Abstract
Toxoplasma gondii is a common protozoan parasite that infects up to one-third of the world’s population. Notably, very little is known about innate immune-sensing mechanisms for this obligate intracellular parasite by human cells. Here, by applying an unbiased biochemical screening approach, we have identified that human monocytes recognized the presence of T. gondii infection via detection of the alarmin S100A11 protein, which is released from parasite-infected cells via caspase-1-dependent mechanisms. S100A11 induced a potent chemokine response to T. gondii via engagement of its receptor RAGE and regulated monocyte recruitment in vivo by inducing expression of the chemokine CCL2. Our experiments have revealed a sensing system for T. gondii by human cells that is based on detection infection-mediated release of alarmin S100A11 and RAGE-dependent induction of CCL2, a crucial chemokine required for host resistance to the parasite.
Innate sensing of infection is a major step required for triggering host defenses against invading pathogens, including protozoan parasites1, 2. Among various classes of the innate immune sensors, Toll-like receptors (TLRs) play a central role in the initiation of interleukin 12 (IL-12)-dependent immune responses to pathogens1. Both humans and mice share similar TLRs required for the recognition of conserved and essential bacterial and viral molecules including the structural components of bacterial cell walls, RNA and DNA1, 3. In striking contrast, the initial steps for the recognition of the common protozoan parasite T. gondii are vastly different between mice and humans4, 5. In mice, the parasite recognition is mediated by TLR11 that directly interacts with T. gondii profilin, a key molecule required for the parasite invasion to the host cells6, 7. T. gondii profilin is a classical pathogen-associated molecular pattern that is unique for T. gondii and other phylogenetically related Apicomplexan parasites including malaria and cryptosporidium6. Profilin is essential for T. gondii survival due to its non-redundant and essential role in regulating actin polymerization during obligatory intracellular parasite entry to the host cells7. Direct interactions between T. gondii profilin and TLR11-TLR12 heterodimer complex result in the induction of IL-12 and CCL2 via MyD88-dependent signaling pathway8, 9, 10, 11. Both IL-12 and CCL2 are essential for host resistance to T. gondii in mice, and the lack of this cytokine and chemokine, respectively, results in acute susceptibility to the infection4, 12. In striking contrast to the murine system, the initial steps for T. gondii recognition by human cells remain largely unknown due to a lack of the functional genes encoding the key innate sensors TLR11 and TLR12 in the human genome13. TLR11 is a pseudogene, and the gene encoding TLR12, which is capable of forming a heterodimer with TLR118, 9, 10, is not present in the human genome14. It is also unknown if T. gondii profilin can be recognized by human cells. While additional innate immune sensors including TLR2, TLR4, NLRP1 (NOD-, LRR- and pyrin domain-containing 1), and NLRP3 can be activated by the parasite, they all play a largely dispensable role in the regulation of host defense and cytokine production4, 15, 16, 17. Thus, while up to one-third of the world’s population is infected with T. gondii and established a long-lasting immunity against the parasite18, how human innate immune cells recognize the parasite remains largely unknown.
Results
T. gondii triggers human CCL2 response
Myeloid cells are sentinels of the immune system and are present in the blood, lymphoid organs and across many tissues. These cells are involved in the early interactions with T. gondii19and we hypothesized that they are capable of the initial parasite recognition and induction of the protective innate immune responses. The lack of knowledge of the human cell types involved in T. gondii recognition prompted us to apply a system biology approach with peripheral human blood cells that are comprised of multiple hematopoietic cells. We aimed to decipher the human innate immune defense program via identification of the mediator of host defense triggered by the parasite.
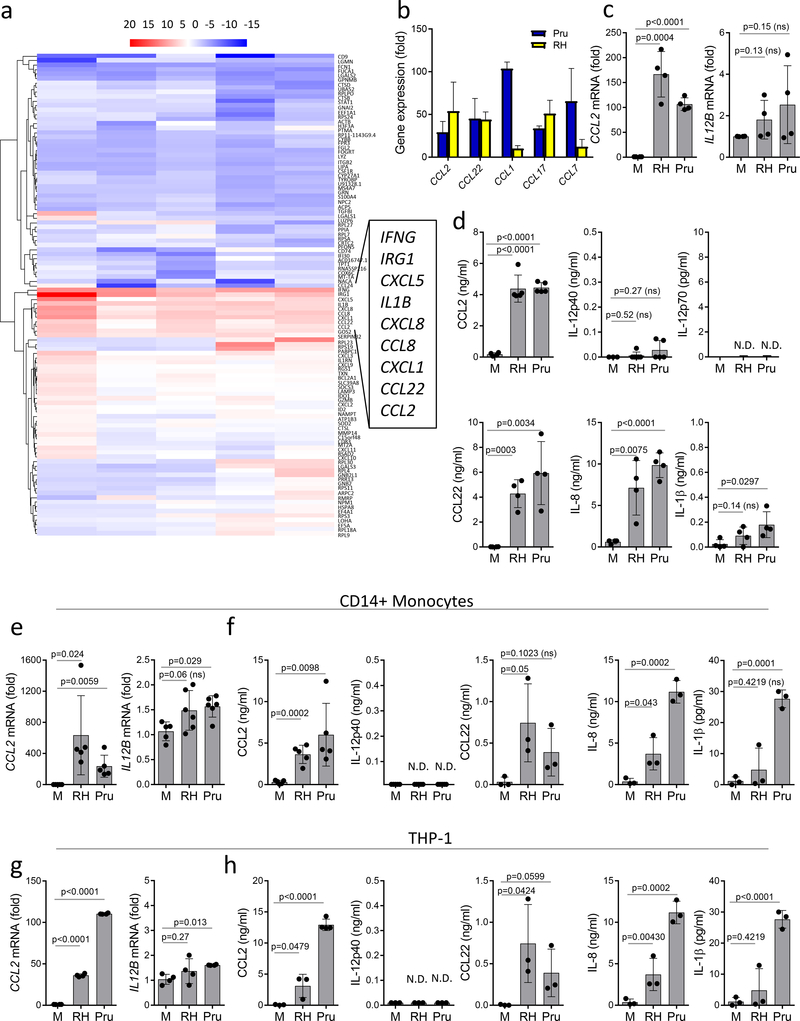
An unbiased RNAseq analysis of human peripheral blood cells revealed that innate responses to T. gondii were primarily characterized by the induction of chemokine expression, including CCL2, rather than IL-12 which was evident by the low induction of IL12B and IL12A (Fig. 1a,b, and Supplementary Fig. 1). These results were observed irrespective of the common laboratory T. gondii strains used for the experimental infection (RH88 and Pru).
Figure 1.
Transcriptome analysis identifies CCL2 as a signature response to T. gondii infection.
(a) Global gene transcriptome analysis of human PBMCs (n=5) infected with Pru tachyzoites (MOI 3:1) for 12 hours. RNA samples were collected and analyzed by RNAseq. (b) Analysis of chemokine expression in PBMCs (n=5) exposed to Pru (blue bars) and RH88 (yellow bars) strains of T. gondii by RNAseq. (c) Quantitative RT-PCR analysis for CCL2 and IL12B expression in human PBMCs (n=4) infected with Pru and RH88 strains of T. gondii. (d) Production of CCL2, IL-12p40, IL-12p70, CCL22, IL-8 and IL-1β by human PBMCs (n=4) infected with Pru and RH88 strains of T. gondii. (e) Analysis of CCL2 and IL12B expression and (f) CCL2, IL-12p40, IL-12p70, CCL22, IL-8 and IL-1β secretion in purified human CD14+ monocytes infected with Pru or RH88 strains of T. gondii. (g) Expression of CCL2 and IL12B in T. gondii-infected THP-1 cells. (h) Secretion of CCL2, IL-12p40, IL-12p70, CCL22, IL-8 and IL-1β by THP-1 cells infected with Pru and RH88 strains of T. gondii. The data shown are representative of (a,b) three, (c-d, g-h) five, (e,f) four independent experiments, and error bars shown represent the mean ± SD. Each symbol represents (c,d) an individual PBMC sample, (e-f) CD14+ monocytes isolated from an individual donor, (g,h) an individual cell culture well with THP-1 cells. Unpaired two-tailed Student’s t test was used for the statistical analysis, ns = not significant, N.D.=not detected.
