This article reports the results of a study evaluating the development of immune‐related adverse events in patients who received nivolumab monotherapy to determine clinical benefit in patients with advanced non‐small cell lung cancer.
Keywords: Nivolumab, Immune‐related adverse event, Immune checkpoint inhibitor, Non‐small‐cell lung cancer, Antithyroid antibody
Abstract
Background.
Immune‐related adverse events (irAEs) are frequently observed with nivolumab monotherapy. This study aimed to evaluate whether the development of irAEs correlates with treatment response in advanced non‐small‐cell lung cancer (NSCLC).
Patients and Methods.
We conducted a retrospective study of patients who received nivolumab monotherapy at Sendai Kousei Hospital (n = 70). The patients were categorized into two groups based on the incidence of irAEs: those with irAEs (irAE group) or those without (non‐irAE group). Treatment efficacy was evaluated in each group. The patients were further categorized into responders and nonresponders, and predictive factors of treatment response were determined.
Results.
The objective response rate was 57% in the irAE group versus 12% in the non‐irAE group. Median progression‐free survival was 12.0 months in the irAE versus 3.6 months in the non‐irAE group. The incidence of both irAEs and pre‐existing antithyroid antibody was significantly higher in responders than in nonresponders. Multivariate analysis identified incidence of irAEs and pre‐existing antithyroid antibody as an independent predictor of treatment response.
Conclusion.
Objective response rate and progression‐free survival were significantly better in the irAE than in the non‐irAE group in patients with advanced NSCLC treated with nivolumab monotherapy. The development of irAEs was associated with clinical efficacy, and the presence of pre‐existing antithyroid antibody might be correlated with treatment response to nivolumab monotherapy.
Implications for Practice.
Immune‐related adverse events (irAEs) are frequently observed with nivolumab monotherapy. This study evaluted whether the development of irAEs correlates with treatment response in advanced non‐small‐cell lung cancer. Results showed that the objective response rate and progression‐free survival were significantly better in the patients who developed irAEs than in the patients who did not develop irAEs, and the incidence of irAEs and positivity for antithyroid antibody at pretreatment were independent predictors of treatment response of nivolumab monotherapy. Therefore, the development of irAEs predicts clinical benefit and suggests that cautious management of irAEs can lead to achieving maximum clinical benefit from nivolumab monotherapy.
摘要
背景。 免疫相关不良事件 (irAE) 经常发生在纳武单抗单药治疗中。本研究旨在评估 irAE 发生是否与晚期非小细胞肺癌 (NSCLC) 的治疗反应相关。
患者和方法。 我们对在 Sendai Kousei 医院 (n=70) 接受纳武单抗治疗的患者进行了一项回顾性研究。根据 irAE 发生率将患者分为两组:出现 irAE 患者组( irAE 组)或未出现 irAE 患者组(非‐irAE 组)。评估各组的治疗效果。将患者进一步分为应答者和无应答者,并确定治疗反应的预测因素。
结果。 irAE 组和非‐irAE 组的客观有效率分别为57%和12%。irAE 组和非‐irAE 组的中位无进展生存期分别为12.0个月和3.6个月。应答者的 irAE 和既往抗甲状腺抗体的发生率均显著高于无应答者。多因素分析发现,irAE 和既往抗甲状腺抗体的发生率是治疗反应的独立预测指标。
结论。 在接受纳武单抗单药治疗的晚期 NSCLC 患者中,irAE 组客观有效率和无进展生存期明显优于非‐irAE 组。irAE 的发生与临床疗效有关,而既往抗甲状腺抗体的存在可能与纳武单抗单药的治疗反应有关。
实践意义: 纳武单抗单药治疗频繁出现免疫相关不良事件 (irAE)。本研究评估了 irAE 进展与晚期非小细胞肺癌治疗反应是否相关。结果表明,客观有效率和无进展生存期在发生 irAE 的患者中明显好于未发生 irAE 的患者,治疗前 irAE 的发生率和抗甲状腺抗体的阳性率是纳武单抗单药治疗效果的独立预测指标。因此,通过 irAE 发生可以预测临床受益,并且表明,谨慎管理 irAE 可以使纳武单抗单药治疗获得最大临床受益。
Introduction
Lung cancer is a leading cause of cancer‐related death worldwide [1]. It is generally classified into two primary categories, small‐cell lung cancer and non‐small‐cell lung cancer (NSCLC), with NSCLC being the more common form, accounting for approximately 85% of all lung cancers. It is divided into two major histological subtypes, adenocarcinoma and squamous cell carcinoma.
Therapies targeting the programmed cell death protein‐1 (PD‐1) pathway have profoundly improved outcomes in patients with a variety of malignancies [2], [3]. PD‐1 is an inhibitory coreceptor primarily expressed on the surface of activated T cells that, upon binding to PD‐ligand 1 (PD‐L1) or PD‐ligand 2, modulates T‐cell effector function, including proliferation [4], cytokine production [5], and survival [6]. Interruption of PD‐1/PD‐L1 signaling by monoclonal antibodies can regenerate T‐cell‐mediated antitumor immunity, producing durable anticancer responses in a subset of patients.
