RUNX1‐RUNX1T1 transcript levels are markers for predicting relapse in patients with t(8;21) acute myeloid leukemia (AML). This article reports the efficacy of minimal residual disease‐directed interferon‐alpha (IFN‐α) treatment in patients with t(8;21) AML who were positive for minimal residual disease after allogeneic hematopoietic stem cell transplantation.
Keywords: Interferon‐α , Hematopoietic stem cell transplantation , Minimal residual disease , Acute myeloid leukemia , RUNX1‐RUNX1T1
Abstract
Background.
RUNX1‐RUNX1T1 transcript levels were established as a powerful marker for predicting relapse in patients with t(8;21) acute myeloid leukemia (AML). We aimed to identify the efficacy of minimal residual disease (MRD)‐directed interferon‐alpha (IFN‐α) treatment in patients with t(8;21) AML who were positive for MRD after allogeneic hematopoietic stem cell transplantation (allo‐HSCT; n=42).
Subjects, Materials, and Methods.
MRD‐positive status was defined as a <4.5‐log reduction from diagnosis in RUNX1‐RUNX1T1 transcripts and/or the loss of a ≥4.5‐log reduction after 3 months after HSCT. Patients with positive MRD received six cycles of IFN‐α treatment (twice or thrice weekly of every 4 weeks cycle).
Results.
The 1‐year cumulative incidence of severe acute and chronic graft‐versus‐host disease after MRD‐directed IFN‐α treatment was 7.1% and 4.8%, respectively. After the treatment, 15 (35.7%), 5 (11.9%), 3 (7.1%), and 9 (21.5%) patients achieved MRD negativity at 1, 2, 3, and >3 months, respectively. Three patients relapsed after the IFN‐α treatment, in which the 1‐year cumulative incidence of relapse was 7.2%. One patient died of severe infection at 460 days after treatment. The 1‐year probabilities of event‐free survival, disease‐free survival, and overall survival after treatment were 76.0%, 92.4%, and 92.5%, respectively. The clinical outcomes in patients who received MRD‐directed IFN‐α treatment were significantly better than those of the MRD‐positive patients without any interventions in the historical cohort.
Conclusion.
MRD‐directed IFN‐α treatment is effective for patients with t(8;21) AML who were MRD‐positive after allo‐HSCT. The study was registered at http://clinicaltrials.gov as NCT02027064.
Implications for Practice.
In patients with t(8;21) acute myeloid leukemia (AML), the presence of post‐allogeneic hematopoietic stem cell transplantation (allo‐HSCT) minimal residual disease (MRD), measured by RUNX1‐RUNX1T1 transcript levels, has been established as a powerful marker for predicting relapse. Interferon‐alpha (IFN‐α) could exert a relatively strong graft‐versus‐leukemia effect, and MRD‐directed IFN‐α treatment is effective for patients with t(8;21) AML who were MRD‐positive after allo‐HSCT.
摘要
背景。研究确定,RUNX1‐RUNX1T1 转录水平是预测 t(8;21) 急性髓性白血病 (AML) 患者复发的有力标志物。我们旨在确定微小残留病 (MRD) 定向干扰素‐α (IFN‐α) 治疗对在异基因造血干细胞移植(allo‐HSCT)后 MRD阳性的 (8;21) AML 患者(n = 42)的效果。
受试者、材料和方法。MRD 阳性状态的定义为通过 RUNX1‐RUNX1T1 转录进行诊断时,对数减少值 <4.5,和/或HSCT 3 个月后,对数减少值 ≥4.5。MRD 阳性患者接受 6 个周期的 IFN‐α 治疗(每周两或三次,每 4 周为一个周期)。
结果。经过 MRD 定向 IFN‐α 治疗后,严重急性和慢性移植物抗宿主病的 1 年累积发病率分别为 7.1% 和 4.8%。
治疗后,分别有 15 (35.7%)、5 (11.9%)、3 (7.1%) 和 9 (21.5%)名患者在 1、2、3 和 >3 个月时 MRD 达到了阴性。三名患者在 IFN‐α 治疗后复发,其中 1 年累积复发率为 7.2%。一名患者在治疗后 460 天死于严重感染。治疗后的 1 年无事件生存期、无病生存期和总生存期百分比分别为 76.0%、92.4% 和 92.5%。接受 MRD 定向 IFN‐α 治疗的患者的临床效果明显优于在历史性队列中未经过任何干预治疗的 MRD 阳性患者。
结论。MRD 定向 IFN‐α 治疗对allo‐HSCT后的 MRD 阳性 t(8;21) AML 患者有效。该研究在 http://clinicaltrials.gov 上的登记编号为 NCT02027064。
对临床实践的提示: 在患有 t(8;21) 急性髓性白血病 (AML) 的患者中,以 RUNX1‐RUNX1T1 转录水平测量的异基因造血干细胞移植 (allo‐HSCT) 微小残留病 (MRD) 的发病率已被确定为预测其复发的有力标志物。干扰素‐α (IFN‐α) 可以发挥较为强大的移植物抗白血病作用,MRD 定向 IFN‐α 治疗对 allo‐HSCT 后 MRD 呈阳性的 t(8;21) AML 患者有效。
Introduction
Although t(8;21) acute myeloid leukemia (AML) is considered to have a good prognosis, relapse occurs in up to 50% of patients treated with chemotherapy, and the long‐term survival is unsatisfactory [1], [2]. Monitoring of minimal residual disease (MRD) during chemotherapy can identify high‐risk patients with t(8;21) [3], [4], and our prospective multicenter study demonstrated that allogeneic hematopoietic stem cell transplantation (allo‐HSCT) significantly improved clinical outcomes of the high‐risk patients [5]. However, nearly 20% of allo‐HSCT recipients still experienced relapse, which led to poor long‐term outcomes [6].
In patients with t(8;21) AML, the presence of post‐allo‐HSCT MRD, measured by RUNX1‐RUNX1T1 transcript levels, has been established as a powerful marker for predicting relapse [7], [8]. Qin et al. [8] reported that a <3‐log reduction of the transcripts within 12 months and/or a <4‐log reduction at ≥12 months were significantly related to a higher 3‐year cumulative incidence of relapse (CIR) in both their entire study cohort (58.4% vs. 2.2%, p < .0001) and the patients with no intervention after HSCT (76.5% vs. 2.0%, p < .0001). Thus, MRD‐directed intervention may be a reasonable option for relapse prophylaxis, of which donor lymphocyte infusion (DLI) is the most important preemptive treatment [9], [10]. Wang et al. [7] showed that for patients with t(8;21) AML who did not achieve a ≥3‐log reduction of RUNX1‐RUNX1T1 until 3 months or lost a ≥3‐log reduction after allo‐HSCT, the 2‐year CIR and leukemia‐free survival with or without DLI was 24% versus 87% (p=.001) and 64% versus 0% (p < .001), respectively.
