Abstract
Biosimilars are biological compounds that contain an active substance that is similar to the active substance in an already approved biologic. This commentary focuses on the barriers to the use of biosimilars in the United States.
The Biologic Price Competition and Innovation Act (BPCIA), a component of the Affordable Care Act, provided an abbreviated regulatory pathway to accelerate the approval process of biosimilars [1]. Biosimilars are biological compounds that contain a highly similar version of the active substance in an already approved biologic, commonly referred to as the “innovator” or “reference product” [2]. To be approved, a biosimilar must establish high resemblance to the innovator in quality characteristics, safety, efficacy, and immunogenicity [2]. Differences are allowed if they are not clinically meaningful. Additionally, regulatory agencies allow extrapolation of safety and efficacy data from one biosimilar indication to another if rigorous and predefined requirements are established [3]. Biosimilars are predicted to lower health care expenditures by entering the market at a price 15%–30% lower than their counterpart reference products and, once in market, further driving price competition [4]. The value proposition of biosimilars is underscored by the continued rise in cancer drug costs, especially as new innovator biologics enter the market and U.S. Food and Drug Administration (FDA) policies accelerate drug approvals [5]. Patent expiration of many of the most prescribed innovator cancer biologics in the U.S. are anticipated to result in a rash of biosimilars entering the U.S. market in the coming years [6]. Four of the top ten prescribed innovator biologics for patients with cancer have biosimilars in queue, including recently approved biosimilars for both bevacizumab (Mvasi, Amgen, Thousand Oaks, CA) [7] and trastuzumab (Ogivri, Mylan, Canonsburg, PA) [8]. Identifying and addressing barriers to market uptake of biosimilars, including regulatory policy, stakeholder perceptions, and provider and patient economics, may be critical to assure the success of these new market entrants. This might be essential in oncology, in which the cost of cancer care continues to rise, and oncologists can learn from their rheumatologist counterparts who have embraced the use of biosimilars. Filgrastim biosimilar has been the only biosimilar approved with an oncology indication in the U.S. but its market uptake has lagged behind Europe, Canada, and Japan [9]. Economic and familiarity with policy and regulatory factors have been suggested as potential obstacles to improved uptake [10]. Understanding barriers to uptake and developing strategies to mitigate them might lead to increased use of biosimilars and indeed lower health care costs.
Nononcology Biosimilars and Use Outside the U.S.
In total, 37 biosimilars have been approved in Europe, with a mean price discount of 15%–40% compared with their reference innovators [11]. The increased use in European markets have been partially attributed to economic policies that incetivize prescribing biosimilars. As an example, more than 50% of biosimilars volume uptake has been observed in Germany [12], [13]. In Norway, negotiating a steep price discount for infliximab biosimilar has led to over 90% market share [14], [15]. The National Institute for Health and Care Excellence has recommended the use of biosimilars to infliximab as opposed to the innovator biologic, which led to a 25% decrease in infliximab with improved patient access [16], [17]. Canada has lagged behind Europe in adopting biosimilars, as payers have encouraged new patients to use biosimilars, but switching existing patients was not incentivized [18], [19]. Starting new patients on biosimilars was pushed and incentivized by Canadian policy makers negotiating with manufacturers and payers. The experience outside the U.S. with nononcology biosimilars suggests that price‐driven incentivization could lead to better uptake and improved use of biosimilars.
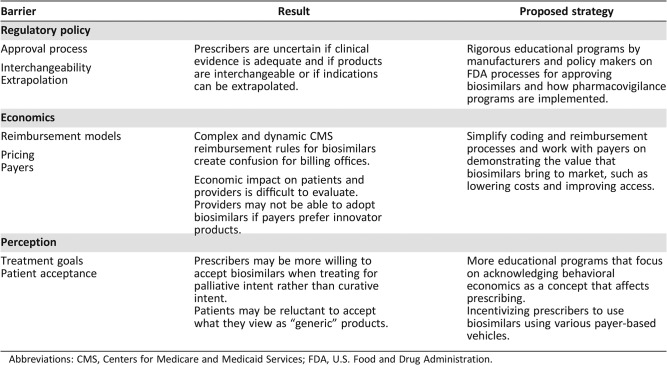
Barriers to Uptake in the U.S. (Table 1)
Table 1. Barriers to biosimilar uptake.
