Abstract
BACKGROUND:
We extend an interrupted time series study design to identify heterogeneous treatment effects using group based trajectory models (GBTM) to identify groups before a new policy and then examine if the effects of the policy has consistent impacts across groups using propensity score weighting to balance individuals within trajectory groups who are and are not exposed to the policy change. We explore this by examining how adherence to endocrine therapy (ET) for women with breast cancer was impacted by reducing copayments for medications by the introduction of generic ETs among women who do not receive a subsidy (the “treatment” group) to those that do receive a subsidy and are not exposed to any changes in copayments (the “control” group).
METHODS:
We examined monthly adherence to ET using the proportion of days covered (PDC) for women diagnosed with breast cancer between 2008–2009 using SEER-Medicare data. To account for baseline trends, we characterize adherence for 1 year before generic approval of ET using GBTM, within each groups we generate inverse probability treatment weights of not receiving a subsidy. We compared adherence after generic entry within each GBTM using a modified Poisson model.
RESULTS:
GBTM for adherence in the one-year pre-generic identified six groups. When comparing patients that did and did not receive a subsidy we found no overall effect of generic introduction. However, 1 of the 6 identified adherence groups post-generic adherence increased (the “consistently low” (RR=1.91; 95% CI=1.34–2.72)).
CONCLUSIONS:
This study describes a new approach to identify heterogeneous effects when using an interrupted time series research design.
INTRODUCTION
Health services researchers are often interested in the effect of a new policy, treatment or program on individuals’ outcomes. While evidence from a randomized controlled trial (RCT) is preferred, it is often not ethical to conduct a trial and even when this is ethical, clinicians and policy makers often need to make decisions much faster than clinical trials can provide answers. Therefore, we are left to use quasi-experimental approaches to evaluate the impact of treatments, policies and programs.
One of the strongest and most widely used approaches is a before and after comparison of individuals that are exposed to a treatment, the treatment group, and those that are not, the comparison group, often referred to as an interrupted time series study design1. This approach allows researchers to control for any secular trends that might bias the estimate. One test researchers often use to ensure that groups are similar is to look at outcomes before treatment or policy change occurs, often referred to as the parallel trends test2,3. In this study, we build on this concept and extend it using a new approach developed in developmental psychology and criminology, group based trajectory models, to ensure those in the treatment and comparison group have similar baseline outcomes and then look at outcomes within each identified group4,5.
Group based trajectory models, an extension of finite mixture models, can capture the complex longitudinal pattern of an outcome by clustering individuals with a similar progression of an outcome over time, overcoming the limitations of summary outcome measures6. This approach allows one to capture differences not only in the average within a group but the different trajectories of use within a group. We then take the identified groups and balance between those who will be exposed to a policy change and those that will not using propensity score weighting. This approach improves upon prior approaches in two ways. First, we are ensuring that the exposed and unexposed groups are similar based on more information than just typical covariates, but also prior outcomes4. Second, this approach allows a new way to identify heterogeneous treatment effects outside of previously described approaches 4. Using this approach, researchers may able to identify subgroups where the treatment is likely to be most effective.
Radical innovation has occurred in breast cancer in the past 25 years. Patients are diagnosed earlier and offered better treatments, resulting in dramatically improved survival. One of the most important additions to treatment regimes has been adjuvant hormonal therapy (HT) for hormone-sensitive breast cancer. These therapies have shown to reduce breast cancer recurrence by 40% and death by over 30%. Hormone therapies include tamoxifen and aromatase inhibitors (anastrozole, letrozole, and exemestane), which can be used together or separately, and are suggested for use, five to ten years post-diagnosis. Despite these clinical benefits, thirty to fifty percent of patients discontinue or poorly adhere to their medications within the first three year of therapy.
Prior research documented the influence of out of pocket costs has on adherence in breast cancer as well as other cancers where orally administered agents are used. The prices are particularly high for standard Medicare Part D beneficiaries, with prior research finding the median monthly out-of-pocket spending was over 30 dollars for ETs when the drugs were under patent. However, in 2010 and 2011 (July 2010 for anastrozole and April 2011 for letrozole and exemestane), ETs lost patent protection and as a result, standard Medicare Part D patient’s out-of-pocket plummeted to a median of under 10 dollars.
Medicare Part D program provides an excellent setting to evaluate the impact of the reducing out-of-pocket spending for ETs, due to the introduction of generics, on adherence for many reasons. First, Medicare requires that all cancer drugs are on all formularies. Second, individuals whose incomes are up to 150% of the federal poverty line are offered the low-income subsidy (LIS). The LIS lowers patient’s premiums, deductibles and copayments. Prior work has shown that LIS beneficiaries out-of-pocket costs showed little variation in response to ETs becoming generic, consistently less than 3 dollars a month. Previous research in this area has found that lower copays for LIS beneficiaries is associated with increased adherence for hypertension and hyperlipidemia drugs when compared to their more affluent counterparts7 and within breast cancer recent research has found that the generic introduction of ETs resulted in increased adherence7.
Group-based trajectory models, as described in the developmental psychology and criminology literature by Haviland and others, will be used to estimate the impact of hormonal therapies becoming generic on adherence for women with breast cancer4. We selected breast cancer adherence to hormonal therapy for many reasons8. First, patients are supposed to be adherent to medication for at least five years after diagnosis9. Second, there is a large body of research that has documented widespread non-adherence to treatment10–13. Third, expected utilization is easy to measure since treatments are administered orally and taken as a daily regimen. Fourth, these medications have recently become generic, with an impact on adherence14,15 in unsubsidized patients. The objective of this study is to examine how adherence to ET for women with breast cancer was impacted by reducing copayments for ETs by the introduction of generic ETs among women who do not receive a subsidy compared to those that do receive a subsidy and are not exposed to any changes in copayments. We examined this using a novel application of group-based trajectory models.
