This article reports the results of a post hoc analysis of a clinical trial, comparing outcomes of 14 patients with advanced nonmetaplastic triple‐negative breast cancer and 59 patients with advanced metaplastic triple‐negative breast cancer treated with targeted therapy using mTOR inhibition (temsirolimus or everolimus) with liposomal doxorubicin and bevacizumab.
Keywords: mTOR inhibitor, Mesenchymal, Metaplastic breast cancer, Triple‐negative breast cancer
Abstract
Background.
Triple‐negative breast cancer (TNBC) is a heterogeneous disease with subtypes having different “targetable” molecular aberrations. Metaplastic breast cancers (MpBCs) are typically TNBCs and commonly have alterations in the PI3K/Akt/mTOR pathway. We previously reported efficacy for an mTOR‐based chemotherapy regimen in MpBC. To determine if tumor subtype influences prognosis, we compared treatment outcomes of patients with MpBC with those of patients with nonmetaplastic TNBC receiving an mTOR‐based systemic therapy regimen.
Patients and Methods.
Patients with advanced MpBC and nonmetaplastic TNBC were treated at our institution from April 16, 2009, through November 4, 2014, using mTOR inhibition (temsirolimus or everolimus) with liposomal doxorubicin and bevacizumab (DAT/DAE). Median progression‐free survival (PFS) and overall survival (OS) were estimated by the Kaplan‐Meier method. Cox regression analyses were used to evaluate associations between tumor histology and outcomes. Multivariable models were adjusted for all covariates.
Results.
Fourteen patients with nonmetaplastic TNBC and 59 patients with advanced MpBC were treated with DAT/DAE. MpBC patients were older (p = .002) and less likely to have a history of bevacizumab use (p = .023). Median PFS for the nonmetaplastic TNBC and MpBC patients was 2.5 months and 4.8 months, respectively. This difference in PFS was statistically significant on univariable (p = .006) but not multivariable analysis (p = .087). Median OS for the nonmetaplastic TNBC and MpBC patients was 3.7 months and 10.0 months, respectively (p = .0003). MpBC remained significantly associated with improved OS on multivariable analysis (p < .0001).
Conclusion.
In our study, DAT/DAE appeared to be more effective in MpBC compared with nonmetaplastic TNBC. These data support patient selection for targeted therapy in TNBC.
Implications for Practice.
Metaplastic breast cancers (MpBCs) represent <1% of all breast cancers, demonstrate mesenchymal differentiation, and are typically resistant to chemotherapy. Patients with advanced MpBC treated with an mTOR‐based systemic therapy regimen had better long‐term outcomes compared with patients with nonmetaplastic triple‐negative breast cancer treated with the same regimen, suggesting that metaplastic histology may predict benefit from agents targeting the PI3K/Akt/mTOR pathway.
摘要
背景。三阴性乳腺癌 (TNBC) 是一种异质性疾病,其亚型具有不同的“可靶向”分子畸变。化生性乳腺癌 (MpBC) 通常是 TNBC,并且 PI3K/Akt/mTOR 通路通常发生改变。我们之前报告了基于 mTOR 的化疗方案对 MpBC 的效果。为了确定肿瘤亚型是否影响预后,我们比较了 MpBC 患者与接受基于 mTOR 的系统治疗方案的非化生性 TNBC 患者的治疗效果。
患者和方法。2009 年 4 月 16 日至 2014 年 11 月 4 日,我们机构采用 mTOR 抑制剂(替西罗莫司或依维莫司)搭配脂质体多柔比星和贝伐珠单抗 (DAT/DAE) 对晚期 MpBC 和非化生性 TNBC 患者进行治疗。我们通过 Kaplan‐Meier 法估算了患者的中位无进展生存期 (PFS) 和总生存期 (OS)。我们采用 Cox 回归分析评估了肿瘤组织学与预
后之间的关系 并针对所有协变量调整了多变量模型。结果。14名非化生性 TNBC 患者与 59 名晚期 MpBC 患者接受了 DAT/DAE 的治疗。MpBC 患者年龄较大 (p = 0.002)和贝伐珠单抗使用史的较少 (p = 0.023)。非化生性 TNBC 和 MpBC 患者的中位 PFS 分别为 2.5 个月和 4.8 个月。PFS 的这种差异在单变量分析中具有统计学意义 (p = 0.006),但在多变量分析则不具有统计学意义 (p = 0.087)。非化生性 TNBC 患者和 MpBC 患者的中位 OS 分别为 3.7 个月和 10.0 个月 (p = 0.000 3)。在多变量分析 (p < 0.0001) 中,MpBC 始终与 OS 的改善关系密切。
结论。我们的研究显示,DAT/DAE 对 MpBC 的效果优于对非化生性 TNBC 的效果。这些数据可帮助 TNBC 患者选择靶向治疗方案。
对临床实践的提示: 化生性乳腺癌 (MpBC) 占所有乳腺癌的比例 <1%,表现出间质分化,并且通常对化疗具有耐药性。采用基于 mTOR 系统治疗方案的晚期 MpBC 患者的长期预后效果优于采用相同治疗方案的非化生三阴性乳腺癌患者的长期预后效果,表明化生组织可能预测针对 PI3K/Akt/mTOR通路的制剂的疗效
Introduction
Metaplastic breast cancers (MpBCs) are a group of histologically heterogeneous breast cancers demonstrating various combinations of poorly differentiated adenocarcinoma, sarcomatoid, and/or squamous components within the same tumor [1], [2], [3], [4], [5], [6]. These tumors are rare, representing <1% of all breast malignancies [7], and are typically triple‐negative breast cancers (TNBCs) with dismal prognosis [8], [9].
The molecular signature of MpBCs most closely resembles the claudin‐low and mesenchymal subtypes of TNBC with an enrichment of genes involved in epithelial‐to‐mesenchymal transition and other stem cell‐associated genes [10], [11]. TNBCs as a group have demonstrated greater sensitivity to cytotoxic chemotherapy compared with non‐TNBCs [12]. However, compared with the basal‐like 1 subtype of TNBC, which has been reported to have a pathologic complete response (pCR) rate of 52% when treated with standard neoadjuvant chemotherapy, the mesenchymal and mesenchymal stem‐like subtypes of TNBC are less sensitive to cytotoxic chemotherapy, with reported pCR rates of only 23%–31% in the neoadjuvant setting [13].
Given the molecular similarities between MpBCs and the mesenchymal and mesenchymal stem‐like subtypes of TNBC, it is therefore not surprising that MpBCs have been reported to be comparatively resistant to cytotoxic chemotherapy [8]. We previously reported the results of a phase I trial evaluating the novel combination of liposomal doxorubicin, bevacizumab, and mammalian target of rapamycin (mTOR) inhibition with temsirolimus or everolimus (DAT or DAE, respectively). We observed promising signs of activity in MpBC, especially in patients who had a molecular aberration affecting the phosphatidylinositol‐3‐kinase (PI3K) pathway [14], [15], [16], [17].