Quantitative real-time PCR analysis of CCL2 and IL12B expression confirmed the screening results and established that T. gondii infection of human blood cells results in much greater fold induction of CCL2 as compared to IL12B (Fig. 1c).
While our results revealed that the induction of CCL2 expression is a common effector mechanism downstream of the innate recognition of the parasite by human cells, we also observed that there were differences in the Pru and RH88 strain-mediated induction of the chemokine responses (Fig. 1 and Supplementary Fig. 1). This observation is most likely due to the differences in virulence factors capable of regulating chemokine response in the infected cells and the pathogenicity of the T. gondii strains20, 21, 22, 23.
Human peripheral blood mononuclear cells (PBMCs) secreted large amounts of CCL2, CCL22 and IL-8 protein, but not IL-12 or IL-1β (Fig. 1d). Furthermore, we observed that, in addition to PBMCs and similar to primary monocytes, the human monocyte cell line THP-1 induced high expression of CCL2 (Fig. 1g) and production of CCL2 protein in response to T. gondii (Fig. 1e-h). This observation suggested that the human recognition system for the parasite is distinct from the mouse recognition system that results in IL-12 production in response to T. gondii profilin. This notion was further evident by the lack of CCL2 or IL12B expression or secretion by human monocytes directly exposed to T. gondii profilin (Fig. 2a,b). Overall, the unbiased system approach with human PBMCs, monocytes and an experimental THP-1 cell line infected with T. gondii revealed that the parasites triggered a potent chemokine response rather than inducing IL-12, a key signature of the mouse innate immune response to T. gondii4. When combined with the lack of the functional genes encoding TLR11 or TLR12 in the human genome, our results formally established that innate recognition of the common parasite T. gondii is mediated by distinct mechanism between human and mouse myeloid cells.
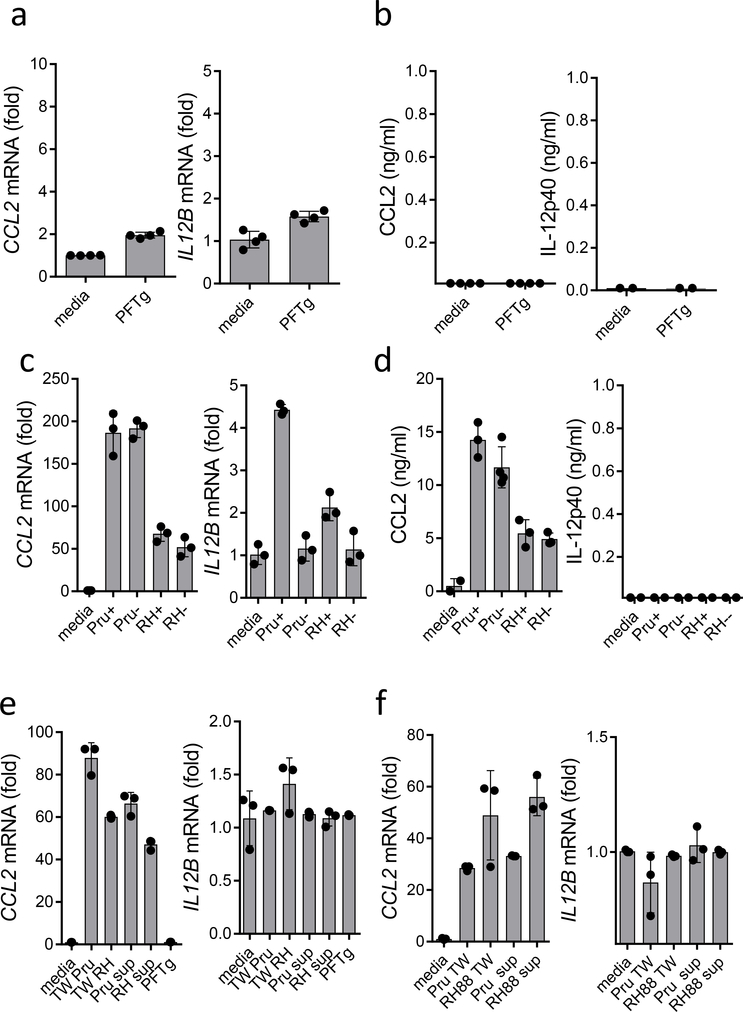
Figure 2.
Soluble mediator elicits CCL2 production in response to T. gondii infection. Lack of CCL2 and IL12B induction (a) and secretion (b) in THP-1 cells stimulated with purified T. gondii profilin (PFTg). (c) THP-1 cells infected with mCherry-expressing Pru and RH88 strains of T. gondii were purified as infected (Pru+ and RH+) and uninfected cells (Pru- and RH-). The expression of CCL2 and IL12B was measured by RT-PCR. (d) Protein secretion of CCL2 and IL-12p40 was measured by ELISA. (e) Analysis of CCL2 and IL12B expression in THP-1 cells exposed to soluble mediators in a transwell system (TW) or by direct stimulation with cell culture supernatants collected from infected monocytes. (f) Analysis of CCL2 and IL12B expression in THP-1 cells exposed to soluble mediators in a transwell system (TW) with infected fibroblasts or by direct stimulation with cell culture supernatants collected from infected fibroblasts. Each symbol represents an individual experimental sample. (a,b) n=4, (c–f) n=3. The data shown are representative of five independent experiments, and error bars shown represent the mean ± SD.
Soluble mediator triggers human CCL2 response
T. gondii is an obligate intracellular parasite, and the growing numbers of intracellular innate immune sensors prompted us to examine if the infection of monocytes is a trigger for CCL2 production in a cell-intrinsic manner. THP-1 cells were infected with mCherry-expressing parasites24, and 12 h later the monocytes were sorted and purified as either infected (mChrerry+) or non-infected (mCherry-) cells based on the presence of the fluorescent parasite. The following quantitative RT-PCR analysis of CCL2 expression revealed that both infected and non-infected monocytes produced similar amounts of CCL2 (Fig. 2c,d). In the infected mCherry+ cells specifically, a small but detectable induction of IL12B expression, but not secretion of IL-12p40 was observed (Fig. 2d), as previously reported25. Taken together, these results suggest that the intracellular presence of T. gondii is dispensable for the induction of CCL2 by monocytes.