Abbreviations: CMS, Centers for Medicare and Medicaid Services; FDA, U.S. Food and Drug Administration.
Policy
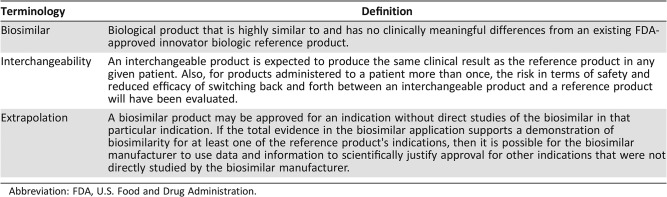
The Hatch‐Waxman Act of 1983 set the rules by which generics would be handled in the marketplace [20]. Similarly, the BPCIA is a critical piece of legislation that establishes the rules by which biosimilars will be handled. The uniqueness of biological synthesis created significant scientific and philosophical issues for regulators and law makers because reference‐brand biologics themselves exhibit chemical variation from batch to batch. This inherent variation of reference biologics challenges many of the assumptions that defined the rules for generic drugs. Interchangeability could not be assumed, and therefore the FDA required randomized clinical trials as a proof of equivalence of efficacy, safety, and immunogenicity between the biosimilar and its reference biologic. In January 2017, the FDA issued a draft guidance for biosimilar interchangeability that would require biosimilar manufacturers to conduct one or more switching studies to assure that switching between the innovator and its biosimilar is safe and does not compromise efficacy [21]. In addition, the biosimilar must demonstrate the same clinical outcomes for all the innovator product indications. The interchangeability designation would allow the biosimilar to be substituted for the reference product without prescriber intervention in states that have approved standards for biosimilar substitution. As of July 2017, 35 states and Puerto Rico allow substitution by a pharmacist if the biosimilar is considered interchangeable and is covered under an insurer's pharmacy benefit [22]. How interchangeability policy will affect providers and patient perceptions of biosimilar comparative effectiveness will be watched closely as products enter the market.
In addition to interchangeability, extrapolation (Table 2) across indications represents another policy decision that may become a barrier to routine biosimilar adoption. Biosimilars may be approved for all indications for which the innovator is approved without clinical testing in those diseases if scientific justification is provided. To date, FDA approvals of biosimilars have leaned toward complete or near‐complete extrapolation in the final approved product labeling. The concept of extrapolation differs from demanding a new clinical trial for every new approved indication. In fact, one can argue that if these studies are demanded, the theoretical health care cost savings with biosimilars will not be realized and will be substantially diminished.
Table 2. Biosimilar definitions.
Abbreviation: FDA, U.S. Food and Drug Administration.
The biosimilar pathway includes a unique process for resolving patent disputes prior to the potential approval of a biosimilar application. In what is referred to as the “patent dance,” biosimilar and reference product sponsors must exchange intellectual property information and work through patent disputes according to a schedule. In theory, the process assures a smoother, more predictable entry for biosimilar products than has been the case with the Hatch‐Waxman generic drug patent challenge system. However, the ground rules for the patent dance have already generated litigation that has been brought to the Supreme Court, which ruled in June 2017, in Sandoz Inc. v. Amgen Inc., that the patent dance is optional under federal law [23]. It is plausible that patent litigation processes might lead to delaying the launch and marketing of biosimilar entrants in the U.S.