Here we report the results of a post hoc analysis of a clinical trial, comparing outcomes of 14 patients with advanced nonmetaplastic TNBC and 59 patients with advanced MpBC treated with targeted therapy using DAT or DAE.
Materials and Methods
Patients
We previously reported our clinical experience with DAT and DAE in MpBC [14], [15], [17], [18]. We identified patients who received DAT or DAE at the University of Texas MD Anderson Cancer Center (MDACC) and compared outcomes in patients with advanced, MpBC to those with advanced, nonmetaplastic TNBC (considered an “unselected cohort” of patients with TNBC). Subspecialized breast pathologists at MDACC reviewed all available tumor material and rendered all histologic diagnoses prior to treatment initiation. MpBC was defined according to the World Health Organization Classification of Tumors of the Breast (4th edition) [19], with invasive breast carcinomas categorized as having metaplastic histology if the invasive carcinoma cells had morphologic evidence of squamous or mesenchymal differentiation, the latter including spindle cell, chondroid, or osseous differentiation. TNBC was defined as a tumor that was estrogen receptor (ER) negative, progesterone receptor negative, and human epidermal growth factor receptor 2 (HER2) negative. ER and progesterone receptor negativity was defined as <10% staining of invasive carcinoma cells of any intensity by immunohistochemistry. HER2 negativity was defined according to the American Society of Clinical Oncology/College of American Pathologists HER2 testing guidelines [20]. Sixty‐three patients received therapy as part of an Institutional Review Board (IRB) approved clinical trial (#2008‐0384), and 10 patients were treated on another IRB‐approved clinical protocol (DR11‐0039) that involved collecting data from patients treated with the same targeted therapy outside of the parent protocol (in this case, #2003‐0384). The results of a dose escalation phase I trial determined the recommended phase II dose to be the following: liposomal doxorubicin, 30 mg/m2 IV every 3 weeks; bevacizumab, 15 mg/kg IV every 3 weeks; and temsirolimus, 10 mg IV weekly, or everolimus, 7.5 mg by mouth daily, using 21‐day cycles (NCT00761644) [15]. Eligibility criteria for protocol #2008‐0384 has been previously described [15], [18]. Patients who had previously received a cumulative doxorubicin dose of greater than 300mg/m2 were excluded from the study, and all patients underwent close cardiac monitoring, as previously described [15]. Treatment assignment to DAT or DAE was determined according to logistical considerations such as patient preference for oral agents, insurance approval, and availability of slots at the time of enrollment. Patients were enrolled from April 16, 2009, through November 4, 2014, and followed for survival outcomes through April 1, 2017.
Efficacy
Restaging scans were obtained every 6 weeks (two cycles) while on protocol and response was assessed using RECIST version 1.1 [21]. All complete and partial responses (CRs and PRs, respectively) were confirmed on at least one subsequent staging evaluation. In patients with a best response of stable disease (SD), the duration of SD was defined as the time from study enrollment to progression or exit from study, whichever occurred first. The overall response rate (ORR) was defined as the percentage of patients with a best response of either a confirmed CR or confirmed PR. The clinical benefit rate (CBR) was defined as the percentage of patients with a best response of a confirmed CR or confirmed PR or having at least 6 months of disease stability (SD ≥6 months).
Progression‐free survival (PFS) was defined as the time from study enrollment to progression or death from any cause, whichever occurred first. For patients not known to have progressed or died, data for PFS were censored at the time of last follow‐up. Overall survival (OS) was defined as the time from study enrollment to death from any cause. Information on the patient's vital status were obtained from our electronic medical records and supplemented by data from the MDACC tumor registry. For patients not known to have died, data for OS were censored at the time the patient was last known to be alive.
Statistical Analysis
Univariable and multivariable logistic regression was used to examine associations between baseline patient characteristics and histologic subtype. Confidence intervals (CI) of proportions were calculated using the exact binomial method. The effect of study covariates on the ORR and CBR were examined using univariable and multivariable logistic regression. Prior taxane and bevacizumab use were excluded from the regression models predicting ORR and CBR because of their joint distribution with outcomes. Estimates of median PFS and OS were calculated using the Kaplan‐Meier method, and time‐to‐event distributions were compared using the log‐rank test. Univariable and multivariable Cox regression analyses were used to determine the effect of study covariates on PFS and OS. Results are expressed as hazard ratios (HR) in univariable analyses and adjusted hazard ratios (aHR) in multivariable models with accompanying 95% CIs.
To account for multiplicity of testing, a two‐sided p value of less than .02 was considered statistically significant when evaluating associations between histology (metaplastic vs. nonmetaplastic) and outcomes. All other comparisons are hypothesis generating, and p values should be interpreted with caution. All data were analyzed using R Statistical Software (version 3.5.0; R Foundation for Statistical Computing, Vienna, Austria).