In 2015, two international, open‐label, randomized phase III studies, CheckMate‐017 [7] and CheckMate‐057 [8], compared the efficacy of nivolumab, a fully humanized immunoglobulin G4 PD‐1 immune checkpoint inhibitor (ICI) antibody, and docetaxel in patients with advanced NSCLC that had progressed during or after platinum‐based chemotherapy. The results showed high response rates and more favorable safety profiles for nivolumab. As such, nivolumab became the new second‐line treatment of choice for advanced NSCLC. However, T‐cell activation can cause immune‐related adverse events (irAEs), such as skin reactions, thyroid dysfunction, pneumonitis, and hepatitis, that do not occur with conventional cytotoxic anticancer agents [9], indicating the need for a different approach in managing adverse events from nivolumab. One study evaluated whether the development of irAEs in patients with nonmelanoma (which included 71 patients with NSCLC) treated with anti‐PD‐1 ICI monotherapy correlated with improved clinical outcomes, and the results indicated that for a subset of patients, in particular those with low‐grade irAEs, the development of irAEs was associated with improved response rates, longer duration between therapies, and increased life span [10]. This suggests an association between the development of irAEs, durable response, and clinical benefit. To our knowledge, however, similar findings have yet to be reported in patients with advanced NSCLC. This study aimed to investigate whether the development of irAEs was associated with clinical benefit in patients with advanced NSCLC treated with nivolumab monotherapy.
Materials and Methods
Patients
Patients with advanced NSCLC who underwent nivolumab monotherapy (3 mg/kg every 2 weeks) at Sendai Kousei Hospital between January 2016 and February 2017 were enrolled in the study. Treatment was given until disease progression, unacceptable toxicity, or withdrawal of consent. All patients were followed up for survival until death, loss to follow‐up, or withdrawal of consent.
Assessment
Objective tumor response to nivolumab monotherapy was confirmed using computed tomography every 8 weeks and determined in accordance with the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines, version 1.1 [11]. One radiologist and two pulmonary physicians (an attending physician and an investigator) measured objective tumor response. The attending physician and a nurse specialist conducted a physical examination, assessed patients for irAEs every 2 weeks throughout the treatment course, and recorded the results. IrAEs were defined according to previous studies [7], [8], [12], [13], [14] as adverse events with a potential immunological basis that require potential intervention with immunosuppressive or endocrine therapy. These include skin reactions, as well as endocrine, gastrointestinal, hepatic, neurological, and pulmonary irAEs. In addition, to reduce bias, we focused only on irAEs that medical professionals could recognize objectively. Infusion reaction was not included among irAEs because infusion reaction can be observed with any monoclonal antibody. Therefore, we do not view infusion reaction as true immune‐mediate event. The clinical severity of irAEs was graded based on the Common Terminology Criteria for Adverse Events, version 4.0. Blood samples were drawn at screening to measure immune safety parameters (thyroid‐stimulating hormone, free T4 level, free T3 level, antithyroglobulin antibody, antithyroid peroxidase antibody, rheumatoid factor, and antinuclear antibody). Progression‐free survival (PFS) was defined as the time from start of treatment to documented disease progression or death due to any cause, and overall survival (OS) was defined as the time from start of treatment to death due to any cause.
The patients were categorized into two groups based on incidence of irAEs: those with irAE (irAE group) or those without (non‐irAE group). Both groups were evaluated with respect to objective response rate (ORR), disease control rate (DCR), and PFS. The patients were further classified as responders, that is, those who achieved complete response (CR) or partial response (PR), and nonresponders, that is, those who developed stable disease (SD) or progressive disease (PD). Predictive factors of treatment response were also analyzed.
Statistical Analysis
Univariate and multivariate logistic regression analyses were used to determine whether patient variables were related to response to nivolumab monotherapy. Categorical variables were tested for significance using the chi‐squared test, Student's t test, the Mann‐Whitney U test, or Welch's t test, as appropriate. PFS and OS were estimated using Kaplan‐Meier curves with a two‐sided log‐rank test. The hazard ratio was estimated using the Cox proportional hazards model. The cutoff date for the survival analysis was June 28, 2017. All p values were two sided, with a value of <.05 considered to indicate statistical significance.
All statistical analyses were performed with EZR (Saitama Medical Centre, Jichi Medical University, Saitama, Japan), a graphical user interface for R (R Foundation for Statistical Computing, Vienna, Austria). Specifically, it is a modified version of R Commander designed to add statistical functions frequently used in biostatistics [15].
The study protocol was approved by the institutional review board of Sendai Kousei Hospital (IRB no. 29–30). The need to obtain informed consent was waived because data were analyzed anonymously.
Results
Patient Characteristics
A total of 70 patients (61 men [87%] and 9 women [13%]) with advanced NSCLC underwent nivolumab monotherapy during the study period (Table 1). The median patient age was 68 years (range, 36–88), and 69 patients (99%) had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1. A total of 27 patients (39%) and 43 patients (61%) were diagnosed with squamous cell carcinoma and nonsquamous NSCLC, respectively. Of the patients with nonsquamous‐cell carcinoma, epidermal growth factor receptor‐mutant NSCLC was diagnosed in five (12%). Thirty‐eight patients (54%) had undergone one prior chemotherapy regimen, 15 (21%) had undergone two, and 17 (25%) had undergone three or more. None had active autoimmune disease. Twenty‐eight patients (40%) developed irAEs, of whom 21 (75%) developed them within 8 weeks. The median onset of irAEs was 5.0 weeks.