Economics
Policy decisions, like the FDA rules governing approval and interchangeability, will have a significant impact on the economics of biosimilars, but their reimbursement will be even more critical. In 2016, the Centers for Medicare and Medicaid Services (CMS) defined a reimbursement structure for biosimilars that included grouping all biosimilar drugs for a common reference biologic under the same billing code (“J code”). The result was a blended J code for biosimilars, analogous to that which is used for small molecule generic products. Furthermore, the policy specified that biosimilars were to be reimbursed at the blended average sales price (ASP) plus 6% of the reference product ASP. As a result, the ASP of a biosimilar would have continued to decline as additional biosimilars to a specific innovator biologic entered the market, creating disincentives for biosimilar competition. CMS solicited comments regarding the impact of its policy of grouping all biosimilar products into the same payment calculation. Based on these comments, effective January 1, 2018, CMS has changed its policy to separately code and pay for biologic biosimilar products under Medicare Part B. With the movement into value‐based care, reimbursement models as exemplified by the CMS bundled payment programs under the Oncology Care Model (OCM) structure, physicians will need to manage the cost of care judiciously so that expenditures fall within a predefined target range. This shift may force physicians to take a more aggressive value stance in formulary decisions, in which payers have historically held the final word. As OCM participants may be responsible for nearly half of Medicare beneficiary cancer care, such a shift in treatment selection could have a formidable impact on drug use. Balancing quality of care with reimbursement challenges may have a significant impact on biosimilar versus brand prescribing decisions.
Because generics have been proven to lower drug costs, there is optimism that biosimilars may do the same. Generics contributed to a steep decline in cancer drug prices in the past decade, in part through interchangeability, leading to 90% of national prescriptions being generics without significant impact on physicians' reimbursement [24]. Although it is unclear to what degree biosimilars will lower costs, early entrants have validated the estimates of a price 15%–30% lower than the reference brand [25]. Payers might embrace biosimilars and switching from innovative biologics so that potential savings can be reinvested in funding newer drugs and entrants. A model that might bend the cost curve downward, although it remains unclear how payers will react.
Despite these potential health care savings, the economics of biosimilar prescribing for both prescribers and patients is more nuanced. Reimbursement to physicians who prescribe biosimilars and cost sharing by patients who receive them may represent real barriers to adoption. Payers' decisions on how to reimburse providers for biosimilars and the benefit design affecting patients is likely to be regional and lack uniformity. Moreover, switching to biosimilars in the midst of active cancer treatment because of changes in insurance coverage is not yet proven safe and effective and will face concerns from providers and patients alike, affecting reimbursement and potentially having economic sequelae.
For patients with commercial coverage, rebate agreements between payers, pharmacy benefit managers (PBMs), and manufacturers represent an additional nuance and complexity in drug adoption. Such agreements create financial incentives for payers, via the contracted PBM, to direct use of preferred drugs via benefit design that restricts either provider prescribing or patient cost contribution. Reference brand manufacturers might employ such strategies to provide an economic incentive for a payer to position an innovator biologic over a biosimilar [22]. These strategies to create drug tiers or step edits that result in hierarchies of drug approvals may reduce cost to a payer without lowering drug price.
Perception
How biosimilars will be perceived by the physicians who prescribe them and the patients to whom they are prescribed is likely to be the most complex of factors in the rate of adoption. Behavioral economic research has shown that our perceptions result from an often‐subconscious set of biases influenced by a myriad of factors [26]. Such complexity is further challenged by the clinical scenarios in which the biosimilars are prescribed. Thresholds for adoption may vary significantly across scenarios and diseases, depending upon such factors as disease activity, prognosis, goals of care, stage of disease, and patient preference, among others.