Results
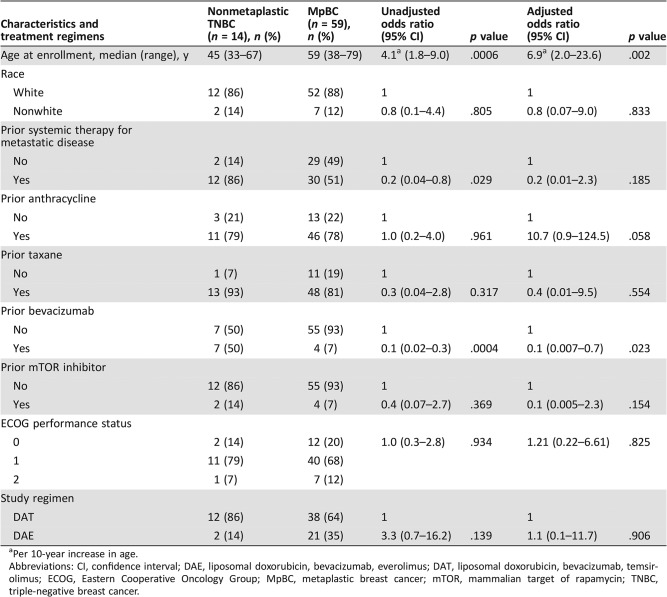
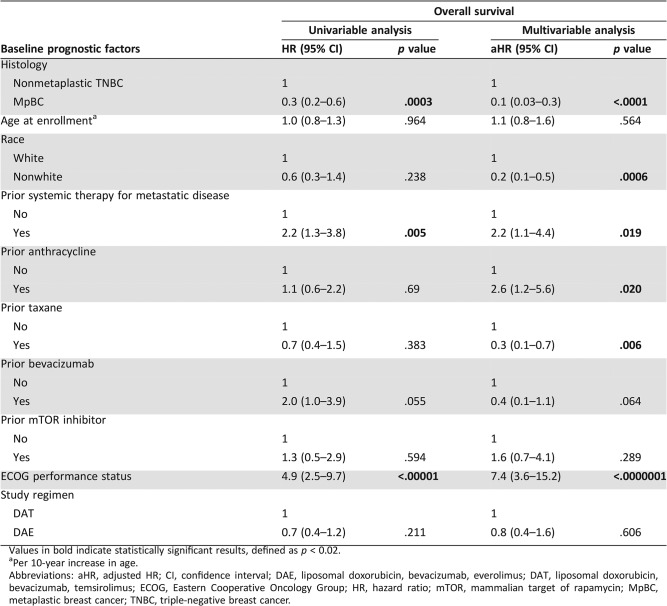
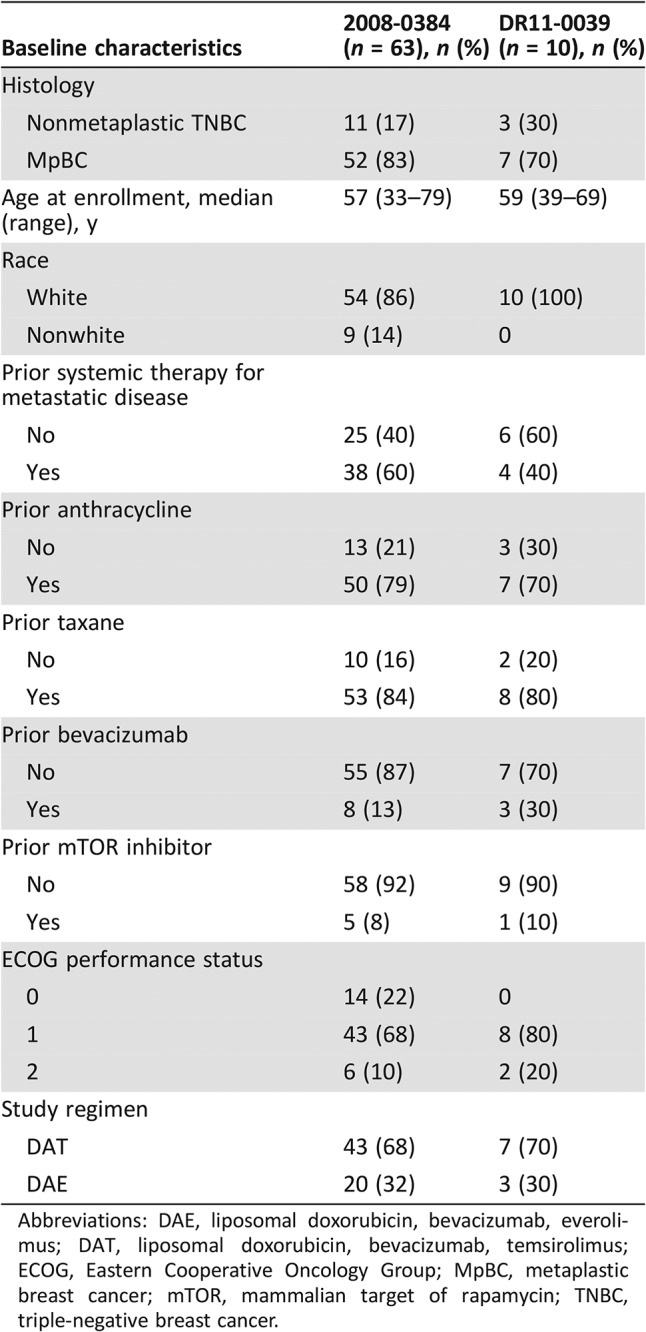
A total of 73 patients were included in this study, of whom 14 patients had advanced nonmetaplastic TNBC (median age, 45 years; range, 33–67 years) and 59 patients had advanced MpBC (median age, 59 years; range, 38–79 years). Of the 59 patients with advanced MpBC, 98% (58/59) had TNBCs. The last patient was ER negative but HER2 positive based on in situ hybridization with an average HER2/Chromosome 17 ratio of 2.5 and an average number of HER2 signals/nucleus of 6.9. This testing was performed outside our institution, and additional archival tumor tissue was not available for repeat testing of HER2 status at MDACC. Notably, this patient developed metastatic disease while receiving adjuvant trastuzumab and developed disease progression after only two cycles of docetaxel, pertuzumab, and trastuzumab as front‐line therapy for metastatic disease. Because this clinical course suggested that her disease was resistant to HER2‐directed therapy, the patient was included in these analyses. Of the 14 patients with advanced nonmetaplastic TNBC, 12 were treated with this regimen because of progression of disease on standard therapy, and the remaining 2 patients received this regimen because of physician and patient preferences. Baseline patient characteristics are summarized in Table 1. On univariable logistic regression, older age was associated with metaplastic histology (odds ratio [OR], 4.1; 95% CI, 1.8–9.0; p = .0006). In contrast, prior systemic therapy in the metastatic setting (OR, 0.2; 95% CI, 0.04–0.8; p = .029) and prior bevacizumab therapy (OR, 0.1; 95% CI, 0.02–0.3; p = .0004) were less likely to be associated with metaplastic histology. Patients with MpBC and nonmetaplastic TNBC received a median of one (range, 0–5) and three (range, 0–6) prior lines of systemic therapy for metastatic disease, respectively. On multivariable logistic regression, older age was associated with metaplastic histology (OR, 6.9; 95% CI, 2.0–23.6; p = .002), whereas prior treatment with bevacizumab was less likely to be associated with metaplastic histology (OR, 0.1; 95% CI, 0.007–0.7; p = .023). Table 2 summarizes and compares the baseline characteristics between the 63 patients who received therapy as part of protocol #2008‐0384 with the 10 patients treated on protocol DR11‐0039.
Table 1. Baseline characteristics and treatment regimens.
Per 10‐year increase in age.
Abbreviations: CI, confidence interval; DAE, liposomal doxorubicin, bevacizumab, everolimus; DAT, liposomal doxorubicin, bevacizumab, temsirolimus; ECOG, Eastern Cooperative Oncology Group; MpBC, metaplastic breast cancer; mTOR, mammalian target of rapamycin; TNBC, triple‐negative breast cancer.
Table 2. Baseline characteristics of patients treated on protocol #2008‐0384 compared with patients treated on protocol DR11‐0039.
Abbreviations: DAE, liposomal doxorubicin, bevacizumab, everolimus; DAT, liposomal doxorubicin, bevacizumab, temsirolimus; ECOG, Eastern Cooperative Oncology Group; MpBC, metaplastic breast cancer; mTOR, mammalian target of rapamycin; TNBC, triple‐negative breast cancer.