Host-parasite interactions result in the presence of a third population of cells that, while not actively infected with the parasite, are exposed to parasitic molecules via contact-dependent injection of virulence factors (uninfected-injected cells)20, 24, 26, 27. To examine whether monocytic production of CCL2 requires cell contact with T. gondii, CCL2 expression was examined in primary monocytes and THP-1 cells that were separated from the parasite in a transwell system. We observed that a lack of direct contact between T. gondii and monocytes failed to prevent induction of CCL2 expression (Fig. 2e,f). Taken together, these results suggested that soluble mediators were responsible for CCL2 induction in response to parasite infection. This hypothesis was confirmed by the induction of CCL2 expression by cell culture supernatants collected from monocytes (Fig. 2e,f). Moreover, supernatants from non-immune fibroblasts induced CCL2 expression in THP-1 cells and primary monocytes in the transwell system; the monocytes and THP-1 cells also underwent direct activation by cell culture supernatants collected from T. gondii-infected cells (Fig. 2e,f). Neither control supernatants collected from non-infected cells nor recombinant T. gondii profilin induced CCL2 expression in human monocytes (Fig. 2e,f and data not shown). These results strongly suggest that a soluble mediator elicits CCL2 production in response to T. gondii infection.
Human alarmin S100A11 induces CCL2
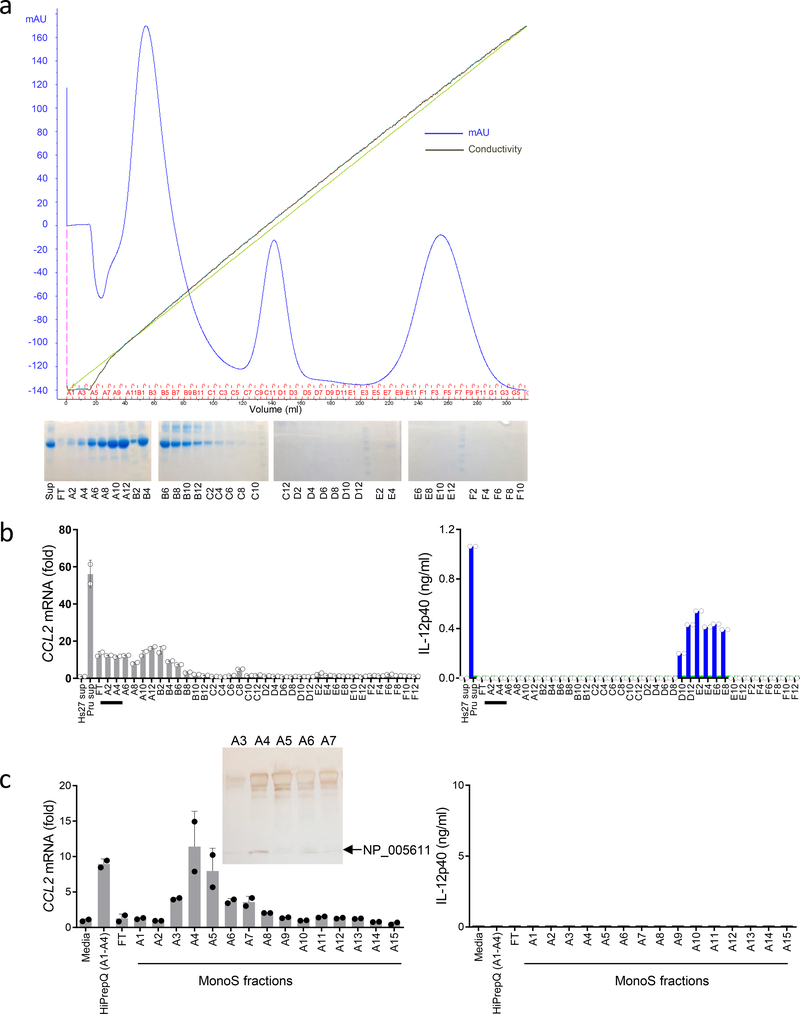
Biochemical purification of a soluble mediator of CCL2 induction was next performed from cell culture supernatants of human fibroblasts infected with T. gondii. Anion-exchange purification revealed that CCL2-inducing fractions were rapidly separated from those inducing IL-12 production from mouse splenocytes via the TLR11-mediated signaling pathway (Fig. 3a,b). These results were obtained by the analysis of CCL2 expression in THP-1 cells. The IL12B-inducing fractions in mouse splenocytes contained T. gondii profilin that was released during infection and stimulated cells via TLR11 (Fig. 3b, and data not shown). These experiments established distinct biochemical properties of CCL2-inducing molecules by human cells from T. gondii profilin-initiated TLR11-dependent induction of IL-12 by mouse cells (Fig. 3b). An additional purification step resulted in a relatively pure fraction, A4, that was capable of inducing potent CCL2 expression in THP-1 cells but not IL-12p40 from mouse splenocytes (Fig. 3c). Combined with the pilot experiments that suggested a low molecular weight protein as the CCL2-inducing molecule (data not shown), mass-spectrometry of the detectable low weight protein band was subsequently performed, resulting in identification of the human S100A11 protein (Fig. 3c, Supplementary Fig. 2a). This protein belongs to a large family of small Ca2+-binding proteins containing two conserved EF-hand motifs that serve pleotropic cellular functions and can also function as a group of damage-associated mediators of inflammation28, 29, 30, 31. Sequence detection by mass-spectrometry revealed that S100A11 is distinct from the remaining S100 proteins (Supplementary Fig. 2a and data not shown).
Figure 3.
Biochemical isolation of S100A11 as a CCL2-inducing molecule.
(a) Initial separation of cell culture supernatants collected from human fibroblasts infected with the Pru strain of T. gondii via HiPrepQ anion-exchange chromatography and (b) assay of individual fractions in duplicates for their ability to induce expression of CCL2 in THP-1 cells (left) and to stimulate IL-12p40 secretion by mouse splenocytes prepared from WT (blue bars) and Tlr11–/– (green bars) mice, right. mAU = milli-Absorbance Units. (c) Mono S cation-exchange chromatography of fractions A1-A4 from the HiPrepQ anion-exchange chromatography. Individual fractions were assayed in duplicates. Each symbol represents an individual experimental sample. The data shown are representative of (a-b) five and (c) three independent experiments, and error bars shown represent the mean ± SD. The inset shows an SDS-PAGE analysis of the Mono S cation-exchange chromatography fractions A3, A4, A5, A6, and A7, with the arrow indicating a band analyzed by mass-spectrometry that revealed the presence of the human S100A11 protein (NP_005611).
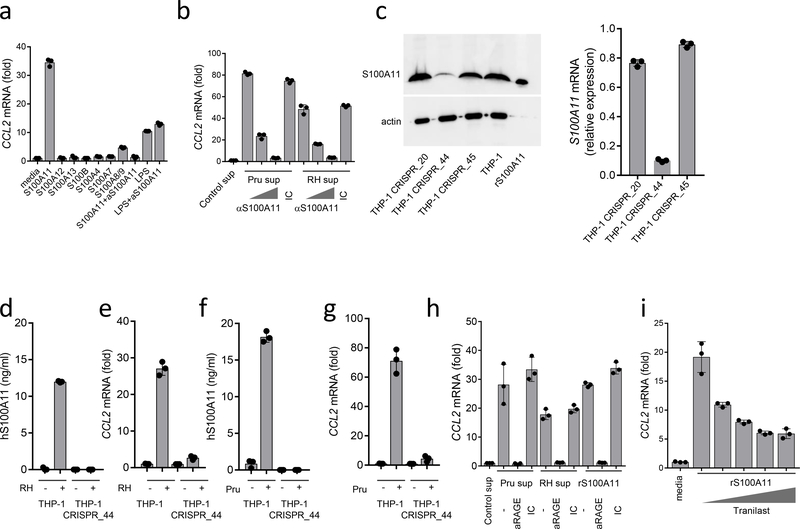
We next directly examined whether S100A11 was sufficient for the induction of CCL2. We observed that highly purified recombinant S100A11 caused potent induction of CCL2 expression in THP-1 cells (Fig. 4a). When several other S100 proteins were used in similar assays, we observed that only the S100A8/A9 complex was capable of inducing substantial CCL2 expression in the stimulated cells, albeit at a lower level when compared to S100A11 (Fig. 4a). In addition, we compared S100A11-inducing CCL2 production with lipopolysaccharide (LPS)-mediated activation of the same chemokine since recombinant S100A11 was expressed in Escherichia coli. We observed that large amounts of LPS induced relatively low amounts of CCL2 compared to S100A11 (Fig. 4a). Furthermore, an S100A11-blocking antibody completely prevented CCL2 induction mediated by S100A11, but not the induction induced by LPS (Fig. 4a). Taken together, these results established that S100A11 alone can induce CCL2 expression by human monocytes. To further examine if soluble S100A11 mediated the CCL2 response, cell culture supernatants were collected from RH88 or Pru-infected fibroblasts and incubated with control or S100A11-blocking antibodies prior to stimulation of human monocytes. We observed that blocking S100A11 prevented CCL2 induction by cell culture supernatants collected from T. gondii-infected fibroblasts (Fig. 4b).
