Abstract
Purpose
To characterize longitudinal trends and factors predictive of depressive symptoms following glaucoma diagnosis in Collaborative Initial Glaucoma Treatment Study (CIGTS) participants.
Design:
Cohort study using follow up data from a clinical trial.
Methods
607 participants with newly-diagnosed open-angle glaucoma were enrolled at 14 clinical centers in the United States from October 1993 through April 1997, randomized to treatment with medication or surgery, and followed every 6 months through 2004.The 8-item Center for Epidemiologic Studies Depression Scale (CES-D) was administered at baseline and follow-up visits. Three outcome measures were investigated: overall CES-D depression score, presence of mild or worse depression (CES-D score≥7), and number of depressive symptoms endorsed.
Results
The average baseline CES-D score was 2.4 (SD=3.8), 12.5% of subjects reported symptomatology associated with mild or worse depression, and 55.3% reported at least one depressive symptom. By one-year post-treatment, depression measures decreased (1.5, 6.7%, and 38.4%, respectively), with modest decreases thereafter. Baseline factors predictive of mild or worse depression included worse vision-related quality of life (VRQOL) (odds ratio, OR=2.41), female sex (OR=1.42), younger age (OR per 10 years younger=1.24), and <high- school education (OR=2.93); other outcomes showed similar results.
Conclusions
Depressive symptomatology decreased considerably during the first year after treatment initiation, but was elevated in those with impaired VRQOL. Given the potential of depression to reduce treatment adherence and thus increase the risk of glaucoma progression, eye-care providers should ask patients about depressive symptoms, provide reassurance when appropriate, and make referrals as necessary.
Introduction
Numerous studies have reported depression and associated symptoms to be frequently found among patients with glaucoma.1–5 Those with more advanced glaucoma had a greater frequency of depressive symptomatology than those with little to no visual field loss.6–8 However, Wilson et al. found no difference in self-reported depressive symptoms between glaucoma patients and controls.9 Evidence is limited and mixed regarding the possibility that depression in glaucoma patients results from the topical medications that are used to treat the condition.10,11 The association of age and depression is unclear, but the 2011–2014 National Health and Nutrition Examination Survey (NHANES) reported past-month antidepressant use in 19% of those aged 60 or older.12 Glaucoma patients with symptoms of depression have been reported to be less adherent to their prescribed medication(s).13–15
In the Collaborative Initial Glaucoma Treatment Study (CIGTS), we administered an abbreviated 8-item version of the 20-item Center for Epidemiologic Studies Depression Scale (CES-D)16 to evaluate depressive symptomatology at the time of open angle glaucoma (OAG) diagnosis among the 607 participants. We reported significant associations at baseline between CES-D responses and participants’ self-reported perception of their visual function from the Visual Activities Questionnaire (VAQ).17 With control for age, gender, race, and co-morbidities, we found more than a 3-fold increased odds (odds ratio (OR), 3.72) of a higher (worse) score on the CES-D with a one-unit higher (worse) score on the VAQ.7 All associations indicated that more difficulty with vision-related tasks was associated with greater depressive symptomatology. We also corroborated the finding by Wang et al.4 of no significant association of depression with clinical measures of glaucoma severity.
Changes over time in depressive symptoms among people being treated for OAG are lacking in the peer-reviewed literature. We know from our study of CIGTS participants’ fear of blindness18that the percentage of participants who expressed this fear was initially substantial but diminished over time. Once diagnosed with glaucoma, patients are often treated for their remaining lifetime. They would benefit to know whether depressive symptoms, if evident initially, are likely to improve over time, and on factors that affect depressive symptoms. Therefore, we evaluated longitudinal trends in depressive symptoms and the potential impact of demographic, clinical, and treatment-related factors on the prevalence of depressive symptoms in CIGTS participants.
Methods
This cohort study involved follow-up of participants involved in the CIGTS, a multicenter clinical trial in which a total of 607 participants with newly-diagnosed OAG were enrolled at 14 clinical centers over a 3.5-year period from October 1993 through April 1997 (ClinicalTrials.gov Identifier #NCT00000149). Approval for this analysis of data collected on its participants was granted by the University of Michigan Investigational Review Board. For each patient, a ‘study eye’ (usually the eye with more glaucomatous changes) was selected for initial treatment. Participants were randomized to receive either topical medications or surgery (trabeculectomy) to treat their glaucoma, and followed after initial treatment at 3 months, 6 months, and every 6 months thereafter for up to 10 years. Fellow eye treatment, when warranted, was specified by protocol to match the study eye treatment. Baseline assessment and follow-up visits included both an in-person clinical examination and a quality of life (QOL) interview administered by telephone shortly after the clinic visit. The clinical examination measured the mean deviation (MD) from visual field testing, intraocular pressure (IOP), and visual acuity (VA), among other glaucoma indicators, to track disease progression. Telephone interviews included assessments of vision-specific QOL (33-item VAQ),17 glaucoma medication adherence (two questions), and depressive symptomatology (8-item CES-D).16 Further details of the CIGTS are given in Musch et al.19 This study was approved by the Investigative Review Board at the University of Michigan, and informed consent was obtained from all participants. In addition, the study was conduced in accordance with Health Insurance Portability and Accountability Act regulations and adhered to the tenets of the Declaration of Helsinki.
