Abstract
Background:
Chlamydial infections are common among young women and can lead to serious reproductive health complications. We assessed the risk of reported repeat chlamydial infection among young women in Louisiana and time interval between infections by age and race/ethnicity.
Methods:
We analyzed surveillance data on chlamydial infections reported among women in Louisiana from January 1, 2000 to December 31, 2015. Multiple reports for the same person were matched using unique codes. Chlamydial infections reported more than 30 days after a previous positive test were considered new infections. Women aged 15–34 years at first infection during 2000–2012 were censored after three years or after they had a repeat infection. Cumulative incidence and incidence rate of repeat chlamydial infection among women were determined by year of first infection. Race- and age-specific results were obtained using stratified analyses.
Results:
One in four women diagnosed with a chlamydial infection at 15–34 years of age in Louisiana had a reported repeat infection in three years or less. Risk of repeat infection increased for younger women, racial/ethnic minorities, and women in more recent cohorts. Young black women aged 15–19 years in 2012 had the highest risk (44%). Black women also had shorter intervals between infections than white women.
Conclusions:
Repeat chlamydial infections were common, especially among young black women. The true number is likely higher because surveillance data only count infections that were detected and reported. Comprehensive prevention strategies are needed to address high rates of repeat chlamydial infections among women.
Keywords: Chlamydia, sexually transmitted infections, sexually transmitted diseases, women, repeat infections, reinfections
INTRODUCTION
Chlamydia trachomatis is the most common notifiable disease in the U.S. In 2016, there were 1,072,719 reported infections among women.1 Untreated infections can lead to serious reproductive health complications such as pelvic inflammatory disease, chronic pelvic pain, ectopic pregnancy, and infertility.2–5 Persons with chlamydial infections are usually asymptomatic3,6 so infections are often not detected or reported.6 This underscores the importance of early detection and treatment for existing chlamydial infections, and prevention efforts to avoid new infections (e.g., expedited partner therapy, risk reduction counseling).7–9 As such, annual screening is recommended for all sexually active women aged <25 years and older women at increased risk for infection, and those who test positive for chlamydia should be screened again three months after treatment to detect repeat infections.2,10
Women who are younger and of a racial/ethnic minority group are disproportionately affected by high rates of chlamydia.1 In 2016, the rate of reported chlamydial infections for young women aged 15–24 years in the U.S. was 3,070.9–3,779.0 per 100,000, almost five-fold higher than the overall national female rate of 657.3 per 100,000. Of note, young black women aged 15-24 years had the highest rate of chlamydial infections (6,485.2–6,747.6 per 100,000) compared to all other racial/ethnic and age groups. In fact, the rate of reported cases among black women was 5.1 times the rate among white women according to the latest U.S. surveillance report.1 Racial/ethnic disparities have been noted elsewhere in the literature.11–13
Repeat infections are common and can affect as many as one in five women treated for chlamydial infection within the previous year.14,15 While risk of repeat chlamydial infections among women has been previously assessed, differences in study design, length of follow-up, and country or population characteristics have yielded variable results.13,14,16–18 Repeat infection rates can be underestimated if women who are not retested are considered uninfected, or overestimated if only retested women are included, and symptomatic women are more likely to be retested. Evaluation of repeat infections by subgroups could help elucidate populations at high-risk who could benefit from targeted public health prevention efforts. In Louisiana, which has the second highest chlamydia rate in the country,1 black women comprise only 17% of the population but account for 52% of all chlamydial infections reported in the state.19,20 Using surveillance data, we assessed the risk of repeat chlamydial infections reported among young women in Louisiana and time interval between infections by age and race/ethnicity.
MATERIALS AND METHODS
For this retrospective cohort study, we obtained surveillance data on all chlamydial infections reported among women in Louisiana from January 1, 2000 to December 31, 2015. Infection with C. trachomatis is directly detected by culture or, most commonly, by nucleic acid amplification testing (NAAT).1,3 Laboratories and/or physicians report positive test results to their local or state health department.21 Health departments remove duplicate case reports before submitting the information to the Centers for Disease Control and Prevention to be compiled into national surveillance reports.