Figure 4.
Role of RAGE in S100A11-induced CCL2
THP-1 cells were stimulated with purified recombinant S100A11, S100A12, S100A13, S100B, S100A4, S100A7, S100A8/9 (all at 10 ng/ml) or with LPS (1 μg/ml), and CCL2 expression was analyzed by RT-PCR 12 hours after stimulation. In several experiments, an anti-S100A11 antibody was added to S100A11 protein (S100A11+α−S100A11) or to LPS (LPS+α−S100A11) for 30 min prior to stimulation. (b) Cell culture supernatants were collected from uninfected (control), Pru- or RH88-infected human fibroblasts and were used to stimulate monocytes directly or in the presence of increasing amounts of anti-S100A11 antibody or the isotype control (IC). CCL2 expression was analyzed by RT-PCR. (c) The knockdown of S100A11 in THP-1 cells (THP-1_CRISPR_44) was revealed by immunoblot and qRT-PCR and resulted in loss of S100A11 release and induction of CCL2 in response to (d,e) RH88 and (f,g) Pru infections. (h) Induction of CCL2 by S100A11 or cell culture supernatants collected from T. gondii-infected cells was prevented by adding an anti-RAGE antibody but not the isotype control (IC), (i) Induction of CCL2 by S100A11 was partially blocked by tranilast. The data shown represent the mean ± SD of assays performed in triplicate and are representative of four independent experiments performed.
To examine if S100A11 is required for CCL2 induction in response to T. gondii infection, we generated S100A11-deficient THP-1 cell lines using the CRISPR/Cas9 genome editing method. The stable S100A11 knockout THP-1-CRISP_44 cells but not the S100A11-expressing THP-1 cells showed a lack of expression of S100A11 measured by immunoblot, RT-PCR and enzyme-linked immunosorbent assay (ELISA), (Fig. 4c,d,f and Supplementary Fig. 2b). The S100A11-deficient THP-1 cells failed to produce significant amounts of CCL2 when infected with RH88 or Pru strains of T. gondii (Fig 4e,g). Similarly, we observed that siRNA sequences against S100A11 severely reduced CCL2 induction in T. gondii-infected cells (Supplementary Fig. 3). Collectively, these results revealed that S100A11 is not only sufficient for induction of the CCL2 response but is in fact required for this activity.
S100A11 induces CCL2 via RAGE
To decipher the mechanism by which S100A11 initiates the CCL2 response, we reanalyzed the RNAseq data with the goal of identifying signaling pathways potentially leading to CCL2 production. The most prominent signaling pathway was a RAGE-induced cellular response to alarmins (Supplementary Fig. 4 and Supplementary Fig. 5). RAGE is known to interact with other S100 family members32, 33, 34, which prompted us to directly examine the requirement for RAGE in the S100A11-mediated induction of CCL2. We found that stimulation of THP-1 cells with purified S100A11 in the presence of a RAGE blocking, but not the isotype control antibody, prevented the induction of CCL2 expression (Fig. 4h). Similar results were obtained when monocytes were stimulated with cell culture supernatants collected from T. gondii-infected, but not control human fibroblasts (Fig. 4h). The presence of RAGE blocking antibodies abrogated the induction of CCL2 by cell culture supernatants produced from the infected cells (Fig. 4h). In addition, CCL2 induction was diminished when monocytes were stimulated with S100A11 in the presence of tranilast, a small molecule inhibitor of the V domain in RAGE, which is involved in interactions with the S100 proteins35 (Fig. 4i). These results revealed that RAGE is a key receptor that mediates S100A11-induced CCL2 production by monocytes.
Caspase-1 mediates S100A11 release
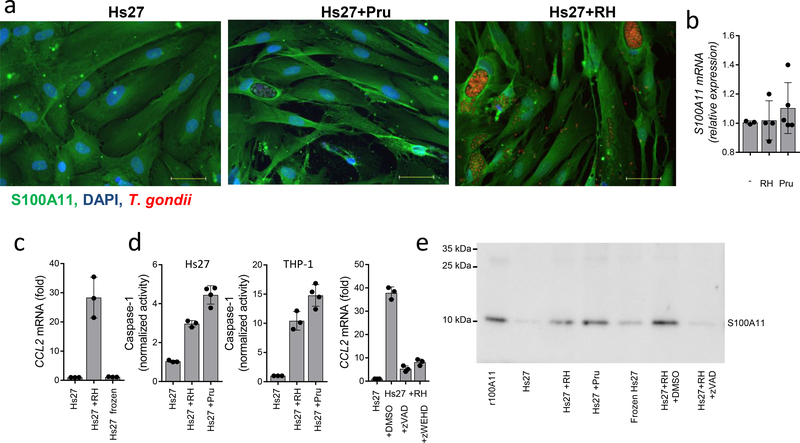
Identification of the human intracellular protein S100A11 as a mediator of the paracrine CCL2 response in T. gondii-infected cells raised questions regarding mechanisms regulating S100A11 release from infected cells. We first examined whether parasite invasion was required for the S100A11-mediated induction of CCL2. We observed that when T. gondii was rendered incapable of invasion due to profilin deficiency7, it failed to induce S100A11-dependent induction of CCL2 expression (Supplementary Fig. 6a). Similarly, blocking parasite invasion with mycalolide B36, 37 prevented T. gondii-induced induction of CCL2 in human monocytes (Supplementary Fig. 6b). These results prompted us to examine whether the cellular lysis ultimately caused by the parasite is a cause of S100A11 release and induction of CCL2. Our experiments revealed that while T. gondii infection did not change the expression or abundance of S100A11 in infected and surrounding cells (Fig. 5a,b), parasite-mediated cellular damage was required for S100A11 release. Cellular death caused by rapid freezing was not sufficient for the liberation of enough S100A11 for the induction of CCL2 (Fig. 5c, Supplementary Fig. 2c). In addition, we observed that not only T. gondii infection resulted in the activation of caspase-1 (Fig. 5d), but also the pan-caspase inhibitor Z-VAD and the caspase-1 inhibitor Z-WHED reduced S100A11 release and abolished CCL2 induction by supernatants collected from T. gondii-infected cells (Fig. 5d,e, and Supplementary Fig. 2c). Caspase-1 (CASP1) deficient THP-1 cells also demonstrated reduced production of CCL2 in response to T. gondii infection (not shown). As expected, CCL2 production by caspase-1-deficient cells was not compromised when THP-1 cells were stimulated with S100A11-containing supernatants (not shown). Overall, these results revealed that an active cellular response to T. gondii infection by an unknown intracellular receptor leads to the caspase-1 depended release of S100A11, which is required and sufficient for the induction of CCL2.