Table 1. Baseline characteristics of the study population (n = 70).
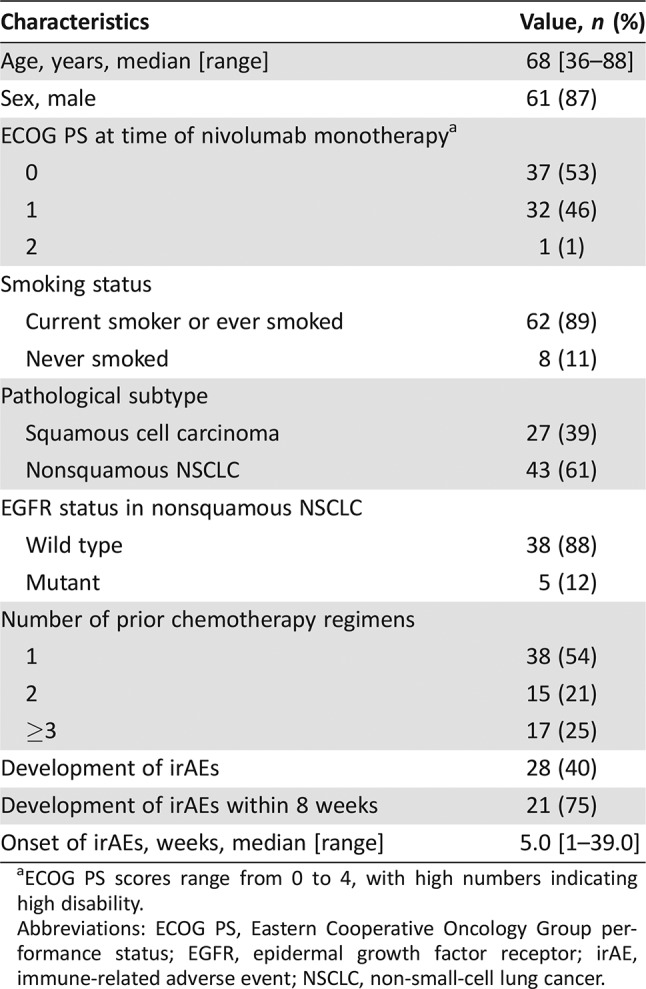
ECOG PS scores range from 0 to 4, with high numbers indicating high disability.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; irAE, immune‐related adverse event; NSCLC, non‐small‐cell lung cancer.
Based on the RECIST version 1.1 criteria, CR was observed in 1 patient (1%), PR in 20 (29%), SD in 23 (33%), and PD in 26 (37%). The ORR was 30% (95% confidence interval [CI], 20–42), and the DCR was 63% (95% CI, 51–74).
Categorization into irAE or Non‐irAE Group
Table 2 shows the categories of irAE incidence. Of the 28 patients who developed irAEs, 22 (79%) had skin reaction, 5 (18%) had myositis or peripheral neuropathy, 6 (21%) had hypothyroidism, 1 (4%) had hyperthyroidism, 5 (18%) had pneumonitis, 1 (4%) had hepatitis, and 2 (7%) had diarrhea. Nivolumab monotherapy had to be discontinued in five patients because of pneumonitis. Systemic steroids were used to treat pneumonitis in four patients (14%). However, no irAE‐related death occurred during the study.
Table 2. Categorization of irAEs.
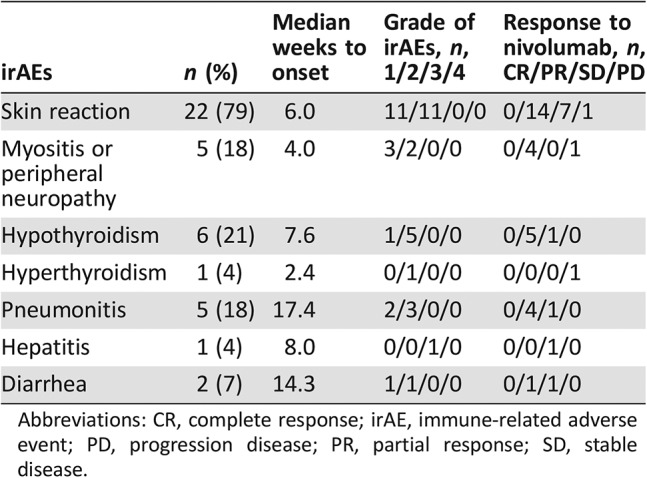
Abbreviations: CR, complete response; irAE, immune‐related adverse event; PD, progression disease; PR, partial response; SD, stable disease.
