Abstract
Purpose:
To test the hypothesis that widely used clinical risk factors for growth of choroidal nevi are associated with malignant transformation.
Methods:
Fine needle biopsy for assignment of gene expression profile (class 1 or class 2) was performed in 207 choroidal melanocytic tumors <3.5 mm in thickness. The class 2 profile was employed as a validated biomarker for malignant transformation. The following data were collected: patient age and sex, tumor diameter and thickness, distance of posterior tumor margin from the optic disc, and the presence or absence of serous retinal detachment, orange lipofuscin pigment, drusen, retinal pigment epithelial fibrosis, retinal pigment epithelial atrophy, visual symptoms, and documented tumor growth.
Results:
Clinical features associated with the class 2 profile included patient age >60 years and tumor thickness >2.25 mm (Fisher exact test, P=.002 for both). Documented growth was not associated with the class 2 profile (P=.5). The odds ratio of a tumor having the class 2 profile was 2.8 (95% confidence interval, 1.3–5.9) for patient age >60 years old and 3.5 (95% confidence interval, 1.4–8.8) for tumor thickness >2.25 mm. For patients with both risk factors, the “number needed to treat” to identify one patient with a class 2 tumor was 4.3 (P=.0002). No other clinical feature or combination of features was associated with the class 2 profile.
Conclusions:
None of the widely used choroidal nevus risk factors for tumor growth, nor documented growth itself, is pathognomonic of malignant transformation as defined by class 2 gene expression profile. Patient age and tumor thickness may be helpful for identifying small choroidal melanocytic tumors that are more likely to have the class 2 profile. Observation for growth prior to treatment continues to be reasonable for most patients with suspicious choroidal nevi.
INTRODUCTION
DISTINGUISHING CHOROIDAL NEVI FROM SMALL CHOROIDAL MELANOMAS
Choroidal melanocytic tumors comprise a spectrum from small flat benign nevi to large elevated high-risk melanomas, with lesions at either extreme being relatively easy to diagnose. However, it has been a long-standing challenge to distinguish between large choroidal nevi that can be safely observed and small choroidal melanomas that should be treated promptly.1 Many names have been proposed for this equivocal category of lesions, including nevoma, indeterminate melanocytic lesion, indeterminate pigmented choroidal tumor, dormant melanoma, and suspicious choroidal nevus.2–5 In his XXXIII Edward Jackson Memorial lecture, Gass referred to these lesions as “melanocytic choroidal tumors of uncertain biologic activity,” and he was among the first to describe clinical features predictive of tumor growth.6,7 The “Doctor Gass” risk factors include visual symptoms, increased tumor size (thickness and diameter), orange lipofuscin pigment, serous retinal detachment, intrinsic tumor vessels, fluorescein angiography hotspots, and the absence of chronic changes induced by the tumor such as drusen, retinal pigment epithelial atrophy and fibrosis, intraretinal pigment migration, choroidal neovascularization, and overlying cystic retina degeneration (Table 1). Remarkably, forty years after Gass’ pioneering work, we still use most of these risk factors. While many subsequent studies have validated Gass’ findings, few have identified new risk factors.
TABLE 1.
“DOCTOR GASS” LIST OF CLINICAL FEATURES PREDICTIVE OF GROWTH OF CHOROIDAL MELANOCYTIC TUMORS
| FIRST LETTER |
CLINICAL FEATURE | CORRELATION WITH GROWTH |
|---|---|---|
| D | Drusen | Negative |
| O | Overlying retinal degeneration | Negative |
| C | Chronic RPE changes | Negative |
| T | Thickness >2mm | Positive |
| O | Orange lipofuscin pigment | Positive |
| R | Reflectivity (low) | Positive |
| G | Girth (diameter) | Positive |
| A | Angiographic hot spots | Positive |
| S | Subretinal fluid | Positive |
| S | Symptoms | Positive |
RPE, retinal pigment epithelium. Chronic RPE changes include atrophy, fibrosis, and choroidal neovascularization. The widely used but arbitrary thickness threshold of 2 mm was suggested by Gass,6,7 whereas this study indicates an optimal threshold of 2.25 mm. Reflectivity refers to low internal reflectivity, also called internal acoustic quiet zone or acoustic hollowness. Symptoms attributable to the tumor can include blurred vision, photopsia, metamorphopsia, and scotoma. All of these features were described by Gass, except low internal reflectivity.6,7
Augsburger and colleagues evaluated 197 “melanocytic choroidal lesions,” 39 of which grew during follow-up, for a 5-year actuarial growth rate of 26%.3 Features predictive of growth included increased tumor thickness, serous retinal detachment, tumor margin within 2 disc diameters of the optic disc, presence of symptoms, and presence of orange pigment. The best combination of features for prediction of lesion growth included tumor thickness, serous retinal detachment, and symptoms. The 5-year rate of growth was 5.8% when none of these factors was present, and 90.6% when all features were present.
Char and colleagues undertook a detailed statistical analysis of 293 “indeterminate pigmented choroidal tumors,” 98 of which grew on follow-up, representing a 36% 5-year actuarial growth rate. Greater tumor thickness, presence of symptoms, orange pigment, internal acoustic quiet zone on B-scan, and hot spots on fluorescein angiography all were significant predictors of tumor enlargement (relative risk of detectable tumor growth, >1.8).4 Interestingly, these investigators found that low internal reflectivity (“internal acoustic quiet zone”) was associated with tumor growth, whereas GASS did not find any ultrasound characteristics that were predictive of growth.6,7 This may be due to Char having access to better equipment than was available in 1977.
The Collaborative Ocular Melanoma Study (COMS) performed a prospective observational study of “small choroidal melanomas.” 8 Among 188 patients enrolled in this study, subsequent growth was documented in 46 cases, with an actuarial growth rate of 21% at 2 years and 31% at 5 years. Features associated with growth included increased tumor thickness and diameter, presence of orange pigment, absence of drusen, and absence of chronic retinal pigment epithelial changes.
Shields and colleagues analyzed 2514 “choroidal nevi” and observed growth in 2%, 9%, and 13% at 1, 5, and 10 years, respectively.9 Factors predictive of growth included tumor thickness greater >2 mm, serous retinal detachment, symptoms, orange pigment, tumor margin within 3 mm of the optic disc, ultrasonographic “hollowness” (low internal reflectivity) and the absence of a “halo” (ring of depigmentation around the tumor). The median hazard ratio associated with 1–2 features was 3; for 3–4 features was 5; for 5–6 features was 9; and for all 7 features was 21. The two “new” features in this report – ultrasonographic hollowness and halo – were previously described by Char and colleagues.4 mentioned above, Char found that ultrasonographic hollowness was associated with tumor growth, but the absence of a halo was not associated with growth. Since a halo is found in only about 6% of choroidal nevi,4 and is of disputed prognostic value, we have not found this feature to be of clinical utility.
GROWTH VERSUS MALIGNANT TRANSFORMATION
A common assumption regarding these choroidal nevus risk factors is that growth is equivalent to malignant transformation, and that these risk factors can identify small choroidal melanomas that should be promptly treated rather than initially observed.9–11 But is this a reasonable assumption?
In a review of 2,627 cases of choroidal melanoma over a 40-year period, Zimmerman and McLean concluded that there was “no support for the concept that dissemination leading to metastasis begins with the onset of … local growth.” 12 Other studies have demonstrated that histopathologically benign choroidal melanocytic tumors can grow.13,14 Furthermore, the definitions of growth used in the literature are arbitrary and inadequate. Why, for example, would lesion enlargement of 0.5 mm be indicative of malignant transformation,9 but 0.4 mm would not? The rate of growth is also important but has rarely been considered in published studies. For example, an increase in thickness of 0.5 mm over one month would be more concerning for malignancy than the same amount of growth occurring over ten years. Taken together, these lines of evidence raise doubts about the use of tumor growth as an indicator of malignant transformation. Yet, it is not feasible to use metastasis – the defining feature of malignant transformation – as the endpoint in studies of choroidal nevi due to the low rate of metastasis and long latency period.