Figure 5.
Role of Caspase-1 in S100A11 release
Analysis of S100A11 protein levels in Pru or RH88-infected fibroblasts by (a) immunofluorescent detection and (b) by expression analysis. Scale bars, 50 μm. Error bars shown represent mean ± SD. (c) CCL2 expression by THP-1 cells stimulated with cell culture supernatants collected from frozen cells in comparison with RH88-infected cells. (d) Analysis of Caspase-1 activity in Hs27 and THP-1 cells infected with RH88 and Pru strains of T. gondii. CCL2 expression in THP-1 cells stimulated with cell culture supernatants collected from human Hs27 fibroblasts infected with RH88 alone or in the presence of zVAD (10 uM) or zWEHD (10 uM) for 72 hours was measured by qRT-PCR. (e) Detection of S1000A11 in cell culture supernatants collected from Hs27 cells, Hs27 cells infected with RH88 (Hs27+RH, MOI 3:1) or Pru (Hs27+Pru, MOI 3:1), Hs27 cells killed by rapid freezing (Frozen Hs27), or Hs27 cells infected with RH88 in the presence of zVAD (Hs27+RH+zVAD). Hs27+RH+DMSO represents an additional vehicle control supernatant for the zVAD-treated cells. Each symbol represents an individual experimental sample, (b-d) n=3 and n=4. The data shown are representative of (a-d) three and (e) five independent experiments, and error bars shown represent the mean ± SD.
S100A11 regulates monocyte recruitment in vivo
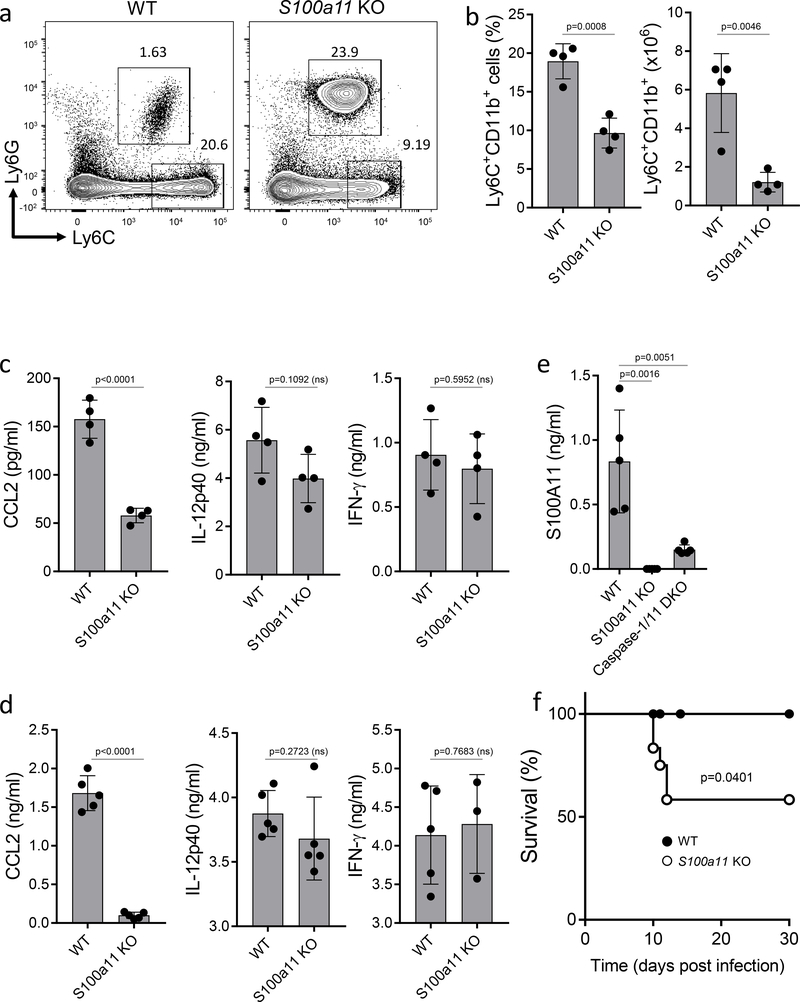
To examine the importance of S100A11 in the regulation of immunity to T. gondii in vivo, S100A11-deficient mice were generated (Supplementary Fig. 7) and infected intraperitoneally with T. gondii. We observed that a lack of S100a11 resulted in compromised monocyte recruitment to the site of infection (Fig. 6a,b), which correlated with reduced concentrations of CCL2 detected in infected mice (Fig. 6c). Concurrently, the lack of S100a11 had no effect on the induction of the IL-12 and interferon-γ (IFN-γ) responses in vivo (Fig. 6c,d). The high concentrations of these cytokines may explain only a partial role for S100A11 in host survival during intraperitoneal T. gondii infection (Fig. 6f). We also observed reduced concentrations of S100A11 in T. gondii infected caspase1/11-deficient mice, confirming that caspase-1 plays a role in S100A11 release in vivo (Fig. 6e).
Figure 6.
S100A11 regulates monocyte recruitment in vivo. (a) WT and S100a11 KO mice were infected intraperitoneally with T. gondii, and the presence of inflammatory monocytes and neutrophils at the site of infection was analyzed by flow cytometry on day 5 post infection. (b) Average frequency and absolute numbers of monocytes in T. gondii-infected WT and S100a11 KO mice on day 5 post infection. The data shown are representative of four independent experiments each involving 4–6 age- and sex-matched mice. (c) CCL2, IL-12p40, and IFN-γ production were measured in the peritoneal cavity on day 5 post infection by ELISA. The data shown are representative of three independent experiments (d) CCL2, IL-12p40, and IFN-γ production were measured in sera on day 5 post infection by ELISA. Each symbol represents an individual experimental muse (e) WT, S100a11 KO, and Caspase-1/11 DKO mice were infected intraperitoneally with T. gondii, and the release of S100A11 was analyzed by ELISA on day 5 post infection. The data shown are representative of three independent experiments. (f) Survival of WT (black circles) and S100a11 KO (open circles) mice infected with T. gondii (20 cysts per mouse). The data shown are representative of four independent experiments each involving 6–8 age- and sex-matched mice. (b-e) Unpaired two-tailed Student’s t test and (f) Mantel-Cox tests were utilized for the statistical analysis, ns = not significant
Oral infection with T. gondii revealed that S100A11 contributes to the induction of CCL2 and migration of monocytes to the site of infection, but not to the IFN-γ mediated response to the parasite (Supplementary Fig. 8). Nevertheless, S100a11 deficiency resulted in decreased intestinal pathology caused by the parasitic infection (Supplementary Fig. 8e,f), indicating that in addition to the protective immunity, the release of S100A11 may lead to the enhanced immunopathological response caused by T. gondii.
Discussion
Herein, we established a biochemical basis for the induction of the chemokine response to T. gondii infection by human cells. Our results revealed that infection with T. gondii leads to the release of S100A11 from infected cells. This soluble mediator is both necessary and sufficient for RAGE-dependent induction of CCL2, a major chemokine required for monocyte-mediated immunity to pathogens38, 39, 40, 41.