Efficacy
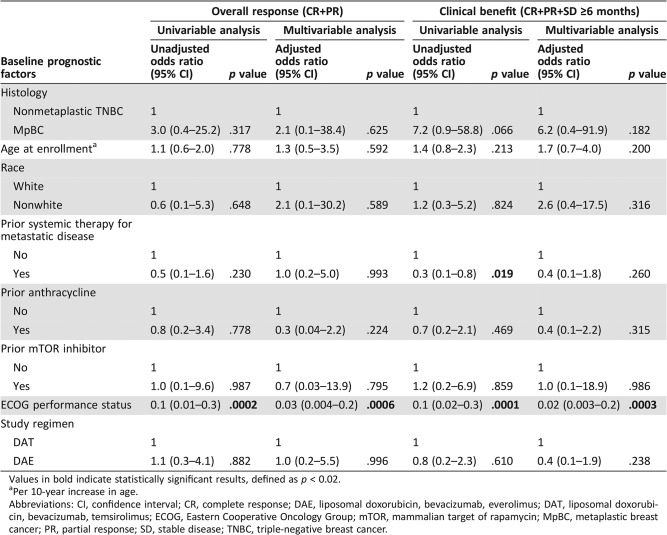
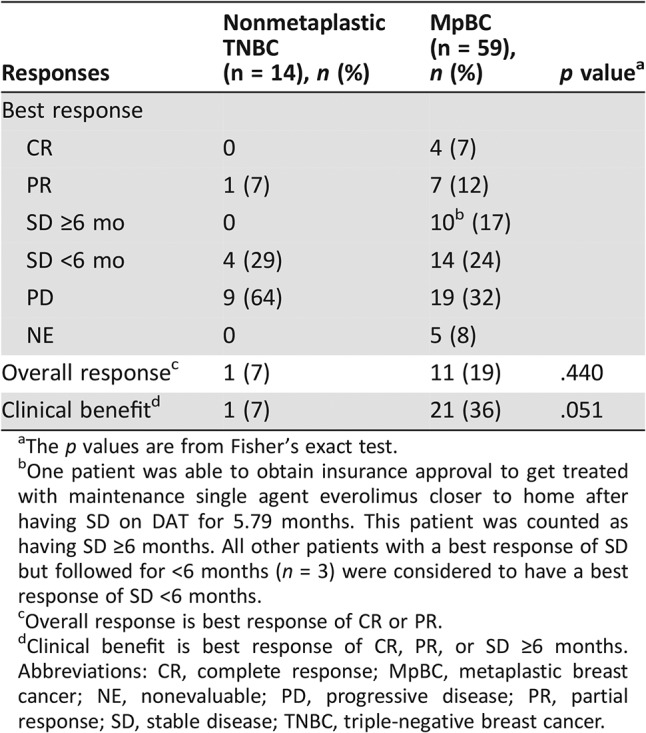
Of the 14 patients with nonmetaplastic TNBC treated, there were no CRs, and one patient had a PR, for an ORR of 7% (95% CI, 0.2–34%; Table 3). In contrast, among the patients with MpBC, there were four CRs and seven PRs for an ORR of 19% (95% CI, 9.7–31%; Table 3). However, this difference was not statistically significant on both univariable (OR, 3.0; 95% CI, 0.4–25.2; p = .317; Table 4) and multivariable logistic regression (OR, 2.1; 95% CI, 0.1–38.4; p = .625; Table 4). Poorer performance status was associated with a lower likelihood of achieving an objective response on both univariable (OR, 0.1; 95% CI, 0.01–0.3; p = .0002; Table 4) and multivariable logistic regression (OR, 0.03; 95% CI, 0.004–0.2; p = .0006; Table 4). Among the 14 patients with nonmetaplastic TNBC, four patients had SD as their best response, but the duration of disease stability was less than 6 months in all four cases, and therefore the CBR was also 7% (95% CI, 0.2–34%; Table 3). Ten patients with MpBC had SD lasting more than 6 months as their best response, and therefore the CBR in patients with MpBC was 36% (95% CI, 24–49%; Table 3). However, this difference did not reach statistical significance using both univariable (OR, 7.2; 95% CI, 0.9–58.8; p = .066; Table 4) and multivariable logistic regression (OR, 6.2; 95% CI, 0.4–91.9; p = .182; Table 4). On univariable logistic regression, prior systemic therapy for metastatic disease (OR, 0.3; 95% CI, 0.1–0.8; p = .019; Table 4) and worse performance status (OR, 0.1; 95% CI, 0.02–0.3; p = .0001, Table 4) were associated with a lower likelihood of clinical benefit. On multivariable analysis, only poorer performance status retained its statistical significance (OR, 0.02; 95% CI, 0.003–0.2; p = .0003; Table 4).
Table 3. Best response.
The p values are from Fisher's exact test.
One patient was able to obtain insurance approval to get treated with maintenance single agent everolimus closer to home after having SD on DAT for 5.79 months. This patient was counted as having SD ≥6 months. All other patients with a best response of SD but followed for <6 months (n = 3) were considered to have a best response of SD <6 months.
Overall response is best response of CR or PR.
Clinical benefit is best response of CR, PR, or SD ≥6 months.
Abbreviations: CR, complete response; MpBC, metaplastic breast cancer; NE, nonevaluable; PD, progressive disease; PR, partial response; SD, stable disease; TNBC, triple‐negative breast cancer.
Table 4. Effect of baseline prognostic factors on overall response and clinical benefit.
Values in bold indicate statistically significant results, defined as p < 0.02.
Per 10‐year increase in age.
Abbreviations: CI, confidence interval; CR, complete response; DAE, liposomal doxorubicin, bevacizumab, everolimus; DAT, liposomal doxorubicin, bevacizumab, temsirolimus; ECOG, Eastern Cooperative Oncology Group; mTOR, mammalian target of rapamycin; MpBC, metaplastic breast cancer; PR, partial response; SD, stable disease; TNBC, triple‐negative breast cancer.