However, some patients cannot receive MRD‐directed DLI because of provider or patient refusal. For these patients, further study is necessary on what other preemptive interventions might be reasonable. Some studies observed that interferon‐alpha (IFN‐α) may be a feasible maintenance therapy for AML patients [11], and could exert a relatively strong immunomodulatory effect [12], [13]. In addition, Singhal et al. [14], Gesundheit et al. [15], and Tang et al. [16] reported that IFN‐α could induce a graft‐versus‐leukemia effect in patients with acute leukemia, and that patients who had relapsed after allo‐HSCT achieved complete remission (CR) on this treatment. In our pilot study, we observed that MRD‐directed IFN‐α treatment was safe for allo‐HSCT recipients, with clinical outcomes comparable to those of MRD‐directed DLI [17]. However, the sample of patients with t(8;21) AML treated in this pilot study was small (n=4). Thus, in this prospective registry study, the efficacy of this treatment was further investigated in patients with t(8;21) AML who had received allo‐HSCT.
Subjects, Materials, and Methods
Patients
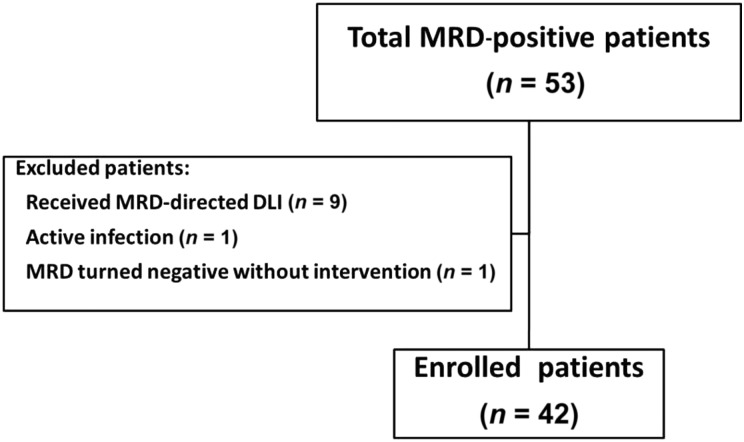
For this prospective, single‐arm registry study, consecutive patients receiving non‐T‐cell‐depleted allo‐HSCT at the Peking University Institute of Hematology were enrolled if they met the following criteria: (a) were ≤65 years old; (b) had AML with t(8;21) and/or RUNX1‐RUNX1T1 transcripts, and had achieved and maintained CR (first or second) before allo‐HSCT; (c) met our published high‐risk criteria (not achieving a ≥3‐log reduction after the second consolidation and/or the loss of a ≥3‐log reduction during the next six consolidation therapies) [5] or showed c‐KIT mutations at the time of diagnosis; and (d) regained MRD positivity after allo‐HSCT. The key exclusion criteria were active graft‐versus‐host disease (GVHD), active infections, severe myelosuppression (white blood cell count <1.0 × 109 cells/L, absolute neutrophil count <0.5 × 109 cells/L, hemoglobin count <65 g/L, or platelet count <25 × 109 cells/L), and organ failure. From October 1, 2013, to February 29, 2016, a total of 42 patients were enrolled (Fig. 1). The final follow‐up visits for endpoint analysis were conducted on November 30, 2017. In order to further compare the clinical outcomes between RUNX1‐RUNX1T1‐positive patients with and without IFN‐α treatment, a historical cohort (including RUNX1‐RUNX1T1‐positive patients without any interventions) that had been reported by Qin et al. [8] was also enrolled (n=21). Informed consent was obtained from all patients or the patients' guardians, and the study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of Peking University People's Hospital. The study was registered at http://clinicaltrials.gov as NCT02027064.
Figure 1.
Diagram of patients enrolled. MRD‐directed DLI was the first choice for patients in the high‐level MRD group, and those who could not receive DLI because of patient refusal (n = 15) or provider refusal (n = 2) received interferon‐α treatment.
Abbreviations: DLI, donor lymphocyte infusion; MRD, minimal residual disease.
Transplant Regimens
Preconditioning consisted of cytarabine (Ara‐C), busulfan (3.2 mg/kg/day administered intravenously on days −8 to −6), cyclophosphamide (1.8 g/m2/day, days −5 to −4), and simustine (250 g/m2/day, day −3). Ara‐C was administered at 4 g/m2/day (days −10 to −9) to the human leukocyte antigen (HLA)‐haploidentical related donor group, at 2 g/m2/day (days −10 to −9) to the HLA‐unrelated donor group, and at 2 g/m2/day (day −9) to the HLA‐identical sibling donor (ISD) group. Rabbit antithymocyte globulin (thymoglobulin, 2.5 mg/kg/day, days −5 to −2; Sanofi, Paris, France) was administered to the HLA‐haploidentical related donor and HLA‐unrelated donor groups [18], [19]. Patients received cyclosporine A, mycophenolate mofetil, and short‐term methotrexate as GVHD prophylaxis [20]. The donor selection, HLA typing, and stem cell harvesting protocols have been described in detail [21].
MRD Monitoring and Definition
Bone marrow samples were collected as part of the treatment protocol. Routine MRD monitoring was performed at 1, 2, 3, 4.5, 6, 9, and 12 months after transplantation and at 6‐month intervals thereafter; however, in patients with 1‐log rising levels of RUNX1‐RUNX1T1 transcripts, monitoring was performed every 2 weeks. MRD was monitored as the level of RUNX1‐RUNX1T1 transcripts, which was quantified by quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR), and the results were expressed as a transcript ratio of [fusion gene/Abelson gene (ABL1)] × 100. The pretreatment baseline level of RUNX1‐RUNX1T1 transcripts was 388% in our laboratory [5]. MRD positivity was defined as a <4.5‐log reduction in RUNX1‐RUNX1T1 transcripts when compared with the pretreatment baseline level and/or the loss of a ≥4.5‐log reduction after 3 months after HSCT. High‐level and low‐level MRDs were respectively defined as <3.5‐log and 3.5–4.5‐log reductions in the transcripts.
MRD‐Directed IFN‐α Treatment Protocol
Based on the MRD status after HSCT and clinical conditions at the time of presence of MRD, patients with positive MRD received six cycles of IFN‐α treatment before hematologic relapse after 3 months after HSCT. Because the efficacy of DLI had been confirmed [7] but the role of IFN‐α treatment was still undefined in high‐level MRD patients, MRD‐directed DLI was the first choice for patients in high‐level MRD group, and those who could not receive DLI because of patient refusal (n=15) or provider refusal (n=2) received IFN‐α treatment.