GENE EXPRESSION PROFILING
Fortunately, recent discoveries in the molecular genetics of uveal melanoma provide a more accurate means of assessing the malignant potential of a choroidal melanocytic tumor. In 2004, we reported that gene expression profiling could be used to predict the risk of metastasis in uveal melanomas treated by enucleation.15 Tumors with the class 1 profile had a low metastatic risk, whereas those with the class 2 profile had a high metastatic risk. Subsequently, our group and others showed that gene expression profiling was superior to clinical, histopathologic and chromosome markers for predicting which uveal melanoma would metastasize.16–19 We refined and migrated the gene expression profile to a highly accurate and reproducible microfluidics real time PCR platform comprising 12 discriminating genes and 3 control genes suitable for analysis of clinical specimens obtained by fine needle biopsy.20,21
Further work has shown that the class 1 profile is indicative of a differentiated melanocytic phenotype that is seen not only in low grade uveal melanomas, but also in benign uveal nevi, melanocytom as and even normal choroidal melanocytes.22,23 In contrast, tumors with the class 2 profile exhibit features of cytologic de-differentiation and expression of cancer stem cell markers.22,24 A large prospective multi-center clinical trial performed by the Collaborative Ocular Oncology Group (COOG) validated the prognostic accuracy of the 15-gene clinical-grade profile, and confirmed its superior prognostic accuracy compared to clinical, histopathologic, and chromosomal features.25 With the subsequent availability and expanding use of the commercially available version of the 15-gene profile (DecisionDx-UM), ocular oncologists have found that accurate molecular prognostic results can readily be obtained by fine needle biopsy in almost all cases, even very small tumors in which biopsy yielded insufficient material for cytopathologic examination.26,27 Thus, for purposes of this thesis, we have defined malignant transformation as having occurred if a choroidal melanocytic tumor exhibits the class 2 gene expression profile.
HYPOTHESIS
The term “malignant transformation” indicates that a primary tumor has undergone a complex series of genetic, molecular, and cellular changes associated with the ability to metastasize.28 Since gene expression profiling of choroidal melanocytic tumors “captures a functional ‘snapshot’ of the tumor’s microenvironment,” we argue that the presence of the class 2 gene expression profile is the most accurate method currently available for assessing whether the complex processes associated with malignant transformation have occurred in a given tumor. Contrariwise, it seems unlikely that such complex changes can be adequately accounted for by simple clinical features. Therefore, we hypothesized that most clinical risk factors for growth of suspicious choroidal melanocytic tumors are not associated with malignant transformation. To test this hypothesis, we analyzed each clinical risk factor, individually and in combination, for statistically significant association with the class 2 gene expression profile. This approach allowed us to overcome the difficulties associated with tumor growth as an indicator of malignant transformation, and it avoided the unfeasibly long time and large number of patients that would be required to use metastasis as the endpoint of a prospective study.
METHODS AND/OR MATERIALS
DATA SOURCES
Data sources included patients from the ocular oncology practice of JWH at Washington University (enrolled between November 22, 2006 and November 12, 2011), the ocular oncology practice of JWH at Bascom Palmer Eye Institute (enrolled between January 23, 2013 and June 20, 2017), and the ocular oncology practice of JJA and ZMC at the University of Cincinnati (enrolled between October 26, 2007 and May 19, 2015). The institutional review boards of Washington University, University of Miami and University of Cincinnati approved this retrospective study before data collection began at each respective institution. Informed consent was obtained from each patient for clinical treatment and participation in research. Patient information was accessed in compliance with the Health Insurance Portability Act (HIPAA).
The study was limited to patients who underwent fine needle biopsy for lesions diagnosed as suspicious choroidal nevus or small choroidal melanoma with tumor thickness ≤3.5 mm that was predominantly (>90%) confined to the choroid. This thickness cut-off was chosen to allow objective determination of the optimal thickness threshold associated with increased risk of malignant transformation, rather than using an arbitrary threshold. Melanocytic tumors confined to the iris and/or ciliary body were excluded because they do not typically exhibit the choroidal nevus clinical features that were the subject of this study. The decision to biopsy was based on either (1) documented growth, or (2) the presence of multiple clinical risk factors such that the risk of malignant was deemed sufficiently high that biopsy was performed without waiting for growth. The following data were collected: patient age at biopsy, patient sex, tumor diameter (measured by indirect ophthalmoscopy and B-scan ultrasonography, whichever was larger), tumor thickness (measured using A- or B-scan ultrasonography), and distance of posterior tumor margin from the optic disc (measure by indirect ophthalmoscopy and/or fundus photography). The presence or absence of the following features was assessed by indirect ophthalmoscopy and fundus biomicroscopy in every case, and also by optical coherence tomography, fundus autofluorescence, and fluorescein angiography as deemed necessary for confirmation in selected cases: serous retinal detachment, orange lipofuscin pigment, drusen, retinal pigment epithelial fibrosis, and retinal pigment epithelial atrophy. The presence of low internal reflectivity was determined using A- or B-scan ultrasonography, and was defined as at least one-third of the tumor interior demonstrating average reflectivity below one-third that of the retina. Symptoms that were judged to be attributable to the tumor by the treating ocular oncologist (JWH, JJA or ZMC) were noted, including blurred vision, metamorphopsia, micropsia, photopsia and/or floaters. Tumor growth and metastasis were not included as endpoints in this study, due to the inherent inaccuracies associated with these variables, as discussed in the Introduction. Features described by Gass that were not analyzed in this study include intraretinal pigment migration, choroidal neovascularization, overlying retinal degeneration and fluorescein angiographic hotspots (Table 1). Intraretinal pigment migration was not routinely noted, and choroidal neovascularization29 was not common enough to be useful. We have shown that overlying retinal degeneration detected by OCT is prognostically favorable,30 but OCT was not performed routinely throughout the entire study period. Similarly, fluorescein angiography is not part of our routine evaluation of choroidal nevi.
DATA ANALYSIS METHODS
The method for performing the 15-gene expression profile has been described in detail elsewhere.31 Briefly, a needle biopsy of the tumor is performed, and the aspirated contents are immediately expelled into an empty tube. Extraction buffer (200 µl) is drawn up into the needle to flush the hub, and is then expelled into the tube containing the tumor sample. RNA is isolated, reverse transcribed into cDNA, pre-amplified, loaded onto microfluidics cards, and analyzed by real-time quantitative PCR. Ct values are calculated for each of the 15 genes in the profile (Table 2), and the results are analyzed by a machine learning algorithm that assigns each new sample to class 1 or class 2. Prior to September 1, 2010, samples were analyzed in the Harbour Laboratory at Washington University. Thereafter, all samples were analyzed at the College of American Pathologists (CAP)-accredited Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory of Castle Biosciences, under the trade name DecisionDx-UM.27,32
TABLE 2.