METHODS
Study Population
We used Surveillance, Epidemiology and End Results (SEER) Medicare linked data for this study. We identified women age 66 years or older with a first diagnosis of breast cancer during 2008 to 2009 who were continuously enrolled in Parts A and B fee-for-service Medicare 1 year before diagnosis and continuously enrolled in Part D until 1 year after a generic version of the drug they initiated treatment was introduced. We excluded patients who were not alive at the end of the study. We excluded women with metastatic disease or unknown stage at diagnosis. We excluded women that initiated tamoxifen as it was generic for the entire study period. We also excluded women that initiated treatment before diagnosis. Lastly, we excluded women that did not have at least one fill prior to the 12 months before generics and did not have at least one fill within the 12 months before generics. (n=2359). This resulted in a final cohort of 3344 women. Based on initial treatment, we categorized women as being an anastrozole user or another HT user (letrozole or exemestane) because the date that their medications became generic varied. For those that initiated anastrozole, we examined their adherence in the 12 months prior to and after when anastrozole became generic, July 1, 2010. Similarly, for those that used a different HT, we examined adherence in the 12 months prior to and after when letrozole and exemestane became generic, April 1, 2011.
Adherence to Hormonal Therapy
We created a supply diary recording if a person had HT supply available for each day after the index prescription fill using the HT dispensing date and the days of supply from the prescription drug event file. If a person had overlapping supply of more than one ET medication (e.g., they filled a prescription for letrozole prior to exhausting their supply of an exemestane), we created separate supply diaries for each drug and merged supply diaries rather than allowing for days supplied to be added across drugs.
For each month during the 12 months prior and 12 months after the ET of interest becoming generic we created an indicator using the proportion of days covered in the month to define adherence. We dichotomized this monthly adherence measure by considering individuals with at least 80% of days covered classified as adherent, as used in prior literature 16,17.
Covariates
Women who received the low-income subsidy were treated as a “control” group since they had consistently low copays. Individuals were classified as receiving the low-income subsidy in one of two ways: 1) if they received a low-income subsidy (LIS) through Medicare or 2) if they qualified for both Medicaid and Medicare (i.e., “dual-eligibles”) at the start of observation period (12 months before a drug became generic). The low-income subsidy dramatically reduces the copays for patients. Prior work has documented that copays remain relatively constant for patient that receive the LIS (quarterly out of pocket spending ranging from 7 to 9 dollars before generics to 2 to 4 dollars after generic introduction), but for patients that do not receive the LIS their copays dramatically decreased (quarterly out of pocket spending ranging from 100 to 200 dollars before generics to 30 to 15 dollars after generic introduction)14,15. Therefore, we compared those that received the LIS and did not to examine how copays influence utilization. Consistent with prior work examining the influence of out-of-pocket spending and adherence to orally administered cancer medications, we included the following covariates in our propensity score model: age at diagnosis, age squared, race, ethnicity, comorbidity (Klabunde comorbidity index 18), SEER urban/rural, diagnosis year, number of other drugs, number of other drugs squared, and time since diagnosis and generic date(see appendix Figure 2 for additional details on when variables were measured)8,19,20.
Statistical Analysis
Adherence Trajectories Before Generic HT
Extending the concept of parallel trends to group-based trajectory models, we first established adherence trajectories during the year prior to generic introductions2,4,5. We modeled longitudinal adherence prior to generic introduction using group-based trajectory models using “Proc Traj,” a free downloadable add-on package to base SAS (SAS, version 9.2, Cary, NC) for our analysis. GBTM simultaneously estimates several models and combines information across all models to maximize the likelihood function21. The primary model assumption is that the within-person correlation structure is fully explained by the trajectory curve for each woman’s group. In addition to the estimated trajectory curves, the model allows us to extract estimated probabilities of group membership for each person and group. For a detailed explanation of using group-based trajectory models refer to prior work 22–25.
The longitudinal outcomes, 12 monthly binary indicators of adherence prior to generic being introduced, were modeled using logistic regression for the group-specific models with time defined by months until HT is generic. For all models we allow third order polynomials to model adherence over time, as recommended for twelve time points 22. We used the Bayesian Information Criteria (BIC) to identify the number of group for our model 26. See Appendix 4 for additional details on model fit criteria. Group-based trajectory models assign a person’s probability of being in a particular group based on their adherence across time. Individuals are assigned to the group where their membership probability is highest, called the maximum probability rule. We used this information to ensure that the groups identified were appropriate by examining the mean maximum probability within each group. A commonly used threshold for membership probabilities is an average of ≥0.7 across all members assigned to the trajectory 23,26. Additionally, in order to ensure that the number of groups is appropriate, we examined the difference between the estimated group probabilities and proportion assigned to each group using the maximum probability rule can also be used to measure model fit with values closer to 0 indicating a better fit.