The 33-item VAQ was the best instrument available at the start of the CIGTS to assess self-reported visual function and impact of vision on daily life among those with glaucoma. Each item of the VAQ asks about the frequency (never, rarely, sometimes, often, always) of a specific vision-related problem. Items are scored from 1 to 5, with higher scores indicating worse visual function; a summary score is computed as the mean of all 33 items.
For each glaucoma medication a patient was taking, adherence was assessed by two questions: (1) Over the last two weeks, how closely did you follow the instructions of your physician for taking your glaucoma medication003F (1–10 scale, 1=not very closely at all, 10=very closely), and (2) Did you miss any dose of your medication yesterday? (Yes, No). For each patient, a summary measure of adherence was calculated for question (1) by taking the minimum of the adherence scores over medications and for question (2) by counting any report of a missed dose on the previous day for any medication.
The 8-item CES-D asks about the presence (yes,no) and frequency over the past week of specific symptoms of depression. Symptom frequency (0–3 scale) reflects the number of days per week that specific symptoms were experienced (0: less than 1 day, 1: 1–2 days, 2: 3–4 days, 3: 5–7 days). Participants who reported the presence of a symptom but at less than 1 day frequency were scored as 0.5 to distinguish from a report of no symptom.
Because CES-D depression symptom scores were highly skewed, we used three summary measures of depressive symptomatology to capture different characteristics of the distribution. First, an overall symptom score was computed as the sum of the 8 symptoms’ frequencies over the past week, with scores ranging from 0 to 24. Second, an indicator for subjects with mild or worse depression was designated as an overall symptom score ≥7. This threshold for mild or worse depression was approximated from the relative position of the threshold used in the 20-item CES-D (overall symptom score ≥16 on a scale from 0–60).20 Lastly, a count of reported symptoms, irrespective of the frequency over the past week, was computed, with a range from 0 to 8. Each summary measure of depressive symptoms was calculated from survey responses at the baseline assessment (post-diagnosis, pre-treatment, pre-randomization) and at follow-up visits (post-treatment).
Statistical Methods
Characteristics of the CIGTS sample were summarized with means and standard deviations (SD) for continuous measures and frequencies and percentages for categorical measures. The frequency of each CES-D symptom was calculated at baseline, year 1 and year 5. The three summary measures of depressive symptoms are graphically presented at baseline and 9-year follow-up using boxplots (for CES-D symptom score), a forest plot (for CES-D score ≥7) , and stacked bar-charts (for number of CES-D symptoms). Additionally, within-subject changes in CES-D symptom scores over time are illustrated using a lasagna plot.21,22 For easier visualization, CES-D scores were grouped as 0–6.9, 7–11.9, 12–17.9, and 18–24.
Linear mixed regression, repeated measures logistic regression, and repeated measures negative binomial regression models were used to investigate predictors of each of the three summary measures of depressive symptoms (overall CES-D symptom score, an indicator for mild or worse depression, and count of depressive symptoms, respectively). All models accounted for the correlation between repeated measures over time within a subject. Alternative strategies to address skewness in the data were explored, including log transformation. The log and raw data models gave similar results and thus we retained the linear mixed model on the raw scale for ease of interpretation. Variables investigated in models included time since diagnosis, patient demographics, clinical measures of glaucoma severity (MD and IOP, both baseline and time-varying), treatment (medicine or surgery), type of medication or medication combination (carbonic anhydrase inhibitor [CAI], beta blocker [BB], prostaglandin analog [PGA]), and baseline VAQ. Variable selection used the method of best subsets.23 Model results are reported as regression coefficient estimates, ORs, or incident rate ratios (IRR), with 95% confidence intervals (CI), for linear, logistic, and negative binomial models, respectively.
Secondary analyses explored glaucoma-related contributions to depression, whether clinically-evaluated severity or self-reported visual function measures. We used linear regression to investigate the association of glaucoma progression (estimated for each subject by the slope of MD over the first 5 years) with depression at 5 years (measured by the CES-D score). This model was adjusted for variables found to be significantly associated with depression in our primary analysis (except for VAQ score because of confounding with MD slope). MD slope was entered as either a continuous variable or a dichotomous variable with cut-points of ≤−0.5 dB/year or ≤−1.0 dB/year. The associations between 5-year VAQ and both MD slope and MD at 5 years were assessed with Pearson correlations.
Lastly, we investigated the association between symptoms of depression and medication adherence for the 307 CIGTS subjects randomized to medication. Repeated measures logistic regression was used to assess the association between CES-D score and probability of missing a dose of any glaucoma medication on the previous day (dichotomous measure). The between- subject and within-subject correlations between CES-D score and the continuous medication adherence score, in the presence of repeated observations, were estimated using the methods of Bland and Altman.24,25 SAS version 9.4 (SAS Institute, Cary, NC) was used for all statistical analyses.
Results
CIGTS participants had an average age of 58.0 years (SD=10.9) at baseline, were 55% male, and 56% White (Table 1). Baseline measures of the designated study eye showed an average mean deviation of −5.4 dB (SD=4.3) and IOP of 27.5 mmHg (SD=5.6). The average vision-related QOL at baseline, as measured by the VAQ total score, was 2.0 (SD=0.7).
Table 1.