Whereas growth factor biosimilars were the first to be approved in the U.S., biosimilars to monoclonal antibodies represent the next wave of biosimilars in oncology. Although oncologists may be comfortable with biosimilars for supportive care, there may be less acceptance when considering biosimilars for cancer treatment, particularly in the curative setting. This requires further reassurance and education to explain the rigor of the approval process and that lower prices do not suggest inferiority. Familiarity with current innovator biologics and how long they have been used might represent a barrier because biosimilars are new entrants that physicians are less familiar with. Reluctance to change to a new market entrant might diminish uptake. Even in medicine, there are laggards. The impact on revenue capture when prescribing an innovator biologic versus a biosimilar is front and center and is increasingly becoming essential to providers as profit margins decline with new reimbursement models and cuts from payers and government agencies. The above theoretical concerns were solidified when we conducted primary market research surveys of more than 500 hematologists and oncologists who voiced their concerns about extrapolation and expedited regulatory approvals. Educational gaps regarding the FDA's rigor in approving biosimilars were identified [10]. In that survey, providers reported that their decisions on prescribing biosimilars are always contingent on payers' agreement and that some payers might not allow biosimilars for a particular disease. Continued education and identification of barriers to prescribing are essential to develop mitigating strategies.
From an operational perspective, managing inventory can be vital to providers' economic health. Payers' decisions on whether biosimilars are preferred formulary may require providers to stock multiple drugs for the same indication. Some payers may exert pressure on health care systems to include biosimilars on formulary, and if patients are incentivized through lower out‐of‐pocket costs, this will add additional pressure on these systems to stock and use biosimilars.
Understanding how biosimilars are named as they get integrated into clinical pathways and electronic medical records is essential to minimize errors and increase uptake. Under the FDA rule, biosimilar names will be a combination of the core name and a specific suffix that is composed of four lowercase letters [27]. Essentially, the goal of the FDA naming convention is twofold. First, it identifies a relationship between the biosimilar and the innovator biologic in terms of therapeutic category and dosing. Second, it differentiates products effectively to support proper pharmacovigilance programs. This ensures that the intended product is administered to the right patient and that adverse events are attributed to the correct product.
Providers and their patients are also likely to be influenced by the support programs offered by the biosimilar and reference brand manufacturers. Patient assistance programs are often critical to a commercially insured patient's ability to initiate or adhere to treatment schedule. Manufacturer‐sponsored patient education programs are also heavily relied upon, given the constraints on practice resources. For adoption barriers to be overcome, it will be critical that providers and patients perceive parity in biosimilar and reference brand access and support services. To that end, understanding the differences in clinical trials required to approve a biosimilar versus those needed for a biologic is critical so that providers understand the rigor involved in the approval process. Our investigation suggests that proper education and knowledge of these studies diminish concerns that providers might have [10].
Until proper education takes place, some patients may assume biosimilars are analogous to generics; those who have been reluctant to use generics may have a similar attitude toward biosimilars. Jacobs et al. reported on major gaps among U.S. and European patients regarding biosimilars [28]. In fact, almost 70% of the 3,198 surveyed individuals had never heard of biosimilars, with a slight edge in knowledge favoring European patients and caregivers. Notably, patients appeared influenced by manufacturers' names and brand awareness, suggesting that their acceptance of biosimilars might depend on who develops the drug [29]. This highlights the need for proper educational platforms and for explaining how biosimilars differ from generics. This can be done through advocacy groups but more importantly through providers and caregivers, who play an equally key role in the process.
Whether patient perceptions differ based on the disease setting and stage remains unknown. One can argue that patients' acceptance of biosimilars might differ when facing a curable disease versus a metastatic incurable cancer. Similar to physician acceptance, biosimilars used as supportive measures might be better received than when used as active anticancer therapy. Explaining the regulatory approval process and FDA requirements should reassure patients, caregivers, and physicians that biosimilars can be used in any disease stage, even when cure is the goal. In addition, education would likely diminish toxicity and efficacy concerns for all stakeholders. Immunogenicity remains the most important safety concern for biosimilars [29]. Ongoing pharmacovigilance programs in the real world are needed to assure that long‐term safety concerns are mitigated [30].