GENES COMPRISING THE 15-GENE EXPRESSION PROFILE
| GENE SYMBOL |
GENE NAME | DIRECTION IN CLASS 2 |
|---|---|---|
| CDH1 | E-Cadherin | Up |
| ECM1 | Extracellular Matrix Protein 1 | Up |
| HTR2B | 5-Hydroxytryptamine (Serotonin) Receptor 2B | Up |
| RAB31 | RAB31, Member RAS Oncogene Family | Up |
| EIF1B | Eukaryotic Translation Initiation Factor 1B | Down |
| FXR1 | Fragile X Mental Retardation, Autosomal Homolog 1 | Down |
| ID2 | Inhibitor of DNA Binding 2 | Down |
| LMCD1 | LIM and Cysteine-Rich Domains 1 | Down |
| LTA4H | Leukotriene A4 Hydrolase | Down |
| MTUS1 | Microtubule-Associated Tumor Suppressor 1 | Down |
| ROBO1 | Roundabout, Axon Guidance Receptor, 1 | Down |
| SATB1 | SATB Homeobox 1 | Down |
| MRPS21 | Mitochondrial Ribosomal Protein S21 | Control |
| RBM23 | RNA-Binding Motif Protein 23 | Control |
| SAP130 | Sin3A-Associated Protein, 130 kDa | Control |
The selection process for choosing these genes was previously described.21
STATISTICAL ANALYSIS
All statistical analyses were performed in MedCalc software (version 15.8; Ostend, Belgium). The main study endpoint was class 2 gene expression profile. The optimal cut-off for continuous variables was determined by receiver operating characteristics analysis. Evaluation of clinical variable for statistically significant association with class 2 gene expression profile was performed using Fisher exact test. The best fit model for describing the relationship between dichotomous clinical variables and class 2 gene expression profile was determined using logistic regression. A P value of ≤.05 was interpreted as statistically significant.
RESULTS
SUMMARY DATA
Baseline clinical characteristics are summarized in Table 3. We identified 207 patients who met the study inclusion criteria, including 112 (54.1%) females and 95 (45.9%) males, with a median age of 62 years (mean 61 years, range 15 – 88 years). Gene expression profile status was class 1 in 163 (78.7%) and class 2 in 39 (21.3%) cases. Median tumor thickness was 2.5 mm (mean 2.4 mm, range 1.0 – 3.5 mm), and LBD was 9.5 mm (mean 9.8 mm, range 2.5 – 17.0 mm). The tumor was located ≤3 mm from the disc in 112 (54.1%) cases. Visual symptoms attributable to the tumor were present in 120 (58.0%) cases. Subretinal fluid was detected in 136 (65.7%) cases, orange lipofuscin pigment in 121 (58.4%), low acoustic internal reflectivity in 60 (29.0%) cases, drusen in 44 (21.0%) cases, retinal pigment epithelial fibrosis in 25 (12.3%) cases, and retinal pigment epithelial atrophy in 44 (21.3%) cases. Biopsy was performed due to documented growth in 95 (45.9%) cases and the presence of multiple clinical risk factors in 112 (54.1%) cases.
TABLE 3.
SUMMARY OF CLINICAL FEATURES AMONG 207 PATIENTS WITH SUSPICIOUS CHOROIDAL MELANOCYTIC TUMORS
| Variable | Summary Data (N=207) |
|---|---|
| Age at diagnosis, years | |
| Mean | 61 |
| Median (range) | 62 (15 to 88) |
| Sex, No. (%) | |
| Female | 112(54.1) |
| Male | 95(45.9) |
| Largest basal diameter (mm) | |
| Mean | 9.8 |
| Median (Range) | 9.5 (2.5 to 17.0) |
| ≤ 12, No. (%) | 162(78.2) |
| > 12, No. (%) | 45(21.7) |
| Thickness (mm) | |
| Mean | 2.4 |
| Median (Range) | 2.5 (1.0 to 3.5) |
| ≤ 2.25, No. (%) | 68(32.9) |
| > 2.25, No. (%) | 139(67.1) |
| Subretinal fluid, No. (%) | |
| Yes | 137(66.1) |
| No | 49(23.7) |
| Not Available | 22(10.6) |
| Visual symptoms, No. (%) | |
| Yes | 120(58.0) |
| No | 86(41.5) |
| Not Available | 1 (.5) |
| Orange pigment, No. (%) | |
| Yes | 121(58.5) |
| No | 84(40.5) |
| Not Available | 2 (1.0) |
| Tumor margin <3 mm from optic disc, No. (%) | |
| Yes | 112(54.1) |
| No | 94(45.4) |
| Not Available | 1 (.5) |
| Drusen, No. (%) | |
| Yes | 44(21.3) |
| No | 161(77.8) |
| Not Available | 2 (1.0) |
| RPE Fibrosis, No. (%) | |
| Yes | 25(12.1) |
| No | 179(86.5) |
| Not Available | 3 (1.4) |
| RPE Atrophy, No. (%) | |
| Yes | 44(21.3) |
| No | 159(76.8) |
| Not Available | 4 (2.0) |
| Low acoustic internal reflectivity, No. (%) | |
| Yes | 60 (29.0) |
| No | 24 (11.6) |
| Not Available | 123 (59.4) |
| Gene expression profile class (GEP), No. (%) | |
| Class 1 | 163 (78.7) |
| Class 1A | 87 (42.0) |
| Class 1B | 40 (19.3) |
| Class 1, sub-classification not available | 36 (17.4) |
| Class 2 | 44 (21.3) |
| Documented growth | |
| Class 1 | |
| Yes | 77 (37.2) |
| No | 86 (41.5) |
| Class 2 | |
| Yes | 18 (8.7) |
| No | 26 (12.6) |
RPE, retinal pigment epithelium. Chronic RPE features include fibrosis, drusen and atrophy.Percentages may not add to 100% due to rounding.
RECEIVER OPERATING CHARACTERISTICS ANALYSIS
Receiver operating characteristics (ROC) analysis was performed to identify optimal cut-offs for continuous variables (patient age, tumor LBD and thickness) (Figure 1). The optimal discriminant threshold for patient age was >62 years (P=.002, Youden index J=.277), and for tumor thickness was >2.25 mm (P=.003, Youden index J=.244). The area under the curve (AUC) for LBD did not reach statistical significance (P=.3, Youden index J=.120). For statistical analysis, we used an age cut-off of >60 years. For LBD, we selected 12 mm, since this cut-off was previously shown to be a significant threshold for metastatic risk in choroidal melanomas.33,34
FIGURE 1.
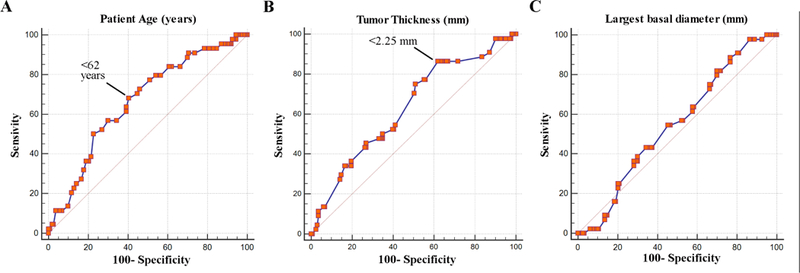
Receiver operating characteristics analysis of (A) patient age, (B) tumor thickness, and (C) largest basal tumor diameter versus class 2 gene expression profile. The indicated point for age and thickness represent the optimal thresholds for sensitivity and specificity using the Youden J index.