The previously defined mechanisms of innate immunity to T. gondii and closely related apicomplexan parasites were chiefly mediated by TLR11-mediated recognition of profilin, an essential molecule for the parasite invasion to the host cells. The TLR11-dependent innate recognition results in a rapid and selective induction of IL-12 which is required for the highly polarized Th1 immunity4. The additional results from our and other laboratories determined that TLR11 forms a heterodimer with TLR12 and detects profilin released from T. gondii within the endolysosomal compartment4. Despite deciphering the molecular events required for the TLR11-dependent activation of MyD88, a major question for TLR11-independent sensing of the parasite still remained. This gap in knowledge needs to be filled, since TLR11 and TLR12 immunity to T. gondii is not functional in human cells. TLR11 is a pseudogene and TLR12 is missing in the human genome13. Additional TLRs, in particular TLR2, and TLR9, as well as the NLRP1 and NLRP3 inflammasomes are engaged by the parasite, but play a minor role in defense against T. gondii16, 42. Combined with the global distribution of T. gondii within the human population and the clinical importance of this parasite, we aimed to define innate immune sensors that could be involved in human defense against T. gondii.
A system biology approach to define innate immune pathways based on the analysis of the effector molecules triggered by T. gondii infection, combined with the biochemical screening revealed that the human recognition system for this parasite is based on the detection of damage-associated molecule S100A11 released from infected cells. The human recognition system is distinct from the previously identified TLR11-mediated immune response to the parasite in mice, which depends on direct sensing of the essential T. gondii protein4, 6. A major downstream effector of S100A11-mediated activation of human monocytes is CCL2, a chemokine regulating monocyte recruitment to the site of infection43. This contrasts the effector soluble mediators released by mouse and human cells in response to T. gondii. Mouse dendritic cells produce large amounts of IL-12, while the human response to T. gondii is predominantly characterized by expression of CCL2. Despite biochemical differences in the recognition systems for T. gondii between mice and humans, it is important to emphasize that both human and murine immunity to T. gondii rely on sensing the active infection rather than the pathogen itself. This is because TLR11 detects profilin released from infected cells but not the parasite itself 44. Moreover , activation of TLR11 by T. gondii profilin is predominantly seen in uninfected cells44. Similarly, human monocytes sense S100A11 released from the surrounding cells infected with the parasite. This finding may represent an innate immune mechanism not sensitive to an array of T. gondii-virulence factors capable of interfering with the host defense pathways and determining the outcome of infection in vivo20, 45, 46, 47. Consistent with this notion, the expression of S100A11 was not affected by parasite infection, suggesting that S100A11 is a pre-made alarmin that initiates rapid CCL2-mediated immune responses to the common intracellular parasite T. gondii.
Our in vivo results in mice suggest that the release of S100A11 from the infected cells plays a role in both host protection against T. gondii and immunopathology mediated by CCL2-dependent defense mechanisms. Additional studies are needed to determine if S100A11-mediated immunity to T. gondii in humans is also primarily regulated via recruitment of monocytes to the site of infection.
Overall, our experiments have revealed that human cells sense T. gondii by detection of infection-mediated release of S100A11. This alarmin engages RAGE and initiate a signaling pathways leading to the induction of CCL2, a crucial chemokine required for host resistance to the parasite.
Methods
Mice
To generate S100a11–/– mice, exons 2 and 3 of the S100A11 gene were targeted by two sgRNAs using CRISPR–Cas9 technology (Supplementary Fig. 7) at the Mouse Genome Editing (MGE) Resource at the University of Rochester Medical Center. For genotyping, the targeted alleles were detected by PCR amplification with a set of primers S100A11 wtF (5′- GAGGCACTGCGCTCCTCTGGCACACT −3′) and S100A11 wtR (5′- CTCCTGCTACCAGCTTCCATGTCAC −3′) that result in PCR products of 2.7 kb for WT mice and 557 bp for the S100a11 KO allele, as a result of deletion of exons 2 and 3. S100a11 KO mice were generated on C57BL/6 background. S100a11 KO mice of both genders were born in Mendelian ratios and gained weight normally throughout development. Sex- and age-matched 6- to 12-week-old mice were used for experiments.
All mice were maintained in the pathogen-free animal facility at the University of Rochester School of Medicine and Dentistry, Rochester, NY. All animal experimentation was conducted in accordance with the guidelines of the University of Rochester’s University Committee on Animal Resources (UCAR), the Institutional Animal Care and Use Committee (IACUC). All animal experimentation in this study was reviewed and approved by the University of Rochester’s University Committee on Animal Resources (UCAR), the Institutional Animal Care and Use Committee (IACUC).
Human blood samples
PBMCs were isolated by Ficoll-plaque density gradient centrifugation from heparinized peripheral blood of 20 healthy volunteers. All donors consented to sample donation consistent with the University of Rochester Institutional Review Board’s recommendations. Both freshly purified and cryopreserved in liquid nitrogen PBMCs were utilized for the study. Cryopreserved PBMCs were thawed in warm complete media (RPMI 1640, 10% FBS, penicillin/streptomycin, L-glutamine) in preparation for in vitro stimulation. All assays were performed in triplicate.
Human primary monocytes were isolated from PBMCs using CD14 MACS microbeads (Miltenyi) or purified as CD14+ cells from freshly prepared PBMCs.
Cell lines
THP-1 cells (TIB-202) and Hs27 fibroblasts (RL-1634) were purchased from ATCC.
Reagents
The following siRNA targeting human S100A11 were used: Sense: 5′-CUUCAUGAAUACAGAACUAUU-3′ , Antisense: 5′-UAGUUCUGUAUUCAUGAAGUU-3′, (CTM-370710); Sense: 5′ -UGAAGAAACUGGACACCAAUU-3′, Antisense: 5′-UUGGUGUCCAGUUUCUUCAUU-3’, (CTM-370711); Sense: 5′-CAACAGUGAUGGUCAGCUAUU-3′, Antisense: 5′-UAGCUGACCAUCACUGUUGUU-3′, (CTM-370712); Sense: 5′-GAAGUAUGCUGGAAAGGAUUU-3′, Antisense: 5′-AUCCUUUCCAGCAUACUUCUU-3′ (CTM-370713). Custom made siRNA against human S100A11 and the control siRNA were ordered from Dharmacon. The knockdown of S100A11 in THP-1 cells was performed by CRISPR/Cas9 (clustered regularly interspaced short palin-drome repeats) guided genome editing. E-CRISP, a web application (http://www.e-crisp.org/E-CRISP/) was utilized to design gRNA sequences (S100A11_44: 5′-GCTGTCTTCCAGAAGTATGC NGG-3′; S100A11_20: 5′-GGGTCCTCAGGTCCGCTTCT NGG-3′; S100A11_45: 5′-GGGCTGGAGATTTTTGCCTT NGG-3′) cloned to lentiCRISPR v2 plasmid (Addgene, #52961) and transduced to THP-1 cells by lentivirus-mediated magnitofection (ViroMag R/L Transduction Reagent, OZ Biosciences).
Caspase-1 Fluorometric Assay
Caspase-1/ICE Fluorometric Assay Kit (R&D Systems, K110–100) was used to measure Caspase-1 activity in T. gondii-infected cells. All assays were performed in triplicate.
Toxoplasma gondii infections
For in vitro experiments, PBMCs, CD14+ monocytes, THP-1 or Hs27 cells were infected with RH88 or Pru T. gondii strains at an MOI=3:1 for the indicated times. In several experiments, either a pan-caspase inhibitor or a caspase-1 inhibitor was added on day 1 post infection. No effect of the inhibitors on the growth of T. gondii in vitro in Hs27 cells was observed by plaque assay.