Baseline patient demographics and clinical measures of the eye (n=607 subjects)
| Continuous Variables | Mean (SD) | Min, Max | Median |
|---|---|---|---|
| Age (years) | 58.0 (10.9) | 28, 75 | 59.2 |
| MD - study eye (dB) | -5.4 (4.3) | -23.5, 3.4 | -4.4 |
| IOP - study eye (mmHg) | 27.5 (5.6) | 19, 50 | 27 |
| VAQ | 2.0 (0.7) | 1.0, 4.4 | 1.9 |
| Categorical Variables | Frequency (Percent) | ||
| Male | 334 (55%) | ||
| Race | |||
| White | 337 (56%) | ||
| Black | 231 (38%) | ||
| Asian | 10 (2%) | ||
| Other | 29 (5%) | ||
| Education | |||
| ≤6th grade | 23 (4%) | ||
| Grade 7–11 | 105 (17%) | ||
| Grade 12 | 167 (28%) | ||
| Some College | 146 (24%) | ||
| College degree | 87 (14%) | ||
| Graduate education | 79 (13%) | ||
| Marital Status | |||
| Never Married | 69 (11%) | ||
| Married | 365 (60%) | ||
| Divorced/Separated | 113 (19%) | ||
| Widowed | 60 (10%) | ||
| Diabetes | 102 (17%) | ||
| Hypertension | 225 (37%) | ||
SD, Standard Deviation; Min, Minimum; Max, Maximum; MD, Mean Deviation; IOP, Intraocular Pressure; VAQ, Visual Activities Questionnaire
The frequency of each CES-D depression symptom at baseline, 1-year, and 5-years post-treatment is shown in Table 2. All symptoms were reported more frequently at baseline than at follow-up. Restless sleep was consistently the most commonly reported symptom over time (30.9% at baseline, 23.0% at year 1, and 18.4% at year 5), followed by feeling sad and feeling depressed.
Table 2.
Frequency of symptoms of depression at baseline (n=606), Year 1 (n=544), and Year 5 (n=485)a
| Baseline | Year 1 | Year 5 | |||||
|---|---|---|---|---|---|---|---|
| CES-D Item | frequency | % | frequency | % | frequency | % | |
| 1 | My sleep was restless | 187 | 30.9 | 125 | 23.0 | 89 | 18.4 |
| 2 | I felt sad | 184 | 30.4 | 80 | 14.7 | 58 | 12.0 |
| 3 | I felt depressed | 168 | 27.7 | 77 | 14.2 | 54 | 11.1 |
| 4 | I could not get going | 131 | 21.6 | 97 | 17.8 | 45 | 9.3 |
| 5 | I felt that everything I did was an effort |
113 | 18.7 | 60 | 11.0 | 36 | 7.4 |
| 6 | I felt that I could not shake off the blues even with the help of my family and friends |
84 | 13.9 | 29 | 5.3 | 21 | 4.3 |
| 7 | I did not feel like eating; my appetite was poor |
74 | 12.2 | 41 | 7.5 | 25 | 5.2 |
| 8 | I had crying spells | 52 | 8.6 | 18 | 3.3 | 15 | 3.1 |
CES-D, Center for Epidemiologic Studies Depression Scale
n=605 for Baseline, items 1 and 6; n=484 for Year 5, item 7
Consistent with the symptom-specific trends described above, Figure 1 shows elevated depressive symptom measures at diagnosis, substantial decreases by one year, and further decreasing trends over follow-up in each of the three CES-D summary depressive symptom measures. The mean CES-D symptom score was 2.4 (SD=3.8) at diagnosis and 1.5 (SD=3.1) at 1 year (top panel); 12.5% of subjects reported a frequency of depressive symptomology consistent with mild or worse depression at diagnosis, but only 6.7% at 1 year (middle panel); 55.3% of subjects reported ≥ 1 depressive symptom at diagnosis and 38.4% at 1 year (bottom panel). Further modest decreases in all three depression measures were observed between 1– 9 years.
Figure 1.
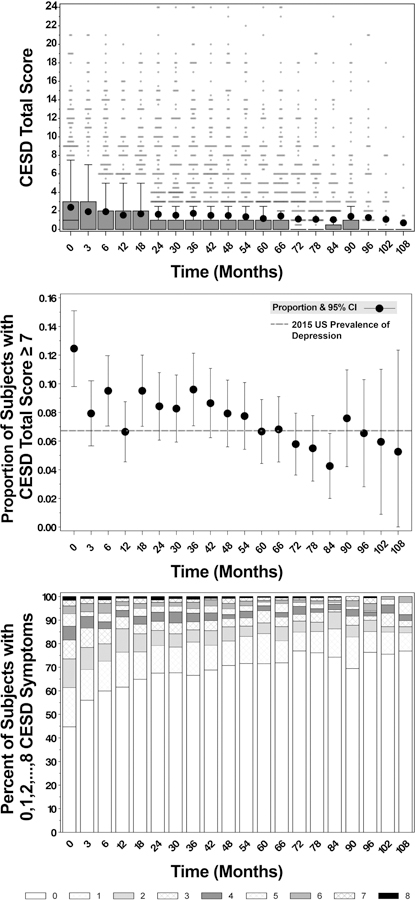
Longitudinal trends in depression as measured by the 8-item CES-D summary depression measures among participants of the CIGTS, including boxplots to display the overall symptom score (top panel), a forest plot to display the prevalence of mild or worse depression over time from baseline (middle panel), and stacked bar-charts to display the percentage of subjects with 0–8 symptoms (bottom panel; e.g., at baseline, 45% of subjects have no symptoms, and the percent is smaller at each higher category, with 2% having all 8 symptoms). CES-D, Center for Epidemiologic Studies Depression Scale; CIGTS, Collaborative Initial Glaucoma Treatment Study
Boxplot displays: the interquartile range (IQR), including the lower quartile/25th percentile (bottom of the box) and the upper quartile/75th percentile (top of the box), the median/50th percentile (line within the box; any median line not visible is equal to zero), the mean (dot within the box), the lower fence/whisker (smallest observation within 1.5*IQR), the upper fence/whisker (largest observation within 1.5*IQR), and outliers (stars; observations located outside 1.5*IQR). Outliers have been jittered to visualize overlying data points.