CLINICAL FEATURES ASSOCIATED WITH CLASS 2 PROFILE
Fisher exact test was performed to identify choroidal nevus clinical features associated with the class 2 gene expression profile (Table 4). In addition to the established choroidal nevus clinical features associated with lesion growth, we analyzed patient age and sex. The only features associated with class 2 gene expression profile were patient age >60 years (P=.002) and tumor thickness >2.25 mm (P=.002) (Figure 2). Indeed, documented tumor growth itself was not associated with the class 2 profile (P=.5). Using logistic regression analysis, the only feature associated with class 2 gene expression profile was age >60 years (P=.006) (Table 5). The odds ratio of a tumor having the class 2 profile was 2.8 (95% confidence interval, 1.3–5.9) for patient age >60 years old and 3.5 (95% confidence interval, 1.4–8.8) for tumor thickness >2.25 mm. A two-factor logistic regression model using age >60 years and tumor thickness >2.25 mm was highly predictive of class 2 gene expression profile (P=.0001). For patients with both of these features versus those with one or none of these features, the odds ratio was 3.9 (95% confidence interval 1.9 to 7.9, P=.0001), and the number needed to treat (NNT) to identify one patient with a class 2 tumor was 4.3 (95% confidence interval 2.9 to 8.1, P=.0002).
TABLE 4.
FISHER EXACT TEST FOR ASSOCIATION BETWEEN CLINICAL FEATURES AND CLASS 2 GENE EXPRESSION PROFILE AMONG 207 SUSPICIOUS CHOROIDAL MELANOCYTIC TUMORS
| VARIABLE | Class 1 N (%) |
Class 2 N (%) |
P-VALUE |
|---|---|---|---|
| Age >60 years | No: 88 (42.5) Yes: 75 (36.2) |
No: 12 (5.7) Yes: 32 (15.5) |
.002 |
| Thickness >2.25 mm | No: 62 (30.0) Yes: 101 (49.0) |
No: 6 (3.0) Yes: 38 (18.3) |
.002 |
| Absence of fibrous metaplasia | No: 139 (68.1) Yes: 22 (10.8) |
No: 40 (19.6) Yes: 3 (1.5) |
.3 |
| Largest basal diameter >12 mm | No: 129(62.3) Yes: 34 (16.4) |
No: 33 (16.0) Yes: 11 (5.3) |
.5 |
| Visual symptoms | No: 69 (33.5) Yes: 93(45.2) |
No: 16 (7.8) Yes 28 (13.6) |
.5 |
| Presence of subretinal fluid | No: 39 (21.0) Yes: 103 (55.4) |
No: 10 (5.4) Yes: 34 (18.3) |
.7 |
| Absence of RPE atrophy | No: 126 (62.0) Yes: 34 (16.7) |
No: 33 (16.0) Yes: 10 (5.0) |
.8 |
| Absence of any chronic feature | No: 100 (48.5) Yes: 62 (30.1) |
No: 28 (13.6) Yes: 16 (7.7) |
.8 |
| Tumor margin <3 mm from disc | No: 74 (36.0) Yes: 88 (42.8) |
No: 20 (9.7) Yes: 24 (11.7) |
1.0 |
| Absence of drusen | No: 126 (61.5) Yes: 35 (17.1) |
No: 35 (17.1) Yes: 9 (4.4) |
1.0 |
| Female sex | No: 76 (36.7) Yes: 87 (42.0) |
No: 19 (9.2) Yes: 25 (12.1) |
1.0 |
| Presence of orange pigment | No: 66 (32.2) Yes: 95 (46.3) |
No: 18 (8.8) Yes: 26 (12.7) |
1.0 |
| Low acoustic internal reflectivity | No: 17 (20.2) Yes: 42 (50.0) |
No: 7 (8.3) Yes: 18 (21.4) |
1.0 |
| Documented tumor growth | No: 86 (41.5) Yes: 77 (37.2) |
No: 26 (12.6) Yes: 18 (8.7) |
0.5 |
RPE, retinal pigment epithelium. Chronic RPE features include fibrosis, drusen and atrophy. Percentages may not add to 100% due to rounding.
FIGURE 2.
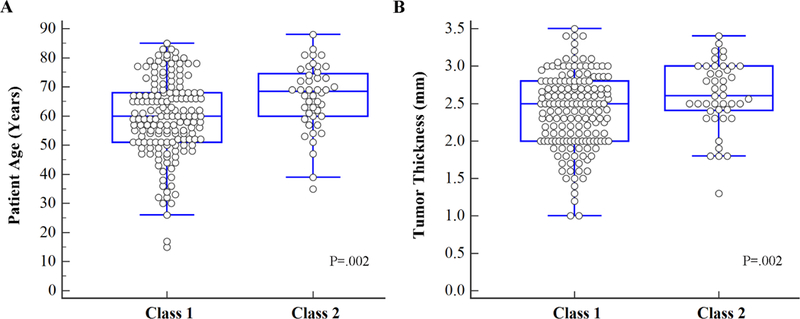
Box and whiskers plots of (A) patient age and (B) tumor thickness versus gene expression profile class. P-value calculated using Fisher exact test.
TABLE 5.
LOGISTIC REGRESSION BEST FIT MODELING OF CLINICAL FEATURES ASSOCIATED WITH CLASS 2 GENE EXPRESSION PROFILE AMONG 207 SUSPICIOUS CHOROIDAL MELANOCYTIC TUMORS
Sample size
| Class 1 | 163 (78.7%) |
| Class 2 | 44 (21.3%) |
CLINICAL FEATURES ASSOCIATED WITH CLASS 1B PROFILE
With increasing application of this technology to analyze smaller tumors, and with longer follow-up, it has been recognized that class 1 tumors can be sub-classified in a biologically meaningful manner into class 1A (minimal metastatic risk) and class 1B (intermediate metastatic risk), based on the expression of two genes (CDH1 and RAB31) in the 15-gene profile.27,35 Strikingly, none of the choroidal nevus clinical features showed statistically significant association with class 1B gene expression profile by Fisher exact test (Table 6), including tumor thickness >2.25 mm (P=.4) and patient age>60 years (P=1.0).
TABLE 6.
FISHER EXACT TEST FOR ASSOCIATION BETWEEN CLINICAL FEATURES AND CLASS 1B GENE EXPRESSION PROFILE AMONG 127 SUSPICIOUS CLASS 1 CHOROIDAL MELANOCYTIC TUMORS
| VARIABLE | Class 1A N (%) |
Class 1B N (%) |
P-VALUE |
|---|---|---|---|
| Tumor thickness >2.25 mm | No: 35 (27.6) Yes: 52 (41.0) |
No: 13 (10.2) Yes: 27 (21.3) |
.4 |
| Female sex | No: 39 (30.7) Yes: 48 (37.8) |
No: 19 (15.0) Yes: 21 (16.5) |
.4 |
| Absence of any chronic RPE features | No: 54 (42.5) Yes: 33 (26.0) |
No: 22 (17.3) Yes: 18 (14.2) |
.6 |
| Tumor margin <3 mm from disc | No: 41 (32.5) Yes: 45 (35.7) |
No: 22 (17.5) Yes: 18 (14.3) |
.6 |
| Visual symptoms | No: 36 (28.4) Yes: 51 (40.2) |
No: 18 (14.2) Yes: 22 (17.3) |
.7 |
| Absence of drusen | No: 69 (54.8) Yes: 18 (14.3) |
No: 30 (23.8) Yes: 9 (7.1) |
.8 |
| Absence of RPE fibrosis | No: 76 (60.3) Yes: 11 (8.7) |
No: 33 (26.2) Yes: 6 (4.8) |
.8 |
| Largest basal tumor diameter >12 mm | No: 67 (52.8) Yes: 20 (15.8) |
No: 32 (25.2) Yes: 8 (6.3) |
.8 |
| Presence of subretinal fluid | No: 22 (19.0) Yes: 57 (49.1) |
No: 9 (7.8) Yes: 28 (24.1) |
.8 |
| Age >60 years | No: 47 (37.1) Yes: 40 (31.5) |
No: 21 (16.5) Yes: 19 (15.0) |
1.0 |
| Absence of RPE atrophy | No: 67 (53.6) Yes: 18 (14.4) |
No: 31 (24.8) Yes: 9 (7.2) |
1.0 |
| Low acoustic internal reflectivity | No: 10 (19.6) Yes: 23 (45.1) |
No: 6 (11.8) Yes: 12 (23.5) |
1.0 |
| Presence of orange pigment | No: 35 (27.8) Yes: 52 (41.3) |
No: 16 (12.7) Yes: 23 (18.3) |
1.0 |
RPE, retinal pigment epithelium. Chronic RPE features include fibrosis, drusen and atrophy. Percentages may not add to 100% due to rounding.