Recombinant human IFN‐α‐2b injections (Anferon; Tianjin Hualida Biotechnology Co., Ltd., Tianjin, People's Republic of China) were administered subcutaneously for six cycles (twice or thrice weekly of every 4 weeks cycle) at dosages of 3 million units for patients older than 16 years, and at 3 million units per square meter for those younger than 16 years (capped by 3 million units). Patients with low‐level MRD received IFN‐α twice weekly, whereas the patients with high‐level MRD were advised to receive IFN‐α trice weekly. Prolonged treatment with IFN‐α was permitted at the request of patients. MRD status was monitored at 1, 2, 3, 4.5, 6, 9, and 12 months after IFN‐α treatment and at 6‐month intervals thereafter. Toxicities of therapy, rated according to the World Health Organization criteria [22], were monitored every 1–2 weeks after IFN‐α treatment. GVHD was excluded as an adverse event. Study medication with IFN‐α was discontinued in any patient with active severe GVHD, severe infection, grade ≥3 toxicity, salvage DLI use, relapse, or nonrelapse mortality (NRM).
The following patients who agreed to receive DLI and did not have active GVHD, active infection, and organ failure were eligible for salvage DLI: patients who regained MRD positivity after achieving MRD‐negative status, or those with persistent and increasing levels of MRD (e.g., 1‐log rising levels of RUNX1‐RUNX1T1 transcripts) after IFN‐α treatment. The DLI protocol has been described in detail elsewhere [10].
Treatment of GVHD After MRD‐Directed IFN‐α Treatment
The treatment of GVHD was in accordance with common international criteria [23], [24]. Acute GVHD (aGVHD) was treated with methylprednisolone (1–2 mg/kg/day) and by resumption of full‐dose cyclosporine A. Second‐ or third‐line immunosuppressive therapies, such as CD25 monoclonal antibody (Basiliximab; Novartis Pharma Stein AG, Basel, Switzerland), mycophenolate mofetil, tacrolimus, or methotrexate, were administered in cases of steroid‐refractory aGVHD. Moderate to severe chronic GVHD (cGVHD) was treated with prednisone (1 mg/kg/day), and cyclosporine A was adjusted to maintain a blood concentration of >150 ng/mL. Second‐ or third‐line immunosuppressive therapies, such as mycophenolate mofetil, methotrexate, penicillamine, azathioprine, or tacrolimus, were administered in cases of steroid‐refractory cGVHD.
Definitions and Assessments
GVHD after MRD‐directed IFN‐α treatment was diagnosed according to accepted international criteria [25], [26]. Relapse was defined as morphologic evidence of disease in samples from the peripheral blood, bone marrow, or extramedullary sites, or by the recurrence and sustained presence of pretransplantation chromosomal abnormalities. Patients who exhibited MRD were not classified as showing relapse. NRM was defined as death without disease progression or relapse. Overall survival (OS) events were defined as death from any cause. Disease‐free survival (DFS) was defined as the survival period with continuous CR. Event‐free survival (EFS) events were defined as salvage DLI, relapse, or death from any cause after MRD‐directed IFN‐α treatment.
Statistical Analysis
All patients who received at least one dose of IFN‐α were included in the analyzed population. The primary endpoint was relapse. The secondary endpoints were NRM, EFS, DFS, and OS. This study was planned to detect a relapse rate of 65% in patients receiving IFN‐α, from the reference rate of our previous study of 87% [7], controlling for type I and II error rates at 5% and 10%, respectively. Considering an expulsion rate of 10%, a total of 42 patients was planned to be enrolled. Data were censored at the time of salvage DLI, relapse, NRM, or last available follow‐up. Continuous variables were compared using the Mann‐Whitney U test; categorical variables were compared using chi‐square and Fisher's exact tests. The Kaplan‐Meier method was used to estimate the probabilities of EFS, DFS, and OS. Competing risk analyses were used to calculate the cumulative incidence of GVHD, relapse, and NRM, using the Gray test for differences between the groups [27]. The competing risks for GVHD were relapse, NRM, and salvage DLI; those for relapse were NRM and salvage DLI; and those for NRM were relapse and salvage DLI. A landmark analysis was performed to assess the effects of MRD‐directed IFN‐α treatment on each outcome, where the post‐transplant day of IFN‐α treatment was defined as the landmark day. Data analyses were primarily conducted with SPSS software (SPSS Inc., Chicago, IL), whereas the R software package (version 2.6.1; http://www.r-project.org) was used for competing risk analysis.
Results
Patient Characteristics
Table 1 summarizes the characteristics of the 42 patients. Full donor chimerism was detected in all the MRD‐positive patients. The median time from transplantation to IFN‐α treatment was 163 (range, 92–710 days), and the median time from MRD positivity to IFN‐α treatment was 8 (range, 0–25 days). Most of the patients with high‐level MRD agreed to receive IFN‐α trice weekly (n=13), and the others (high‐level: 4; low‐level: 25) received IFN‐α twice weekly. The median cycles of IFN‐α treatment was 4.5 (range, 0.5–17 cycles), and the reasons for discontinuing IFN‐α treatment included treatment completed (n=18), salvage DLI (n=9), grade ≥3 toxicity (n=6), GVHD (n=5), relapse (n=3), and NRM (n=1). Eleven patients continued on IFN‐α treatment beyond the planned six cycles. The median duration of follow‐up after IFN‐α treatment was 676 days (range, 15–1,340 days).
Table 1. Patient characteristics.