Adherence After Generic ET
We next examined adherence after HT became generic within each of the pre-generic trajectory groups. We compared those who received the LIS subsidy and those that did not to examine how reducing copayments will impact adherence. Using the output of a propensity score model, we created standardized inverse treatment probability weighting (IPTW) for each group identified in the GBTM to compare LIS and non-LIS women – meaning we estimated separate propensity score models for each adherence trajectory group that were standardized 4,27. We stabilized the propensity score weights by multiplying the IPTW weights by the marginal prevalence of the treatment they received, providing an estimate of the treatment effect in the population. The variables included in the propensity score model were: age at diagnosis, age squared, race, ethnicity, comorbidity (Klabunde comorbidity index), SEER urban/rural, diagnosis year, number of other drugs, number of other drugs squared, and time since diagnosis and generic date. In addition to standard approach of examining the p-values, we assessed the differences between LIS and non-LIS groups after apply the IPTW using standardized differences, with a greater than 0.10 difference interpreted as meaningful 27
Generalized estimating equations with IPTW were used to examine the impact of generic HT on patient adherence to HT. The unit of analysis is the person month, with each individual contributing 12 months of post-generic adherence information. We used modified Poisson model (a log link and Poisson distribution) to estimate the adjusted risk ratios (aRRs) with 95% confidence intervals (CI) 28. Statistical significance was defined as p=0.05. We ran separate models for each trajectory group adjusting for LIS status and pooled models which included all groups and interacted group assignment and LIS status. These two approaches resulted in consistent estimates and we will display results from the separate model analysis (the pooled model results can be found in Appendix 1, Table 1).
Table 1.
Descriptive characteristics of the sample
| Consistently High | Slow Decline, Increase | Rapid Decline, Increase | Slow Decline | Rapid Decline | Consistently Low | Overall | |
|---|---|---|---|---|---|---|---|
| N | 1571 | 463 | 341 | 381 | 162 | 426 | 3344 |
| Age at DX, Mean (SD) | 74.3(6.1) | 74.1(5.8) | 73.8(5.8) | 74.5(6.0) | 74.5(5.8) | 74.9(6.0) | 74.3(6.0) |
| Race | |||||||
| White | 84.3% | 89.6% | 90.0% | 88.5% | 89.5% | 91.5% | 87.3% |
| Ethnicity | |||||||
| Spanish/Hispanic | 6.4% | 5.4% | 3.8% | 7.1% | 5.6% | N.S.* | 6.0% |
| Urbanicity | |||||||
| Metro | 83.3% | 83.6% | 82.7% | 83.5% | 85.2% | 85.9% | 83.7% |
| Non-Metro | 16.7% | 16.4% | 17.3% | 16.5% | 14.8% | 14.1% | 16.2% |
| Year Diagnosed | |||||||
| 2008 | 68.0% | 73.0% | 66.3% | 65.9% | 58.0% | 68.3% | 67.8% |
| 2009 | 32.0% | 27.0% | 33.7% | 34.1% | 42.0% | 31.7% | 32.2% |
| Days Between Diagnosis and Generic Introduction, Mean (SD) | 765.1 (176.1) | 758.9 (172.4) | 764.1 (181.8) | 779.0 (195.5) | 785.1 (192.4) | 770.6 (171.9) | 767.4 (178.8) |
| Comorbidity | |||||||
| 0 | 50.4% | 54.4% | 59.2% | 47.8% | 56.2% | 50.9% | 51.9% |
| 1+ | 49.6% | 45.6% | 40.8% | 52.2% | 43.8% | 49.1% | 48.1% |
| LIS | 40.0% | 20.1% | 14.7% | 31.8% | 14.8% | 18.1% | 29.7% |
| Number of Other Drugs | 10.0 (6.3) | 8.7 (4.9) | 8.0 (5.0) | 9.9 (6.2) | 9.0 (5.6) | 9.7 (5.7) | 9.5 (5.9) |
| Exemestene or Lenezotrole | 43.1% | 38.4% | 47.5% | 51.4% | 61.7% | 48.8% | 45.5% |
| Anastrozole | 56.9% | 61.6% | 52.5% | 48.6% | 38.3% | 51.2% | 54.5% |
Note: LIS identified at the start of the 12 month period prior to introduction of a generic. Number of drugs identified 12 month time period. Type of HT identified as the first drug used. N.S. not stated due to sample size restrictions.
Sensitivity Analysis
We conducted many sensitivity analyses to assess the robustness of our results. First, we ran a doubly robust model which included all covariates from the propensity score model in the modified Poisson model to ensure that no measured covariates are confounding the result (Appendix 1, Table 1). Second, we ran separate models for anastrozole users and other HT users. In these analyses, we estimated new GBTM for users of each medication (anastrozole and other HTs), estimated IPTW for each identified group and then GEE models to estimate the effect of access to generic medication on adherence. Third, we conducted an additional sensitivity analysis that only included individuals who did not switch to a different ET throughout the 24-month study period. Again, for this sensitivity analysis we estimated new GBTM, estimated IPTW for each group and GEE models to estimate the effect of generic medication on adherence (see Appendix 2). Fourth, to ensure that our results were not due to treating adherence as a binary variable, we assessed if the results were consistent when adherence was treated as a continuous variable. For this sensitivity analysis we estimated new GBTM using a normal distribution, estimated IPTW for each group and GEE models with a log link and binomial distribution to estimate the effect of generic medication on adherence (see Appendix 3).
RESULTS
After applying our inclusion criteria, we have a sample of 3,344 women (Appendix 1, Figure 1). Characteristics of the 3,344 women included in our cohort are presented in Table 1. The mean age at diagnosis was 74.3 (SD:6.0), 87% were white and 71.3% did not receive LIS. Roughly half did not have comorbidity and almost two-thirds of the sample used more than 6 drugs in the prior year.