Conclusion
The rapid increase in health care costs and the patent expiration of various top‐selling innovator biologics have paved the way for biosimilar development in the U.S. Although abbreviated, regulatory processes to approve biosimilars are rigorously gauged to assure safety, efficacy, and success across various indications. However, understanding barriers to commercial success is critical to design strategies that overcome these barriers and allow patient access to biosimilars. Identifying standardized metrics that allow stakeholders to compare the value of biosimilars with other biologics, such as quality‐adjusted life‐year [31], is critical to assess biosimilars' cost‐saving potential. Acceptance by patients, payers, and prescribers of the value of biosimilars might eventually lead to lower overall cost of care, but longer follow‐up is needed to see if this is accomplished.
Disclosures
Chadi Nabhan: Cardinal Health (E, OI); Amy Valley: Cardinal Health (E, OI); Bruce A. Feinberg: Cardinal Health (E, OI).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Weise M, Bielsky MC, De Smet K et al. Biosimilars: What clinicians should know. Blood 2012;120:5111–5117. [DOI] [PubMed] [Google Scholar]
- 2.Nabhan C, Parsad S, Mato AR et al. Biosimilars in oncology in the United States: A review. JAMA Oncol 2018;4:241–247. [DOI] [PubMed] [Google Scholar]
- 3.Weise M, Kurki P, Wolff‐Holz E et al. Biosimilars: The science of extrapolation. Blood 2014;124:3191–3196. [DOI] [PubMed] [Google Scholar]
- 4.Malik AN, Keeping K, Fletcher‐Louis M. U.S. payer expectations for reimbursement of biosimilars. Value Health 2015;18:A545. [Google Scholar]
- 5.Yang YT, Chen B, Bennett CL. Biosimilars‐Curb your enthusiasm. JAMA Oncol 2017;3:1467–1468. [DOI] [PubMed] [Google Scholar]
- 6.Hakim A, Ross JS. Obstacles to the adoption of biosimilars for chronic diseases. JAMA 2017;317:2163–2164. [DOI] [PubMed] [Google Scholar]
- 7.Thatcher N, Thomas M, Paz‐Ares L et al. Randomized, double‐blind, phase 3 study evaluating efficacy and safety of ABP 215 compared with bevacizumab in patients with non‐squamous NSCLC. J Clin Oncol 2016;34(suppl 15):9095. [Google Scholar]
- 8.Rugo HS, Barve A, Waller CF et al. Effect of a proposed trastuzumab biosimilar compared with trastuzumab on overall response rate in patients with ERBB2 (HER2)‐positive metastatic breast cancer: A randomized clinical trial. JAMA 2017;317:37–47. [DOI] [PubMed] [Google Scholar]
- 9.Courage N, Parsons A. The comparability conundrum: Biosimilars in the United States, Europe and Canada. Food Drug Law J 2011;66:203–224, i–ii. [PubMed] [Google Scholar]
- 10.Nabhan C, Jeune‐Smith Y, Valley A et al. Community oncologists' perceptions and acceptance of biosimilars in oncology. J Clin Pathways 2018;4:43–47. [Google Scholar]
- 11.Zuñiga L, Calvo B. Regulatory aspects of biosimilars in Europe. Trends Biotechnol 2009;27:385–387. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan E, Piercy J, Waller J et al. Assessing gastroenterologist and patient acceptance of biosimilars in ulcerative colitis and Crohn's disease across Germany. PLoS One 2017;12:e0175826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waller J, Sullivan E, Piercy J et al. Assessing physician and patient acceptance of infliximab biosimilars in rheumatoid arthritis, ankylosing spondyloarthritis and psoriatic arthritis across Germany. Patient Prefer Adherence 2017;11:519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jahnsen J, Kaasen Jørgensen K. Experience with biosimilar infliximab (Remsima) in Norway. Dig Dis 2017;35:83–90. [DOI] [PubMed] [Google Scholar]
