In 2017, the Food and Drug Administration granted accelerated approval of blinatumomab for the treatment of relapsed or refractory precursor B‐cell acute lymphoblastic leukemia. This article focuses on evidence to support conversion from accelerated to regular approval of blinatumomab.
Keywords: Acute lymphoblastic leukemia, Blinatumomab, Philadelphia chromosome, Drug approval
Abstract
On July 11, 2017, the Food and Drug Administration granted approval for blinatumomab for the treatment of relapsed or refractory (R/R) precursor B‐cell acute lymphoblastic leukemia (ALL). Blinatumomab is a bispecific CD19‐directed CD3 T‐cell engager. The basis for the approval included results from two clinical trials, TOWER and ALCANTARA. TOWER, a randomized trial comparing overall survival in patients with Philadelphia chromosome (Ph)‐negative R/R ALL receiving blinatumomab versus standard‐of‐care (SOC) chemotherapy, demonstrated a hazard ratio of 0.71 favoring blinatumomab (p = .012; median survival, 7.7 months with blinatumomab and 4.0 months with SOC chemotherapy). Complete remission (CR) rates were 34% for patients receiving blinatumomab and 16% for those receiving SOC. Adverse events were consistent with those observed in prior trials, with cytokine release syndrome and some neurologic events, including tremor, encephalopathy, peripheral neuropathy, and depression, observed more frequently in the blinatumomab arm, whereas neutropenia and infection were less common among patients receiving blinatumomab. Depression emerged as a rare but potentially severe neurologic event associated with blinatumomab. In ALCANTARA, a single‐arm trial of blinatumomab in patients with Ph‐positive R/R ALL, the CR rate was 31%, and adverse events were similar to those observed previously in Ph‐negative R/R ALL. These results support conversion from accelerated to regular approval of blinatumomab for R/R ALL and broadening of the intended population to include both Ph‐positive and Ph‐negative precursor B‐cell R/R ALL.
Implications for Practice.
In TOWER, a randomized trial in patients with relapsed or refractory Philadelphia chromosome (Ph)‐negative precursor B‐cell acute lymphoblastic leukemia (ALL), treatment with blinatumomab showed superiority over conventional chemotherapy for complete remission (CR) rate (34% vs. 16%) and survival (3.7‐month improvement in median; hazard ratio, 0.71). In ALCANTARA, a single‐arm trial of blinatumomab for treatment of relapsed or refractory Ph‐positive precursor B‐cell ALL, the CR rate was 31%. Blinatumomab is now approved for treatment of relapsed or refractory precursor B‐cell ALL that is Ph positive or Ph negative.
Introduction
Blinatumomab is a bispecific CD19‐directed CD3 T‐cell engager that mediates formation of a synapse between the CD3+ T cell and the CD19+ target cell, resulting in upregulation of cell adhesion molecules, production of cytolytic proteins, and release of cytokines [1]. The U.S. Food and Drug Administration (FDA) granted blinatumomab accelerated approval for treatment of Philadelphia chromosome (Ph)‐negative relapsed or refractory (R/R) precursor B‐cell acute lymphoblastic leukemia (ALL) in 2014 based on complete remission (CR) rate, duration of CR, and the proportion of patients with a minimal residual disease (MRD)‐negative CR or CR with partial hematologic recovery (CRh) within two cycles of treatment in a single‐arm study (NCT01466179) [2]. Cytokine release syndrome was reported initially in 11% of patients, but it was rarely life threatening or fatal. About half of the patients had a neurologic event, including encephalopathy and seizures. Additional common adverse reactions identified in the initial experience included pyrexia, headache, peripheral edema, febrile neutropenia, nausea, hypokalemia, tremor, rash, and constipation [3].