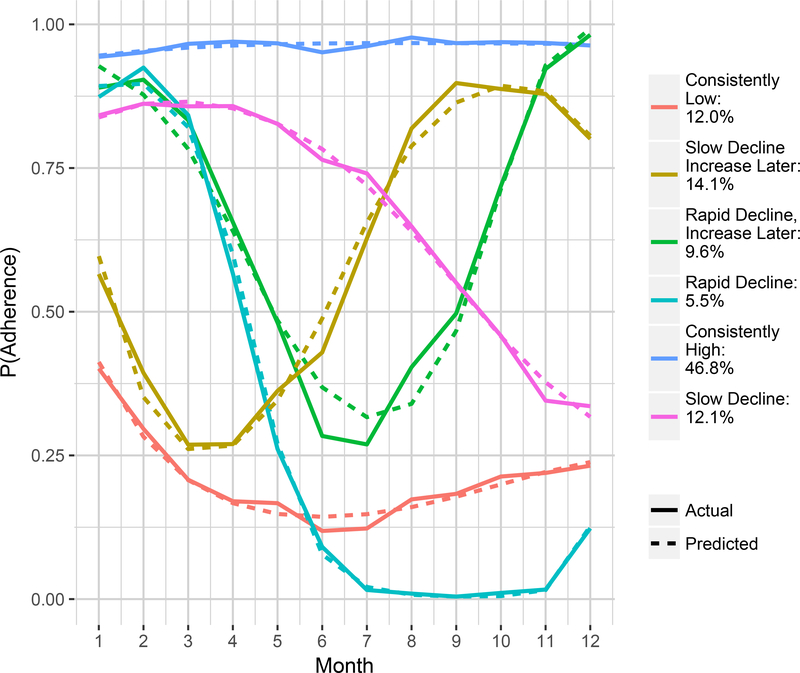
Figure 1. Adherence Trajectories in the 12 Months Prior to Generic Introduction.
Note: The percentages in the legend indicate the proportion of the sample that is within each group.
Adherence to ET over the one year pre-generic is presented in Figure 1. After evaluating model fit, we identified that a six-group adherence trajectory model was best suited for our sample of those tested (see Appendix for model fit and performance details). Adherence groups appeared to fit six patterns: 1) consistently high (N=1571), 2) slow decline, then increase (N=463), 3) rapid decline, then increase (N=341), 4) slow decline (N=381), 5) rapid decline (N=162), and 6) consistently low (N=426), Figure 1.
When comparing characteristics of women assigned to each of the trajectory groups we found that women classified as belonging to the “consistently high” adherence group had the highest rate of receiving the LIS, being black or a non-white race (Table 1). The “consistently low” group was more likely to use more medications and were older on average. When comparing those who receive the LIS within each group (Table 2) we find applying IPTWs there are many significant differences between groups, however, after applying IPTWs there are not any statically significant differences within each group between LIS and non-LIS beneficiaries. However, when examining the standardized differences, we identified some comparisons where the standardized difference is larger than 0.10 indicating covariate imbalance between groups (Table 3).
Table 2:
LIS Compared to Non-LIS within Trajectory Group Before Propensity Score Weighting
| Consistently High | Slow Decline, Increase Later | Rapid Decline, Increase Later | Slow Decline | Rapid Decline | Consistently Low | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LIS | No LIS | Std Diff | P-Value | LIS | No LIS | Std Diff | P-Value | LIS | No LIS | Std Diff | P-Value | LIS | No LIS | Std Diff | P-Value | LIS | No LIS | Std Diff | P-Value | LIS | No LIS | Std Diff | P-Value | |
| N | 629 | 942 | 93 | 370 | 50 | 291 | 121 | 260 | 24 | 138 | 77 | 349 | ||||||||||||
| Age, Mean(SD) | 74.5 (5.9) | 74.1 (6.2) | 0.07 | 0.19 | 74.3 (6.1) | 74.0 (5.7) | 0.05 | 0.65 | 74.6 (6.1) | 73.7 (5.8) | 0.15 | 0.30 | 75.1 (5.6) | 74.3 (6.2) | 0.14 | 0.23 | 73.8 (6.0) | 74.6 (5.8) | −0.14 | 0.52 | 74.6 (6.2) | 75.0 (6.0) | −0.06 | 0.64 |
| Race | 0.52 | <0.0001 | 0.51 | <0.0001 | 0.70 | <0.0001 | 0.39 | <0.0001 | 0.47 | 0.012 | 0.54 | <0.0001 | ||||||||||||
| White | 72.8% | 92.0% | 75.3% | 93.2% | 68.0% | 93.8% | 79.3% | 92.7% | N.S.* | 92.0% | 76.6% | 94.8% | ||||||||||||
| Ethnicity | 0.46 | <0.0001 | 0.43 | <0.0001 | 0.65 | <0.0001 | 0.56 | <0.0001 | 0.71 | <0.0001 | 0.42 | <0.0001 | ||||||||||||
| Hispanic | 13.5% | 1.7% | 15.1% | 3.0% | N.S.* | N.S.* | 18.2% | N.S.* | N.S.* | N.S.* | N.S.* | N.S.* | ||||||||||||
| Urbanicity | 0.09 | 0.08 | −0.20 | 0.10 | −0.04 | 0.79 | 0.13 | 0.24 | −0.42 | 0.11 | 0.10 | 0.44 | ||||||||||||