- 15.Reinaud F, Ando G. Have infliximab discounted prices in Norway had an impact on prices around the world? Value Health 2015;18:A518. [Google Scholar]
- 16.Wailoo A, Bansback N, Chilcott J. Infliximab, etanercept and adalimumab for the treatment of ankylosing spondylitis: Cost‐effectiveness evidence and NICE guidance. Rheumatology (Oxford) 2008;47:119–120. [DOI] [PubMed] [Google Scholar]
- 17.Limdi JK, Shaffer JL. How “NICE” were we with infliximab? Inflamm Bowel Dis 2005;11:705–706. [DOI] [PubMed] [Google Scholar]
- 18.Furlanetto A, Purcell N. Biologics and biosimilars: A legal perspective from Canada. Pharm Pat Anal 2016;5:79–81. [DOI] [PubMed] [Google Scholar]
- 19.Kay J, Feagan BG, Guirguis MS et al. Health Canada/BIOTECanada Summit on regulatory and clinical topics related to subsequent entry biologics (biosimilars), Ottawa, Canada, 14 May 2012. Biologicals 2012;40:517–527. [DOI] [PubMed] [Google Scholar]
- 20.Gupta R, Kesselheim AS, Downing N et al. Generic drug approvals since the 1984 Hatch‐Waxman Act. JAMA Intern Med 2016;176:1391–1393. [DOI] [PubMed] [Google Scholar]
- 21.Center for Biologics Evaluation and Research, Center for Drug Evaluation and Research U.S. Food and Drug Administration . Considerations in Demonstrating Interchangeability with a Reference Product: Guidance for Industry [draft guidance]. Silver Spring, MD: U.S. Food and Drug Administration; January 2017. [Google Scholar]
- 22.Conti RM. Biosimilars: Reimbursement issues in your oncology practice. J Oncol Pract 2017;13(suppl 9):12s–14s. [DOI] [PubMed] [Google Scholar]
- 23.Baghdadi R. Biosimilars. Health Aff (Millwood) Policy Brief, July 21, 2017. DOI: 10.1377/hpb20170721.487227. Accessed May 15, 2018. [DOI]
- 24.Bate R, Mathur A, Lever HM et al. Generics substitution, bioequivalence standards, and international oversight: Complex issues facing the FDA. Trends Pharmacol Sci 2016;37:184–191. [DOI] [PubMed] [Google Scholar]
- 25.Mullard A. Bracing for the biosimilar wave. Nat Rev Drug Discov 2017;16:152–154. [DOI] [PubMed] [Google Scholar]
- 26.Nabhan C, Feinberg BA. Behavioral economics and the future of biosimilars. J Natl Compr Canc Netw. 2017;15:1449–1451. [DOI] [PubMed] [Google Scholar]
- 27.Center for Biologics Evaluation and Research, Center for Drug Evaluation and Research, Food and Drug Administration, U.S. Department of Health and Human Services . Nonproprietary Naming of Biological Products: Guidance for Industry. January 2017. Available at https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM459987.pdf. Accessed January 24, 2018.
- 28.Jacobs I, Singh E, Sewell KL et al. Patient attitudes and understanding about biosimilars: An international cross‐sectional survey. Patient Prefer Adherence 2016;10:937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rifkin RM, Peck SR. Biosimilars: Implications for clinical practice. J Oncol Pract 2017;13(suppl 9):24s–31s. [DOI] [PubMed] [Google Scholar]
- 30.Liu PM, Zou L, Sadhu C et al. Comparative immunogenicity assessment: A critical consideration for biosimilar development. Bioanalysis 2015;7:373–381. [DOI] [PubMed] [Google Scholar]
- 31.Neumann PJ, Cohen JT, Weinstein MC. Updating cost‐effectiveness‐‐The curious resilience of the $50,000‐per‐QALY threshold. N Engl J Med 2014;371:796–797. [DOI] [PubMed] [Google Scholar]