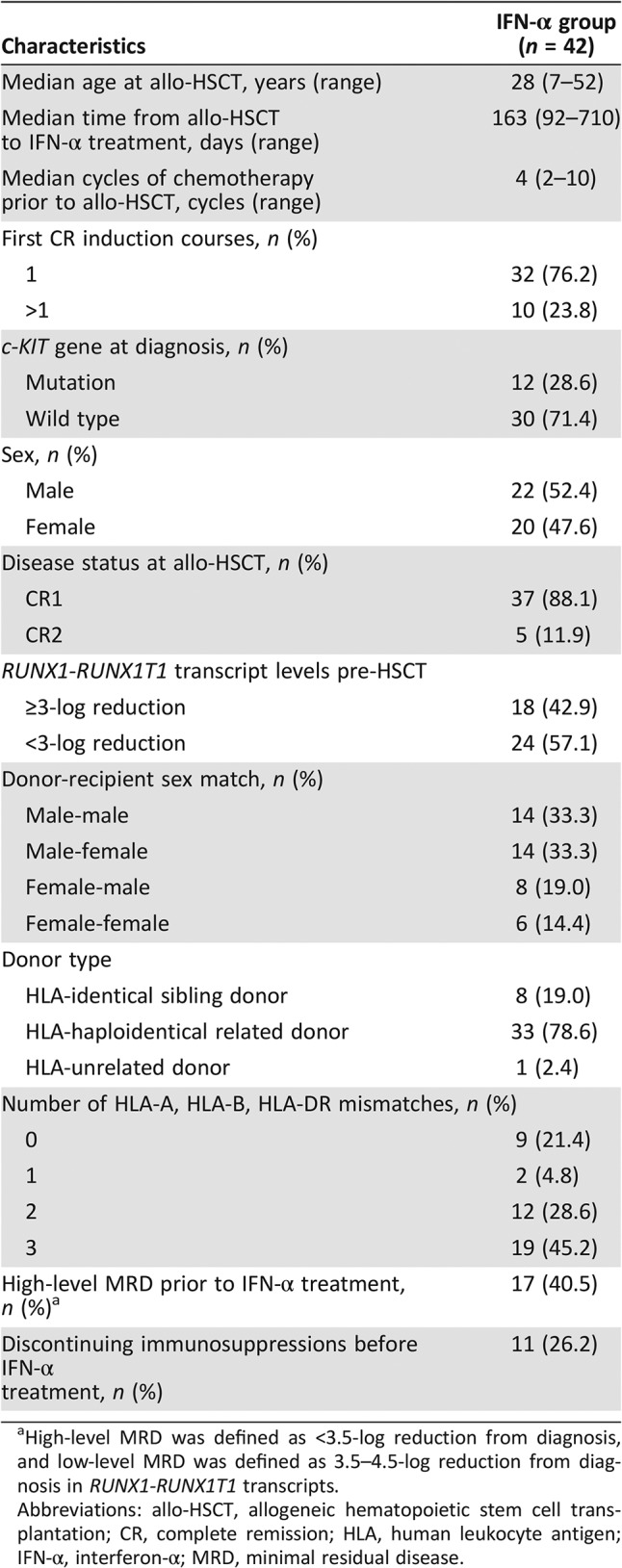
High‐level MRD was defined as <3.5‐log reduction from diagnosis, and low‐level MRD was defined as 3.5–4.5‐log reduction from diagnosis in RUNX1‐RUNX1T1 transcripts.
Abbreviations: allo‐HSCT, allogeneic hematopoietic stem cell transplantation; CR, complete remission; HLA, human leukocyte antigen; IFN‐α, interferon‐α; MRD, minimal residual disease.
GVHD After IFN‐α Treatment
After IFN‐α treatment, grades 1, 2, 3, and 4 aGVHD were observed in five, five, two, and one patient, respectively. The median time from treatment to occurrence of aGVHD was 40 days (range, 13–129 days). The 1‐year cumulative incidence of total aGVHD and severe aGVHD (≥grade 3) after treatment was 31.0% (95% confidence interval [CI], 16.8%–45.2%) and 7.1% (95% CI, 0.0%–15.0%), respectively.
Mild, moderate, and severe cGVHD were observed in 4, 16, and 2 patients, respectively, after treatment. The median time from treatment to occurrence of cGVHD was 97 days (range, 5–727 days). Eighteen cGVHD patients received systemic immunosuppressive treatments after IFN‐α treatment. The 1‐year cumulative incidence of total cGVHD and severe cGVHD after IFN‐α treatment was 61.0% (95% CI, 42.2%–79.8%) and 4.8% (95% CI, 0.0%–11.4%), respectively.
Toxicities and NRM After IFN‐α Treatment
All patients reported transient fevers; however, their maximum temperatures did not exceed 39.5°C and the fevers decreased spontaneously. Six patients showed grade ≥3 toxicity (hematologic: n=2; infectious: n=4). None of the other grade ≥3 toxicities were reported. One patient died of severe infection at 460 days after treatment.
Relapse After IFN‐α Treatment
Three patients relapsed at 15, 19, and 281 days, respectively, after MRD‐directed IFN‐α treatment. The 1‐year CIR after treatment was 7.2% (95% CI, 0.0%–15.2%).
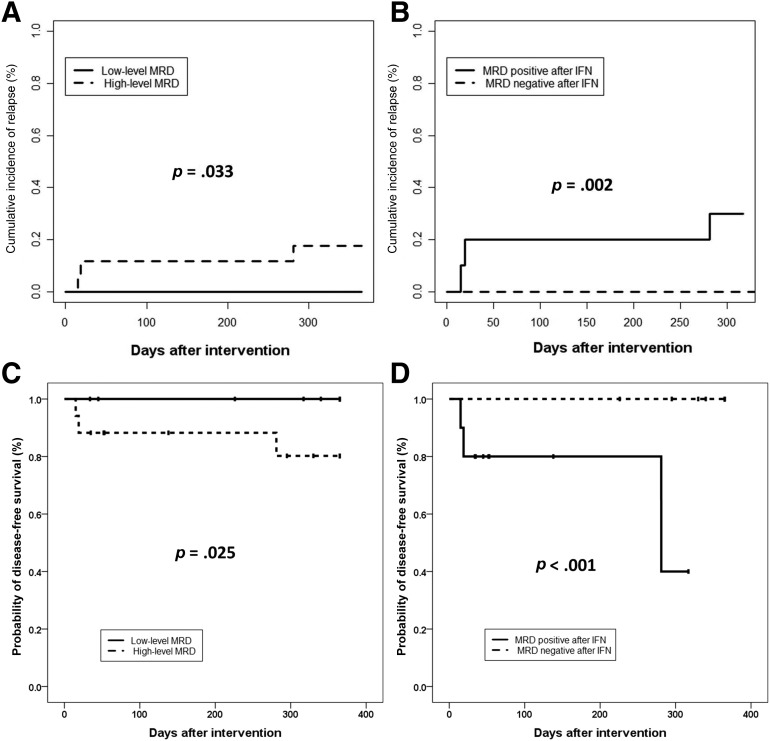
Twenty‐five and seventeen patients were in the low‐level and high‐level MRD groups, respectively (Table 1). The 1‐year CIR after IFN‐α treatment was lower in the low‐level MRD group (0.0%) than in the high‐level group (17.6%, 95% CI, 0.0%–36.4%, p=.033; Fig. 2A).
Figure 2.
Clinical outcomes at 1 year after MRD‐directed IFN‐α treatment. Cumulative incidence of relapse at 1 year after MRD‐directed IFN‐α treatment, according to MRD status prior to IFN‐α treatment (0.0% vs. 17.6%, p = .033; A) and MRD status after IFN‐α treatment (0.0% vs. 30.0%, p = .002; B). Probabilities of disease‐free survival at 1 year after MRD‐directed IFN‐α treatment, according to MRD status prior to IFN‐α treatment (100.0% vs. 80.2%, p = .025; C) and MRD status after IFN‐α treatment (100.0% vs. 40.0%, p < .001; D).
