, Inc.).
- Ganetespib was dosed based on body surface area (mg/m2) and administered intravenously, over 1 hour, weekly, on days 1, 8, and 15 of each 28‐day cycle. Ziv‐aflibercept was dosed based on body weight (mg/kg) and administered intravenously, over 1 hour, every 2 weeks, on days 1 and 15 of each 28‐day cycle. The escalation portion of the trial was planned as a standard 3 + 3 design, in which patients were to be dose‐escalated to the next dose level (DL) in cohorts of 3 until a dose‐limiting toxicity was observed. The starting dose level (DL 1) was ganetespib at 100 mg/m2 and ziv‐aflibercept at 4 mg/kg. The trial included an expansion phase to be opened once the MTD was established; this phase was designed to enroll 10 additional patients for the collection of tumor biopsies to be used for the assessment of pharmacodynamic endpoints.
- AEs were graded according to Common Terminology Criteria for Adverse Events, version 4.0. Dose‐limiting toxicity was based on events observed in the first cycle of therapy and was defined as an AE that was related (possibly, probably, or definitely) to administration of study drugs and fulfilled one of the following criteria: grade ≥3 nonhematological toxicity (except grade 3 diarrhea, nausea, vomiting responsive to supportive therapy); grade ≥3 rise in creatinine (except grade 3 able to be corrected to grade 1 with IV fluids within 48 hours); grade ≥3 metabolic toxicity (except asymptomatic toxicities able to be corrected to grade 1 or baseline within 48 hours; for hypokalemia or hyperkalemia, grade ≥2 toxicities were considered dose‐limiting if unable to be corrected); grade 4 QTc prolongation; grade 4 thrombocytopenia or grade 3 thrombocytopenia associated with bleeding; grade 4 neutropenia ≥5 days or febrile neutropenia; grade 3 fatigue of greater than 1 week duration; or failure to tolerate 100% of the dosing in the first cycle. Any degree of anemia, leukopenia in the absence of grade 4 neutropenia ≥5 days, or lymphopenia were not considered dose‐limiting. Any degree of alopecia was not considered dose‐limiting.
- Radiologic response assessments by computed tomography scans were performed at baseline and every two cycles to evaluate tumor response based on the RECIST, version 1.0.
- This trial was conducted under a National Cancer Institute‐sponsored investigational new drug application with institutional review board approval. Protocol design and conduct followed all applicable regulations, guidance, and local policies.
- Safety Assessments
- History and physical examination were performed at baseline, at the start of every cycle, and on the 8th and 15th days of each cycle. Complete blood counts with differential and serum chemistries were performed at baseline, weekly during cycles 1 and 2, and at the start of every subsequent cycle. During the first cycle, electrocardiograms were done before each ganetespib administration and approximately 24 hours after drug administration during each week of treatment; for subsequent cycles, electrocardiograms were performed before each ganetespib administration and as clinically indicated after treatment. All study participants were required to have a baseline eye exam by a qualified ophthalmologist within 4 weeks prior to enrollment and as clinically indicated thereafter.
- Investigator's Analysis
Poorly tolerated/not feasible
Drug Information
- Drug 1
- Generic/Working Name
Ziv‐aflibercept
- Trade Name
Zaltrap
- Company Name
sanofi‐aventis U.S. LLC
- Drug Type
Recombinant fusion protein
- Drug Class
Angiogenesis—VEGF
- Dose
3 or 4 mg/kg
- Route
IV
- Schedule of Administration
Over 1 hour on days 1 and 15 of each 28‐day cycle (every 2 weeks)
- Drug 2
- Generic/Working Name
Ganetespib
- Company Name
Synta Pharmaceuticals (now Madrigal Pharmaceuticals, Inc.)