CES-D symptom scores over time for the same subject are shown in Figure 2 for each of the 607 CIGTS subjects. Each subject’s score is displayed as one layer (row) of the plot. CIGTS subjects who reported the most depressive symptoms at baseline (darker gray sections at the top left corner of the plot) also reported more symptoms over follow-up, but intermittently and mostly to a decreasing extent. Subjects who reported less frequent or no symptoms of depression at baseline reported similar levels throughout their follow-up (center light gray section). Subjects with less frequent or no symptoms of depression at baseline, followed by missing data, were sorted to appear in the lower part of the plot.
Figure 2.
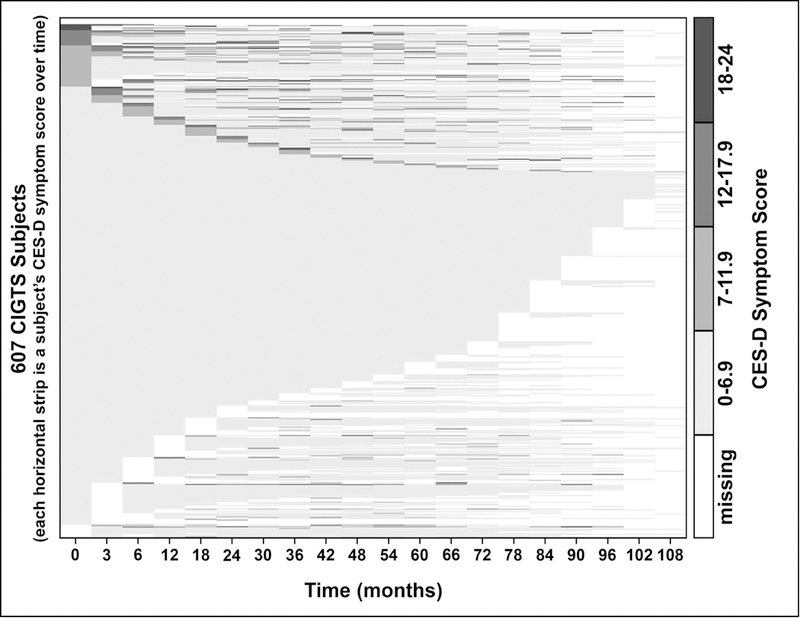
Display of the CES-D overall symptom score within a subject over time for each of the 607 CIGTS subjects. In this ‘lasagna plot’, each row shows a subject’s symptom score history from enrollment (time=0) to 108 months (9 years). Darker grey represents higher CES-D symptom score categories (see category intervals at right), and white areas represent missing data at that time point. At time=0, subjects are ordered from lowest to highest depression score category, and for those within the same score category at time=0, patients are ordered from lowest to highest depression score at 3 months, etc.
CIGTS, Collaborative Initial Glaucoma Treatment Study;CES-D Center for Epidemiologic Studies Depression Scale
Regression model results for each of the 3 depressive symptom measures are displayed in Table 3. Similar effects were observed in each of the 3 models, so for brevity we describe only results for the logistic regression model predicting mild or worse depression (CES-D score ≥ 7). As observed in the descriptive results and plots, there was a strong effect of follow-up time on risk for mild or worse depression. At initial diagnosis (baseline), subjects had a 41% increased odds of mild or worse depression (OR=1.41; p-value=0.0072) relative to Year 1. For every additional year of follow-up, subjects had a 6% decrease in odds of mild or worse depression (OR=0.94; p-value=0.0066). Other factors investigated and found to be consistently associated with an increased odds of mild or worse depression included younger age (OR=1.24 for every 10 year decrease in age; p-value=0.0031), female sex (OR=1.42 versus male; p- value=0.0726), race (OR=2.20 Other versus White race, p-value=0.0077; OR=0.08 Asian versus White race, p-value=0.0046), less education (OR=2.93 for < high school education versus graduate education; p-value=0.0001), and worse baseline vision-related QOL (OR=2.41 for a 1-unit increase (worsening) in VAQ score (1–5 scale); OR=5.79 for a 2-unit increase; p- value<0.0001). Other variables investigated in adjusted models but not found to have significant associations with the summary depressive symptom measures included baseline or time-varying MD and IOP of the study eye, initial treatment (medicine vs. surgery), indicators for a visit within one year prior to cataract surgery or prior to argon laser trabeculoplasty, comorbidities (diabetes and hypertension), marital status, season in which the follow-up visit occurred, clinical center where treated, type of medication or medication combination (for CAI, BB, and PGA medications) and two interactions (center by season, and age by gender).
Table 3.