For in vivo experiments, age- and sex-matched 6- to 12-week-old WT and S100a11−/− mice were intraperitoneally infected with 20 T. gondii brain cysts (ME49 strain) for the duration of the experiments. For the analysis of monocyte recruitment to the site of infection, peritoneal exudate cells were collected on day 5 post infection and analyzed by flow cytometry. CD11b (clone M1/70) and CD45 (clone 30-F11) antibodies were purchased from BD Bioscience; Ly6C (clone HK1.4) and Ly6G (clone 1A8) were purchased from eBioscience (ThermoFisher).
Isolation of lamina propria cells was performed as follows. The small intestine was removed on day 7 post infection and carefully cleaned of the mesentery and Peyer patches. The intestine was then opened longitudinally, washed of fecal contents, cut into smaller sections and subjected to 2 sequential incubations in PBS with 5 mM EDTA and 1mM DTT at 37°C with agitation to remove epithelial cells. The solution was discarded between incubation steps and replaced. The remaining tissue was agitated in PBS and then filtered through a 100-μm strainer. The tissue was then incubated for 30 min with gentle agitation in 0.4 mg/ml of Collagenase D and 50 mg/ml of DNase I at 37 °C. The samples were then washed through a strainer (100 μm).
The severity of intestinal pathology was analyzed based on the following additive scoring system48, 49, 50. For crypt and villi integrity: 0, normal; 1, irregular villi and crypts; 2, mildly inflamed; 3, severe villi and crypt loss; 4, complete villi and crypt loss with an intact epithelial cell layer; 5, complete loss of villi and crypts and surface epithelium. For infiltration of inflammatory cells into the mucosa: 0, normal; 1, mild; 2, modest; and 3, severe. For infiltration of the submucosa: 0, normal; 1, mild; 2, modest; and 3, severe. For infiltration of the muscle: 0, normal; 1, mild; 2, modest; and 3, severe. These scores were added, resulting in a total scoring range of 0 to 14.
Cytokine measurements
Cytokines CCL2, IL-12p40 and IFN-γ were measured with ELISA kits (eBioscience) and by Milliplex MAP Human Cytokine/Chemokine Magnetic Bead Panel (IL-12p40, IL-12p70, IL-1β, IL-8/CXCL8, MCP-1/CCL2, MIP-1α/CCL3, MDC/CCL22, MCP3/CCL7).
S100A11 isolation, purification, and in vitro assays.
Cell culture supernatants were collected from RH88- and Pru-infected HS27 cells cultured in complete media (RPMI 1640, 10% FBS, penicillin/streptomycin, L-glutamine). The cell culture supernatants were first filtered through a 0.22 μm filter and then separated by a HiPrepQ chromatography column by applying a linear gradient of 0–1 M NaCl in 20 mM Tris, pH 8.0. The collected fractions were analyzed on 12% SDS-PAGE gels (BioRad) and tested for their ability to trigger CCL2 expression in THP-1 cells. The active fractions A1, A2, A3, and A4 were pooled and subjected to cation-exchange chromatography on a Mono S column. The active FPLC fraction were analyzed by SDS-Page and subjected to MS-MS analysis. The identified S100A11 peptides are shown in Supplementary Fig. 2a.
For protein expression, the synthetic S100A11 gene (NM_005620) was prepared at IDT and inserted into the pET-17b vector (Novagen) using its NdeI and XhoI restriction sites, and then expressed in E. coli strain BL21(DE3) pLysS cells. The resulting recombinant protein was purified to homogeneity (as judged by SDS-PAGE) by a combination of anion exchange and Sepahacryl S-100 chromatography. Lipopolysaccharide (LPS) levels in the recombinant protein preparations were analyzed using the LAL Chromogenic Endotoxin Quantification kit (Thermo) per the manufacturer’s instructions.
The CCL2-inducing activity of cell culture supernatants and recombinant S100A11 protein was assayed by THP-1 cells, PBMCs, or CD14+ monocytes with serial dilutions of the test sample, followed by RNA extraction and measurement of CCL2 and IL12B expression by RT-PCR.
For immunofluorescence, control and T. gondii-infected cells were fixed in 4% paraformaldehyde in PBS for 4 hours. Cells were permeabilized with 0.2% Triton-X in PBS, and blocked with 0.1% Triton-X with 5% Normal Goat Serum in PBS. Permeabilized cells were incubated with rabbit polyclonal anti-S100A11 followed by secondary Alexa Fluor 488 conjugated antibody (GE healthcare) for 1 hour at room temperature, and counterstained with DAPI. Slides were imaged with an inverted microscope (Leica DMi8) using a Leica 40X objective.
Quantitative Real-Time PCR
Total RNA was isolated from experimental cells using a Purelink RNA mini kit (Life Technologies) and subjected to first-strand cDNA synthesis using an iScript Reverse Transcription Supermix for RT-qPCR (BioRad). Real-time PCR was performed using Ssofast Eva Green Supermix (BioRad). The relative expression of each sample was determined after normalization to the housekeeping gene GAPDH using the ddCt method. The following primers were used for gene expression analysis: GAPDH (5′-GAGCCCGCAGCCTCCCGCTT-3′ and 5′-CCCGCGGCCATCACGCCACAG-3′); CCL2 (5′-CCCCAGTCACCTGCTGTTAT-3′ and 5′-TGGAATCCTGAACCCACTTC-3′); IL12B (5′-AGAGGCTCTTCTGACCCCCAAG-3′ and 5′-CTCTTGCTCTTGCCCTGGACCTG-3′).
RNAseq data and signaling pathway analyses
Five randomly chosen human PBMC samples (out of n=20) were prepared for RNA sequencing experiments. For each sample, PBMC were divided into three aliquots (0.5×106 cells per sample) and were (i) left untreated, (ii) infected with T gondii Pru strain at MOI 3 (iii) infected with T. gondii RH strain at MOI 3. After 12 h post infection a total of 15 samples were subjected to RNA sequencing. Total RNA was extracted and used for library preparation following manufacturer’s protocols. Samples were sequenced on the Illumina HiSeq 1000 with 20 × 106 80-bp single-read per sample. Raw reads were analyzed within Galaxy platform, trimmed for adaptor sequence, masked for low-complexity or low-quality sequence, followed by mapping to hg38 whole human genome using TopHat v2.1.0 (default parameters) and analyzed by Cuffdiff v2.2.1 (default parameters) for differential gene expression between T. gondii infected and non-infected samples. The results of the RNAseq data analysis pipeline were uploaded to IPA software to identify signaling pathways shown in Supplementary Fig 4 and 5. The pheatmap R package was used to build heatmaps where the genes were filtered to satisfy the following conditions: (i) at least for one sample expression values in FPKM units were greater than 200 in one or more conditions (infected/non infected), and (ii) fold change ≥ 4. Only genes with logarithmic fold differences greater than two were used for heatmaps.Accession codes: RNAseq data, GSE119835.
Supplementary Material
Acknowledgements
This work was supported by NIAID Grants R01AI136538, R01AI121090, and the Burroughs Wellcome Foundation.
Footnotes
Competing interests
The authors declare no competing financial interests
Statistical analysis
All data were analyzed with Prism (version 6; Graphpad). These data were considered statistically significant when P values were less than 0.05 by two tailed t test.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The materials, data, and any associated protocols that support the findings of this study are available from the corresponding authors upon request.
Accession Codes All RNA-seq data generated in this study are deposited in Gene Expression Omnibus (GEO) under accession code GSE119835.