Abbreviations: IFN‐α, interferon‐α; MRD, minimal residual disease.
Fifteen (35.7%), five (11.9%), three (7.1%), and nine (21.5%) patients achieved MRD negativity at 1, 2, 3, and >3 months, respectively, after MRD‐directed IFN‐α treatment, and none of them relapsed. Of 10 (23.8%) patients who did not achieve MRD negativity after treatment, 3 relapsed. The post‐treatment 1‐year CIR was significantly lower in patients who achieved MRD negativity (0.0%) than in those with persistent MRD (30.0%, 95% CI, 0.0%–63.3%, p=.002; Fig. 2B).
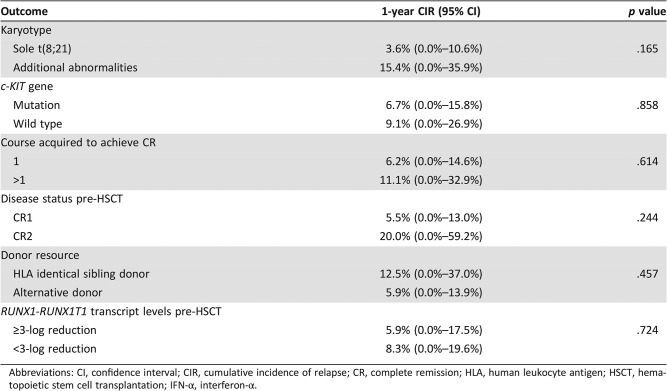
Other variables, including karyotype, c‐KIT mutation at diagnosis, courses acquired to achieve CR, disease status prior to HSCT, donor resource, and RUNX1‐RUNX1T1 transcript levels prior to HSCT, were not related to a higher 1‐year CIR (Table 2).
Table 2. The impact of factors other than the RUNX1‐RUNX1T1 transcript on relapse after IFN‐α treatment.
Abbreviations: CI, confidence interval; CIR, cumulative incidence of relapse; CR, complete remission; HLA, human leukocyte antigen; HSCT, hematopoietic stem cell transplantation; IFN‐α, interferon‐α.
EFS, DFS, and OS After IFN‐α Treatment
Nine patients received salvage DLI after MRD‐directed IFN‐α treatment (two patients regained MRD positivity after achieving MRD‐negative status; seven patients showed persistent and increasing levels of MRD). Five (20.0%) were in the low‐level MRD group, and four (23.5%) were in the high‐level group (p=1.000). The 1‐year probability of EFS after IFN‐α treatment was 76.0% (95% CI, 62.8%–89.2%), being higher in the low‐level (87.8%, 95% CI, 74.6%–100.0%) than in the high‐level MRD group (58.8%, 95% CI, 34.5%–83.1%, p=.026), and significantly higher in patients who achieved MRD negativity than in those with persistent MRD (100.0% vs. 0.0%, p < .001).
The 1‐year probability of DFS after IFN‐α treatment was 92.4% (95% CI, 83.9%–100.0%), and was higher in the low‐level MRD group (100.0%) than in the high‐level group (80.2%, 95% CI, 58.9%–100.0%, p=.025), and significantly higher in patients who achieved MRD negativity (100.0%) than in those with persistent MRD (40.0%, 95% CI, 13.8%–66.2%, p < .001; Fig. 2C, 2D).
The 1‐year probability of OS after IFN‐α treatment was 92.5% (95% CI, 84.2%–100.0%), and was higher in the low‐level MRD group (100.0%) than in the high‐level group (80.9%, 95% CI, 60.5%–100.0%, p=.028), and significantly higher in patients who achieved MRD negativity (100.0%) than in those with persistent MRD (63.0%, 95% CI, 24.9%–100.0%, p < .001).
Clinical Outcomes of MRD‐Positive Patients with and Without Preemptive Intervention
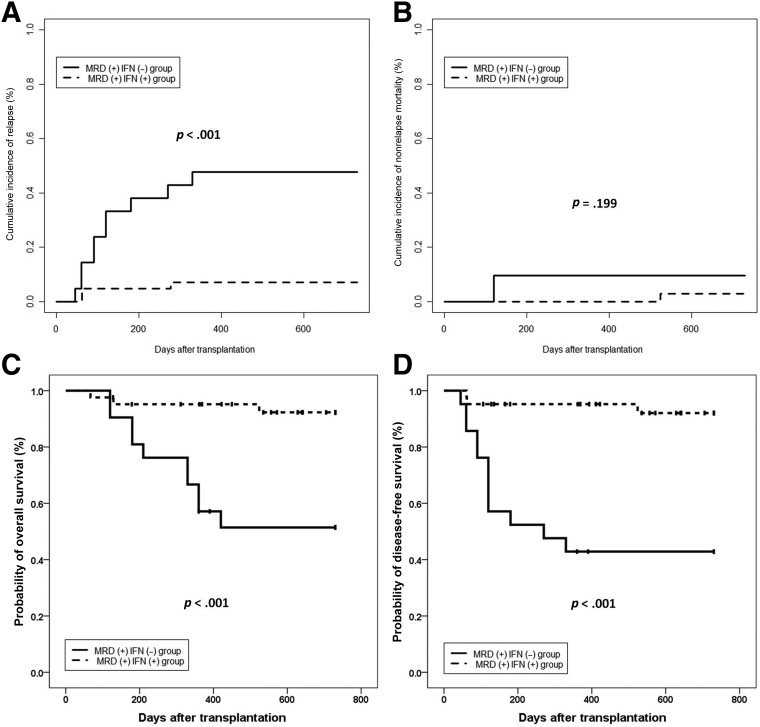
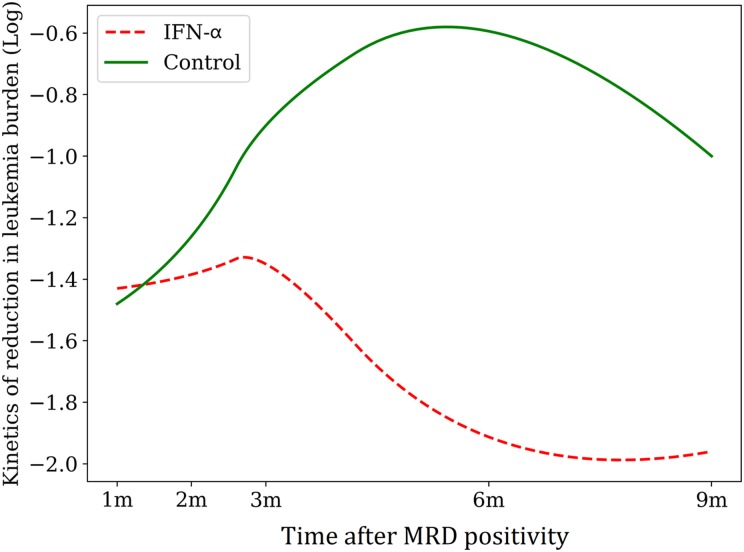
Twenty‐one patients who showed RUNX1‐RUNX1T1‐positive (i.e., a <4.5‐log reduction in RUNX1‐RUNX1T1 transcripts) without any interventions in the historical cohort were included in this analysis. The protocols of conditioning regimen, graft source, and GVHD prophylaxis of the historical control group were all the same as those who received MRD‐directed IFN‐α treatment. In addition, the disease and patient characteristics were comparable between the groups, except that more patients without MRD‐directed interventions received ISD HSCT (supplemental online Table 1). The 2‐year cumulative incidences of relapse, DFS, and OS after allo‐HSCT were 7.1%, 92.1%, and 92.3%, respectively, in patients who received MRD‐directed IFN‐α treatment, which were significantly better than those of the MRD‐positive patients without any interventions in the historical cohort (2‐year relapse, 47.6%, p < .001; 2‐year DFS, 42.9%, p < .001; 2‐year OS, 51.4%, p < .001). The 2‐year cumulative incidence of NRM after allo‐HSCT was comparable between the groups (Fig. 3A–3D). Of 21 patients in the historical cohort, 2 turned MRD negative without any interventions; however, the MRD evolution over time showed the superiority of IFN‐α treatment in MRD‐positive patients after allo‐HSCT (Fig. 4; supplemental online Table 2).