DISCUSSION
Forty years after Gass’ landmark treatise,6,7 the choroidal nevus clinical features that he described have stood the test of time as predictors of tumor growth. Yet, he understood that growth was not equivalent to malignant transformation, and he shared the view of many experts that most choroidal nevi have “very low malignant potential” and can be safely observed for growth prior to treatment, regardless of risk factors.36,37 An alternative view posits that “two or more risk factors probably represent small choroidal melanomas” and “early treatment is generally indicated” so that “better prognosis can be achieved.” 38 However, there is very little high quality evidence in the literature to support this position. To address this controversy, we used the class 2 gene expression profile as a validated biomarker for choroidal melanocytic tumors that have undergone malignant transformation, rather than relying on the crude and problematic endpoint of tumor growth. Strikingly, we found that none of the traditional risk factors for choroidal nevus growth, except for tumor thickness, was associated with the class 2 profile. Further, we found that increased patient age (not one of the traditional risk factors) was the strongest predictor of class 2 profile. These findings raise important questions.
SHOULD CLINICAL RISK FACTORS BE USED TO MAKE TREATMENT DECISIONS?
Several studies have shown that class 2 uveal melanomas with diameter less than 12 mm have much lower risk of metastasis than those with larger diameter,34,39 suggesting that early ablative treatment of class 2 tumors when they are still small might improve survival. However, only about 1 in 5 suspicious choroidal nevi have the class 2 profile. By stratifying patients by age >60 years and tumor thickness >2.25 mm, we can reduce the number of lesions needed to treat to ~4 for each one with the class 2 profile. Consequently, when tumor location would allow ablative treatment with only a small threat to vision, this “number needed to treat” may be acceptable. On the other hand, when ablative treatment would pose a high risk for vision loss, it is less clear whether this “number needed to treat” is acceptable. In this case, a fine needle aspiration biopsy to ascertain gene expression profile class may be appropriate to avoid ablative treatment in class 1 tumors where the benefit is questionable. This risk-benefit analysis could change with the development of noninvasive tests that more accurately predict class 2 profile (i.e., lower the number needed to treat) or the development of ablative therapies that pose less risk of vision loss. The use of non-ablative treatment aimed at reducing symptomatic serous retinal detachment is beyond the scope of this thesis.40 Our results suggest that treating patients with choroidal nevi based on clinical risk factors alone could result in many patients with benign lesions receiving potentially unnecessary vision-threatening treatment. As such, we do not view any of these choroidal nevus risk factors as pathognomonic of malignant transformation, although patient age and tumor thickness may be helpful for identifying small choroidal melanocytic tumors that are more likely to have the class 2 profile.
SHOULD ALL CLASS 1 TUMORS BE OBSERVED WITHOUT TREATMENT?
The 5-year metastatic rate for class 1 uveal melanomas >12 mm in diameter is only about 10%.33 For class 1 tumors <12 mm, this metastatic rate is much lower. Thus, initial observation for growth would seem appropriate in most suspicious choroidal nevi ≤2.25 mm thickness and in patients ≤60 years old, since most will have the class 1 profile. But what if a tumor is found to have the class 1 profile, then subsequently grows. Shouldn’t that tumor receive ablative treatment to prevent it from “transforming” to the class 2 profile? Recent discoveries in our laboratory have shed new light on this question (Figure 3). All types of benign choroidal melanocytic lesions (e.g., nevi, melanocytomas, melanocytosis, benign diffuse uveal melanocytic proliferation) initially have the class 1 gene expression profile, which signifies melanocytic differentiation.22 If the nascent lesion then acquires a mutation in EIF1AX (Eukaryotic Translation Initiation Factor 1A, X-Linked) or SF3B1 (Splicing Factor 3B Subunit 1), it may progress and grow, but it retains the class 1 profile (class 1A or class 1B, respectively). Alternatively, if the tumor undergoes biallelic mutational inactivation of BAP1 (BRCA1 Associated Protein 1), the class 1 profile is overtaken by the class 2 profile, which denotes a loss of melanocytic differentiation and replacement by a cancer stem cell-like state.22,24 Interestingly, while BAP1 is differentially expressed in uveal melanoma (increased in class 1 and decreased in Class 2),41 it did not pass the rigorous filtering process to be included in the final 15-gene profile, which was optimized for dynamic range, microfluidic PCR, and other characteristics needed for optimal performance for needle biopsy samples. Further, the gene expression profile has prognostic accuracy that is superior to sequencing for BAP1 mutations because current sequencing methods cannot detect all BAP1 mutations.42
FIGURE 3.
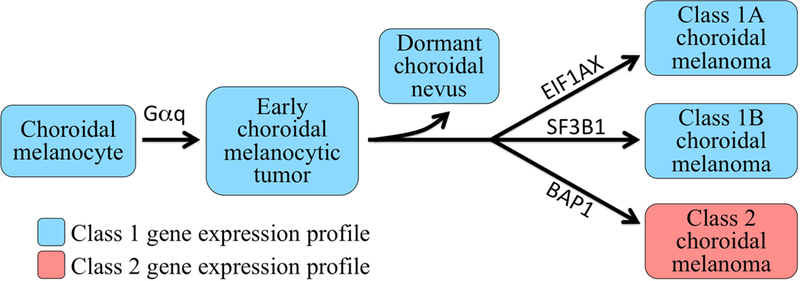
Schematic diagram depicting current understanding of how choroidal melanoma progresses. First, choroidal melanocytes acquire a Gαq mutation that leads to development of an early choroidal melanocytic neoplasm.58,59 The vast majority of such nevi are arrested by tumor suppressor and/or immune surveillance mechanisms and driven into a dormant state. Some lesions progress past this checkpoint to the “BSE node,” in which they acquire mutations in either BAP1 (BRCA1 Associated Protein 1), SF3B1 (Splicing Factor 3B Subunit 1), or EIF1AX (Eukaryotic Translation Initiation Factor 1A, X-Linked), in a mutually exclusive manner. Tumors that acquire an SF3B1 or EIF1AX mutation retain the class 1 profile (class 1A or class 1B, respectively), whereas those that undergo BAP1 inactivation acquire a class 2 profile. Gαq mutations include mutual exclusive hemizygous activating point mutations in GNAQ (G protein subunit alpha Q), GNA11 (G Protein Subunit Alpha 11), PLCB4 (Phospholipase C Beta 4) and CYSLTR2 (Cysteinyl Leukotriene Receptor 2).