References
- 1.Iwasaki A & Medzhitov R Control of adaptive immunity by the innate immune system. Nat Immunol 16, 343–353 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Man SM, Karki R & Kanneganti TD Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev 277, 61–75 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawai T & Akira S The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11, 373–384 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Yarovinsky F Innate immunity to Toxoplasma gondii infection. Nat Rev Immunol 14, 109–121 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Pifer R & Yarovinsky F Innate responses to Toxoplasma gondii in mice and humans. Trends Parasitol 27, 388–393 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yarovinsky F et al. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 308, 1626–1629 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Plattner F et al. Toxoplasma profilin is essential for host cell invasion and TLR11-dependent induction of an interleukin-12 response. Cell Host Microbe 3, 77–87 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Raetz M et al. Cooperation of TLR12 and TLR11 in the IRF8-dependent IL-12 response to Toxoplasma gondii profilin. J Immunol 191, 4818–4827 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koblansky AA et al. Recognition of profilin by Toll-like receptor 12 is critical for host resistance to Toxoplasma gondii. Immunity 38, 119–130 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrade WA et al. Combined action of nucleic acid-sensing Toll-like receptors and TLR11/TLR12 heterodimers imparts resistance to Toxoplasma gondii in mice. Cell Host Microbe 13, 42–53 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neal LM & Knoll LJ Toxoplasma gondii profilin promotes recruitment of Ly6Chi CCR2+ inflammatory monocytes that can confer resistance to bacterial infection. PLoS Pathog 10, e1004203 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dupont CD, Christian DA & Hunter CA Immune response and immunopathology during toxoplasmosis. Semin Immunopathol 34, 793–813 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roach JC et al. The evolution of vertebrate Toll-like receptors. Proc Natl Acad Sci U S A 102, 9577–9582 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muller UB & Howard JC The impact of Toxoplasma gondii on the mammalian genome. Curr Opin Microbiol 32, 19–25 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Debierre-Grockiego F et al. Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J Immunol 179, 1129–1137 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Ewald SE, Chavarria-Smith J & Boothroyd JC NLRP1 is an inflammasome sensor for Toxoplasma gondii. Infect Immun 82, 460–468 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clay GM, Sutterwala FS & Wilson ME NLR proteins and parasitic disease. Immunol Res 59, 142–152 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Black MW & Boothroyd JC Lytic cycle of Toxoplasma gondii. Microbiol Mol Biol Rev 64, 607–623 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denkers EY, Schneider AG, Cohen SB & Butcher BA Phagocyte responses to protozoan infection and how Toxoplasma gondii meets the challenge. PLoS Pathog 8, e1002794 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hakimi MA, Olias P & Sibley LD Toxoplasma Effectors Targeting Host Signaling and Transcription. Clin Microbiol Rev 30, 615–645 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gay G et al. Toxoplasma gondii TgIST co-opts host chromatin repressors dampening STAT1-dependent gene regulation and IFN-gamma-mediated host defenses. J Exp Med 213, 1779–1798 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olias P, Etheridge RD, Zhang Y, Holtzman MJ & Sibley LD Toxoplasma Effector Recruits the Mi-2/NuRD Complex to Repress STAT1 Transcription and Block IFN-gamma-Dependent Gene Expression. Cell Host Microbe 20, 72–82 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naor A et al. MYR1-Dependent Effectors Are the Major Drivers of a Host Cell’s Early Response to Toxoplasma, Including Counteracting MYR1-Independent Effects. MBio 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koshy AA et al. Toxoplasma co-opts host cells it does not invade. PLoS Pathog 8, e1002825 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tosh KW et al. The IL-12 Response of Primary Human Dendritic Cells and Monocytes to Toxoplasma gondii Is Stimulated by Phagocytosis of Live Parasites Rather Than Host Cell Invasion. J Immunol 196, 345–356 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christian DA et al. Use of transgenic parasites and host reporters to dissect events that promote interleukin-12 production during toxoplasmosis. Infect Immun 82, 4056–4067 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melo MB, Jensen KD & Saeij JP Toxoplasma gondii effectors are master regulators of the inflammatory response. Trends Parasitol 27, 487–495 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donato R S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol 33, 637–668 (2001). [DOI] [PubMed] [Google Scholar]
- 29.Donato R et al. Functions of S100 proteins. Curr Mol Med 13, 24–57 (2013). [PMC free article] [PubMed] [Google Scholar]
- 30.Ulas T et al. S100-alarmin-induced innate immune programming protects newborn infants from sepsis. Nat Immunol 18, 622–632 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Gross SR, Sin CG, Barraclough R & Rudland PS Joining S100 proteins and migration: for better or for worse, in sickness and in health. Cell Mol Life Sci 71, 1551–1579 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kierdorf K & Fritz G RAGE regulation and signaling in inflammation and beyond. J Leukoc Biol 94, 55–68 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Leclerc E, Fritz G, Vetter SW & Heizmann CW Binding of S100 proteins to RAGE: an update. Biochim Biophys Acta 1793, 993–1007 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Koch M et al. Structural basis for ligand recognition and activation of RAGE. Structure 18, 1342–1352 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penumutchu SR, Chou RH & Yu C Structural insights into calcium-bound S100P and the V domain of the RAGE complex. PLoS One 9, e103947 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hori M et al. Mycalolide-B, a novel and specific inhibitor of actomyosin ATPase isolated from marine sponge. FEBS Lett 322, 151–154 (1993). [DOI] [PubMed] [Google Scholar]
- 37.Lavine MD & Arrizabalaga G Exit from host cells by the pathogenic parasite Toxoplasma gondii does not require motility. Eukaryot Cell 7, 131–140 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunay IR et al. Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity 29, 306–317 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serbina NV, Jia T, Hohl TM & Pamer EG Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol 26, 421–452 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi C & Pamer EG Monocyte recruitment during infection and inflammation. Nat Rev Immunol 11, 762–774 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim YG et al. The Nod2 sensor promotes intestinal pathogen eradication via the chemokine CCL2-dependent recruitment of inflammatory monocytes. Immunity 34, 769–780 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gov L, Schneider CA, Lima TS, Pandori W & Lodoen MB NLRP3 and Potassium Efflux Drive Rapid IL-1beta Release from Primary Human Monocytes during Toxoplasma gondii Infection. J Immunol 199, 2855–2864 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robben PM, LaRegina M, Kuziel WA & Sibley LD Recruitment of Gr-1+ monocytes is essential for control of acute toxoplasmosis. J Exp Med 201, 1761–1769 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pifer R, Benson A, Sturge CR & Yarovinsky F UNC93B1 is essential for TLR11 activation and IL-12-dependent host resistance to Toxoplasma gondii. J Biol Chem 286, 3307–3314 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saeij JP et al. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature 445, 324–327 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reese ML, Zeiner GM, Saeij JP, Boothroyd JC & Boyle JP Polymorphic family of injected pseudokinases is paramount in Toxoplasma virulence. Proc Natl Acad Sci U S A 108, 9625–9630 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Butcher BA et al. Toxoplasma gondii rhoptry kinase ROP16 activates STAT3 and STAT6 resulting in cytokine inhibition and arginase-1-dependent growth control. PLoS Pathog 7, e1002236 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burger E et al. Loss of Paneth Cell Autophagy Causes Acute Susceptibility to Toxoplasma gondii-Mediated Inflammation. Cell Host Microbe 23, 177–190 e174 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopez-Yglesias AH, Burger E, Araujo A, Martin AT & Yarovinsky F T-bet-independent Th1 response induces intestinal immunopathology during Toxoplasma gondii infection. Mucosal Immunol 11, 921–931 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raetz M et al. Parasite-induced TH1 cells and intestinal dysbiosis cooperate in IFN-gamma-dependent elimination of Paneth cells. Nat Immunol 14, 136–142 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.