| Metro | 81.2% | 84.6% | N.S.* | 82.2% | N.S.* | 82.5% | 80.2% | 85.0% | N.S.* | 83.3% | N.S.* | 86.5% | ||||||||||||
| Not Metro | 18.8% | 15.4% | N.S.* | 17.8% | N.S.* | 17.5% | 19.8% | 15.0% | N.S.* | 16.7% | N.S.* | 13.5% | ||||||||||||
| Year Diagnosed | 0.01 | 0.77 | 0.15 | 0.20 | −0.04 | 0.78 | −0.03 | 0.77 | −0.11 | 0.63 | 0.02 | 0.87 | ||||||||||||
| 2008 | 67.6% | 68.3% | 67.7% | 74.3% | 68.0% | 66.0% | 66.9% | 65.4% | N.S.* | 57.2% | 67.5% | 68.5% | ||||||||||||
| 2009 | 32.4% | 31.7% | 32.3% | 25.7% | 32.0% | 34.0% | 33.1% | 34.6% | N.S.* | 42.8% | 32.5% | 31.5% | ||||||||||||
| Days Between Diagnosis and Generic Introduction, Mean (SD) | 765.1 (183.3) | 765.1 (171.2) | <−0.01 | 0.9996 | 768.1 (181.6) | 756.5 (170.2) | 0.07 | 0.56 | 742.2 (174.3) | 767.9 (183.0) | −0.14 | 0.36 | 779.8 (193.7) | 778.7 (196.7) | 0.01 | 0.96 | 723.1 (159.1) | 795.9 (196.1) | −0.41 | 0.087 | 763.1 (156.9) | 772.2 (175.2) | −0.05 | 0.67 |
| Comorbidity | 0.54 | <0.0001 | 0.34 | 0.003 | 0.67 | <0.0001 | 0.45 | <0.0001 | 0.45 | 0.046 | 0.33 | 0.01 | ||||||||||||
| 0 | 34.8% | 60.8% | 40.9% | 57.8% | 32.0% | 63.9% | 33.1% | 54.6% | N.S.* | 59.4% | 37.7% | 53.9% | ||||||||||||
| 1+ | 65.2% | 39.2% | 59.1% | 42.2% | 68.0% | 36.1% | 66.9% | 45.4% | N.S.* | 40.6% | 62.3% | 46.1% | ||||||||||||
| Number of Other Drugs Mean (SD) | 12.6 (6.9) | 8.3 (5.3) | 0.70 | <0.0001 | 11.3 (5.8) | 8.0 (4.4) | 0.65 | <0.0001 | 12.4 (6.4) | 7.2 (4.3) | 0.94 | <0.0001 | 11.9 (7.5) | 9.0 (5.3) | 0.44 | <0.0001 | 13.1 (7.0) | 8.2 (5.1) | 0.79 | <0.0001 | 12.8 (7.3) | 9.1 (5.0) | 0.59 | <0.0001 |
| Exemestene or Lenezotrole | 45.0% | 41.8% | −0.06 | 0.21 | 50.5% | 35.4% | −0.31 | 0.0073 | 36.0% | 49.5% | 0.28 | 0.078 | 50.4% | 51.9% | 0.03 | 0.78 | N.S.* | 65.2% | 0.49 | 0.029 | 48.1% | 49.0% | 0.02 | 0.88 |
| Anastrozole | 55.0% | 58.2% | 49.5% | 64.6% | 64.0% | 50.5% | 49.6% | 48.1% | N.S.* | 34.8% | 51.9% | 51.0% | ||||||||||||
Note: N.S.=note stated due to sample size restrictions. Std. Diff. = standardized differences.
Table 3:
LIS Compared to Non-LIS within Trajectory Group After Propensity Score Weighting
| Consistently High | Slow Decline, Increase Later | Rapid Decline, Increase Later | Slow Decline | Rapid Decline | Consistently Low | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LIS | No LIS | Std Diff | P-Value | LIS | No LIS | Std Diff | P-Value | LIS | No LIS | Std Diff | P-Value | LIS | No LIS | Std Diff | P-Value | LIS | No LIS | Std Diff | P-Value | LIS | No LIS | Std Diff | P-Value | |
| N | 629 | 942 | 93 | 370 | 50 | 291 | 121 | 260 | 24 | 138 | 77 | 349 | ||||||||||||
| Age, Mean(SD) | 74.4 (6.1) | 74.2 (6.0) | 0.02 | 0.65 | 73.6 (6.0) | 73.8 (5.8) | −0.04 | 0.76 | 73.5 (4.9) | 73.7 (5.8) | −0.04 | 0.82 | 74.4 (6.0) | 74.4 (6.0) | −0.01 | 0.94 | 74.7 (5.1) | 74.4 (5.8) | 0.07 | 0.81 | 75.5 (5.9) | 75.0 (6.0) | 0.08 | 0.53 |
| Race | 0.03 | 0.56 | −0.05 | 0.67 | 0.11 | 0.50 | 0.02 | 0.88 | 0.24 | 0.31 | <0.01 | 0.96 | ||||||||||||
| White | 84.2% | 85.3% | N.S.* | 88.4% | N.S.* | 88.1% | 89.0% | 89.5% | N.S.* | 87.9% | N.S.* | 90.5% | ||||||||||||