The accelerated approval in 2014 was contingent on demonstrating an overall survival (OS) benefit for blinatumomab versus standard‐of‐care (SOC) chemotherapy in a phase III randomized clinical trial (TOWER) in patients with R/R Ph‐negative ALL. An additional single‐arm trial (ALCANTARA) was conducted to establish the activity of blinatumomab for treatment of R/R Ph‐positive ALL. Herein we provide a summary of the FDA review of the marketing application for conversion to regular approval of blinatumomab for the treatment of R/R precursor B‐cell ALL.
Assessment of the TOWER Trial
Design
TOWER (NCT02013167) [4] was a phase III, randomized, open‐label, multicenter trial comparing blinatumomab with conventional chemotherapy in patients with R/R Ph‐negative ALL [4]. Eligible patients were adults with primary refractory disease who had a first relapse with first remission with a duration of ≤12 months, second or greater relapse, or relapse at any time after allogeneic hematopoietic stem cell transplantation (HSCT). Patients were randomized 2:1 to either single‐agent blinatumomab or one of four SOC chemotherapy options.
Blinatumomab was administered at 9 mcg per day on days 1–7 and 28 mcg per day on days 8–28 for Cycle 1, and 28 mcg per day on days 1–28 for Cycles 2–5 in 42‐day cycles and for Cycles 6–9 in 84‐day cycles. Dose adjustment was possible in case of adverse events. SOC chemotherapy included combinations based on fludarabine, cytarabine arabinoside, and granulocyte colony‐stimulating factor; high‐dose cytarabine arabinoside alone or in combination regimens; high‐dose methotrexate‐based combinations; or clofarabine alone or in combination regimens. Off‐treatment criteria included toxicity, failure to achieve a marrow response within two cycles, relapse after CR/CRh or CR with incomplete hematologic recovery (CRi), and intent to receive other treatment, including allogeneic HSCT.
The primary endpoint was OS. Additional prespecified endpoints used in the assessment of efficacy included CR within 12 weeks of treatment initiation and MRD‐negative CR/CRh. MRD was assessed in patients who achieved CR, CRh, or CRi by flow cytometry or polymerase chain reactions using assays with sensitivities ≤0.01%. An MRD response was defined as an MRD level <0.01%.
Demographics and Treatment Characteristics
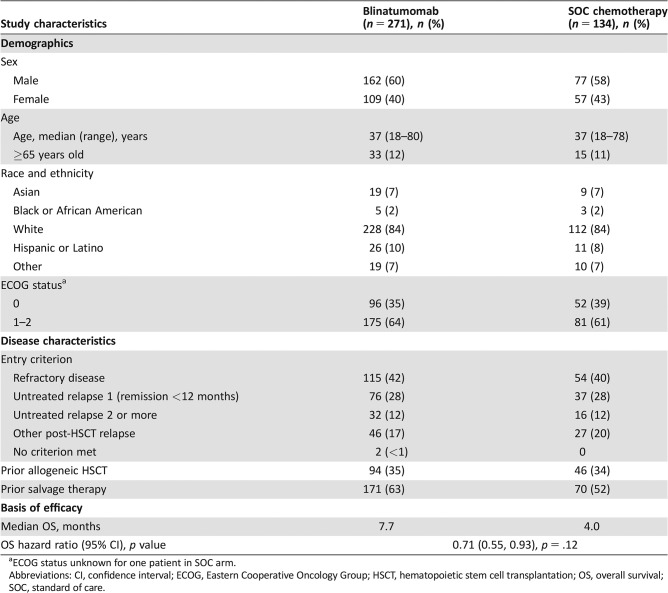
Baseline demographic characteristics of the randomized patients are presented in Table 1. A total of 405 patients, 271 in the blinatumomab arm and 134 in the SOC arm, were included in the intent‐to‐treat population, of whom 376 patients (267 blinatumomab, 109 SOC) received at least one dose of the assigned medication and were included in the safety population. The arms were well balanced with respect to demographics and disease characteristics, including the stratification factors (age <35 vs. ≥35, prior allogeneic HSCT, and prior salvage therapy; Table 1). The median number of cycles given differed between the two arms, being two cycles (range, one to nine cycles) for blinatumomab and one cycle (range, one to four cycles) for SOC. Fifty (18%) patients in the blinatumomab arm and 18 (13%) patients in the SOC arm underwent allogeneic HSCT after treatment initiation.