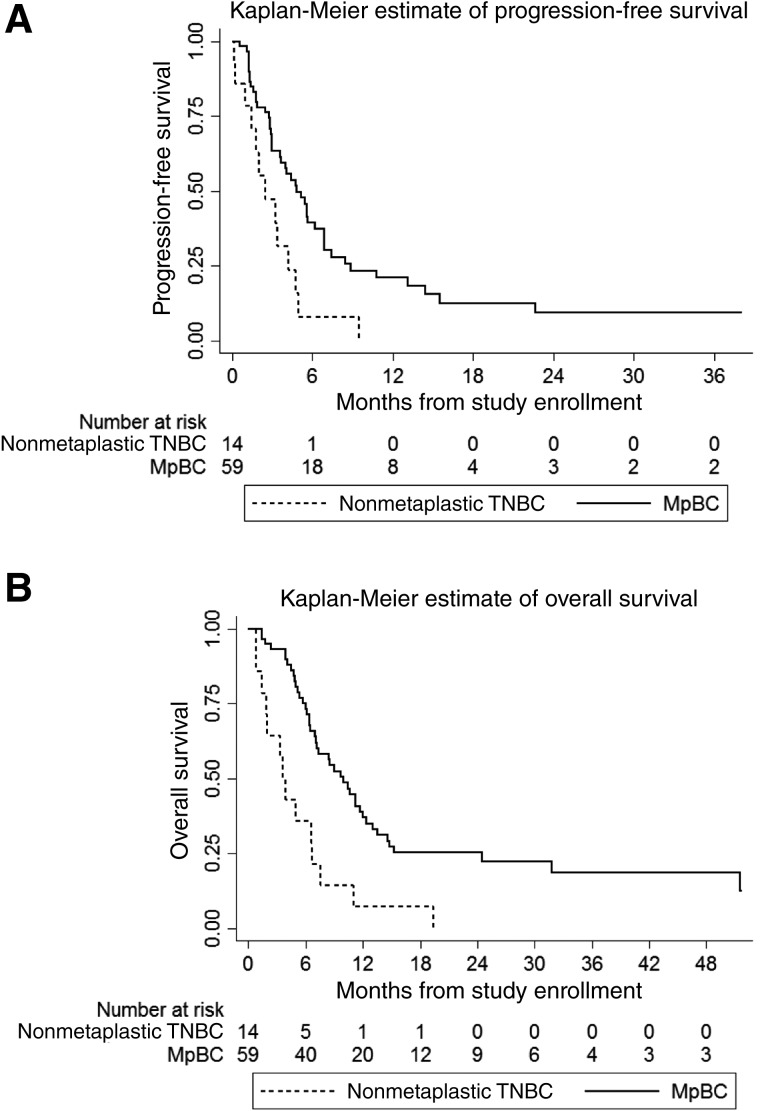
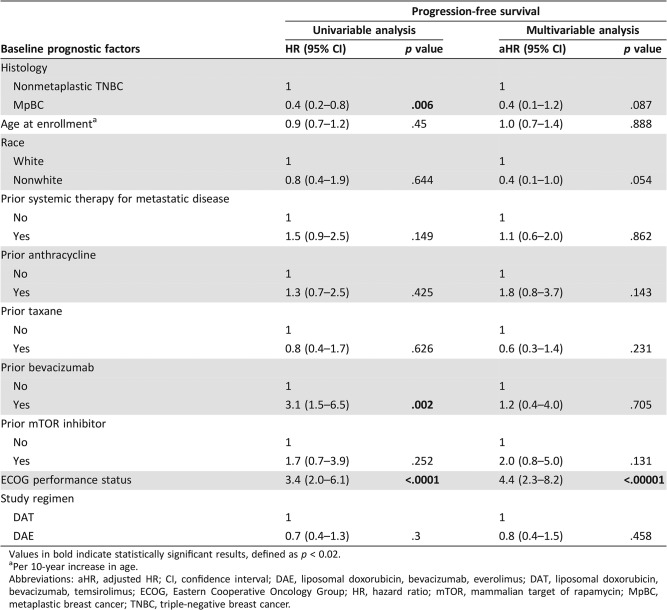
The median follow‐up for all patients was 29.0 months (interquartile range, 21.3–51.6 months). The estimated median PFS of the patients with nonmetaplastic TNBC and the patients with MpBC was 2.5 months (95% CI, 1.0–4.2 months) and 4.8 months (95% CI, 3.0–6.9 months), respectively (Fig. 1A). On univariable analysis, MpBC (HR, 0.4; 95% CI, 0.2–0.8; p = .006; Table 5) was associated with improved PFS, whereas prior bevacizumab therapy (HR, 3.1; 95% CI, 1.5–6.5; p = .002; Table 5) and worse performance status (HR, 3.4; 95% CI, 2.0–6.1; p < .0001) were associated with worse PFS. On multivariable analysis, only worse performance status retained its statistical significance (aHR, 4.4; 95% CI, 2.3–8.2; p < .00001; Table 5).
Figure 1.
Compared with patients with MpBC, patients with nonmetaplastic TNBC had a shorter progression‐free survival (PFS) and overall survival (OS). Kaplan‐Meier plots of PFS (A) and OS (B) are shown, stratified by histologic subtype. Compared with patients with MpBC (n = 59, solid lines) treated with the combination of mTOR inhibition, liposomal doxorubicin and bevacizumab, patients with nonmetaplastic TNBC (n = 14, dashed lines) treated with the same regimen had a poorer PFS (p = .006) and OS (p = .0003) on univariable analysis. On multivariable analysis, the difference in OS between the two groups of patients remained statistically significant (p < .0001), whereas the difference in PFS was no longer statistically significant (p = .087).
Abbreviations: MpBC, metaplastic breast cancer; TNBC, triple‐negative breast cancer.
Table 5. Effect of baseline prognostic factors on progression‐free survival.
Values in bold indicate statistically significant results, defined as p < 0.02.
Per 10‐year increase in age.
Abbreviations: aHR, adjusted HR; CI, confidence interval; DAE, liposomal doxorubicin, bevacizumab, everolimus; DAT, liposomal doxorubicin, bevacizumab, temsirolimus; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; mTOR, mammalian target of rapamycin; MpBC, metaplastic breast cancer; TNBC, triple‐negative breast cancer.
The estimated median OS of the patients with nonmetaplastic TNBC and the patients with MpBC was 3.7 months (95% CI, 1.5–6.7 months) and 10.0 months (95% CI, 6.9–12.0 months), respectively (Fig. 1B). On univariable analysis, MpBC was associated with improved OS (HR, 0.3; 95% CI, 0.2–0.6; p = .0003; Table 6), whereas prior systemic therapy for metastatic disease (HR, 2.2; 95% CI, 1.3–3.8; p = .005; Table 6) and worse performance status (HR, 4.9; 95% CI, 2.5–9.7; p < .00001; Table 6) were associated with worse OS. On multivariable analysis, MpBC (aHR, 0.1; 95% CI, 0.03–0.3; p < .0001; Table 6), nonwhite ethnicity (aHR, 0.2; 95% CI, 0.1–0.5; p = .0006; Table 6), and prior taxane therapy (aHR, 0.3; 95% CI, 0.1–0.7; p = .006; Table 6) were associated with improved OS, whereas prior systemic therapy for metastatic disease (aHR, 2.2; 95% CI, 1.1–4.4; p = .019; Table 6), prior anthracycline therapy (aHR, 2.6; 95% CI, 1.2–5.6; p = .020; Table 6), and worse performance status (aHR, 7.4; 95% CI, 3.6–15.2; p < .0000001; Table 6) were associated with worse OS.
Table 6. Effect of baseline prognostic factors on overall survival.
Values in bold indicate statistically significant results, defined as p < 0.02.
Per 10‐year increase in age.