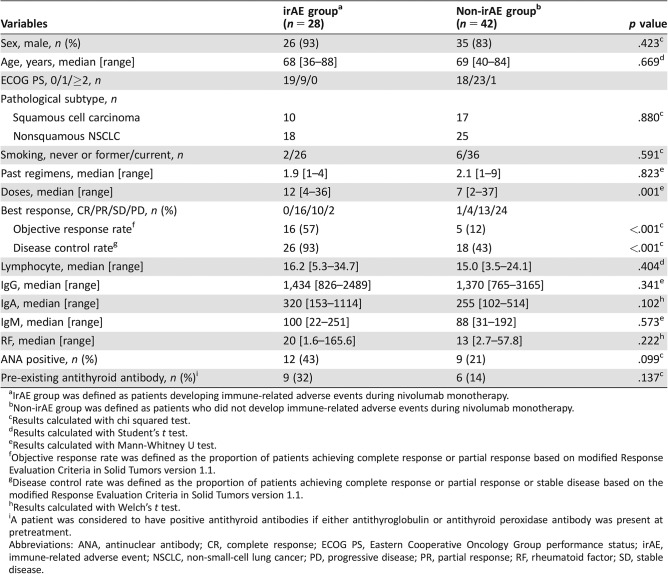
Table 3 shows the comparison of patient characteristics between the irAE and non‐irAE groups. No significant difference was observed in terms of sex, age, ECOG PS, frequency of pathological subtype, smoking history, the number of past regimens, or any of the blood parameters investigated between the two groups. In the irAE group, 16 patients (57%) showed PR, 10 (36%) SD, and 2 (7%) PD. None of the patients showed CR. Of the 42 patients who did not develop irAEs, 1 (2%) showed CR, 4 (10%) PR, 13 (31%) SD, and 24 (57%) PD. The ORR was significantly higher in the irAE group than in the non‐irAE group (57% vs. 12%, respectively; p < .001). The DCR was also significantly higher in the irAE group than in the non‐irAE group (93% vs. 43%, respectively; p < .001).
Table 3. Characteristics of patients in the irAE and non‐irAE groups (n = 70).
IrAE group was defined as patients developing immune‐related adverse events during nivolumab monotherapy.
Non‐irAE group was defined as patients who did not develop immune‐related adverse events during nivolumab monotherapy.
Results calculated with chi squared test.
Results calculated with Student's t test.
Results calculated with Mann‐Whitney U test.
Objective response rate was defined as the proportion of patients achieving complete response or partial response based on modified Response Evaluation Criteria in Solid Tumors version 1.1.
Disease control rate was defined as the proportion of patients achieving complete response or partial response or stable disease based on the modified Response Evaluation Criteria in Solid Tumors version 1.1.
Results calculated with Welch's t test.
A patient was considered to have positive antithyroid antibodies if either antithyroglobulin or antithyroid peroxidase antibody was present at pretreatment.
Abbreviations: ANA, antinuclear antibody; CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; irAE, immune‐related adverse event; NSCLC, non‐small‐cell lung cancer; PD, progressive disease; PR, partial response; RF, rheumatoid factor; SD, stable disease.
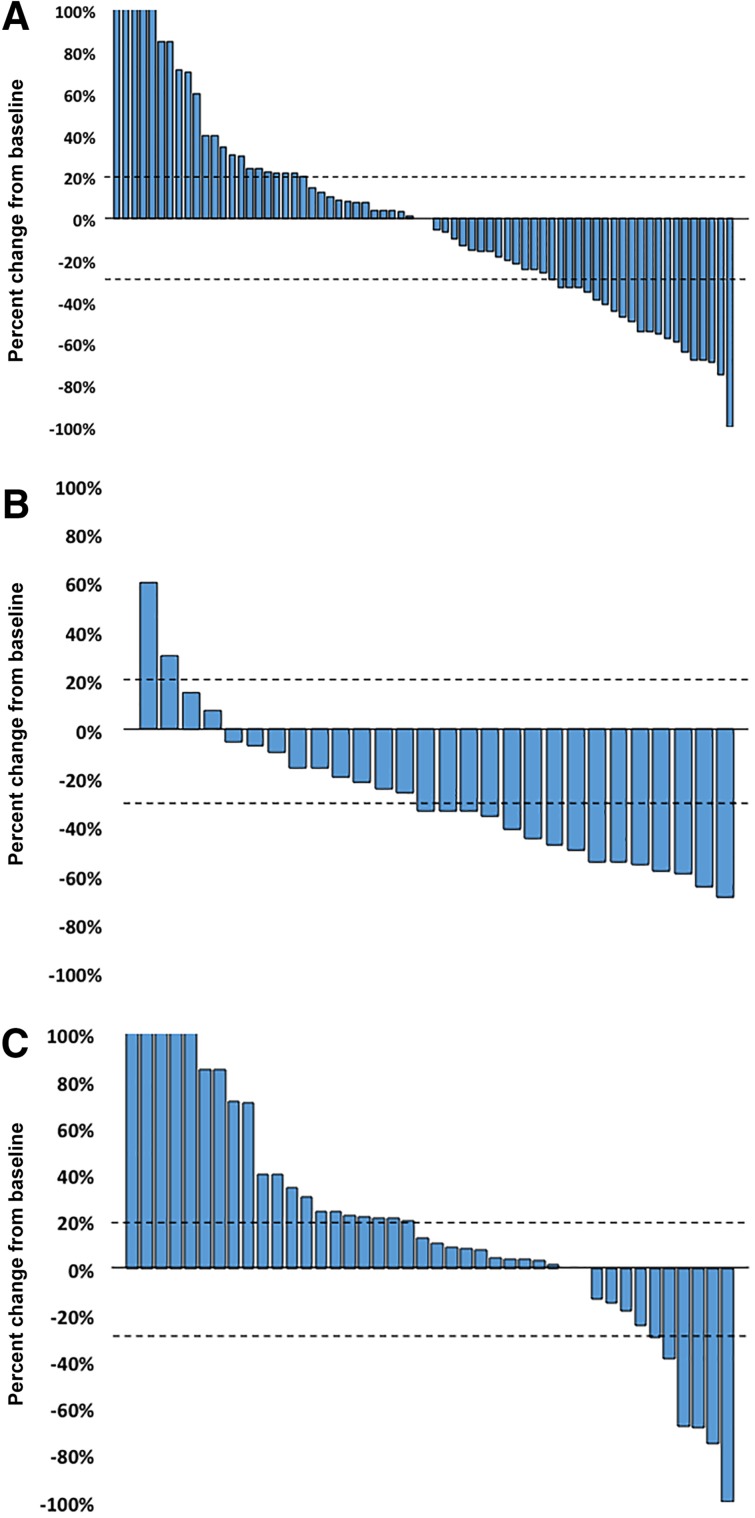
The best response to nivolumab monotherapy is shown in Figure 1. The frequency of tumor reduction was significantly higher in the irAE group than in the non‐irAE group.