Table 1. The TOWER study.
ECOG status unknown for one patient in SOC arm.
Abbreviations: CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HSCT, hematopoietic stem cell transplantation; OS, overall survival; SOC, standard of care.
Efficacy Results
The determination of efficacy was based on OS (Table 1). In TOWER, OS in the blinatumomab arm was superior to that in the control (hazard ratio 0.71; 95% confidence interval [CI], 0.55–0.93). Median OS for patients on the blinatumomab arm was 7.7 months (95% CI, 5.6–9.6) versus 4.0 months (95% CI, 2.9–5.3) in the SOC arm. No outliers were observed across subgroups with respect to treatment effect on OS.
A significantly greater percentage of patients on the blinatumomab arm achieved a CR within 12 weeks of treatment initiation, with 91 (34%; 95% CI, 28–40) patients on the blinatumomab arm achieving CR compared with 21 (16%; 95% CI, 10–23) patients on the SOC arm. CRh was achieved by an additional 24 patients in the blinatumomab arm and 6 patients in the SOC arm. MRD‐negative CR/CRh was achieved by 73 (27%; 95% CI, 22–32) patients in the blinatumomab arm versus 14 (10%; 95% CI, 5–16) in the SOC arm.
Safety
The most common adverse reactions (≥20%) in the blinatumomab arm in TOWER were infections, pyrexia, headache, infusion‐related reactions, anemia, febrile neutropenia, thrombocytopenia, and neutropenia. Serious adverse reactions were reported in 62% of patients. The most common serious adverse reactions (≥2%) included febrile neutropenia, pyrexia, sepsis, pneumonia, overdose, septic shock, cytokine release syndrome, bacterial sepsis, device‐related infection, and bacteremia. Adverse reactions of grade 3 or higher were reported in 87% of patients. Discontinuation of therapy because of adverse reactions occurred in 12% of patients treated with blinatumomab; neurologic events and infections were the most frequently reported reasons for discontinuation of treatment because of an adverse reaction. Fatal adverse events occurred in 16% of patients. The majority of fatal events were infections.
Because of differences in the median number of cycles given in each arm of TOWER, comparisons between arms for adverse events were limited to the first cycle only (42 days). The incidences of major safety events were similar between the blinatumomab arm and the SOC arm, including serious adverse events (46% vs. 39%), fatal adverse events (14% vs. 15%), and all‐cause mortality at day 29 (11% vs. 14%) and day 60 (24% vs. 27%). Of note, however, for patients who proceeded to HSCT without additional intervening therapy after blinatumomab, mortality 100 days after HSCT was numerically higher in the blinatumomab arm at 12% versus 0% in the SOC arm. No single cause of death predominated among these patients, with both progressive disease and complications of transplant being observed as causes of death.
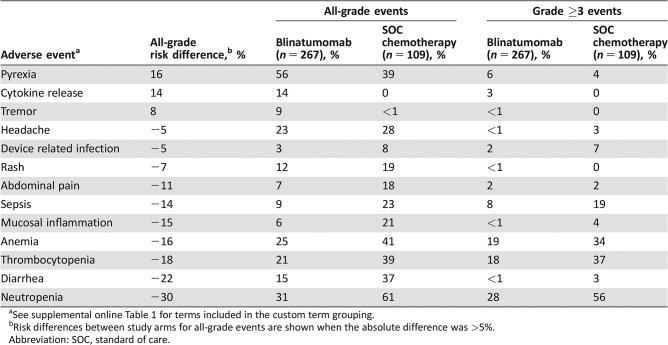
Table 2 shows the adverse events in the first cycle that differed between arms by at least 5%; the events are order in decreasing difference in incidence between arms for all‐grade events. Patients receiving blinatumomab experienced substantially more pyrexia, cytokine release syndrome, and tremor than those treated with SOC, but substantially less cytopenias, diarrhea, mucositis, and sepsis (Table 2). In the shift analysis of laboratory abnormalities, fewer patients receiving blinatumomab worsened from baseline grade 0–2 to maximal grade 3–4 for thrombocytopenia (47% vs. 85%), anemia (29% vs. 43%), and neutropenia (57% vs. 68%) than those treated with SOC, but the patients on the blinatumomab arm had a higher shift for increased aspartate aminotransferase (8% vs. 4%) with little difference for bilirubin (5% vs. 4%).