Multivariable longitudinal models of the 3 CES-D summary depression measures (CES-D symptom score, CES-D symptom score ≥7, Count of CES-D symptoms)
| Linear Mixed Model of CESD Total Score |
Logistic Regression of CESD Total Score ≥ 7 |
Negative Binomial Regression of # CESD Symptoms |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Multivariable models | Estimatea | 95% CI | P-value | ORa | 95% CI | P-value | IRRa | 95% CI | P-value |
| Timeb | |||||||||
| baseline vs year 1 | 0.51 | (0.29, 072) | <0.0001 | 1.41 | (1.10, 1.82) | 0.0072 | 1.45 | (1.30, 1.61) | <0.0001 |
| 1–8 years (per 1 year) | -0.09 | (−0.12, −0.06) | <0.0001 | 0.94 | (0.91, 0.98) | 0.0066 | 0.92 | (0.90, 0.94) | <0.0001 |
| Age (per 10 years younger) | 0.21 | (0.39, 0.04) | 0.0159 | 1.24 | (1.08, 1.44) | 0.0031 | 1.12 | (1.04, 1.22) | 0.0031 |
| Gender (Female vs Male) | 0.55 | (0.17, 0.94) | 0.0052 | 1.42 | (0.97, 2.09) | 0.0726 | 1.47 | (1.20, 1.80) | 0.0002 |
| Race (vs White) | |||||||||
| Black | 0.29 | (−0.13, 0.71) | 0.1769 | 1.21 | (0.83, 1.76) | 0.3177 | 1.16 | (0.94, 1.44) | 0.1633 |
| Asian | -0.96 | (−2.43, 0.51) | 0.2019 | 0.08 | (0.01, 0.45) | 0.0046 | 0.46 | (0.25, 0.85) | 0.0130 |
| Other | 1.51 | (0.59, 2.42) | 0.0012 | 2.20 | (1.23, 3.92) | 0.0077 | 2.11 | (1.45, 3.06) | <0.0001 |
| Education (vs Graduate Education) | |||||||||
| <HS | 0.9 | (0.20, 1.61) | 0.0125 | 2.93 | (1.69, 5.10) | 0.0001 | 1.41 | (1.01, 1.98) | 0.0442 |
| HS | -0.12 | (−0.76, 0.51) | 0.7080 | 1.50 | (0.89, 2.54) | 0.1304 | 0.90 | (0.66, 1.21) | 0.4756 |
| Some College | 0.40 | (−0.24, 1.05) | 0.2167 | 2.30 | (1.29, 4.10) | 0.0045 | 1.14 | (0.83, 1.56) | 0.4247 |
| College Degree | 0.10 | (−0.61, 0.80) | 0.7854 | 1.64 | (0.85, 3.17) | 0.1381 | 1.11 | (0.77, 1.60) | 0.5726 |
| Baseline VAQ (per 1-unit increase on 1–5 scale, higher is worse) |
1.26 | (0.99, 1.54) | <0.0001 | 2.41 | (1.91, 3.04) | <0.0001 | 1.89 | (1.67, 2.15) | <0.0001 |
CESD, Center for Epidemiologic Studies Depression Scale; CI, Confidence Interval; OR, Odds Ratio; IRR, Incident Rate Ratio; HS, High School; VAQ, Visual Activities Questionnaire.
Increased risk of depression is indicated by Estimate>0, OR>1 or IRR>1.
Because the drop in depression scores from baseline to 1 year was larger than predicted by a linear relationship between 1 and 8 years in all 3 models, time since diagnosis was modeled with a linear term for all time points as well as an indicator variable for deviation from the line at the baseline time point.
Note: Linear mixed regression used the compound symmetry covariance structure to adjust for the correlation between repeated measures. Repeated measures logistic and negative binomial regressions used generalized estimating equations to adjust for the correlation between repeated measures.
Exploration of predictors of depressive symptoms related to glaucoma revealed that clinical measures of glaucoma (MD, IOP) showed little or no correlation with depressive symptoms, whereas self-reported visual function (VAQ) showed strong correlation with these symptom measures (Figure 3). Both the 5-year MD and glaucoma progression as measured by 0–5 year MD slope showed no significant correlation with CES-D score at 5 years (r=−0.08, p=0.0953 and r=−0.00, p=0.9656, respectively). Testing these associations in models adjusting for age, sex, race and education yielded no significant association between 5-year CES-D score and either 0–5 year MD slope (p=0.6754) or 5-year MD (p=0.0787). Further, no significant effect on depressive symptom measures was seen when MD slope was dichotomized at −0.5 dB/year (p=0.0696), or at −1.0 dB/year (p=0.4609). In contrast, VAQ score was significantly associated with depressive symptom measures at 5 years (r=0.32, p<0.0001). The individual VAQ item most predictive of depressive symptoms was “I have problems carrying out activities that require a lot of visual concentration and attention” (r=0.34, p<0.0001).
Figure 3.