| Ethnicity | −0.09 | 0.10 | 0.01 | 0.91 | 0.15 | 0.29 | 0.01 | 0.91 | 0.14 | 0.57 | 0.04 | 0.73 | ||||||||||||
| Hispanic | 6.6% | 8.9% | N.S.* | N.S.* | N.S.* | N.S.* | N.S.* | 6.8% | N.S.* | 4.4% | N.S.* | 5.1% | ||||||||||||
| Urbanicity | −0.02 | 0.67 | 0.14 | 0.21 | −0.12 | 0.50 | 0.04 | 0.73 | −0.01 | 0.97 | 0.06 | 0.63 | ||||||||||||
| Metro | 82.8% | 81.9% | 77.6% | 83.2% | N.S.* | 83.0% | 82.5% | 84.0% | N.S.* | 85.2% | 82.6% | 84.9% | ||||||||||||
| Not Metro | 17.2% | 18.1% | 22.4% | 16.8% | N.S.* | 17.0% | 17.5% | 16.0% | N.S.* | 14.8% | 17.4% | 15.1% | ||||||||||||
| Year Diagnosed | 0.05 | 0.37 | 0.04 | 0.76 | −0.01 | 0.93 | 0.01 | 0.91 | 0.06 | 0.82 | 0.03 | 0.83 | ||||||||||||
| 2008 | 67.8% | 69.9% | 69.9% | 71.5% | 67.8% | 67.1% | 64.9% | 65.6% | N.S.* | 57.9% | 67.6% | 68.9% | ||||||||||||
| 2009 | 32.2% | 30.1% | 30.1% | 28.5% | 32.2% | 32.9% | 35.1% | 34.4% | N.S.* | 42.1% | 32.4% | 31.1% | ||||||||||||
| Days Between Diagnosis and Generic Introduction, Mean (SD) | 769.4 (177.4) | 773.9 (179.7) | −0.03 | 0.63 | 740.9 (181.5) | 757.4 (175.2) | −0.09 | 0.42 | 799.3 (159.8) | 765.0 (180.8) | 0.20 | 0.26 | 779.4 (185.1) | 781.0 (190.3) | −0.01 | 0.94 | 747.1 (127.4) | 785.9 (194.6) | −0.24 | 0.43 | 761.2 (161.4) | 771.3 (172.8) | −0.06 | 0.65 |
| Comorbidity | <0.01 | 0.94 | 0.13 | 0.25 | 0.36 | 0.03 | 0.03 | 0.77 | 0.34 | 0.20 | 0.11 | 0.38 | ||||||||||||
| 0 | 49.8% | 50.0% | 61.0% | 54.4% | 41.5% | 59.1% | 46.5% | 48.1% | N.S.* | 57.0% | 44.5% | 50.2% | ||||||||||||
| 1+ | 50.2% | 50.0% | 39.0% | 45.6% | 58.5% | 40.9% | 53.5% | 51.9% | N.S.* | 43.0% | 55.5% | 49.8% | ||||||||||||
| Number of Other Drugs Mean (SD) | 10.1 (6.3) | 10.1 (6.4) | −0.01 | 0.88 | 8.7 (5.1) | 8.8 (5.2) | −0.02 | 0.87 | 9.5 (4.7) | 7.9 (4.8) | 0.33 | 0.057 | 10.1 (6.2) | 9.5 (5.7) | 0.09 | 0.43 | 12.1 (5.4) | 8.9 (5.5) | 0.59 | 0.026 | 10.1 (5.5) | 9.7 (5.4) | 0.09 | 0.51 |
| Exemestene or Lenezotrole | 44.1% | 43.6% | −0.01 | 0.85 | 38.9% | 40.5% | −0.03 | 0.77 | 55.2% | 47.0% | −0.17 | 0.34 | 50.2% | 52.2% | 0.04 | 0.72 | 50.3% | 61.0% | 0.22 | 0.41 | 53.0% | 49.0% | −0.08 | 0.54 |
| Anastrozole | 55.9% | 56.4% | 61.1% | 59.5% | 44.8% | 53.0% | 49.8% | 47.8% | 49.7% | 39.0% | 47.0% | 51.0% | ||||||||||||
Note:
N.S.=not stated dues to sample size restrictions. Std. Diff. = standardized difference
When comparing non-LIS to LIS beneficiaries’ overall adherence, the generic introduction did not impact a patients’ adherence. However, when looking within specific groups (Table 4) we found that the adherence of beneficiaries categorized as “consistent low” (aRR=1.91, 95% CI=1.34, 2.72; p=<0.01) improved in response to generic introduction of HT. In sensitivity analysis, for anastrozole users we found that that generic status decreased adherence for non-LIS beneficiaries among those who adherence was inconsistently high (aRR=0.89, 95 CI%=0.81, 0.98; p=0.02). Among those using other HTs, we found that generic introduction was associated with improved adherence among those whose adherence was categorized at “consistently low” (aRR=2.14, 95 CI%=1.14, 4.01; p=0.02).