- Drug Type
Small molecule
- Drug Class
Hsp90
- Dose
100 mg/m2
- Route
IV
- Schedule of Administration
Over 1 hour on days 1, 8, and 15 of each 28‐day cycle (3 weeks on/1 week off)
Dose Escalation Table for Phase I Combination Treatment
Patient Characteristics
- Number of Patients, Male
2
- Number of Patients, Female
3
- Stage
Metastatic/advanced
- Age
Median (range): 60 (50–67)
- Number of Prior Systemic Therapies
Median (range): 8 (5–15)
- Performance Status: ECOG
-
0 — 0
1 — 5
2 — 0
3 — 0
Unknown — 0
- Cancer Types or Histologic Subtypes
-
Colon adenocarcinoma 3
Duodenum adenocarcinoma 1
Rectal adenocarcinoma 1
Primary Assessment Method
- Title
Total patient population
- Number of Patients Enrolled
5
- Number of Patients Evaluable for Toxicity
5
- Number of Patients Evaluated for Efficacy
4
- Evaluation Method
RECIST 1.0
- Response Assessment CR
n = 0 (0%)
- Response Assessment PR
n = 0 (0%)
- Response Assessment SD
n = 3 (75%)
- Response Assessment PD
n = 1 (25%)
- Response Assessment OTHER
n = 0 (0%)
- (Median) Duration Assessments Response Duration
4 months
- (Median) Duration Assessments Duration of Treatment
4 months
- Outcome Notes
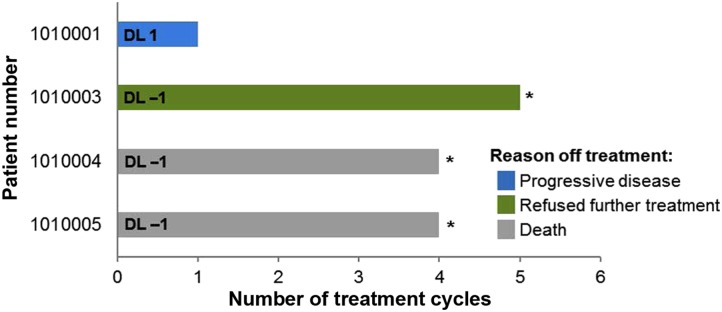
- Patients were treated with ganetespib and ziv‐aflibercept for one to five cycles (Figure 1). Four patients were evaluable for response; three had a best response of stable disease for a median of 4 months and one had progressive disease. One patient who achieved stable disease for five treatment cycles chose to withdraw from the study. The other two patients with stable disease died on study but did not show signs of disease progression on their last completed restaging scans.
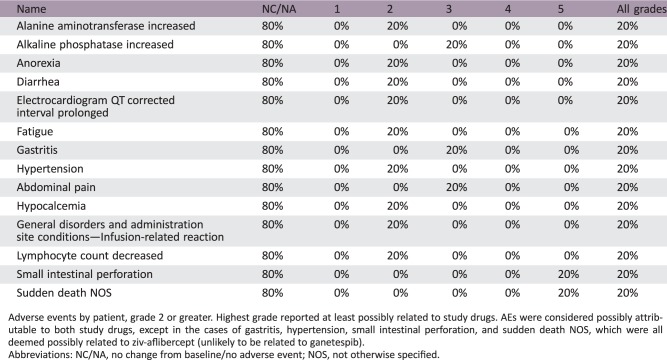
Phase I Combination Treatment Adverse Events
All Cycles
Adverse events by patient, grade 2 or greater. Highest grade reported at least possibly related to study drugs. AEs were considered possibly attributable to both study drugs, except in the cases of gastritis, hypertension, small intestinal perforation, and sudden death NOS, which were all deemed possibly related to ziv‐aflibercept (unlikely to be related to ganetespib).
Abbreviations: NC/NA, no change from baseline/no adverse event; NOS, not otherwise specified.
Serious Adverse Events
Serious adverse events possibly related to study treatment. Both events were considered to be possibly related to ziv‐aflibercept (unlikely to be related to ganetespib).
Abbreviation: NOS, not otherwise specified.