Recently, we showed that once a choroidal melanocytic tumor acquires a “BSE” ( BAP1, SF3B1 or EIF1AX) mutation, its evolutionary trajectory generally becomes fixed, such that it does not acquire another BSE mutation or switch gene expression profiles.42 Thus, it may be unnecessary to treat an EIF1AX-mutant class 1 tumor (regardless of the presence of risk factors for growth) since its metastatic risk is minimal and its likelihood of acquiring a BAP1 mutation and switching to the class 2 profile is exceedingly low. The same argument could possibly be made for an SF3B1-mutant class 1 tumor (regardless of the presence of risk factors for growth), even though the risk of metastasis is slightly higher. The growing class 1 tumors that are most likely to benefit from treatment are those that have neither a EIF1AX or SF3B1 mutation, as these tumors are not yet evolutionarily stabilized and may go on to acquire a BAP1 mutation. We anticipate that gene expression profiling and BSE mutation profiling will play complementary roles in precision medicine for patients with suspicious choroidal nevi. However, until these concepts have been prospectively validated (see below), we still favor observation for evidence of growth for most suspicious choroidal nevi due to the low risk of malignant transformation, regardless of the presence of clinical risk factors.
WHY IS AGE ASSOCIATED WITH MALIGNANT TRANSFORMATION?
To our knowledge, this is the first study to identify age as a choroidal nevus risk factor that can be used to assess the risk of malignant transformation in individual patients. A previous epidemiologic study using a public database found an association between increased patient age and the ratio of individuals in the U.S. population with a choroidal melanoma versus those with a choroidal nevus, but this was only a very rough approximation of malignant transformation.43 What might explain the relationship between age and the class 2 gene expression profile? The class 2 profile is strongly associated with inactivating mutations in the tumor suppressor BAP1.41 Indeed, it is likely that loss of BAP1 is the trigger for development of the class 2 profile by causing a loss of melanocytic differentiation.41,44 These findings suggest that increased patient age is associated with an increased risk of BAP1 mutations in suspicious choroidal nevi, as it is in overt uveal melanomas.45 One possible explanation is that BAP1 mutations occur more commonly with increasing age, perhaps due to less efficient DNA damage repair. Although we cannot rule this out completely at the current time, our recent analysis of a large number of primary uveal melanomas using next generation sequencing did not find an aging signature associated with BAP1 mutations.42 Another possible explanation is that with increasing age, the cellular microenvironment of the uveal tract becomes less able to limit the growth of a uveal melanocyte that develops a BAP1 mutation. Indeed, there is growing evidence that aging-associated tissue changes provide a substrate for previously latent mutant cells to overgrow.46 Likely contributors to this altered milieu are the resident choroidal macrophages, which have been shown to undergo senescent changes contributing to uveal melanoma47 and age-related macular degeneration.48 The failure of metastatic uveal melanoma to respond to T-cell checkpoint inhibitor immunotherapy may be due, at least in part to this altered immune microenvironment.49 Research into the interplay between aging immune cells and metastasis may lead to improved strategies for immunotherapy in uveal melanoma,50,51 as the current immunotherapeutic approaches have been less effective in uveal melanoma compared to cutaneous melanoma.
LIMITATIONS AND FURTHER WORK
One limitation of this work is that it did not include some newer diagnostic modalities that could potentially increase the ability to distinguish class 2 tumors noninvasively. We and others have shown that OCT can accurately differentiate serous retinal detachment from chronic overlying retinal cystoid degeneration, which have different prognostic implications,30 but routine performance of OCT on all patients was not standard care at the time of this study. Further, the OCT technology available today is more advanced than at the time some of the earlier patients in this study were treated. For example, swept source OCT angiography can now elucidate intricate details of the choroidal circulation that may have prognostic value in choroidal nevi,52 but this technique is only now becoming widely available. Fundus autofluorescence may improve our ability to detect orange lipofuscin pigment and to distinguish it from orange coloration that can be due to RPE atrophy.53 Studies are now underway in our center and others to evaluate the prognostic value of these and other emerging diagnostic imaging methods. It is important in future studies to use validated molecular biomarkers of malignant transformation, such as the class 2 gene expression profile, rather than problematic surrogates for malignant transformation such as tumor growth.
Another limitation is the lack of uniform criteria for biopsy and the potential bias in patient selection, both of which are common shortcomings of retrospective studies. Of the 207 patients in the study, 93 (45%) were observed for growth prior to biopsy, and all patients were biopsied based on the presence of one or more risk factors. In cases that were observed for growth prior to biopsy, documented growth was the principal reason for biopsy in most cases. In the 55% of cases in which biopsy was performed without waiting for growth, the constellation of clinical risk factors was deemed to be of sufficiently high risk not to observe first. As such, the factors determining which patients would undergo biopsy were not standardized. However, since the patients were managed by a small number of ocular oncologists using uniform criteria, systematic bias should be small. Importantly, older patients were not observed for a longer period of time on average than younger patients, which could be a confounding factor in our finding of increased age as a factor predictive of class 2 gene expression profile. Our findings in this study point out the critical need for prospective randomized controlled studies to further address the management of small suspicious choroidal melanocytic tumors.
A potential criticism of this study is that we used the 15-gene expression profile as a marker for malignant transformation. It has been claimed that malignant transformation in choroidal melanoma can be assessed by tumor growth or by histopathologic examination,9,54 but these claims have been disputed by Gass, Zimmerman, and others.6,7,13,14 The one unequivocal manifestation of malignant transformation in uveal melanoma is distant metastasis, and the 15-gene expression profile is the only biomarker for metastasis in uveal melanoma that has been validated in a prospective, multicenter study and is more accurate for this purpose than clinical, histopathologic or chromosomal markers.25,34 Thus, we submit that the class 2 gene expression profile is the best available biomarker for malignant transformation in suspicious choroidal melanocytic tumors, as argued in another recent study that showed similar findings.35
Nevertheless, we continue to search for increasingly more accurate biomarkers for malignant transformation and metastatic risk in choroidal melanocytic tumors. To this end, we have initiated a new multi-center prospective clinical study called the Collaborative Ocular Oncology Group Study Number 2 (COOG2). This is a National Cancer Institute-funded study being conducted at over 20 leading ocular oncology centers in North America, with Dr. Harbour as the Principal Investigator. Inclusion criteria include a clinical diagnosis of uveal melanoma arising in the choroid and/or ciliary body (including small suspicious choroidal melanocytic tumors) that will be treated with standard methods. The primary tumors undergo fine needle biopsy prior to treatment, and the biopsy sample is subjected to gene expression profiling (class 1A, 1B or 2), PRAME expression status,55–57 and mutation profiling for all common uveal melanoma driver mutations.45 Patients are monitored with careful systemic surveillance for detection of metastatic disease. The primary outcome measure is metastasis, and the secondary outcome measure is melanoma specific mortality. The study goal is to identify the optimal combinatorial use of these molecular prognostic biomarkers, all of which are obtained from a single fine needle biopsy, for precision medicine in these patients.
SUMMARY
There are several practical applications of these findings. First, our findings indicate that no clinical feature or combination of features is pathognomonic for malignant transformation, defined as class 2 gene expression profile, in small suspicious choroidal melanocytic tumors. Thus, caution is urged in making treatment decisions based these features in the absence of documented growth. Nevertheless, these features (especially tumor thickness, subretinal fluid and orange pigment) are valuable for gauging the frequency of visits to monitor for growth. Second, the only commonly-used choroidal nevus risk factor that was associated with malignant transformation was tumor thickness, emphasizing the importance of careful thickness measurements by an experienced echographer. Since there is some inter- and intra-observer variability in ultrasound thickness measurements, it may be prudent to re-check tumors with borderline significant thickness (2.0 – 2.5 mm) prior to making a treatment decision. Third, although the risk of a small tumor having the class 2 profile increased with age, it is important to monitor younger patients periodically for tumor growth. Finally, our results suggest that the vast majority of small suspicious choroidal melanocytic tumors can be observed safely without treatment, as long as growth is not documented. Future improvements in non-invasive imaging may improve the ability to detect small choroidal melanocytic tumors that have undergone malignant transformation without prognostic biopsy.