Table 4:
Adjusted Risk Ratios Between the Association of LIS compared to non-LIS within Pre Generic Adherence Group
| aRR | 5% | 95% | P-Value | |
|---|---|---|---|---|
| Consistently High | 0.97 | 0.95 | 1.00 | 0.07 |
| Slow Decline, Following Increase | 1.01 | 0.90 | 1.13 | 0.87 |
| Rapid Decline, Following Increase | 0.98 | 0.86 | 1.11 | 0.73 |
| Slow Decline | 1.01 | 0.84 | 1.21 | 0.95 |
| Rapid Decline | 1.99 | 0.84 | 4.76 | 0.12 |
| Consistently Low | 1.91 | 1.34 | 2.72 | <0.01 |
When using a doubly robust estimation, we found similar results with non-LIS beneficiaries categorized as “consistent low” (aRR=1.92, 95% CI=1.35, 2.73; p=<0.01) adherence increasing compared to LIS beneficiaries. Additionally, we found “consistently high” non-LIS adherence decrease following the introduction of generics (aRR=0.98, 95% CI=0.95, 1.00; p=0.05) when compared to LIS beneficiaries in the same trajectory group. This result is similar to the baseline result, but now statistically significant.
DISCUSSION
In this article, we describe an approach new to health services research which aims at improving inference regarding the impact of a policy, program or intervention. This approach aims to fully utilize the richness of longitudinal data that is often a key feature of health services research studies using claims. GBTM are used to identify subgroups whose longitudinal outcomes before an intervention or policy or program change are similar and for who treatment effect may or may not vary. Following identification of groups, we then use propensity score methods to balance measured covariates between individuals within trajectory groups. This not only results in balance on observable covariates but also balance in longitudinal outcomes between the treatment and comparison group before the treatment group received treatment. This approach not only improves balance between those that receive treatment and the comparison group, but it also aids in transparency of the findings. As previously described by Haviland and others, this approach results in being able to present and communicate easy to interpret findings, which can be particularly challenging when trying to communicate heterogeneous treatment effects as we found in this study4,5. However, it should be noted that this approach has a similar threat to validity as other interrupted time series approaches, specifically there is bias if there are secular trends that differentially impact the treatment and comparison group in period after the intervention occurs.
When using this approach, it is important to keep “time in order” 5. Specifically, we must make sure that the outcomes we measure using trajectories occur before the patient receives treatment. It should be noted that many observational studies will compare patients which have made choices. Such as how receiving a surgery is associated with quarterly health care expenditures. We believe that in these cases this method may be particularly informative since the analyst can examine any heterogeneity of treatment effect by prior expenditure trajectory. Additionally, this approach can be used when there are not average baseline parallel trends in the overall population. Other applications of this approach could include settings where patients are followed longitudinally and then exposed to an intervention. For example, in a crossover trial, patients’ trajectories of any longitudinal outcome could be measured to identify subgroups before the cross over occurs. This approach could be used as a post-hoc analysis of trial data to identify heterogenous treatment effects. It is unlikely that this approach could be used when designing a trial since identification of trajectory groups is a data driven process and may vary from one sample to another. Like typical interrupted time series studies, non-observed factors may drive any differences between groups and restrict the research from making causal claims. However, this approach may help control for some non-observed differences if they are captured in the trajectories.
Our research findings contribute to the larger body of research focused on the importance of copays in orally administered oncology drugs. Prior research has found high copays can reduce adherence and delay initiation for some targeted therapies20,29,30. This research has been consistent within breast cancer and ETs. A prior study on younger women and Medicare Advantage patients found that the introduction of generics increased adherence. A different study looking at women in fee-for-service Medicare plans also found there was less variability in adherence throughout the year for non-LIS beneficiaries due to the introduction of generics. This novel approach has been able to identify a long-lasting impact on adherence for those with the lowest adherence. However, this method needs to be applied in other settings to examine if consistent results are found. Our results suggest that, at least for this population, costs were limiting the use among patients with the lowest adherence.
This study has limitations. First, there are limitations due to the nature of pharmacy claims, specifically we do not assess patients that never initiated treatment and among those that use the medication of interest, we do not know if a patient actually uses the medication. We also require that participants live through the entire study period which may bias the finding if HT adherence and LIS is associated with short term survival. However, the impact of HTs on survival is typically seen at least five years after initiation. Therefore, conditioning on a future event of being alive for this specific treatment likely results in very little if any bias. Additionally, we were not able to assess recurrence which would result in patients discontinuing ETs. It is not clear if this should vary by receipt of LIS. The second class of limitations in this study stems from our modelling approach. This approach cannot account for non-observable characteristics driving differences. However, unless these differences were time-varying, they would be controlled for because the study design exploits a “natural experiment” of ETs becoming generic.
This study introduces a new approach to health services researchers to better understand the effects of policies, programs and treatments using observational data by combining GBTM and propensity score weighting. This approach can be used to examine if treatment effect is consistently found within a population or if treatment effect varies within subgroups which can be important both when an overall effect is found and not found. This approach can be easily implemented in all major statistical packages (Stata, SAS and R) and generates intuitive output. This approach is particularly well suited for much of health services research since we often have longitudinal data from claims databases and allows us to fully use the richness of these data sources to create comparable treatment and comparison groups to estimate the population effect as well as identify heterogeneous treatment effects for subgroups identified from the GBTM. We believe that this approach could be used in many other areas of health services research.
Supplementary Material
Acknowledgments
Funding: This work was supported by the American Cancer Society RSG-11–098-01-CPHPS
Footnotes
The authors have no conflict of interests to report.
Contributor Information
Aaron N Winn, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53226, awinn@mcw.edu 414-955-2890.
Nicole M Fergestrom, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53226, nfergestrom@mcw.edu.
Joan M Neuner, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53226, jneuner@mcw.edu.