Assessment, Analysis, and Discussion
- Completion
Study terminated before completion
- Terminated Reason
Company stopped development
- Investigator's Assessment
Poorly tolerated/not feasible
A critical feature of cancer progression is tumor angiogenesis, the process whereby malignant cells drive the formation of new blood vessels [1], [2]. One of the antiangiogenic drugs that have been developed for cancer treatment is ziv‐aflibercept, a U.S Food and Drug Administration‐approved fusion protein with high binding affinity for vascular endothelial growth factor (VEGF)‐A, VEGF‐B, and placental growth factor. These factors are potent signaling proteins secreted by tumors to stimulate blood vessel formation [3], [4]. Ziv‐aflibercept has been shown to be tolerable and effective when combined with chemotherapy, and phase I dose escalation studies established the recommended phase II dose (RP2D) as 4 mg/kg administered intravenously (IV) every 2 weeks [5], [6].
Although ziv‐aflibercept and other antiangiogenic drugs have demonstrated clinically significant antitumor activity, the ability of these agents to produce durable, long‐term responses is limited [7]. Hypoxia in tumor tissue induces a cellular response that activates oncogenic signaling pathways, which can result in resistance to therapy and promote tumor growth [8], [9]. One potential treatment strategy is to combine antiangiogenic treatment with inhibition of heat shock protein 90 (Hsp90), a molecular chaperone that assists in the stabilization of multiple oncogenic proteins, including those involved in tumor invasion, metastasis, and avoidance of apoptosis [10]. Importantly, Hsp90 plays an essential role in angiogenesis by not only modulating VEGF signaling and vessel formation but also by stabilizing the hypoxia‐inducible factor‐1 α (HIF‐1α) dimerization complex required for endothelial cell proliferation [11], [12], [13]. Combining an Hsp90 inhibitor with an antiangiogenic agent therefore allows for the targeting of multiple oncogenic pathways, including those that promote angiogenesis and those that drive tumor invasion in response to antiangiogenic therapy.
Ganetespib is an Hsp90 inhibitor that leads to the misfolding and degradation of Hsp90‐associated proteins [14], [15]. A first‐in‐human phase I dose‐escalation study established the RP2D to be 200 mg/m2 given IV on days 1, 8, and 15 of each 4‐week cycle; ganetespib was well tolerated on this dosing schedule [16]. Furthermore, clinical activity of ganetespib monotherapy was demonstrated in phase II studies of patients with non‐small cell lung carcinomas and metastatic breast cancer [17], [18].
Our phase I study to assess the safety of combining the antiangiogenic agent ziv‐aflibercept with the Hsp90 inhibitor ganetespib demonstrated serious toxicity and suggested that this combination is intolerable on the tested 28‐day schedule. Ziv‐aflibercept is known to be associated with gastrointestinal perforation and hemorrhage [19], and ganetespib also carries the risk of gastrointestinal toxicity [14]; in combination, these agents were associated with several gastrointestinal adverse events (AEs), ranging from grade 2 to grade 5 in severity. Further preclinical studies are needed to determine if similar adverse events would be observed with combinations of different VEGF and Hsp90 inhibitors and whether adjusting the dose or treatment schedule would make this treatment strategy feasible. The inclusion of pharmacodynamic assays in future clinical studies, such as the measurement of HIF‐1α and Hsp90 client proteins in tumor tissue before and after treatment, may provide evidence of antitumor activity and further support for optimizing this combination [20] (unfortunately, in the present study, these biomarker measurements were planned only for the expansion cohort and were therefore not obtained).
Drug studies that combine established treatments with novel agents or with new applications of known agents represent an exciting area of clinical research. Due to tumor cell heterogeneity, targeted agents like ganetespib and ziv‐aflibercept—although less harmful to normal cells than conventional chemotherapies—have been shown to have limited single‐agent antitumor activity and may need to be administered in combination in order to achieve meaningful clinical benefit. Combination therapy with agents that target complementary pathways may, in addition to preventing the development of treatment resistance, demonstrate synergistic antitumor activity compared with either agent alone [21], [22]. However, as our results show, tolerability is a significant issue to consider when developing novel combination regimens with drugs that may have overlapping toxicities. Although gastrointestinal perforation is noted as a risk of both ganetespib and ziv‐aflibercept, these events are extremely rare, and it is not clear if the one grade 5 small bowel perforation in the current trial was coincidental (and its significance inflated due to the small number of patients) or truly representative of synergistic toxicity. This difficulty in determining the independent actions of individual drugs, and differentiating them from the direct results of administering drugs in combination, has been recognized and discussed in the literature and remains a challenge when interpreting the results of combination studies [23], [24]. Furthermore, the incidence of AEs in combination studies can be affected by pharmacokinetic and pharmacodynamic interactions between drugs, which can alter drug exposure and potentially lead to an increase in the frequency and severity of known AEs as well as to unexpected toxicities [25], [26], [27].