Abbreviations: aHR, adjusted HR; CI, confidence interval; DAE, liposomal doxorubicin, bevacizumab, everolimus; DAT, liposomal doxorubicin, bevacizumab, temsirolimus; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; mTOR, mammalian target of rapamycin; MpBC, metaplastic breast cancer; TNBC, triple‐negative breast cancer.
Discussion
Although modern molecular profiling has identified subtypes of TNBC with different targetable molecular aberrations [22], there are limited data demonstrating that subtyping TNBC will improve clinical outcomes. Additionally, the simultaneous activation of multiple signaling pathways in a tumor may explain low clinical response rates when single‐agent targeted therapeutic strategies are employed, and combining multiple agents may help overcome this therapeutic barrier.
In our study, patients with advanced MpBC treated with the combination of liposomal doxorubicin, bevacizumab, and mTOR inhibition had better OS compared with patients with nonmetaplastic or “unselected” TNBC patients treated with the same regimen on both univariable and multivariable analysis. On univariable analysis, patients with MpBC also had better PFS compared with patients with nonmetaplastic TNBC. However, the difference in PFS between the two groups was not statistically significant on multivariable analysis. In addition, although our data suggested that patients with MpBC had better ORR and CBR rates compared with patients with nonmetaplastic TNBC, these differences were not statistically significant on both univariable and multivariable analyses. Furthermore, the trend towards lower ORR and CBR in patients with nonmetaplastic TNBC compared with patients with MpBC could potentially be explained by differences in baseline characteristics between the two groups, including higher rates of prior systemic therapy in the metastatic setting and prior bevacizumab use in the 14 patients with nonmetaplastic TNBC.
Several reasons could potentially explain the better OS outcomes observed in patients with MpBC. First, MpBC could be inherently more sensitive to mTOR inhibition because of hyper‐activation of PI3K/Akt/mTOR pathway components [11]. Notably, MpBCs have gene expression profiles resembling those of cancer stem cells (CSCs), in which increased activity of the PI3K pathway features prominently [11], [17], [23], [24], [25], [26], [27]. Additionally, preclinical data have suggested that breast CSC survival and tumorigenesis can be inhibited by administration of rapamycin [17], [26], [27]. Furthermore, MpBCs are commonly associated with PI3KCA mutations and loss of PTEN [11], which could also account for increased sensitivity to mTOR inhibitors. Second, the use of bevacizumab, a vascular endothelial growth factor (VEGF) inhibitor, in this novel combination could explain in part its higher efficacy in MpBCs, which are characterized by increased levels of angiogenesis and expression of VEGF as well as hypoxia‐inducible factor 1 (HIF‐1) [24], [25]. Additionally, in vitro studies have shown that inhibition of mTOR leads to decreased levels of HIF‐1 and VEGF [28], [29], [30], [31], which could potentially enhance the efficacy of bevacizumab when used in combination with mTOR inhibition. However, it is important to note that bevacizumab has not been shown to be more effective in patients with increased levels of angiogenesis markers, and our inferences regarding the utility of bevacizumab in MpBC remain hypothesis generating.
It could also be argued that factors other than appropriate selection of targeted therapy influenced patient outcomes in this study. Although survival data for MpBC are limited because of its rarity, this subtype of breast cancer has been associated with worse survival compared with TNBC [32] or cancers with ductal or lobular histologies [33], suggesting that the MpBC is, in general, more aggressive than nonmetaplastic TNBC, making it highly unlikely that the better PFS and OS observed in the MpBC cohort is the result of a more indolent biology in MpBC. Additionally, it is notable that prior bevacizumab use was significantly higher in the nonmetaplastic TNBC cohort and associated with poorer PFS on univariable analysis. Because bevacizumab was one of the three drugs used in this study, the development of compensatory pathways and resistance in patients previously treated with bevacizumab [34] could arguably be the cause of poorer survival outcomes in nonmetaplastic TNBC. However, prior bevacizumab use was neither an independent predictor of PFS on multivariable analysis nor significantly associated with OS outcomes, consistent with most published trials in which the addition of bevacizumab to standard chemotherapy has not been shown to impact OS in breast cancer [35], [36], [37].
Although multivariable logistic regression did not reveal any statistically significant differences in Eastern Cooperative Oncology Group (ECOG) performance status or the use of prior systemic therapy in the metastatic setting between the MpBC and nonmetaplastic TNBC cohorts, poorer ECOG performance status and the use of prior systemic therapy in the metastatic setting independently predicted worse OS on multivariable analysis. In addition, poorer ECOG performance status was also an independent predictor of worse PFS on multivariable analysis. These findings are consistent with prior publications, suggesting that the patients studied displayed similar patterns of survival seen in advanced breast cancer. As previously published, the ECOG performance status predicts the efficacy of systemic therapy, as patients with poorer functional status are less likely to adhere to therapy and more likely to require dose interruptions or reductions while on therapy [38], [39], [40], [41]. The observed association between prior systemic therapy for metastatic disease and poorer outcomes is also consistent with other studies evaluating systemic therapy in metastatic breast cancer [42], [43], [44] and is thought to be due to development of increasing resistance from sequential exposure to cytotoxic agents [45]. In support of this, a pooled analysis of four prospective trials of liposomal doxorubicin in patients with metastatic breast cancer found that patients who were less heavily pretreated had a higher CBR on univariable analysis but not on multivariable analysis [44], consistent with the findings from our study.
In this study, prior anthracycline use was found to be an independent predictor of poorer OS on multivariable analysis. We hypothesize that this finding is a reflection of anthracycline resistance and reduced efficacy of liposomal doxorubicin in this patient population. Interestingly, prior taxane use and nonwhite ethnicity were independently associated with improved OS in this study on multivariable but not univariable analyses. The significance of these observations is unclear and needs to be evaluated in future studies with larger patient cohorts.
Our study has a few limitations. First, there were several differences in baseline characteristics between patients with MpBC and nonmetaplastic TNBC. However, through multivariable modeling, we adjusted for all known study covariates to account for these baseline differences, and metaplastic histology remained a statistically significant predictor of improved OS. Second, our relatively small sample size, in particular the limited number of patients with nonmetaplastic TNBC in this study, may have restricted our ability to detect statistically significant differences in ORR or CBR. In addition, the observation that metaplastic histology was predictive of improved PFS on univariable but not multivariable analysis could also be due to our limited sample size. Third, the lack of a contemporary control group of patients with MpBC treated with alternative regimens limited our ability to draw conclusions on the relative benefit of this regimen in MpBC. Fourth, as all patients were treated with the combination of DAT or DAE, it was not possible to determine the relative contribution of each agent to efficacy in this study. However, to the best of our knowledge, our study is the largest series of patients with MpBC and nonmetaplastic TNBC treated with a targeted therapy regimen. Furthermore, despite the small sample size, we observed a statistically significant difference in OS between patients with MpBC and those with nonmetaplastic TNBC, which is intriguing, considering the aggressive clinical course and shortened OS associated with advanced MpBC.