Figure 3.
Cumulative incidences of clinical outcomes at 2 years after allo‐HSCT in MRD positive patients who received IFN‐α treatment and those who did not receive any interventions. (A) relapse; (B) nonrelapse mortality; (C) overall survival; and (D) disease‐free survival.
Abbreviations: IFN‐α, interferon‐α; MRD, minimal residual disease.
Figure 4.
MRD evolution over time in patients with and without IFN‐α treatment.
Abbreviations: IFN‐α, interferon‐α; MRD, minimal residual disease.
Outcomes of Patients Who Received Salvage DLI
Of nine patients who received salvage DLI, four achieved MRD negativity (three of them achieved DFS, and one died of severe pneumonia). Five patients had persistent MRD after salvage DLI, and two died after relapse.
Discussion
In our previous study, we observed that MRD‐directed IFN‐α treatment can decrease the risk of relapse and improve the survival of AML patients after allo‐HSCT [28]. In the present trial of patients with t(8;21) AML, we observed a 1‐year CIR of 7.2%, and 1‐year EFS, DFS, and OS probabilities of 76.0%, 92.4%, and 92.5%, respectively, after IFN‐α treatment. The clinical outcomes in patients who received MRD‐directed IFN‐α treatment were significantly better than those of the MRD‐positive patients without any interventions in the historical cohort. This prospective registry study provides an opportunity for exploring the currently undefined role of MRD‐directed IFN‐α treatment for patients with t(8;21) AML after allo‐HSCT.
In this study, we observed that MRD‐directed IFN‐α treatment can significantly decrease the CIR and improve the survival compared with MRD‐positive patients without any interventions, which suggests that MRD‐directed IFN‐α treatment is effective. In addition, we observed the clinical outcomes in the low‐level MRD group to be significantly better than those in the high‐level group, which suggested that IFN‐α treatment may more appropriate for low‐level MRD patients.
Although several studies observed that MRD monitoring by qRT‐PCR allows risk stratification and predicts relapse in t(8;21) AML, the thresholds and timing of RUNX1‐RUNX1T1 transcripts for the prediction of relapse after chemotherapy were different [2], [3], [4], [5], [29]. Qin et al. [8] observed that over 90% of allo‐HSCT recipients had a ≥3‐log reduction in transcripts from the first month and a ≥4‐log reduction at ≥12 months after HSCT. In addition, these two frequencies were similar at each time point starting at 12 months. Thus, ≥3‐log and ≥4‐log reductions in RUNX1‐RUNX1T1 transcripts were chosen as the thresholds for predicting relapse within the first year and starting at 12 months after HSCT, respectively, and were also used to direct the DLI in our previous study [7]. However, we further observed that a <4.5‐log reduction from diagnosis and/or the loss of a ≥4.5‐log reduction after 3 months after HSCT also significantly predicted relapse after allo‐HSCT, although some of them received preemptive immunotherapies (2.6% vs. 27.4%, p < .001; Qin et al., data unpublished). Some authors suggested that IFN‐α treatment should preferably be started in leukemia patients with a relatively low tumor burden [13]. On the other hand, because the safety of MRD‐directed IFN‐α treatment had previously been proven [17], [28], [30], we defined a <4.5‐log reduction as MRD positivity for directing the IFN‐α treatment in this study.
In the present study, only one patient showed NRM after MRD‐directed IFN‐α treatment, and six patients showed grade ≥3 toxicity, which were in accordance with the results of previous studies [17], [28], [30]. In addition, the incidence of patients with severe aGVHD was only 7.1% in the present trial. On the other hand, although we used short‐term immunosuppressive agents after DLI, the incidences of grades 2–4 and 3–4 aGVHD were 32.7% and 8.2%, respectively [31]. In addition, more than 70% of the DLI recipients experienced grade ≥3 hematologic toxicity [17], [32]. Thus, considering the safety, IFN‐α treatment is suitable for MRD‐positive patients, particularly those with low‐level MRD.
Although both DLI and IFN‐α treatments can clear MRD, some patients showed persistent MRD after immunotherapy, which was significantly associated with an increased risk of relapse and poorer survival [17], [33]. In this study, 23.8% of patients who did not achieve MRD negativity after IFN‐α treatment had poor outcomes. We observed that four out of nine patients who received salvage DLI achieved MRD negativity. We also observed that the outcomes of patients subjected to salvage IFN‐α treatment after DLI were significantly better than those with persistent MRD without IFN‐α treatment [34], [35]. Thus, it is suggested that IFN‐α may clear MRD through different mechanisms [36], [37] and it may have a synergistic effect with DLI [38]. Thus, it is worth exploring whether the combination of DLI and IFN‐α can help to improve the outcomes of patients with persistent MRD.
This study had several limitations. First, it was a single‐center study; a future multicenter study may further confirm our current findings. Second, the observation period was relatively short; an extended study would evaluate the long‐term relapse after treatment. Lastly, we observed that 2 out of 21 patients became MRD negative without any interventions. However, the RUNX1‐RUNX1T1 transcript levels in patients receiving IFN‐α decreased faster than that of those without any interventions, which suggested that MRD‐positive patients can benefit from IFN‐α treatment.