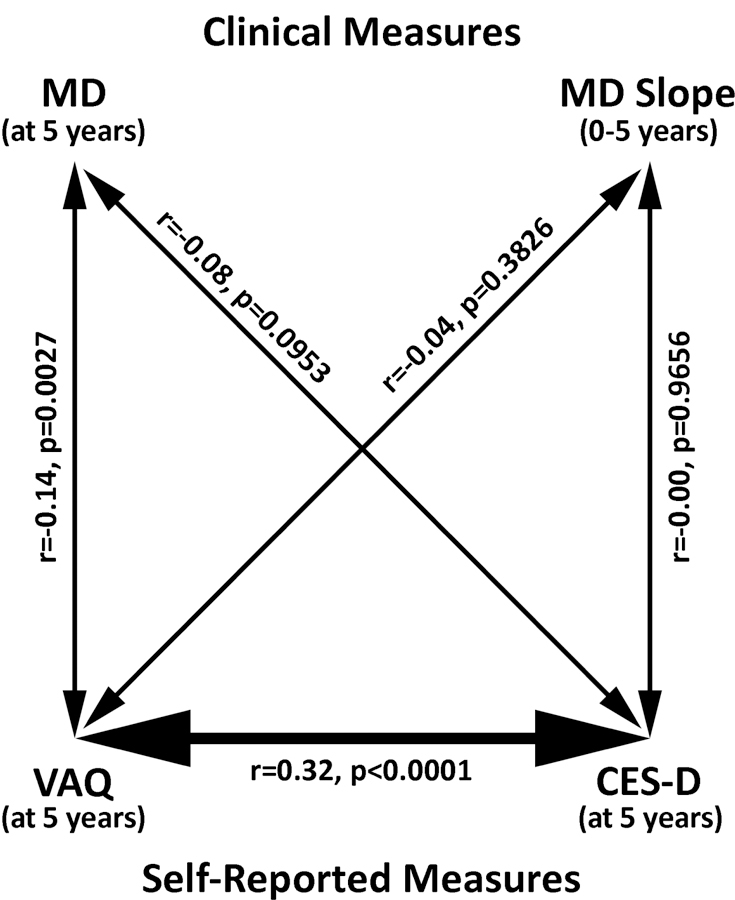
Relationships among clinical measures (Mean Deviation [MD] and 0–5 year MD slope), self-reported Visual Activities Questionnaire (VAQ), and self-reported Center for Epidemiologic Studies Depression Scale (CES-D) scores. These correlations show that the strongest glaucoma-related predictor of depression was self-assessment of difficulty with visual activities, not clinical measures of visual function or progression. r: correlation, p: p-value.
The CES-D score was found to be significantly associated with medication adherence. For every one-unit increase in the CES-D score, there was a 4% increase in the odds of missing a dose of medication during the previous day (OR=1.04, 95% CI=1.01, 1.08, p=0.0062), and a 35% increased odds of missing a dose for every 7-unit increase in the CES-D score (OR=1.35, 95% CI=1.09–1.67). The continuous measures of adherence and depressive symptoms had an overall between-subjects correlation of −0.24 (p<0.0001), i.e., subjects with higher (worse) CES- D scores tended to have lower adherence. The within-subject correlations between adherence and CES-D score had an interquartile range from −0.29 to 0.23, indicating a modest tendency for those with more depressive symptoms at a given time point to have more non-adherence at that time point.
Discussion
CIGTS participants reported the most depressive symptoms and were more likely to have a depression score indicative of mild or worse depression at or near the time of the clinic visit at which they were initially diagnosed with OAG, and informed that they would likely need lifetime treatment for this condition. By one year post-treatment, these same participants reported fewer and less frequent depressive symptoms and lower prevalence of mild or worse depression. These findings closely parallel the decline in fear of blindness over time since diagnosis that we reported previously,18 as well as the responses over time of patients informed of other potentially serious conditions.26 This trend likely relates to an initially elevated concern when patients are informed of having a chronic, vision-threatening condition. The level of concern and associated depressive symptoms in glaucoma are substantially ameliorated with extended time under treatment, during which most patients were told that the treatment they had been receiving was effective in reducing IOP, stabilizing visual field loss, and controlling their OAG. However, there is evidence that poorer glaucoma control is associated with more depressive symptoms, while depressive symptoms are in turn related to non-adherence and possibly poorer glaucoma control. Diniz-Filho et al.27 reported that fast visual field progression was associated with a greater occurrence of depressive symptoms in 102 patients with glaucoma, and Freeman et al.28 found that a maladaptive coping strategy (denial) led to a higher risk of glaucomatous progression.
Personal factors associated with a higher frequency of depressive symptoms included younger age, lower educational attainment, female gender, race, and baseline VAQ. The literature documents a greater tendency of females than males to report depression.29,30 Pratt et al. reported that 24.4% of women and 12.6% of men aged 60 or older in NHANES household interviews had taken an antidepressant medication in the past month.12 Zhou et al. found that both younger age and female gender were independent predictors of anxiety but not depression in their study of 506 Chinese patients with glaucoma.5 Our finding of a 42% increased odds of mild or worse depression in female versus male CIGTS participants is consistent with, although somewhat less than, that reported in the general medical literature. The fact that those with an educational attainment less than high school had higher rates of depression may relate to unmeasured factors, such as the extent to which the patient understood glaucoma and its treatment and lower health literacy. The latter has been associated with perceived risk that exceeds actual risk, leading to undue worry in spite of reassurances of minimal risk.31 Race effects showed no difference between blacks and whites. The significant effects seen in Asians (n=10) and Other races (n=29) compared to whites were based on sample sizes too small for meaningful inference. Participants who reported worse vision-related QOL at baseline were more likely to report depressive symptoms, which fits well with past reports on this association.5,7,32,33
While these findings are based on a large group of newly-diagnosed glaucoma patients who were evaluated in a standardized manner over extended follow-up during treatment for glaucoma, we lack information on the participants’ depressive symptoms prior to being informed of their OAG diagnosis. Further, we did not collect information on medications or other treatment for depression. Some participants may have had pre-existing depressive symptoms, and some may have required treatment for depression during follow-up, and so our findings need to be considered with these caveats in mind.