Figure 1.
Waterfall plot of treatment showing best response in patients treated with nivolumab monotherapy compared with pretreatment baseline. (A): The response in the overall population. (B): The response in the immune‐related adverse events (irAE) group. (C): The response in the non‐irAE group.
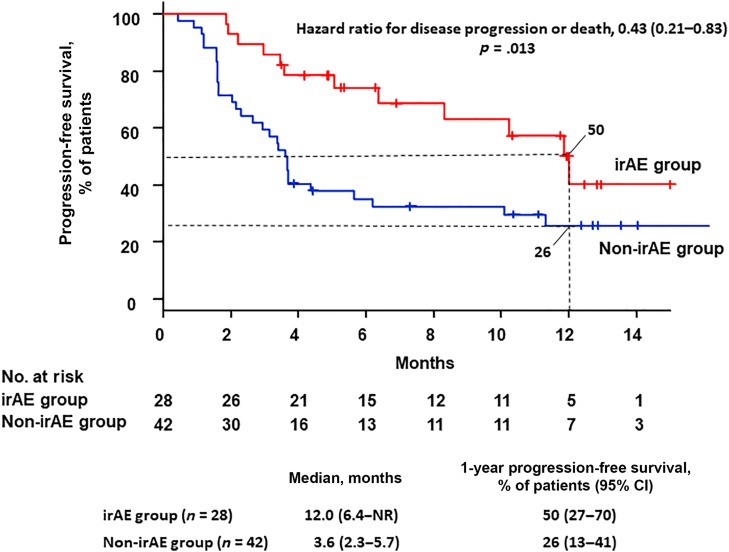
The median PFS was 12.0 months (95% CI, 6.4 to not reached) in the irAE group and 3.6 months (95% CI, 2.3–5.7) in the non‐irAE group. The 12‐month PFS was 50% (95% CI, 27–70) in the irAE group and 26% (95% CI, 13–41) in the non‐irAE group. The hazard ratio for progression disease or death was 0.43 (95% CI, 0.21–0.83; p = .013; Fig. 2). PFS was statistically significantly better in the irAE group than in the non‐irAE group.
Figure 2.
Rate of progression‐free survival in the study population. Kaplan‐Meier curves are shown for progression‐free survival. Ticks indicate patients for whom data were censored at the data cutoff point (June 28, 2017).
Abbreviations: CI, confidence interval; irAEs, immune‐related adverse events; NR, not reached.
Categorization as Responder or Nonresponder
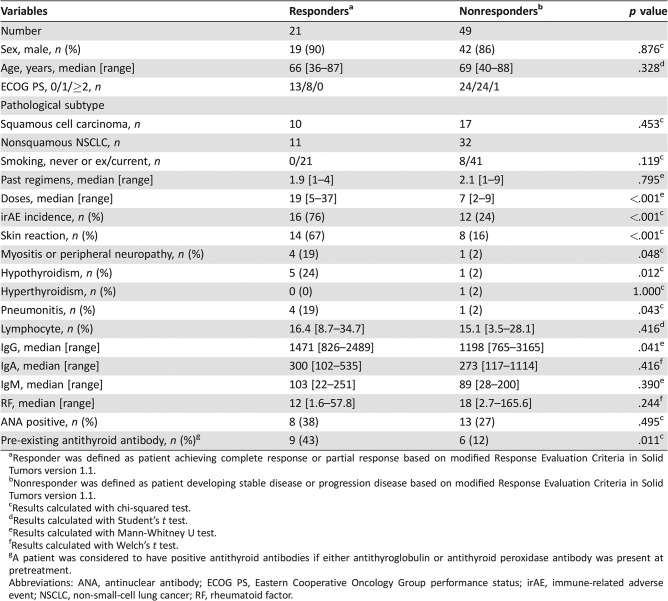
Table 4 shows a comparison of patient characteristics between responders and nonresponders. No significant difference was observed in terms of sex, age, ECOG PS, frequency of pathological subtype, smoking history, or the number of past regimens between the two groups. The number of doses was significantly higher in responders than in nonresponders (19 vs. 7, respectively; p < .001). The incidence of irAEs was significantly higher in responders than in nonresponders (76% vs. 24%, respectively; p < .001). Univariate analysis identified a significantly higher occurrence of irAEs, pre‐existing antithyroid antibody, and higher IgG in responders than in nonresponders. Factors identified significantly associated with treatment response to nivolumab monotherapy in the univariate analysis were subjected to multivariate analysis. The results revealed that the development of irAEs (odds ratio [OR], 0.11; 95% CI, 0.03–0.43; p < .001) and pre‐existing antithyroid antibody (OR, 0.22; 95% CI, 0.06–0.88; p = .033) were independent predictors of treatment response (Table 5).