Data were de-identified but unique profile numbers specific to each woman allowed us to match multiple records for the same person in lieu of not having names, addresses, or other identifying information. Women aged 15–34 years at first infection were censored after they had a repeat infection or after three years of follow up, whichever came first. Chlamydial infections reported more than 30 days after a previous positive test were considered new incident infections because repeat testing less than 4 weeks after completing therapy is often false positive due to detection of residual nucleic acid from nonviable bacteria.2
We examined the 1) cumulative incidence of repeat chlamydial infection, and 2) incidence rate of repeat chlamydial infection among women by calendar year of first reported infection for 2000–2012. The year 2012 was the maximum limit to allow three-years of potential follow up through 2015. The cumulative incidence describes the probability or risk of an outcome over a specific period of time. Thus, the cumulative incidence of a repeat infection over a three-year period was estimated by dividing the number of repeat infections reported at least 30 days from the first positive test by the total number of women first diagnosed with chlamydial infection in the respective year. Women were considered to be uninfected if no subsequent positive test was reported. Next, since the incidence rate is a measure of the number of new cases per unit of time, we calculated rates for each year by dividing the number of women with repeat infections reported within three years of their first positive report by the number of total person-years (PY) of follow up. Given the high morbidity in Louisiana, all rates were expressed as the number of repeat infections per 1000 PY.
To explore risk of repeat infections among various subgroups, we evaluated the incidence and rate of repeat infection by race (white, black, other, and unknown) age group (15–19 years, 20–24 years, 25–29 years, and 30–34 years) and year of first infection (2000, 2007, and 2012) using stratified analyses. The year 2007 was considered for more stable estimates following the devastating impact of Hurricane Katrina in August 2005 on population displacement, unstable housing, and inadequate access to health care.22,23 Lastly, we assessed timing of repeat chlamydial infection from the first reported infection (i.e., ≤3 months, ≤6 months, ≤12 months, ≤24 months, and ≤36 months) by race and age. Due to the small sample size in other racial/ethnic groups, age-specific estimates were obtained for only white and black women. The Centers for Disease Control and Prevention made the determination that this study was not human subjects research and would be exempt from review by the Institutional Review Board because it used routinely collected surveillance data after personal identifiers were removed.
RESULTS
From 2000 to 2012, there were 142,763 women aged 15–34 years at first chlamydial infection, and 24.4% had a repeat infection in three years or less. The cumulative incidence of repeat infections among young women in Louisiana for years 2000 to 2012 ranged from as low as 19.9% in 2004 to as high as 27.7% in 2010 (Figure 1). Among women first diagnosed with a chlamydial infection in 2012, the risk of having a repeat infection within three years was 27.4%. In 2000 to 2012, the rate of repeat chlamydia infection increased 22% (9.0 per 100 PY to 11.0 per 100 PY). Overall repeat infection rates among women gradually decreased from 2000 to 2004 (9.0 per 100 PY to 7.5 per 100 PY), then increased 43% by 2008 (10.7 per 100 PY). Rates stayed fairly stable with minor fluctuations in 2008 to 2012 (10.4–11.0 per 100 PY).
Figure 1:
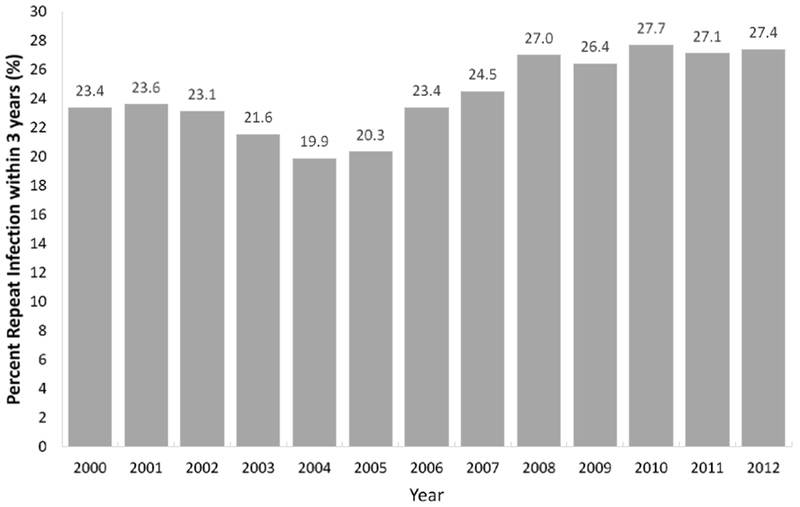
Percent repeat chlamydial infections within three years among women aged 15-34 years – Louisiana. 2000-2012
Note: graph shows cumulative incidence of repeat chlamydial infection by year of first reported infection.