Table 2. The TOWER study: comparison of cycle 1 treatment‐emergent adverse events.
See supplemental online Table 1 for terms included in the custom term grouping.
Risk differences between study arms for all‐grade events are shown when the absolute difference was >5%.
Abbreviation: SOC, standard of care.
Because of evidence of neurotoxicity identified in earlier studies of blinatumomab, a more detailed comparison of neurologic events in TOWER was performed. Overall, slightly more patients in the blinatumomab arm experienced neurologic or psychiatric adverse events (51% vs. 47%) in the first cycle. The difference between arms varied for specific type of neuropsychiatric disorders, with tremor (9% vs. 0%), peripheral neuropathy (7% vs. 1%), encephalopathy (11% vs. 6%), and depression (3% vs. 0%) being more common in the blinatumomab arm, whereas headache (23% vs. 28%) and seizure (2% vs. 5%) were more common in the SOC arm.
Assessment of the ALCANTARA Trial
ALCANTARA (NCT02000427) was a multicenter, single‐arm trial examining the complete remission rate in patients with R/R Ph‐positive precursor B‐cell ALL [5]. Patients were eligible if they had relapsed or were refractory to at least one second‐generation tyrosine kinase inhibitor (TKI) or were intolerant to second‐generation TKIs and refractory to imatinib. Blinatumomab was administered at 9 mcg per day on days 1–7 and 28 mcg per day on days 8–28 for Cycle 1, and 28 mcg per day on days 1–28 for subsequent cycles. Dose adjustment was possible in case of adverse events. The finding of efficacy was based on the CR rate, duration of CR, and proportion of patients with an MRD‐negative CR/CRh within two cycles of treatment.
The study population included 45 patients who received at least one infusion of blinatumomab. The median number of treatment cycles was two (range, one to five). The demographics and baseline characteristics are shown in Table 3. Fourteen patients (31%) achieved a CR, and the median duration of CR was 6.7 months. Overall, fourteen patients (31%) achieved MRD‐negative CR/CRh. Treatment‐emergent adverse events (TEAEs) were similar to those previously observed in patients with Ph‐negative ALL, with pyrexia being the most common TEAE and cytokine release syndrome and neurologic events being notable serious adverse events.
Table 3. The ALCANTARA study (n = 45).