Conclusion
The underlying molecular heterogeneity of TNBC has in part limited the success of targeted therapies [22], and although small numbers limit the strength of inferences that can be drawn from this comparison, our results suggest that metaplastic histology is associated with improved OS as compared with “unselected” TNBC in patients with advanced disease receiving mTOR‐based systemic therapy regimens. Additionally, in the absence of data from prospective randomized trials in MpBC, our results are among the best reported for patients with advanced MpBC. Because metaplastic histology has been associated with distinct mesenchymal molecular signatures [11], these interesting observations support the concept of molecular subtyping in TNBC for the purposes of clinical trial design. However, the utility of molecular subtyping needs to be confirmed in larger, prospective studies before becoming standard of care for patient management. The overlap and similarities between MpBC and the mesenchymal subtypes of TNBC also support testing agents targeting the PI3K/Akt/mTOR pathway in clinical trials for these subtypes of TNBC.
Acknowledgments
The authors acknowledge the support of the Triple‐Negative Breast Cancer Foundation and the Conquer Cancer Foundation of the American Society of Clinical Oncology (to R.K.B), the Allison and Brian Grove Fellowship for Breast Medical Oncology (to C.Y.), the Susan Papizan Dolan Fellowship in Breast Oncology (to C.Y.), and the numerous private donors who contribute to research in metaplastic and triple‐negative breast cancer at The University of Texas MD Anderson Cancer Center. The authors also wish to thank John M. Stewart, M.D., for his assistance in processing archival tumor specimens for this study.
G.N.H. was the principal investigator of the BOLERO‐2 trial and received research funding from Novartis for conducting the trial and honoraria for chairing the protocol steering committee.
Contributed equally
Author Contributions
Conception/design: Clinton Yam, Filip Janku, Stacy L. Moulder
Provision of study material or patients: Michael Gilcrease, Rashmi K. Murthy, Daniel D. Karp, Funda Meric‐Bernstam, Vicente Valero, Constance Albarracin, Jennifer K. Litton, Mariana Chavez‐MacGregor, David Hong, Razelle Kurzrock, Gabriel N. Hortobagyi, Filip Janku, Stacy L. Moulder
Collection and/or assembly of data: Reva K. Basho, Clinton Yam, Thorunn Helgason, Stacy L. Moulder
Data analysis and interpretation: Clinton Yam, Kenneth R. Hess, Stacy L. Moulder
Manuscript writing: Reva K. Basho, Clinton Yam, Michael Gilcrease, Rashmi K. Murthy, Thorunn Helgason, Daniel D. Karp, Funda Meric‐Bernstam, Kenneth R. Hess, Vicente Valero, Constance Albarracin, Jennifer K. Litton, Mariana Chavez‐MacGregor, David Hong, Razelle Kurzrock, Gabriel N. Hortobagyi, Filip Janku, Stacy L. Moulder
Final approval of manuscript: Reva K. Basho, Clinton Yam, Michael Gilcrease, Rashmi K. Murthy, Thorunn Helgason, Daniel D. Karp, Funda Meric‐Bernstam, Kenneth R. Hess, Vicente Valero, Constance Albarracin, Jennifer K. Litton, Mariana Chavez‐MacGregor, David Hong, Razelle Kurzrock, Gabriel N. Hortobagyi, Filip Janku, Stacy L. Moulder
Disclosures
Gabriel N. Hortobagyi: Novartis (RF, H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Tse GM, Tan PH, Putti TC et al. Metaplastic carcinoma of the breast: A clinicopathological review. J Clin Pathol 2006;59:1079–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wargotz ES, Norris HJ. Metaplastic carcinomas of the breast. I. Matrix‐producing carcinoma. Hum Pathol 1989;20:628–635. [DOI] [PubMed] [Google Scholar]
- 3.Wargotz ES, Deos PH, Norris HJ. Metaplastic carcinomas of the breast. II. Spindle cell carcinoma. Hum Pathol 1989;20:732–740. [DOI] [PubMed] [Google Scholar]
- 4.Wargotz ES, Norris HJ. Metaplastic carcinomas of the breast. III. Carcinosarcoma. Cancer 1989;64:1490–1499. [DOI] [PubMed] [Google Scholar]
- 5.Wargotz ES, Norris HJ. Metaplastic carcinomas of the breast. IV. Squamous cell carcinoma of ductal origin. Cancer 1990;65:272–276. [DOI] [PubMed] [Google Scholar]
- 6.Wargotz ES, Norris HJ. Metaplastic carcinomas of the breast: V. Metaplastic carcinoma with osteoclastic giant cells. Hum Pathol 1990;21:1142–1150. [DOI] [PubMed] [Google Scholar]
- 7.Abouharb S, Moulder S. Metaplastic breast cancer: Clinical overview and molecular aberrations for potential targeted therapy. Curr Oncol Rep 2015;17:431. [DOI] [PubMed] [Google Scholar]
- 8.Rayson D, Adjei AA, Suman VJ, et al. Metaplastic breast cancer: Prognosis and response to systemic therapy. Ann Oncol 1999;10:413–419. [DOI] [PubMed] [Google Scholar]
- 9.Jung SY, Kim HY, Nam BH et al. Worse prognosis of metaplastic breast cancer patients than other patients with triple‐negative breast cancer. Breast Cancer Res Treat 2010;120:627–637. [DOI] [PubMed] [Google Scholar]
- 10.Lehmann BD, Bauer JA, Chen X et al. Identification of human triple‐negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011;121:2750–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hennessy BT, Gonzalez‐Angulo AM, Stemke‐Hale K et al. Characterization of a naturally occurring breast cancer subset enriched in epithelial‐to‐mesenchymal transition and stem cell characteristics. Cancer Res 2009;69:4116–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liedtke C, Mazouni C, Hess KR et al. Response to neoadjuvant therapy and long‐term survival in patients with triple‐negative breast cancer. J Clin Oncol 2008;26:1275–1281. [DOI] [PubMed] [Google Scholar]
- 13.Masuda H, Baggerly KA, Wang Y et al. Differential response to neoadjuvant chemotherapy among 7 triple‐negative breast cancer molecular subtypes. Clin Cancer Res 2013;19:5533–5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basho RK, Gilcrease M, Murthy RK et al. Targeting the PI3k/Akt/mTOR pathway for the treatment of mesenchymal triple‐negative breast cancer: Evidence from a phase 1 trial of mTOR inhibition in combination with liposomal doxorubicin and bevacizumab. JAMA Oncol 2017;3:509–515. [DOI] [PubMed] [Google Scholar]
- 15.Moroney J, Fu S, Moulder S et al. Phase I study of the antiangiogenic antibody bevacizumab and the mTOR/hypoxia‐inducible factor inhibitor temsirolimus combined with liposomal doxorubicin: Tolerance and biological activity. Clin Cancer Res 2012;18:5796–5805. [DOI] [PubMed] [Google Scholar]