References
- 1.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309. [DOI] [PubMed] [Google Scholar]
- 2.Zhou H, Taber C, Arcona S, Li Y. Difference-in-Differences Method in Comparative Effectiveness Research: Utility with Unbalanced Groups. Appl Health Econ Health Policy. 2016;14(4):419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crown WH. Propensity-score matching in economic analyses: comparison with regression models, instrumental variables, residual inclusion, differences-in-differences, and decomposition methods. Appl Health Econ Health Policy. 2014;12(1):7–18. [DOI] [PubMed] [Google Scholar]
- 4.Haviland A, Nagin DS, Rosenbaum PR, Tremblay RE. Combining group-based trajectory modeling and propensity score matching for causal inferences in nonexperimental longitudinal data. Dev Psychol. 2008;44(2):422–436. [DOI] [PubMed] [Google Scholar]
- 5.Haviland A, Nagin DS, Rosenbaum PR. Combining propensity score matching and group-based trajectory analysis in an observational study. Psychol Methods. 2007;12(3):247–267. [DOI] [PubMed] [Google Scholar]
- 6.Nagin DS, Odgers CL. Group-Based Trajectory Modeling in Clinical Research. Annu Rev Clin Psycho. 2010;6:109–138. [DOI] [PubMed] [Google Scholar]
- 7.Li P, McElligott S, Bergquist H, Schwartz JS, Doshi JA. Effect of the Medicare Part D coverage gap on medication use among patients with hypertension and hyperlipidemia. Annals of internal medicine. 2012;156(11):776–784, W-263, W-264, W-265, W-266, W-267, W-268, W-269. [DOI] [PubMed] [Google Scholar]
- 8.Winn AN, Dusetzina SB. The association between trajectories of endocrine therapy adherence and mortality among women with breast cancer. Pharmacoepidemiology and drug safety. 2016;25(8):953–959. [DOI] [PubMed] [Google Scholar]
- 9.Howell A, Cuzick J, Baum M, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365(9453):60–62. [DOI] [PubMed] [Google Scholar]
- 10.Ziller V, Kalder M, Albert US, et al. Adherence to adjuvant endocrine therapy in postmenopausal women with breast cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2009;20(3):431–436. [DOI] [PubMed] [Google Scholar]
- 11.Hadji P Improving compliance and persistence to adjuvant tamoxifen and aromatase inhibitor therapy. Critical reviews in oncology/hematology. 2010;73(2):156–166. [DOI] [PubMed] [Google Scholar]
- 12.Reeder-Hayes KE, Meyer AM, Dusetzina SB, Liu H, Wheeler SB. Racial disparities in initiation of adjuvant endocrine therapy of early breast cancer. Breast cancer research and treatment. 2014;145(3):743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wheeler SB, Kohler RE, Reeder-Hayes KE, et al. Endocrine therapy initiation among Medicaid-insured breast cancer survivors with hormone receptor-positive tumors. J Cancer Surviv. 2014;8(4):603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nattinger AB, Pezzin LE, McGinley EL, Charlson JA, Yen TW, Neuner JM. Patient costs of breast cancer endocrine therapy agents under Medicare Part D vs with generic formulations. Springerplus. 2015;4:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neuner JM, Kamaraju S, Charlson JA, et al. The introduction of generic aromatase inhibitors and treatment adherence among Medicare D enrollees. Journal of the National Cancer Institute. 2015;107(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast cancer research and treatment. 2011;126(2):529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yood MU, Owusu C, Buist DS, et al. Mortality impact of less-than-standard therapy in older breast cancer patients. Journal of the American College of Surgeons. 2008;206(1):66–75. [DOI] [PubMed] [Google Scholar]
- 18.Klabunde CN, Legler JM, Warren JL, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17(8):584–590. [DOI] [PubMed] [Google Scholar]
- 19.Riley GF, Warren JL, Harlan LC, Blackwell SA. Endocrine therapy use among elderly hormone receptor-positive breast cancer patients enrolled in Medicare Part D. Medicare Medicaid Res Rev. 2011;1(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doshi JA, Li P, Huo H, et al. High cost sharing and specialty drug initiation under Medicare Part D: a case study in patients with newly diagnosed chronic myeloid leukemia. Am J Manag Care. 2016;22(4 Suppl):s78–86. [PubMed] [Google Scholar]
- 21.Nagin D Group-Based Modeling of Development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- 22.Franklin JM, Shrank WH, Pakes J, et al. Group-based trajectory models: a new approach to classifying and predicting long-term medication adherence. Medical care. 2013;51(9):789–796. [DOI] [PubMed] [Google Scholar]
- 23.Nagin DS. Group-based trajectory modeling: an overview. Annals of nutrition & metabolism. 2014;65(2–3):205–210. [DOI] [PubMed] [Google Scholar]
- 24.Nagin DS, Odgers CL. Group-Based Trajectory Modeling (Nearly) Two Decades Later. Journal of quantitative criminology. 2010;26(4):445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annual review of clinical psychology. 2010;6:109–138. [DOI] [PubMed] [Google Scholar]
- 26.Jones BL, Nagin DS, Roeder K. A SAS Procedure Based on Mixture Models for Estimating Developmental Trajectories. Sociological Methods and Research. 2001;29(3):374–393. [Google Scholar]
- 27.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 29.Dusetzina SB, Winn AN, Abel GA, Huskamp HA, Keating NL. Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(4):306–311. [DOI] [PubMed] [Google Scholar]
- 30.Winn AN, Keating NL, Dusetzina SB. Factors Associated With Tyrosine Kinase Inhibitor Initiation and Adherence Among Medicare Beneficiaries With Chronic Myeloid Leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(36):4323–4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.