Patterns of depressive symptoms observed over follow-up of CIGTS participants are encouraging, as the higher symptom frequency at diagnosis decreased to a lower, stable level over time. However, 11% of patients continued to affirm the CES-D item indicating that they felt depressed even at 5 years after diagnosis. The association of such symptoms with lack of adherence to medications previously reported13–15 and validated herein, may be due to maladaptive strategies of coping such as denial.28 The possible consequence of faster glaucomatous progression27 warrants attention to the glaucoma patient’s psychological status and better risk communication by eye care providers. Clinicians might consider using the VAQ or a single VAQ item to assess the patient’s glaucoma burden and possible related depression. In keeping with Rabins’ advice,34 continued assessment and management of patients’ anxiety about glaucoma is a necessary addition to the standard clinical reminders about the importance of treatment adherence. Signs of depression, even if unrelated to glaucoma, can lead to low medication adherence and warrant referral for care by a qualified health care professional.
Highlights.
In this study of 607 newly-diagnosed glaucoma patients, depressive symptoms initially evident in 12.5% of participants diminished over time (6.7% at one year), and remained lower over 9-year follow-up.
Participants who were younger, female, less educated, or had poorer vision-related quality of life were more likely to experience depressive symptoms.
While depressive symptoms diminish over time after diagnosis, care providers need to monitor for these symptoms and be aware of who are more likely to experience them.
Acknowledgments
Funding/Support
This study was supported by a research grant from the National Institutes of Health/National Eye Institute (grant number EY025719), and by a departmental grant from Research to Prevent Blindness (RPB), New York, NY. Dr. Musch is a recipient of RPB’s Lew R. Wasserman Merit Award. The sponsors had no role in the study design; in the collection, analysis, and interpretation of the data; in the writing of the report; or in the decision to submit the article for publication.
Financial Disclosures
Dr. Musch is a member of independent data and safety monitoring boards for Glaukos, Inc. (San Clemente, CA), InnFocus, Inc. (Miami,FL), and Ivantis, Inc. (Irvine, CA), and a consultant to Editas Medicine (Boston, MA), Iridex, Inc. (Mountainview, CA), and Notal Vision, Inc. (Manassas, VA).
Ms. Niziol, Dr. Gillespie, and Dr. Janz have no financial disclosures.
Other Acknowledgments
Presented in part at the annual meeting of the Association for Research in Vision and Ophthalmology, Baltimore, MD, May 2017.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
David C. Musch, Dept. of Ophthalmology and Visual Sciences, Medical School; Dept. of Epidemiology, School of Public Health; University of Michigan, Ann Arbor, MI
Leslie M. Niziol, Dept. of Ophthalmology and Visual Sciences, Medical School; University of Michigan, Ann Arbor, MI
Nancy K. Janz, Dept. of Health Behavior and Health Education, School of Public Health, University of Michigan, Ann Arbor, MI
Brenda W. Gillespie, Dept. of Biostatistics, School of Public Health, University of Michigan, Ann Arbor, MI
References
- 1.Mabuchi F, Yoshimura K, Kashiwagi K, et al. High prevalence of anxiety and depression in patients with primary open-angle glaucoma. J Glaucoma 2008;17(7):552–557. [DOI] [PubMed] [Google Scholar]
- 2.Lin H-C, Chien C-W, Hu C-C, Ho J-D. Comparison of comorbid conditions between open- angle glaucoma patients and a control cohort: a case-control study. Ophthalmology 2010;117(11):2088–2095. [DOI] [PubMed] [Google Scholar]
- 3.Popescu ML, Boisjoly H, Schmaltz H, et al. Explaining the relationship between three eye diseases and depressive symptoms in older adults. Invest Ophthalmol Vis Sci 2012;53(4):2308–2313. [DOI] [PubMed] [Google Scholar]
- 4.Wang SY, Singh K, Lin SC. Prevalence and predictors of depression among participants with glaucoma in a nationally-representative population sample. Am J Ophthalmol 2012;154(3):436–444.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou C, Qian S, Wu P, Qiu C. Anxiety and depression in Chinese patients with glaucoma: sociodemographic, clinical, and self-reported correlates. J Psychosom Res 2013;75(1):75–82. [DOI] [PubMed] [Google Scholar]
- 6.Cumurcu T, Cumurcu BE, Celikel FC, Etikan I. Depression and anxiety in patients with pseudoexfoliative glaucoma. Gen Hosp Psychiatr 2006;28(6):509–515. [DOI] [PubMed] [Google Scholar]
- 7.Jampel HD, Frick KD, Janz NK, et al. Depression and mood indicators in newly diagnosed glaucoma patients. Am J Ophthalmol 2007;144(2):238–244. [DOI] [PubMed] [Google Scholar]
- 8.Skalicky S, Goldberg I. Depression and quality of life in patients with glaucoma: a cross- sectional analysis using the Geriatric Depression Scale-15, assessment of function related to vision, and the Glaucoma Quality of Life-15. J Glaucoma 2008;17(7):546–551. [DOI] [PubMed] [Google Scholar]