Overall Model Fit
| Null model −2 Log Likelihood | 214.174 |
| Full model −2 Log Likelihood | 195.659 |
| Chi-squared | 18.515 |
| Significance level | P = .0001 |
Coefficients and standard Errors
| Variable | Coefficient | Std. Error | Wald | P-Value |
|---|---|---|---|---|
| Patient age >60 | 1.03544 | .38007 | 7.4219 | .006 |
| Tumor thickness >2.25 mm | 1.24331 | .47471 | 6.8596 | .009 |
| Constant | -2.87033 | .49237 | 33.9839 | <.0001 |
Odds Ratios and 95% Confidence Intervals
| Variable | Odds ratio | 95% CI |
|---|---|---|
| Patient age >60 | 2.8163 | 1.3371 to 5.9321 |
| Tumor thickness >2.25 mm | 3.4671 | 1.3673 to 8.7912 |
Acknowledgments
A. Funding/Support: This work was supported by grants from the National Cancer Institute to Dr. Harbour (R01 CA125970, Research to Prevent Blindness, Inc. Senior Scientific Investigator Award, Melanoma Research Foundation, Melanoma Research Alliance, Ocular Melanoma Foundation and the Sylvester Comprehensive Cancer Center), and to the Bascom Palmer Eye Institute (NIH Core Grant P30EY014801, Research to Prevent Blindness Unrestricted Grant, and Department of Defense Grant #W81XWH-09-1-0675).
B. Financial Disclosures: Dr. Harbour is the inventor of intellectual property related to the gene expression profile technology used in the study and intellectual property related to the discovery of BAP1 mutations in uveal melanoma. Dr. Harbour is a paid consultant for Castle Biosciences, which licensed this intellectual property, and he receives royalties from its commercialization. Dr. Harbour is a member of the scientific advisory board for Aura Biosciences and Immunocore, Ltd.
D. Other Acknowledgments: The authors would like to thank Christina E. Decatur for administrative support for the study and William J. Feuer for statistical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No other authors disclose a conflict of interest.
Contributor Information
J. William Harbour, Ocular Oncology Service, Bascom Palmer Eye Institute and Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine.
Manuel Paez-Escamilla, Ocular Oncology Service, Bascom Palmer Eye Institute and Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine.
Louis Cai, Ocular Oncology Service, Bascom Palmer Eye Institute and Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine.
Scott D. Walter, Ocular Oncology Service, Bascom Palmer Eye Institute and Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine.
James J. Augsburger, Department of Ophthalmology, University of Cincinnati College of Medicine.
Zelia M. Correa, Wilmer Eye Institute, Johns Hopkins University.
REFERENCES
- 1.Augsburger JJ, Correa ZM, Trichopoulos N, Shaikh A. Size overlap between benign melanocytic choroidal nevi and choroidal malignant melanomas. Invest Ophthalmol Vis Sci 2008;49(7):2823– 2828. [DOI] [PubMed] [Google Scholar]
- 2.Shields JA, Augsburger JJ, Brown GC, Stephens RF. The differential diagnosis of posterior uveal melanoma. Ophthalmology 1980;87(6):518–522. [DOI] [PubMed] [Google Scholar]
- 3.Augsburger JJ, Schroeder RP, Territo C, Gamel JW, Shields JA. Clinical parameters predictive of enlargement of melanocytic choroidal lesions. Br J Ophthalmol 1989;73(11):911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler P, Char DH, Zarbin M, Kroll S. Natural history of indeterminate pigmented choroidal tumors. Ophthalmology 1994;101(4):710–716; discussion 717 [DOI] [PubMed] [Google Scholar]
- 5.Gragoudas E, Lane AM, Kim I. Indeterminate Melanocytic Lesions of the Choroid. In: Schmidt- Erfurth U, Kohnen T, eds. Encyclopedia of Ophthalmology Berlin: Springer-Verlag Berlin Heidelberg; 2015:1–3. [Google Scholar]
- 6.Gass JD. Problems in the differential diagnosis of choroidal nevi and malignant melanomas. The XXXIII Edward Jackson Memorial Lecture. Am J Ophthalmol 1977;83(3):299–323. [DOI] [PubMed] [Google Scholar]
- 7.Gass JD. Problems in the differential diagnosis of choroidal nevi and malignant melanoma. XXXIII Edward Jackson Memorial lecture. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol 1977;83(1):19–48. [PubMed] [Google Scholar]
- 8.COMS. Factors predictive of growth and treatment of small choroidal melanoma: COMS Report No. 5. The Collaborative Ocular Melanoma Study Group. Arch Ophthalmol 1997;115(12):1537– 1544. [DOI] [PubMed] [Google Scholar]
- 9.Shields CL, Furuta M, Berman EL, et al. Choroidal nevus transformation into melanoma: analysis of 2514 consecutive cases. Arch Ophthalmol 2009;127(8):981–987. [DOI] [PubMed] [Google Scholar]
- 10.Shields JA. Treating some small melanocytic choroidal lesions without waiting for growth. Arch Ophthalmol 2006;124(9):1344–1346. [DOI] [PubMed] [Google Scholar]
- 11.Kaiserman I, Kaiserman N, Pe’er J. Long term ultrasonic follow up of choroidal naevi and their transformation to melanomas. Br J Ophthalmol 2006;90(8):994–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmerman LE, McLean IW. Do growth and onset of symptoms of uveal melanomas indicate subclinical metastasis? Ophthalmology 1984;91(6):685–691. [DOI] [PubMed] [Google Scholar]
- 13.Abramson DH. Growing melanocytic tumor is not always cancer. Arch Ophthalmol 2005;123(10):1457–1458. [DOI] [PubMed] [Google Scholar]
- 14.Elner VM, Flint A, Vine AK. Histopathology of documented growth in small melanocytic choroidal tumors. Arch Ophthalmol 2004;122(12):1876–1878. [DOI] [PubMed] [Google Scholar]
- 15.Onken MD, Worley LA, Ehlers JP, Harbour JW. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res 2004;64:7205–7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Worley LA, Onken MD, Person E, et al. Transcriptomic versus chromosomal prognostic markers and clinical outcome in uveal melanoma. Clin Cancer Res 2007;13(5):1466–1471. [DOI] [PubMed] [Google Scholar]
- 17.Petrausch U, Martus P, Tonnies H, et al. Significance of gene expression analysis in uveal melanoma in comparison to standard risk factors for risk assessment of subsequent metastases. Eye 2007;22(8):997–1007. [DOI] [PubMed] [Google Scholar]
- 18.Singh AD, Sisley K, Xu Y, et al. Reduced expression of autotaxin predicts survival in uveal melanoma. Br J Ophthalmol 2007;91(10):1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Gils W, Lodder EM, Mensink HW, et al. Gene expression profiling in uveal melanoma: two regions on 3p related to prognosis. Invest Ophthalmol Vis Sci 2008;49(10):4254–4262. [DOI] [PubMed] [Google Scholar]
- 20.Onken MD, Worley LA, Davila RM, Char DH, Harbour JW. Prognostic testing in uveal melanoma by transcriptomic profiling of fine needle biopsy specimens. J Mol Diagn 2006;8(5):567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onken MD, Worley LA, Tuscan MD, Harbour JW. An accurate, clinically feasible multi-gene expression assay for predicting metastasis in uveal melanoma. J Mol Diagn 2010;12(4):461–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Onken MD, Ehlers JP, Worley LA, Makita J, Yokota Y, Harbour JW. Functional gene expression analysis uncovers phenotypic switch in aggressive uveal melanomas. Cancer Res 2006;66(9):4602–4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Alba MA, Villegas VM, Gold AS, et al. Clinical findings and genetic expression profiling of three pigmented lesions of the optic nerve. Case Rep Ophthalmol Med 2015;2015:590659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang SH, Worley LA, Onken MD, Harbour JW. Prognostic biomarkers in uveal melanoma: evidence for a stem cell-like phenotype associated with metastasis. Melanoma Res 2008;18(3):191–200. [DOI] [PubMed] [Google Scholar]