Table 4. Characteristics of patients in the responders and nonresponders groups (n = 70).
Responder was defined as patient achieving complete response or partial response based on modified Response Evaluation Criteria in Solid Tumors version 1.1.
Nonresponder was defined as patient developing stable disease or progression disease based on modified Response Evaluation Criteria in Solid Tumors version 1.1.
Results calculated with chi‐squared test.
Results calculated with Student's t test.
Results calculated with Mann‐Whitney U test.
Results calculated with Welch's t test.
A patient was considered to have positive antithyroid antibodies if either antithyroglobulin or antithyroid peroxidase antibody was present at pretreatment.
Abbreviations: ANA, antinuclear antibody; ECOG PS, Eastern Cooperative Oncology Group performance status; irAE, immune‐related adverse event; NSCLC, non‐small‐cell lung cancer; RF, rheumatoid factor.
Table 5. Multivariate analysis of predictive factors of response to nivolumab monotherapy (n = 70).
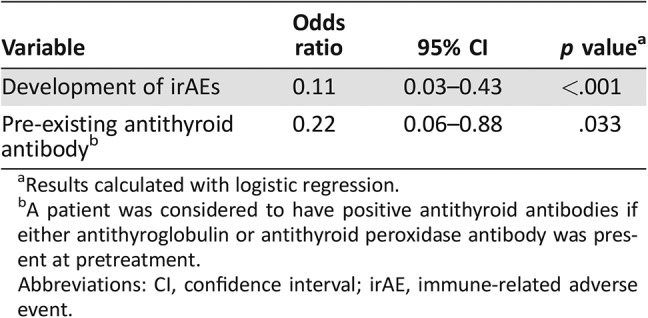
Results calculated with logistic regression.
A patient was considered to have positive antithyroid antibodies if either antithyroglobulin or antithyroid peroxidase antibody was present at pretreatment.
Abbreviations: CI, confidence interval; irAE, immune‐related adverse event.
Categorization as Those with Pre‐Existing Antithyroid Antibody and Those Without
Supplemental online Table 1 shows a comparison of patient characteristics between those with pre‐existing antithyroid antibody and those without. In our study, 15 patients had pre‐existing antithyroid antibody. Of the 15 patients, 6 (40%) had thyroid dysfunction, and the development of thyroid dysfunction was significantly higher in those with pre‐existing antithyroid antibody than in those without (40% vs. 2%, respectively; p < .001).
Discussion
This was a single‐center, retrospective study of patients with advanced NSCLC treated with nivolumab monotherapy in a clinical setting. The purpose of this study was to investigate whether the development of irAEs was associated with clinical benefit and to search for predictors of treatment response in nivolumab monotherapy.
Predictive markers of effective response to nivolumab monotherapy have been previously proposed. In the CheckMate‐057 trial [8], PD‐L1 expression was significantly associated with improved OS. Moreover, in the KEYNOTE‐010 trial [12], treatment with pembrolizumab—a highly selective IgG4‐κ humanized monoclonal antibody directed against the human cell surface receptor PD‐1— significantly prolonged OS compared with docetaxel treatment in previously treated, PD‐L1‐positive, patients with advanced NSCLC. Additionally, in the KEYNOTE‐024 trial, pembrolizumab was significantly associated with longer OS than platinum‐based combination chemotherapy in patients with previously untreated advanced NSCLC and a PD‐L1 tumor proportion score of ≥50% [13]. However, some studies did not reveal the same correlation [7], [16]. Therefore, PD‐L1 expression alone may be not be able to predict response to nivolumab monotherapy. Alternatively, Kobayashi et al. reported that another predictive marker of response to nivolumab in patients with advanced NSCLC may be the treatment response obtained immediately before nivolumab monotherapy [17]. However, other factors predictive of response to nivolumab monotherapy in patients with NSCLC remain unknown. In our study, the results of multivariate analysis revealed that the development of irAEs and the presence of pre‐existing antithyroid antibody were independent predictors of treatment response; therefore, we think that the development of irAEs was associated with clinical efficacy, and the presence of pre‐existing antithyroid antibody might be correlated with the treatment response of nivolumab monotherapy. However, the correlation between PD‐L1 expression and the development of irAEs remains unclear.
The present study also found that patients who developed irAEs had a better ORR and DCR as well as longer PFS than those who did not, indicating a strong association between the development of irAEs and better treatment efficacy. Several studies have investigated the correlation between the development of irAEs and clinical efficacy of ICIs in various kinds of cancer [10], [14], [18], but few have done so in lung cancer. Among these studies, two recent retrospective analyses of patients with advanced NSCLC who received ICIs found a correlation between the development of irAEs and clinical efficacy of the drug. One of these studies reported that among patients with NSCLC treated with pembrolizumab monotherapy, OS was significantly longer in those who developed immune‐related thyroid dysfunction than those who did not [19]. The other study reported a positive correlation between skin irAEs and tumor response in patients with NSCLC treated with nivolumab monotherapy [20]. The present analysis also found a strong correlation between the development of irAEs and clinical efficacy in patients with advanced NSCLC treated with nivolumab monotherapy.