Conclusion
These data confirmed the potential of MRD‐directed IFN‐α treatment for patients with t(8;21) AML who were MRD‐positive after allo‐HSCT; the treatment efficacy should be further confirmed by large‐scale, multicenter clinical studies.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
We thank Editage for their editorial assistance, and thank Dr. Dao‐Xing Deng for his assistance in collecting the data for this manuscript. This work was supported by the Beijing Talents fund (grant number 2015000021223ZK39), the Capital's Funds for Health Improvement and Research (grant number 2018‐4‐4089), the Key Program of the National Natural Science Foundation of China (grant number 81530046), the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (grant number 81621001), the Science and Technology Project of Guangdong Province of China (grant number 2016B030230003), and the project of health collaborative innovation of Guangzhou city (grant number 201704020214).
Footnotes
For Further Reading: André Bosly, Andrew Grigg, Harald Holte et al. Versus No Treatment as Consolidation After High Dose Therapy and Autologous Stem Cell Transplantation for Patients with Relapsed Lymphoma. The Oncologist 2013;18:1189.
Abstract
Background. Patients with lymphoma who have experienced a first relapse or progression and have disease deemed sensitive to salvage chemotherapy nevertheless have a high likelihood of having a second relapse. To decrease the likelihood of a second relapse after high‐dose therapy (HDT) and autologous stem cell transplantation (ASCT), interferon (IFN) α‐2b was given in a prospective randomized international trial.
Methods. In this trial, 221 patients with varying histologic diagnoses (8 small lymphocytic, 37 follicular, 9 mantle, 90 diffuse large B‐cell, 20 peripheral T‐cell, 3 high‐grade B‐cell non‐Hodgkin lymphoma, and 54 Hodgkin lymphoma) were randomly assigned to receive no further treatment (arm A: 117 patients) or IFNα‐2b, 3 MU three times weekly, for 18 months (arm B: 104 patients).
Results. In arm B, 21 patients (20%) did not receive IFNα‐2b because of early progression or absence of hematologic recovery, 29 patients (28%) completed the 18 months of treatment, and 54 patients (52%) interrupted treatment because of progression (23%) or toxicity (29%). Event‐free survival and overall survival were not different between the two arms on an intent‐to‐treat analysis and also if analysis was restricted to patients who were alive and had not experienced disease progression three months after transplantation. The study was not sufficiently powered to evaluate effects in histologic subtypes.
Conclusion. In this trial, post‐autograft IFNα‐2b did not improve outcomes in a heterogeneous group of patients with lymphoma.
Author Contributions
Conception/design: Xiao‐Dong Mo, Yu Wang, Xiao‐Jun Huang
Provision of study material or patients: Xiao‐Hui Zhang, Lan‐Ping Xu, Chen‐Hua Yan, Huan Chen, Yu‐Hong Chen, Kai‐Yan Liu
Collection and/or assembly of data: Xiao‐Dong Mo, Ya‐Zhen Qin
Data analysis and interpretation: Xiao‐Dong Mo, Xiao‐Jun Huang
Manuscript writing: Xiao‐Dong Mo, Xiao‐Jun Huang
Final approval of manuscript: Xiao‐Dong Mo, Yu Wang, Xiao‐Hui Zhang, Lan‐Ping Xu, Chen‐Hua Yan, Huan Chen, Yu‐Hong Chen, Ya‐Zhen Qin, Kai‐Yan Liu, Xiao‐Jun Huang
Disclosures
The authors indicated no financial relationships.
References
- 1.Marcucci G, Mrózek K, Ruppert AS et al. Prognostic factors and outcome of core binding factor acute myeloid leukemia patients with t(8;21) differ from those of patients with inv(16): A Cancer and Leukemia Group B study. JClin Oncol 2005;23:5705–5717. [DOI] [PubMed] [Google Scholar]
- 2.Jourdan E, Boissel N, Chevret S et al. Prospective evaluation of gene mutations and minimal residual disease in patients with core binding factor acute myeloid leukemia. Blood 2013;121:2213–2223. [DOI] [PubMed] [Google Scholar]
- 3.Weisser M, Haferlach C, Hiddemann W et al. The quality of molecular response to chemotherapy is predictive for the outcome of AML1‐ETO‐positive AML and is independent of pretreatment risk factors. Leukemia 2007;21:1177–1182. [DOI] [PubMed] [Google Scholar]
- 4.Yin JA, O'Brien MA, Hills RK et al. Minimal residual disease monitoring by quantitative RT‐PCR in core binding factor AML allows risk stratification and predicts relapse: Results of the United Kingdom MRC AML‐15 trial. Blood 2012;120:2826–2835. [DOI] [PubMed] [Google Scholar]
- 5.Zhu HH, Zhang XH, Qin YZ et al. MRD‐directed risk stratification treatment may improve outcomes of t(8;21) AML in the first complete remission: Results from the AML05 multicenter trial. Blood 2013;121:4056–4062. [DOI] [PubMed] [Google Scholar]
- 6.Yoon JH, Kim HJ, Kim JW et al. Identification of molecular and cytogenetic risk factors for unfavorable core‐binding factor‐positive adult AML with post‐remission treatment outcome analysis including transplantation. Bone Marrow Transplant 2014;49:1466–1474. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Wu DP, Liu QF et al. In adults with t(8;21)AML, posttransplant RUNX1/RUNX1T1‐based MRD monitoring, rather than c‐KIT mutations, allows further risk stratification. Blood 2014;124:1880–1886. [DOI] [PubMed] [Google Scholar]
- 8.Qin YZ, Wang Y, Xu LP et al. The dynamics of RUNX1‐RUNX1T1 transcript levels after allogeneic hematopoietic stem cell transplantation predict relapse in patients with t(8;21) acute myeloid leukemia. JHematol Oncol 2017;10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dominietto A, Pozzi S, Miglino M et al. Donor lymphocyte infusions for the treatment of minimal residual disease in acute leukemia. Blood 2007;109:5063–5064. [DOI] [PubMed] [Google Scholar]
- 10.Yan CH, Liu DH, Liu KY et al. Risk stratification‐directed donor lymphocyte infusion could reduce relapse of standard‐risk acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Blood 2012;119:3256–3262. [DOI] [PubMed] [Google Scholar]
- 11.McSweeney EN, Worman CP, Tsakona CP et al. Low‐dose recombinant alfa‐2a‐interferon: A feasible maintenance therapy in acute myeloid leukaemia in the older patient. Acta Haematol 1993;89:1–5. [DOI] [PubMed] [Google Scholar]