Many of the challenges of designing tolerable combinations stem from a lack of understanding of how novel targeted therapies affect normal cells. Preclinical studies performed in tumor models often focus only on the evaluation of tumor cell signaling without considering the consequences of the drug on normal cells or tissue [28]. Although combination regimens are thought to be effective due to the ability to target multiple aberrant signaling pathways, this approach carries significant risk of toxicity to normal cells with unaltered survival mechanisms. The toxicity of targeted agent combinations is typically not explored in preclinical models, with investigators relying instead on single‐agent data to determine the safety of novel combinations. Due to the lack of preclinical data on the toxicity of specific combinations, the design of these trials requires vigilance and careful selection of treatment doses and schedules [27].
The results presented here emphasize the need to use caution when conducting studies of novel drug combinations. However, in cases in which side effects can be successfully managed, the potential benefits of drug combination trials are likely to outweigh the risks. Rationally designed combination trials represent a promising avenue for combating resistance to anticancer therapies and improving the options for treatment. As in the case of this trial, careful monitoring of expected and unexpected toxicity is required to achieve an acceptable risk/benefit ratio.
Figures and Tables
Figure 1.
Number of treatment cycles for the four evaluable patients. The dose level for each patient is shown (DL 1 or DL −1). The reason for the patient going off treatment is indicated in the legend. Abbreviations: *, patients who achieved a best response of stable disease; DL, dose level.
Acknowledgments
This project was funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract Number HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. We thank Dr. Laura K. Fogli, Kelly Services, for medical writing support in the preparation of this manuscript.
Footnotes
ClinicalTrials.gov Identifier: NCT02192541
Sponsor: National Cancer Institute
Principal Investigator: Alice P. Chen
IRB Approved: Yes
Disclosures
The authors indicated no financial relationships.
References
- 1. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 2. Ziyad S, Iruela‐Arispe ML. Molecular mechanisms of tumor angiogenesis. Genes Cancer 2011;2:1085–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Holash J, Davis S, Papadopoulos N et al. VEGF‐Trap: A VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A 2002;99:11393–11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Verheul HM, Hammers H, van Erp K et al. Vascular endothelial growth factor trap blocks tumor growth, metastasis formation, and vascular leakage in an orthotopic murine renal cell cancer model. Clin Cancer Res 2007;13:4201–4208. [DOI] [PubMed] [Google Scholar]
- 5. Isambert N, Freyer G, Zanetta S et al. Phase I dose‐escalation study of intravenous aflibercept in combination with docetaxel in patients with advanced solid tumors. Clin Cancer Res 2012;18:1743–1750. [DOI] [PubMed] [Google Scholar]
- 6. Lockhart AC, Rothenberg ML, Dupont J et al. Phase I study of intravenous vascular endothelial growth factor trap, aflibercept, in patients with advanced solid tumors. J Clin Oncol 2010;28:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bergers G, Hanahan D. Modes of resistance to antiangiogenic therapy. Nat Rev Cancer 2008;8:592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eckerich C, Zapf S, Fillbrandt R et al. Hypoxia can induce c‐Met expression in glioma cells and enhance SF/HGF‐induced cell migration. Int J Cancer 2007;121:276–283. [DOI] [PubMed] [Google Scholar]
- 9. Xu L, Nilsson MB, Saintigny P et al. Epidermal growth factor receptor regulates MET levels and invasiveness through hypoxia‐inducible factor‐1alpha in non‐small cell lung cancer cells. Oncogene 2010;29:2616–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stravopodis DJ, Margaritis LH, Voutsinas GE. Drug‐mediated targeted disruption of multiple protein activities through functional inhibition of the Hsp90 chaperone complex. Curr Med Chem 2007;14:3122–3138. [DOI] [PubMed] [Google Scholar]