The present findings may provide important insights into the immunobiology of PD‐1 and autoimmunity in general. Apart from their effect on T‐cell functioning, PD‐1 and PD‐1 inhibitors have also been suggested to play an important role in regulating humoral immune response. One study found high PD‐1 expression in activated B cells [21], which has also been reported to be modulated via interaction with both T‐cell‐independent and ‐dependent mechanisms [22], [23], [24]. Some earlier studies using PD‐1 in preclinical models had suggested an antibody mediation of immune‐related toxicity [25], [26]; however, this needs to be further investigated in humans. T‐cells enhance the treatment effect of PD‐1 antibodies that may in turn induce auto‐antibodies via B cells, thereby promoting the development of irAEs. Some studies reported that the autoantibodies mediate irAEs [27], [28]. This means that the development of irAEs and positivity for auto‐antibody, particularly antithyroid antibody, may correlate with treatment response.
The present analysis revealed median onset times of 6.0 weeks for skin reaction, 4.0 weeks for myositis or peripheral neuropathy, 5.3 weeks for thyroid dysfunction, and 17.4 weeks for pneumonitis. These results are consistent with those observed in previous trials of nivolumab in patients with advanced NSCLC [7], [8], [29]. In the irAE group, 21 patients (75%) developed irAEs within 8 weeks from the start of nivolumab monotherapy. This early onset of irAEs suggests that any association with treatment is unlikely to be due to longer periods of therapy leading to a high risk of toxicity.
In this study, “responder” was defined as patient achieving CR or PR based on RECIST version 1.1. However, in a previous study, “responder” was defined in terms of durable clinical benefit, that is, being alive and without disease progression (CR + PR + SD) at 6 months [30]. Thus, we think that further consideration will be needed to define this term in the context of ICI therapy.
This study had several limitations. First, PD‐L1 expression was not routinely assayed, as no diagnostic kits were commercially available in Japan during this study. Therefore, the potential impact of PD‐L1 on treatment response or the development of irAEs to nivolumab monotherapy was assumed to be minimal. Second, this was a retrospective, nonrandomized study performed at a single center. Third, the sample size was small. However, to the best of our knowledge, we believe that this is the first study to report that the presence of pre‐existing antithyroid antibody might correlate with clinical response to nivolumab monotherapy.
Conclusion
In patients with advanced NSCLC treated with nivolumab monotherapy, ORR, DCR, and PFS were significantly better in the irAE group than in the non‐irAE group. The incidences of irAEs and pre‐existing antithyroid antibody were identified as independent predictors of treatment response. The development of irAEs and the presence of pre‐existing antithyroid antibody might be useful predictors of clinical response to nivolumab in patients with NSCLC. We think that cautious management of irAEs can lead to achieving maximum clinical benefit from nivolumab monotherapy. However, further studies with large patient samples are needed to validate these findings.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
The authors thank Professor Jeremy Williams of Tokyo Medical University for his review of this manuscript, Associate Professor Masataka Taguri of Yokohama City University School of Medicine for his statistical evaluation, Mayuko Doi, Radiology Department of Sendai Kousei Hospital, Chieko Hattori, chief nurse of Sendai Kousei Hospital and all members of Frontline Immunotherapy team (FIT) in Sendai Kousei Hospital.
Footnotes
For Further Reading: Julia Judd, Matthew Zibelman, Elizabeth Handorf et al. Immune‐Related Adverse Events as a Biomarker in Non‐Melanoma Patients Treated with Programmed Cell Death 1 Inhibitors. The Oncologist 2017;22:1232–1237.
Implications for Practice: This study evaluated whether the development of immune‐related adverse events in non‐melanoma patients treated with programmed cell death 1 checkpoint inhibitors correlates with improved clinical outcomes. The results indicate that for a subset of patients, in particular those with low‐grade immune‐related adverse events, immune‐related adverse events predicted for an improved response rate and longer time to next therapy or death.