- 9.Wilson MR, Coleman AL, Yu F, et al. Depression in patients with glaucoma as measured by self-report surveys. Ophthalmology 2002;109(5):1018–1022. [DOI] [PubMed] [Google Scholar]
- 10.Schweitzer I, Maguire K, Tuckwell V. Antiglaucoma medication and clinical depression. Austr NZ J Psychiatry. 2001;35(5):569–571. [DOI] [PubMed] [Google Scholar]
- 11.Kaiserman I, Kaiserman N, Elhayany A, Vinker S. Topical beta-blockers are not associated with an increased risk of treatment for depression. Ophthalmology 2006;113(7):1077–1080. [DOI] [PubMed] [Google Scholar]
- 12.Pratt LA, Brody DJ, Gu Q. Antidepressant use among persons aged 12 and over: United States, 2011–2014. NCHS Data Brief 2017, No. 283. [PubMed]
- 13.Pappa C, Hyphantis T, Pappa S, et al. Psychiatric manifestations and personality traits associated with compliance with glaucoma treatment. J Psychosom Res 2006;61(5):609–617. [DOI] [PubMed] [Google Scholar]
- 14.Jayawant SS, Bhosle MJ, Anderson RT, Balkrishnan R. Depressive symptomatology, medication persistence, and associated healthcare costs in older adults with glaucoma. J Glaucoma 2007;16(6):513–520. [DOI] [PubMed] [Google Scholar]
- 15.Lim MC, Watnik MB, Imson KR, et al. Adherence to glaucoma medication: the effect of interventions and association with personality type. J Glaucoma 2013;22(6):439–446. [DOI] [PubMed] [Google Scholar]
- 16.Radloff LS. The CES-D scale: A self report depression scale for research in the general population. Applied Psychological Measurement 1977;1(3):385–401. [Google Scholar]
- 17.Sloane ME, Ball K, Owsley C, et al. The Visual Activities Questionnaire: developing an instrument for assessing problems in everyday visual tasks. Tech Dig Noninvasive Assess Vis Syst 1992;1:26–29. [Google Scholar]
- 18.Janz NK, Wren PA, Guire KE, et al. Fear of blindness in the Collaborative Initial Glaucoma Treatment Study: patterns and correlates over time. Ophthalmology 2007;114(12):2213–2220. [DOI] [PubMed] [Google Scholar]
- 19.Musch DC, Lichter PR, Guire KE, et al. The Collaborative Initial Glaucoma Treatment Study (CIGTS): study design, methods, and baseline characteristics of enrolled patients. Ophthalmology 1999;106(4):653–662. [DOI] [PubMed] [Google Scholar]
- 20.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiological Studies- Depression Scale (CES-D) as a screening instrument for depression among community- residing older adults. Psychology and Aging 1997;12(2):277–287. [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson L, Friendly M. The history of the cluster heat map. Am Stat 2009;63(2):179–184. [Google Scholar]
- 22.Swihart BJ, Caffo B, James BD, et al. Lasagna plots: a saucy alternative to spaghetti plots. Epidemiology 2010;21(5):621–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosmer DW, Jovanovic B, Lemeshow S. Best subsets logistic regression. Biometrics 1989;45(4):1265–1270. [Google Scholar]
- 24.Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: Part 1--Correlation within subjects. BMJ 1995;310(6977):446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: Part 2--Correlation between subjects. BMJ 1995;310(6980):633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maunsell E, Brisson J, Deschenes L. Psychological distress after initial treatment of breast cancer. Assessment of potential risk factors. Cancer 1992;70(1):120–125. [DOI] [PubMed] [Google Scholar]
- 27.Diniz-Filho A, Abe RY, Cho HJ, et al. Fast visual field progression is associated with depressive symptoms in patients with glaucoma. Ophthalmology 2016;123(4):754–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freeman EE, Lesk MR, Harasymowycz P, et al. Maladaptive coping strategies and glaucoma progression. Medicine 2016;95(35):e4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Djernes JK. Prevalence and predictors of depression in populations of elderly: a review. Acta Psychiatr Scand 2006;113(5):372–387. [DOI] [PubMed] [Google Scholar]
- 30.Vink D, Aartsen MJ, Schoevers RA. Risk factors for anxiety and depression in the older: a review. J Affect Disorder 2008;106(1–2):29–44. [DOI] [PubMed] [Google Scholar]
- 31.Hawley ST, Janz NK, Griffith KA, et al. Recurrence risk perception and quality of life following treatment of breast cancer. Breast Cancer Res Treat 2017;161(3):557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brody BL, Garnst AC, Williams RA, et al. Depression, visual acuity, comorbidity, and disability associated with age-related macular degeneration. Ophthalmology 2001;108(10); 1893–1901. [DOI] [PubMed] [Google Scholar]
- 33.Hahm B-J, Shin Y-W, Shim E-J, et al. Depression and the vision-related quality of life in patients with retinitis pigmentosa. Br J Ophthalmol 2008;92(5):650–654. [DOI] [PubMed] [Google Scholar]
- 34.Rabins PV. Depressive symptoms in ophthalmology patients. JAMA Ophthalmol 2016;134(9):1015. [DOI] [PubMed] [Google Scholar]