- 11. Bohonowych JE, Gopal U, Isaacs JS. Hsp90 as a gatekeeper of tumor angiogenesis: Clinical promise and potential pitfalls. J Oncol 2010;2010:412985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kawanami D, Mahabeleshwar GH, Lin Z et al. Kruppel‐like factor 2 inhibits hypoxia‐inducible factor 1alpha expression and function in the endothelium. J Biol Chem 2009;284:20522–20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mabjeesh NJ, Post DE, Willard MT et al. Geldanamycin induces degradation of hypoxia‐inducible factor 1alpha protein via the proteosome pathway in prostate cancer cells. Cancer Res 2002;62:2478–2482. [PubMed] [Google Scholar]
- 14. Jhaveri K, Modi S. Ganetespib: Research and clinical development. Onco Targets Ther 2015;8:1849–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ying W, Du Z, Sun L et al. Ganetespib, a unique triazolone‐containing Hsp90 inhibitor, exhibits potent antitumor activity and a superior safety profile for cancer therapy. Mol Cancer Ther 2012;11:475–484. [DOI] [PubMed] [Google Scholar]
- 16. Goldman JW, Raju RN, Gordon GA et al. A first in human, safety, pharmacokinetics, and clinical activity phase I study of once weekly administration of the Hsp90 inhibitor ganetespib (STA‐9090) in patients with solid malignancies. BMC Cancer 2013;13:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jhaveri K, Chandarlapaty S, Lake D et al. A phase II open‐label study of ganetespib, a novel heat shock protein 90 inhibitor for patients with metastatic breast cancer. Clin Breast Cancer 2014;14:154–160. [DOI] [PubMed] [Google Scholar]
- 18. Socinski MA, Goldman J, El‐Hariry I et al. A multicenter phase II study of ganetespib monotherapy in patients with genotypically defined advanced non‐small cell lung cancer. Clin Cancer Res 2013;19:3068–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saif MW, Relias V, Syrigos K et al. Incidence and management of ziv‐aflibercept related toxicities in colorectal cancer. World J Clin Oncol 2014;5:1028–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parchment RE, Doroshow JH. Pharmacodynamic endpoints as clinical trial objectives to answer important questions in oncology drug development. Semin Oncol 2016;43:514–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Humphrey RW, Brockway‐Lunardi LM, Bonk DT et al. Opportunities and challenges in the development of experimental drug combinations for cancer. J Natl Cancer Inst 2011;103:1222–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dancey JE, Chen HX. Strategies for optimizing combinations of molecularly targeted anticancer agents. Nat Rev Drug Discov 2006;5:649–659. [DOI] [PubMed] [Google Scholar]
- 23. Doroshow JH, Simon RM. On the design of combination cancer therapy. Cell 2017;171:1476–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palmer AC, Sorger PK. Combination cancer therapy can confer benefit via patient‐to‐patient variability without drug additivity or synergy. Cell 2017;171:1678–1691 e1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lopez JS, Banerji U. Combine and conquer: Challenges for targeted therapy combinations in early phase trials. Nat Rev Clin Oncol 2017;14:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paller CJ, Bradbury PA, Ivy SP et al. Design of phase I combination trials: Recommendations of the Clinical Trial Design Task Force of the NCI Investigational Drug Steering Committee. Clin Cancer Res 2014;20:4210–4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park SR, Davis M, Doroshow JH et al. Safety and feasibility of targeted agent combinations in solid tumours. Nat Rev Clin Oncol 2013;10:154–168. [DOI] [PubMed] [Google Scholar]
- 28. Ocana A, Pandiella A, Siu LL et al. Preclinical development of molecular‐targeted agents for cancer. Nat Rev Clin Oncol 2010;8:200–209. [DOI] [PubMed] [Google Scholar]