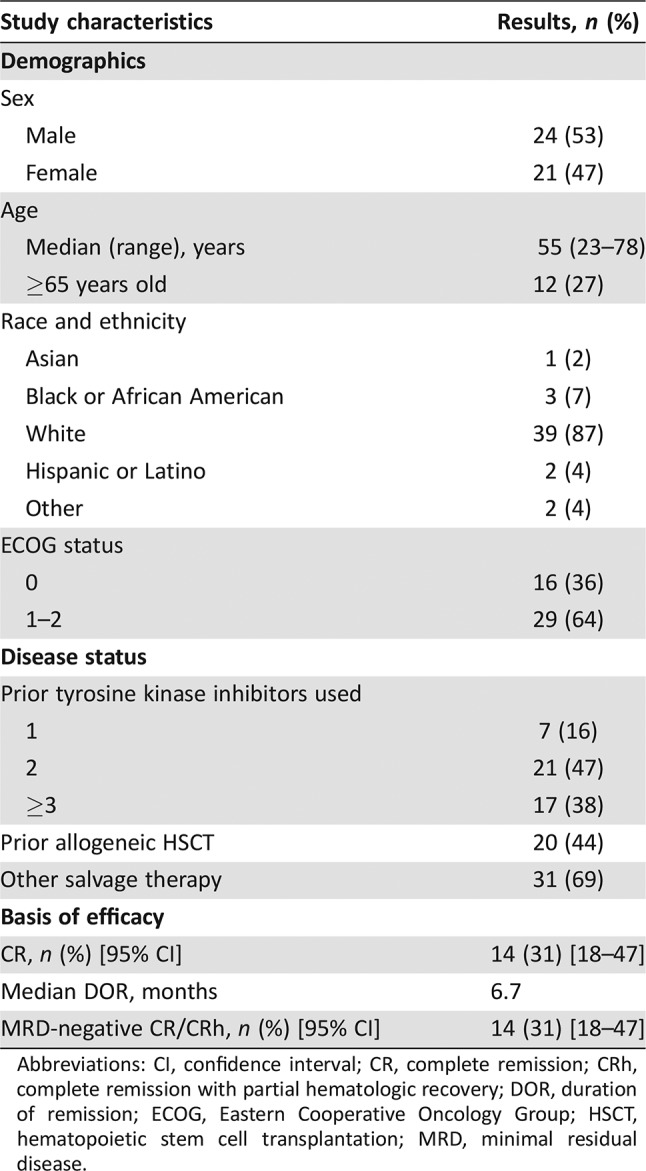
Abbreviations: CI, confidence interval; CR, complete remission; CRh, complete remission with partial hematologic recovery; DOR, duration of remission; ECOG, Eastern Cooperative Oncology Group; HSCT, hematopoietic stem cell transplantation; MRD, minimal residual disease.
Integrated Assessment of Efficacy
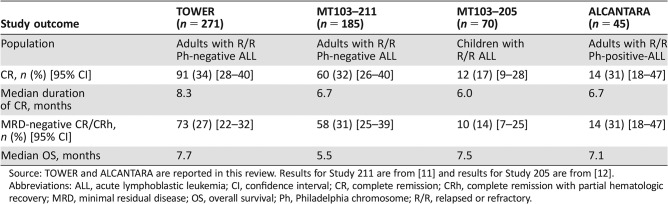
There were two trials of blinatumomab for treatment of R/R ALL in addition to TOWER and ALCANTARA. Study MT103–211 (NCT01466179) was a single‐arm multicenter trial in adults with Ph‐negative R/R ALL [2], and Study MT103–205 (NCT01471782) was a single‐arm multicenter trial in pediatric patients with Ph‐positive or Ph‐negative R/R ALL [5]. Table 4 shows a side‐by‐side listing of outcomes by study. Despite the variability in the study populations with regard to prognostic factors, the rates of achievement of CR and MRD‐negative CR are consistent across the studies. MT103–205, the pediatric study, had the lowest CR rate, but this protocol accrued largely patients in relapse after allogeneic HSCT or with refractory disease, factors known to be associated with poorer prognosis [6]. Nonetheless, for those who achieved CR, the durability of CR and median OS were similar to those seen in the adult trials.
Table 4. Outcomes in studies of blinatumomab for R/R ALL.
Source: TOWER and ALCANTARA are reported in this review. Results for Study 211 are from [11] and results for Study 205 are from [12].
Abbreviations: ALL, acute lymphoblastic leukemia; CI, confidence interval; CR, complete remission; CRh, complete remission with partial hematologic recovery; MRD, minimal residual disease; OS, overall survival; Ph, Philadelphia chromosome; R/R, relapsed or refractory.
It was notable that the duration of CR was longest in TOWER, which was also the only protocol that allowed for up to five cycles for induction and consolidation followed by four additional cycles of continued therapy. Although there was no randomized trial to assess the benefit of the four additional cycles of continued therapy as used in TOWER, the safety of the additional therapy was acceptable. Hence, the recommended dosing was revised to include up to a total of nine cycles of blinatumomab.