- 16.Moulder S, Helgason T, Janku F et al. Inhibition of the phosphoinositide 3‐kinase pathway for the treatment of patients with metastatic metaplastic breast cancer. Ann Oncol 2015;26:1346–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moulder S, Moroney J, Helgason T et al. Responses to liposomal doxorubicin, bevacizumab, and temsirolimus in metaplastic carcinoma of the breast: Biologic rationale and implications for stem‐cell research in breast cancer. J Clin Oncol 2011;29:e572–e575. [DOI] [PubMed] [Google Scholar]
- 18.Moroney JW, Schlumbrecht MP, Helgason T et al. A phase I trial of liposomal doxorubicin, bevacizumab, and temsirolimus in patients with advanced gynecologic and breast malignancies. Clin Cancer Res 2011;17:6840–6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinn HP, Kreipe H. A brief overview of the WHO Classification of Breast Tumors, 4th edition, focusing on issues and updates from the 3rd edition. Breast Care (Basel) 2013;8:149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolff AC, Hammond ME, Hicks DG et al. Recommendations for human epidermal growth factor. receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997–4013. [DOI] [PubMed] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST guideline (version 1.1). Eur J. Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 22.Yam C, Mani SA, Moulder SL. Targeting the molecular subtypes of triple negative breast cancer: Understanding the diversity to progress the field. The Oncologist 2017;22:1086–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Creighton CJ, Li X, Landis M et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor‐initiating features. Proc Natl Acad Sci USA 2009;106:13820–13825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellino R, Arisio R, D'Addato F et al. Metaplastic breast carcinoma: Pathology and clinical outcome. Anticancer Res 2003;23:669–673. [PubMed] [Google Scholar]
- 25.Kochhar R, Howard EM, Umbreit JN et al. Metaplastic breast carcinoma with squamous differentiation: Molecular and clinical analysis of six cases. Breast J 2005;11:367–369. [DOI] [PubMed] [Google Scholar]
- 26.Zhou J, Wulfkuhle J, Zhang H et al. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem‐like cells is required for viability and maintenance. Proc Natl Acad Sci USA 2007;104:16158–16163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mungamuri SK, Yang X, Thor AD et al. Survival signaling by Notch1: Mammalian target of rapamycin (mTOR)‐dependent inhibition of p 53. Cancer Res 2006;66:4715–4724. [DOI] [PubMed] [Google Scholar]
- 28.Pantuck AJ, Zeng G, Belldegrun AS et al. Pathobiology, prognosis, and targeted therapy for renal cell carcinoma: Exploiting the hypoxia‐induced pathway. Clin Cancer Res 2003;9:4641–4652. [PubMed] [Google Scholar]
- 29.Del Bufalo D, Ciuffreda L, Trisciuoglio D et al. Antiangiogenic potential of the mammalian target of rapamycin inhibitor temsirolimus. Cancer Res 2006;66:5549–5554. [DOI] [PubMed] [Google Scholar]
- 30.Huynh H, Teo CC, Soo KC. Bevacizumab and rapamycin inhibit tumor growth in peritoneal model of human ovarian cancer. Mol Cancer Ther 2007;6:2959–2966. [DOI] [PubMed] [Google Scholar]
- 31.Huynh H, Chow PK, Palanisamy N et al. Bevacizumab and rapamycin induce growth suppression in mouse models of hepatocellular carcinoma. J Hepatol 2008;49:52–60. [DOI] [PubMed] [Google Scholar]
- 32.Bae SY, Lee SK, Koo MY et al. The prognoses of metaplastic breast cancer patients compared to those of triple‐negative breast cancer patients. Breast Cancer Res Treat 2011;126:471–478. [DOI] [PubMed] [Google Scholar]
- 33.Okada N, Hasebe T, Iwasaki M et al. Metaplastic carcinoma of the breast. Hum Pathol 2010;41:960–970. [DOI] [PubMed] [Google Scholar]
- 34.Bergers G, Hanahan D. Modes of resistance to anti‐angiogenic therapy. Nat Rev Cancer 2008;8:592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bell R, Brown J, Parmar M et al. Final efficacy and updated safety results of the randomized phase III BEATRICE trial evaluating adjuvant bevacizumab‐containing therapy in triple‐negative early breast cancer. Ann Oncol 2017;28:754–760. [DOI] [PubMed] [Google Scholar]
- 36.Li Q, Yan H, Zhao P et al. Efficacy and safety of bevacizumab combined with chemotherapy for managing metastatic breast cancer: A meta‐analysis of randomized controlled trials. Sci Rep 2015;5:15746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumler I, Christiansen OG, Nielsen DL. A systematic review of bevacizumab efficacy in breast cancer. Cancer Treat Rev 2014;40:960–973. [DOI] [PubMed] [Google Scholar]
- 38.Nash CH 3rd, Jones SE, Moon TE et al. Prediction of outcome in metastatic breast cancer treated with adriamycin combination chemotherapy. Cancer 1980;46:2380–2388. [DOI] [PubMed] [Google Scholar]
- 39.Sjostrom J, Blomqvist C. Predictive factors for response to cytotoxic treatment in advanced breast cancer: A review. Acta Oncol 1996;35 (suppl 5):84–90. [DOI] [PubMed] [Google Scholar]
- 40.Robain M, Pierga JY, Jouve M et al. Predictive factors of response to first‐line chemotherapy in 1426 women with metastatic breast cancer. Eur J. Cancer 2000;36:2301–2312. [DOI] [PubMed] [Google Scholar]
- 41.Yates JW, Chalmer B, McKegney FP. Evaluation of patients with advanced cancer using the Karnofsky performance status. Cancer 1980;45:2220–2224. [DOI] [PubMed] [Google Scholar]
- 42.Aogi K, Iwata H, Masuda N et al. A phase II study of eribulin in Japanese patients with heavily pretreated metastatic breast cancer. Ann Oncol 2012;23:1441–1448. [DOI] [PubMed] [Google Scholar]
- 43.Maeda S, Saimura M, Minami S et al. Efficacy and safety of eribulin as first‐ to third‐line treatment in patients with advanced or metastatic breast cancer previously treated with anthracyclines and taxanes. Breast 2017;32:66–72. [DOI] [PubMed] [Google Scholar]
- 44.Al‐Batran SE, Guntner M, Pauligk C et al. Anthracycline rechallenge using pegylated liposomal doxorubicin in patients with metastatic breast cancer: A pooled analysis using individual data from four prospective trials. Br J. Cancer 2010;103:1518–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Persidis A. Cancer multidrug resistance. Nat Biotechnol 1999;17:94–95. [DOI] [PubMed] [Google Scholar]