Author Contributions
Conception/design: Yukihiro Toi, Shunichi Sugawara
Collection and/or assembly of data: Yukihiro Toi, Shunichi Sugawara, Yosuke Kawashima, Tomoiki Aiba, Sachiko Kawana, Ryohei Saito, Kyoji Tsurumi, Kana Suzuki, Hisashi Shimizu, Jun Sugisaka, Hirotaka Ono, Yutaka Domeki, Keisuke Terayama, Atsushi Nakamura, Shinsuke Yamanda, Yuichiro Kimura, Yoshihiro Honda
Data analysis and interpretation: Yukihiro Toi, Shunichi Sugawara
Manuscript writing: Yukihiro Toi
Final approval of manuscript: Yukihiro Toi, Shunichi Sugawara, Yosuke Kawashima, Tomoiki Aiba, Sachiko Kawana, Ryohei Saito, Kyoji Tsurumi, Kana Suzuki, Hisashi Shimizu, Jun Sugisaka, Hirotaka Ono, Yutaka Domeki, Keisuke Terayama, Atsushi Nakamura, Shinsuke Yamanda, Yuichiro Kimura, Yoshihiro Honda
Disclosures
Shunichi Sugawara: Ono Pharmaceutical Co., Ltd., Bristol‐Myers Squibb, Merck Sharp & Dohme (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Bray F, Jemal A, Torre LA et al. Long‐term realism and cost‐effectiveness: Primary prevention in combatting cancer and associated inequalities worldwide. J Natl Cancer Inst 2015;107:djv273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ribas A, Hamid O, Daud A et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 2016;315:1600–1609. [DOI] [PubMed] [Google Scholar]
- 3. Garon EB, Rizvi NA, Hui R et al. Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med 2015;372:2018–2028. [DOI] [PubMed] [Google Scholar]
- 4. Parry RV, Chemnitz JM, Frauwirth KA et al. CTLA‐4 and PD‐1 receptors inhibit T‐cell activation by distinct mechanisms. Mol Cell Biol 2005;25:9543–9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bennett F, Luxenberg D, Ling V et al. Program death‐1 engagement upon TCR activation has distinct effects on costimulation and cytokine‐driven proliferation: Attenuation of ICOS, IL‐4, and IL‐21, but not CD28, IL‐7, and IL‐15 responses. J Immunol 2003;170:711–718. [DOI] [PubMed] [Google Scholar]
- 6. Ishida Y, Agata Y, Shibahara K et al. Induced expression of PD‐1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992;11:3887–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brahmer J, Reckamp KL, Baas P et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Borghaei H, Paz‐Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weber JS, Kähler KC, Hauschild A. Management of immune‐related adverse events and kinetics of response with ipilimumab. J Clin Oncol 2012;30:2691–2697. [DOI] [PubMed] [Google Scholar]
- 10. Judd J, Zibelman M, Handorf E et al. Immune‐related adverse events as a biomarker in non‐melanoma patients treated with programmed cell death 1 inhibitors. The Oncologist 2017;22:1232–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 12. Herbst RS, Baas P, Kim DW et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016;387:1540–1550. [DOI] [PubMed] [Google Scholar]
- 13. Reck M, Rodríguez‐Abreu D, Robinson AG et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016;375:1823–1833. [DOI] [PubMed] [Google Scholar]
- 14. Freeman‐Keller M, Kim Y, Cronin H et al. Nivolumab in resected and unresectable metastatic melanoma: Characteristics of immune‐related adverse events and association with outcomes. Clin Cancer Res 2016;22:886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013;48:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rittmeyer A, Barlesi F, Waterkamp D et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kobayashi H, Omori S, Nakashima K et al. Response to the treatment immediately before nivolumab monotherapy may predict clinical response to nivolumab in patients with non‐small cell lung cancer. Int J Clin Oncol 2017;22:690–697. [DOI] [PubMed] [Google Scholar]
- 18. Downey SG, Klapper JA, Smith FO et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL‐associated antigen‐4 blockade. Clin Cancer Res 2007;13:6681–6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Osorio JC, Ni A, Chaft JE et al. Antibody‐mediated thyroid dysfunction during T‐cell checkpoint blockade in patients with non‐small‐cell lung cancer. Ann Oncol 2017;28:583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hasan Ali O, Diem S, Markert E et al. Characterization of nivolumab‐associated skin reactions in patients with metastatic non‐small cell lung cancer. Oncoimmunology 2016;5:e1231292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Velu V, Titanji K, Zhu B et al. Enhancing SIV‐specific immunity in vivo by PD‐1 blockade. Nature 2009;458:206–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thibult ML, Mamessier E, Gertner‐Dardenne J et al. PD‐1 is a novel regulator of human B‐cell activation. Int Immunol 2013;25:129–137. [DOI] [PubMed] [Google Scholar]
- 23. Kawamoto S, Tran TH, Maruya M et al. The inhibitory receptor PD‐1 regulates IgA selection and bacterial composition in the gut. Science 2012;336:485–489. [DOI] [PubMed] [Google Scholar]
- 24. Sage PT, Francisco LM, Carman CV et al. The receptor PD‐1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol 2013;14:152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nishimura H, Nose M, Hiai H et al. Development of lupus‐like autoimmune diseases by disruption of the PD‐1 gene encoding an ITIM motif‐carrying immunoreceptor. Immunity 1999;11:141–151. [DOI] [PubMed] [Google Scholar]
- 26. Okazaki T, Tanaka Y, Nishio R et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD‐1‐deficient mice. Nat Med 2003;9:1477–1483. [DOI] [PubMed] [Google Scholar]
- 27. Williams TJ, Benavides DR, Patrice KA et al. Association of autoimmune encephalitis with combined immune checkpoint inhibitor treatment for metastatic cancer. JAMA Neurol 2016;73:928–933. [DOI] [PubMed] [Google Scholar]
- 28. Suzuki S, Ishikawa N, Konoeda F et al. Nivolumab‐related myasthenia gravis with myositis and myocarditis in Japan. Neurology 2017;89:1127–1134. [DOI] [PubMed] [Google Scholar]
- 29. Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev 2016;44:51–60. [DOI] [PubMed] [Google Scholar]
- 30. Qin A, Street L, Cease K et al. Clinical determinants of durable clinical benefit of pembrolizumab in veterans with advanced non‐small‐cell lung cancer. Clin Lung Cancer 2017;18:559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]