The TOWER trial confirms that the CR benefit observed in earlier studies translated to an OS benefit. A consistent pattern of benefit was observed in patients receiving blinatumomab across four clinical trials. These results demonstrate that blinatumomab is effective in both Ph‐positive and Ph‐negative R/R ALL and supported regular approval of blinatumomab and broadening the indication.
Discussion
Treatment for patients with relapsed and refractory ALL continues to be suboptimal, with poor outcomes and high rates of toxicity, including life‐threatening toxicities. Blinatumomab became an option for treatment of patients with R/R ALL in 2014 when it received accelerated approval contingent on completion of a randomized phase III trial with OS as the primary endpoint. The TOWER trial has now been completed and demonstrated increased OS for patients receiving blinatumomab versus those receiving SOC, with a 3.7‐month increase in median OS. The results in the SOC arm are as would be expected with chemotherapy alone [7]; thus, the trial confirms clinical benefit and justifies regular approval of blinatumomab.
Prior studies of TKIs reported CR rates of 19%–34% for patients with R/R Ph‐positive ALL [8], [9], [10]. In the ALCATARA trial, the patients with TKI‐refractory R/R Ph‐positive ALL had a CR rate of 31%, and the responses were durable, but there was no randomized trial comparing SOC chemotherapy with blinatumomab in Ph‐positive disease. The FDA may consider confirmation of benefit in randomized trials in related diseases to support regular approval based on a single‐arm trial. Because the mechanism of action of blinatumomab is the same for Ph‐positive and Ph‐negative ALL, TOWER was accepted as the confirmatory trial for the Ph‐positive indication. The lack of an alternative beneficial therapy for R/R Ph‐positive ALL was also taken into consideration for this decision. Overall, these results support broadening the indication to include patients with R/R Ph‐positive ALL.
The safety profile of blinatumomab has been characterized previously. In the current study, cytokine release syndrome, pyrexia, encephalopathy, and tremor were notably more common among patients receiving blinatumomab than those receiving SOC chemotherapy. Conversely, cytopenias, including neutropenia, and related infections occurred less frequently among patients receiving blinatumomab compared with those receiving SOC chemotherapy. Depression emerged as a new but rare and potentially fatal event in patients receiving blinatumomab; it is unclear if depression is a manifestation of neurologic toxicity of blinatumomab.
The finding that mortality 100 days after HSCT was numerically higher in the blinatumomab arm than in the SOC arm (12% vs. 0%) is of concern. Because the case numbers were too small to support a definitive conclusion concerning whether this represented a true increase in risk, further evaluation is warranted. To that end, at the time of this approval, a postmarketing requirement was issued to characterize the effect, if any, of administration of blinatumomab as salvage therapy prior to allogeneic HSCT on the early safety outcomes after HSCT, including the risk of day‐100 mortality or acute graft‐versus‐host disease.
In summary, the data indicate that the benefit‐risk ratio of treatment with blinatumomab is favorable for patients with R/R ALL. Potential safety concerns, including risk of cytokine release syndrome, neurologic events including mood disturbances, and immunosuppression due to neutropenia or low immunoglobulins, are confirmed in this study.
Conclusion
The TOWER trial confirmed the clinical benefit of blinatumomab in patients with R/R ALL, demonstrating a higher OS compared with SOC chemotherapy with a manageable toxicity profile. The ALCANTARA trial demonstrated a response rate benefit in patients with Ph‐positive disease, providing grounds for the expansion of the indication to this patient population. The safety profile identifies some advantages for blinatumomab over chemotherapy, and blinatumomab remains the only new drug that has demonstrated a survival advantage for the treatment of relapsed or refractory ALL over chemotherapy.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Footnotes
For Further Reading: Marcela V. Maus, Bruce L. Levine. Chimeric Antigen Receptor T‐Cell Therapy for the Community Oncologist. The Oncologist 2016;21:608–617.