- 12.Smits EL, Anguille S, Berneman ZN. Interferon α may be back on track to treat acute myeloid leukemia. Oncoimmunology 2013;2:e23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anguille S, Lion E, Willemen Y et al. Interferon‐α in acute myeloid leukemia: An old drug revisited. Leukemia 2011;25:739–748. [DOI] [PubMed] [Google Scholar]
- 14.Singhal S, Powles R, Treleaven J et al. Sensitivity of secondary acute myeloid leukemia relapsing after allogeneic bone marrow transplantation to immunotherapy with interferon‐alpha 2b. Bone Marrow Transplant 1997;19:1151–1153. [DOI] [PubMed] [Google Scholar]
- 15.Gesundheit B, Shapira MY, Resnick IB et al. Successful cell‐mediated cytokine‐activated immunotherapy for relapsed acute myeloid leukemia after hematopoietic stem cell transplantation. Am J Hematol 2009;84:188–190. [DOI] [PubMed] [Google Scholar]
- 16.Tang X, Song YH, Sun A et al. Successful treatment of relapsed acute myeloid leukemia without chemotherapy. JClin Oncol 2016;34:e117–e119. [DOI] [PubMed] [Google Scholar]
- 17.Mo XD, Zhang XH, Xu LP et al. Interferon‐α: A potentially effective treatment for minimal residual disease in acute leukemia/myelodysplastic syndrome after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2015;21:1939–1947. [DOI] [PubMed] [Google Scholar]
- 18.Xiao‐Jun H, Lan‐Ping X, Kai‐Yan L et al. Partially matched related donor transplantation can achieve outcomes comparable with unrelated donor transplantation for patients with hematologic malignancies. Clin Cancer Res 2009;15:4777–4783. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Liu QF, Xu LP et al. Haploidentical vs identical‐sibling transplant for AML in remission: A multicenter, prospective study. Blood 2015;125:3956–3962. [DOI] [PubMed] [Google Scholar]
- 20.Huang XJ, Liu DH, Liu KY et al. Haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of hematological malignancies. Bone Marrow Transplant 2006;38:291–297. [DOI] [PubMed] [Google Scholar]
- 21.Huang XJ, Liu DH, Liu KY et al. Treatment of acute leukemia with unmanipulated HLA‐mismatched/haploidentical blood and bone marrow transplantation. Biol Blood Marrow Transplant 2009;15:257–265. [DOI] [PubMed] [Google Scholar]
- 22.Miller AB, Hoogstraten B, Staquet M et al. Reporting results of cancer treatment. Cancer 1981;47:207–214. [DOI] [PubMed] [Google Scholar]
- 23.Dignan FL, Clark A, Amrolia P et al. Diagnosis and management of acute graft‐versus‐host disease. Br J Haematol 2012;158:30–45. [DOI] [PubMed] [Google Scholar]
- 24.Dignan FL, Amrolia P, Clark A et al. Diagnosis and management of chronic graft‐versus‐host disease. Br J Haematol 2012;158:46–61. [DOI] [PubMed] [Google Scholar]
- 25.Przepiorka D, Weisdorf D, Martin P et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995;15:825–828. [PubMed] [Google Scholar]
- 26.Filipovich AH, Weisdorf D, Pavletic S et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft‐versus‐host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005;11:945–956. [DOI] [PubMed] [Google Scholar]
- 27.Gooley TA, Leisenring W, Crowley J et al. Estimation of failure probabilities in the presence of competing risks: New representations of old estimators. Stat Med 1999;18:695–706. [DOI] [PubMed] [Google Scholar]
- 28.Mo XD, Zhang XH, Xu LP et al. Interferon‐Α is effective for treatment of minimal residual disease in patients with acute leukemia after allogeneic hematopoietic stem cell transplantation: Results of a registry study. Biol Blood Marrow Transplant 2017;23:1303–1310. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Li Q, Li W et al. Monitoring of minimal residual disease in acute myeloid leukemia with t(8;21)(q22;q22). Int J Hematol 2013;97:786–792. [DOI] [PubMed] [Google Scholar]
- 30.Klingemann HG, Grigg AP, Wilkie‐Boyd K et al. Treatment with recombinant interferon (alpha‐2b) early after bone marrow transplantation in patients at high risk for relapse [corrected]. Blood 1991;78:3306–3311. [PubMed] [Google Scholar]
- 31.Yan C, Xu L, Liu D et al. Immunosuppression for 6‐8 weeks after modified donor lymphocyte infusion reduced acute graft‐versus‐host disease without influencing graft‐versus‐leukemia effect in haploidentical transplant. Chin Med J (Engl) 2014;127:3602–3609. [PubMed] [Google Scholar]
- 32.Mo XD, Zhang XH, Xu LP et al. Comparison of outcomes after donor lymphocyte infusion with or without prior chemotherapy for minimal residual disease in acute leukemia/myelodysplastic syndrome after allogeneic hematopoietic stem cell transplantation. Ann Hematol 2017;96:829–838. [DOI] [PubMed] [Google Scholar]
- 33.Mo XD, Zhang XH, Xu LP et al. Salvage chemotherapy followed by granulocyte colony‐stimulating factor‐primed donor leukocyte infusion with graft‐vs.‐host disease control for minimal residual disease in acute leukemia/myelodysplastic syndrome after allogeneic hematopoietic stem cell transplantation: Prognostic factors and clinical outcomes. Eur J Haematol 2016;96:297–308. [DOI] [PubMed] [Google Scholar]
- 34.Mo X, Zhao X, Xu L et al. Interferon α: The salvage therapy for patients with unsatisfactory response to minimal residual disease‐directed modified donor lymphocyte infusion. Chin Med J (Engl) 2014;127:2583–2587. [PubMed] [Google Scholar]
- 35.Mo XD, Zhang XH, Xu LP, et al. Interferon‐α salvage treatment is effective for patients with acute leukemia/myelodysplastic syndrome with unsatisfactory response to minimal residual disease‐directed donor lymphocyte infusion after allogeneic hematopoietic stem cell transplantation. Front Med 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 36.Rohatiner AZ. Growth inhibitory effects of interferon on blast cells from patients with acute myelogenous leukaemia. Br J Cancer 1984;49:805–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morecki S, Revel‐Vilk S, Nabet C et al. Immunological evaluation of patients with hematological malignancies receiving ambulatory cytokine‐mediated immunotherapy with recombinant human interferon‐alpha 2a and interleukin‐2. Cancer Immunol Immunother 1992;35:401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grigg A, Kannan K, Schwarer AP et al. Chemotherapy and granulocyte colony stimulating factor‐mobilized blood cell infusion followed by interferon‐alpha for relapsed malignancy after allogeneic bone marrow transplantation. Intern Med J 2001;31:15–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.