- 25.Onken MD, Worley LA, Char DH, et al. Collaborative Ocular Oncology Group report number 1: prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology 2012;119(8):1596–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Correa ZM, Augsburger JJ. Sufficiency of FNAB aspirates of posterior uveal melanoma for cytologic versus GEP classification in 159 patients, and relative prognostic significance of these classifications. Graefes Arch Clin Exp Ophthalmol 2014;252(1):131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plasseraud KM, Wilkinson JK, Oelschlager KM, et al. Gene expression profiling in uveal melanoma: technical reliability and correlation of molecular class with pathologic characteristics. Diagn Pathol 2017;12(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 29.Callanan DG, Lewis ML, Byrne SF, Gass JD. Choroidal neovascularization associated with choroidal nevi. Arch Ophthalmol 1993;111(6):789–794. [DOI] [PubMed] [Google Scholar]
- 30.Espinoza G, Rosenblatt B, Harbour JW. Optical coherence tomography in the evaluation of retinal changes associated with suspicious choroidal melanocytic tumors. Am J Ophthalmol 2004;137(1):90–95. [DOI] [PubMed] [Google Scholar]
- 31.Harbour JW. A prognostic test to predict the risk of metastasis in uveal melanoma based on a 15- gene expression profile. Methods Mol Biol 2014;1102:427–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harbour JW, Chen R. The DecisionDx-UM Gene Expression Profile Test Provides Risk Stratification and Individualized Patient Care in Uveal Melanoma. PLoS Curr 2013;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walter SD, Chao DL, Feuer W, Schiffman J, Char DH, Harbour JW. Prognostic implications of tumor diameter in association with gene expression profile for uveal melanoma. JAMA Ophthalmol 2016;134(7):734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Correa ZM, Augsburger JJ. Independent prognostic significance of gene expression profile class and largest basal diameter of posterior uveal melanomas. Am J Ophthalmol 2016;162:20–27 e21.26596399 [Google Scholar]
- 35.Nguyen BT, Kim RS, Bretana ME, Kegley E, Schefler AC. Association between traditional clinical high-risk features and gene expression profile classification in uveal melanoma. Graefes Arch Clin Exp Ophthalmol 2017. [DOI] [PubMed]
- 36.Lane AM, Egan KM, Kim IK, Gragoudas ES. Mortality after diagnosis of small melanocytic lesions of the choroid. Arch Ophthalmol 2010;128(8):996–1000. [DOI] [PubMed] [Google Scholar]
- 37.Murray TG, Sobrin L. The case for observational management of suspected small choroidal melanoma. Arch Ophthalmol 2006;124(9):1342–1344. [DOI] [PubMed] [Google Scholar]
- 38.Shields CL, Demirci H, Materin MA, Marr BP, Mashayekhi A, Shields JA. Clinical factors in the identification of small choroidal melanoma. Can J Ophthalmol 2004;39(4):351–357. [DOI] [PubMed] [Google Scholar]
- 39.Harbour JW. Prognostic Implications of the largest basal tumor diameter vs the TNM staging system in association with the gene expression profile for uveal melanoma-Reply. JAMA Ophthalmol 2017. [DOI] [PMC free article] [PubMed]
- 40.Fabian ID, Stacey AW, Papastefanou V, et al. Primary photodynamic therapy with verteporfin for small pigmented posterior pole choroidal melanoma. Eye (Lond) 2017;31(4):519–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010;330(6009):1410–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Field MG, Durante MA, Anbunathan H, et al. Punctuated evolution of canonical genomic aberrations in uveal melanoma. Nat Commun 2018;9(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh AD, Kalyani P, Topham A. Estimating the risk of malignant transformation of a choroidal nevus. Ophthalmology 2005;112(10):1784–1789. [DOI] [PubMed] [Google Scholar]
- 44.Matatall KA, Agapova OA, Onken MD, Worley LA, Bowcock AM, Harbour JW. BAP1 deficiency causes loss of melanocytic cell identity in uveal melanoma. BMC Cancer 2013;13(1):371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Decatur CL, Ong E, Garg N, et al. Driver mutations in uveal melanoma: associations with gene expression profile and patient outcomes. JAMA Ophthalmol 2016;134(7):728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeGregori J Challenging the axiom: does the occurrence of oncogenic mutations truly limit cancer development with age? Oncogene 2013;32(15):1869–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ly LV, Baghat A, Versluis M, et al. In aged mice, outgrowth of intraocular melanoma depends on proangiogenic M2-type macrophages. J Immunol 2010;185(6):3481–3488. [DOI] [PubMed] [Google Scholar]
- 48.Sene A, Khan AA, Cox D, et al. Impaired cholesterol efflux in senescent macrophages promotes age-related macular degeneration. Cell Metab 2013;17(4):549–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heppt MV, Steeb T, Schlager JG, et al. Immune checkpoint blockade for unresectable or metastatic uveal melanoma: A systematic review. Cancer Treat Rev 2017;60:44–52. [DOI] [PubMed] [Google Scholar]
- 50.Chandran SS, Somerville RPT, Yang JC, et al. Treatment of metastatic uveal melanoma with adoptive transfer of tumour-infiltrating lymphocytes: a single-centre, two-stage, single-arm, phase 2 study. The Lancet Oncology 2017. [DOI] [PMC free article] [PubMed]
- 51.Rothermel LD, Sabesan AC, Stephens DJ, et al. Identification of an immunogenic subset of metastatic uveal melanoma. Clin Cancer Res 2016;22(9):2237–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michalewska Z, Michalewski J, Nawrocki J. Swept source optical coherence tomography of choroidal nevi. Can J Ophthalmol 2016;51(4):271–276. [DOI] [PubMed] [Google Scholar]
- 53.Lavinsky D, Belfort RN, Navajas E, Torres V, Martins MC, Belfort R Jr. Fundus autofluorescence of choroidal nevus and melanoma. Br J Ophthalmol 2007;91(10):1299–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naumann G, Yanoff M, Zimmerman LE. Histogenesis of malignant melanomas of the uvea. I. Histopathologic characteristics of nevi of the choroid and ciliary body. Arch Ophthalmol 1966;76(6):784–796. [DOI] [PubMed] [Google Scholar]
- 55.Field MG, Decatur CL, Kurtenbach S, et al. PRAME as an independent biomarker for metastasis in uveal melanoma. Clin Cancer Res 2016;22(5):1234–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Field MG, Durante MA, Decatur CL, et al. Epigenetic reprogramming and aberrant expression of PRAME are associated with increased metastatic risk in Class 1 and Class 2 uveal melanomas. Oncotarget 2016;7(37):59209–59219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gezgin G, Luk SJ, Cao J, et al. PRAME as a potential target for immunotherapy in metastatic uveal melanoma. JAMA Ophthalmol 2017;135(6):541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harbour JW, Chao DL. A molecular revolution in uveal melanoma: implications for patient care and targeted therapy. Ophthalmology 2014;121(6):1281–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med 2010;363(23):2191–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]