Implications for Practice: The present report describes the current status of chimeric antigen receptor (CAR) T lymphocytes as an immunotherapy for patients with relapsed or refractory B‐cell malignancies. CAR T cells targeting CD19, a protein expressed on many B‐cell malignancies, typically induce high complete response rates in patients with B‐cell leukemia or lymphoma who have very limited therapeutic options. Recent clinical trial results of CD19 CAR T‐cell therapies and the management of CAR T‐cell‐associated adverse events are discussed. The present report will therefore inform physicians regarding the efficacy and safety of CAR T cells as a therapy for B‐cell malignancies.
Author Contributions
Conception/design: E. Dianne Pulte, Jonathon Vallejo, Donna Przepiorka, Lei Nie, Ann T. Farrell
Collection and/or assembly of data: E. Dianne Pulte, Jonathon Vallejo
Data analysis and interpretation: E. Dianne Pulte, Jonathon Vallejo, Donna Przepiorka, Lei Nie, Ann T. Farrell
Manuscript writing: E. Dianne Pulte, Jonathon Vallejo, Donna Przepiorka, Lei Nie, Ann T. Farrell, Kirsten B. Goldberg, Amy E. McKee, Richard Pazdur
Final approval of manuscript: E. Dianne Pulte, Jonathon Vallejo, Donna Przepiorka, Lei Nie, Ann T. Farrell, Kirsten B. Goldberg, Amy E. McKee, Richard Pazdur
Disclosures
The authors indicated no financial relationships.
References
- 1. Klinger M, Brandl C, Zugmaier G et al. Immunopharmacologic response of patients with B‐lineage acute lymphoblastic leukemia to continuous infusion of T cell‐engaging CD19/CD3‐bispecific BiTE antibody blinatumomab. Blood 2012;119:6226–6233. [DOI] [PubMed] [Google Scholar]
- 2. Topp MS, Gökbuget N, Stein AS et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B‐precursor acute lymphoblastic leukaemia: A multicentre, single‐arm phase 2 study. Lancet Oncol 2015;16:57–66. [DOI] [PubMed] [Google Scholar]
- 3. Przepiorka D, Ko CW, Deisseroth A et al. FDA approval: Blinatumomab. Clin Cancer Res 2015;21:4035–4039. [DOI] [PubMed] [Google Scholar]
- 4. Kantarjian H, Stein A, Gökbuget N et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Eng J Med 2017;376:836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martinelli G, Boissel N, Chevallier P et al. Complete hematologic and molecular response in adult patients with relapsed/refractory Philadelphia chromosome‐positive B‐precursor acute lymphoblastic leukemia following treatment with blinatumomab: Results from a phase II, single‐arm, multicenter study . J Clin Oncol 2017;35:1795–1802. [DOI] [PubMed] [Google Scholar]
- 6. von Stackelberg A, Locatelli F, Zugmaier G et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol 2016;34:4381–4389. [DOI] [PubMed] [Google Scholar]
- 7. Gökbuget N, Dombret H, Ribera JM et al. International reference analysis of outcomes in adults with B‐precursor Ph‐negative relapsed/refractory acute lymphoblastic leukemia. Haematologica 2016;101:1524–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gleevec (imatinib mesylate) tablets [U.S. prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; September 2017. [Google Scholar]
- 9.Sprycel (dasatinib) tablets [U.S. prescribing information]. Princeton, NJ: Bristol‐Myers Squibb Company; November 2017. [Google Scholar]
- 10.Iclusig (ponatinib hydrochloride) tablets [U.S. prescribing information]. Cambridge, MA: Ariad Pharmaceuticals, Inc; November 2016. [Google Scholar]
- 11.Przepiorka D, Deisseroth A. BLA 125557Orig1s000: Blincyto (blinatumomab) Medical Review. Available at https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/125557Orig1s000MedRedt.pdf. Accessed November 21, 2014.
- 12.Krauss A, Przepiorka D. BLA 125557Supplements005: Blincyto (blinatumomab) Clinical Review. Available at https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/125557Orig1s005MedRedt.pdf. Accessed August 11, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.