The percentage of women with repeat infections within three years of a first infection and incidence rate of repeat chlamydial infections increased over time for women of all racial/ethnic and age groups (Table 1). At each year of interest (i.e., 2000, 2007, and 2012), black women had the highest risk of repeat infection. In 2012, the rate of repeat infection for black women (14.6 per 100 PY) was 1.8 times the rate among white women (8.1 per 100 PY), and 1.5 times the rate among women of other racial/ethnic groups (9.6 per 100 PY). Age at first infection was inversely related to the risk of having a repeat chlamydial infection regardless of race/ethnicity. The risk of a repeat infection among white women aged 15–19 years old was 28.8% whereas for 25–29 year olds it was 13.8%. The risk of repeat infection among black women was 44.4% for 15–19 year olds and 16.9% for 25–29 year olds.
Table 1:
Incidence rate and cumulative incidence of repeat chlamydial infections among women by year, age, and race – Louisiana, 2000, 2007, 2012
| Year 2000 | Age group |
Year 2007 | Age group |
Year 2012 | Age group |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 15-19 years | 20-24 years | 25-29 years | 30-34 years | Total | 15-19 years | 20-24 years | 25-29 years | 30-34 years | Total | 15-19 years | 20-24 years | 25-29 years | 30-34 years | |
| White | |||||||||||||||
| First Infection (N) | 1,799 | 851 | 688 | 202 | 58 | 1,600 | 729 | 585 | 217 | 69 | 2,647 | 1,043 | 1,079 | 384 | 141 |
| Reinfection Rate per 100 PY | 5.6 | 7.2 | 4.7 | 2.8 | 3.7 | 7.3 | 10.8 | 4.9 | 4.8 | 2.6 | 8.1 | 11.6 | 6.5 | 5.1 | 4.9 |
| Repeat Infection Within 3 Years (%) | 15.2 | 19.2 | 12.9 | 7.9 | 10.3 | 19.4 | 27.2 | 13.5 | 13.4 | 7.2 | 21.1 | 28.8 | 17.3 | 13.8 | 13.5 |
| Black | |||||||||||||||
| First Infection (N) | 8,919 | 4,127 | 3,439 | 1,029 | 324 | 5,826 | 2,928 | 1,888 | 724 | 286 | 6,543 | 3,328 | 2,210 | 700 | 305 |
| Reinfection Rate per 100 PY | 10.7 | 14.5 | 9.1 | 5.0 | 2.8 | 12.7 | 18.2 | 9.7 | 5.0 | 4.3 | 14.6 | 20.2 | 11.6 | 6.3 | 3.6 |
| Repeat Infection Within 3 Years (%) | 27.2 | 35.0 | 23.7 | 13.8 | 8.0 | 31.3 | 41.7 | 24.9 | 13.7 | 11.9 | 34.6 | 44.4 | 28.8 | 16.9 | 10.2 |
| Other race | |||||||||||||||
| First Infection (N) | 88 | 230 | 505 | ||||||||||||
| Reinfection Rate per 100 PY | 6.3 | 4.4 | 9.6 | ||||||||||||
| Repeat Infection Within 3 Years (%) | 17.0 | 12.2 | 24.2 | ||||||||||||
| Unknown race | |||||||||||||||
| First Infection (N) | 1,159 | 2,255 | 1,607 | ||||||||||||
| Reinfection Rate per 100 PY | 2.4 | 4.2 | 3.4 | ||||||||||||
| Repeat Infection Within 3 Years (%) | 7.0 | 11.8 | 9.6 | ||||||||||||
Overall, the risk of chlamydial repeat infection increased as the duration of time since the first infection increased (Figure 2). In 2012, the proportion of women with repeat infection within three months was higher in young black women (6.8%) than young white women (5.1%) aged 15–19 years. However, among older women, risk of repeat infection was slightly higher among white women in the first few months following a first infection. Over longer periods of time, the risk of repeat infection was more pronounced among black women. A repeat chlamydial infection by 12 months was more common among young black women aged 15–19 years (22.6%) than white women (15.2%). At 24 months, the risk of repeat infection for 15–19 year olds was 23.5% (white) and 36.4% (black); for 20–24 year olds it was 14.6% (white) and 23.9% (black); for 25–29 year olds it was 12.8% (white) and 15.0% (black); and for 30–34 year olds it was 10.6% (white) and 9.5% (black). In addition, the time between infections were shorter among black women than white women. For instance, the risk of repeat infection for black women at ≤12 months (17.8%) was akin to the risk for white women at ≤24 months (17.6%, data not shown). Similar findings were observed for black women at ≤6 months (10.4%) and white women at ≤12 months (11.7%).
Figure 2:
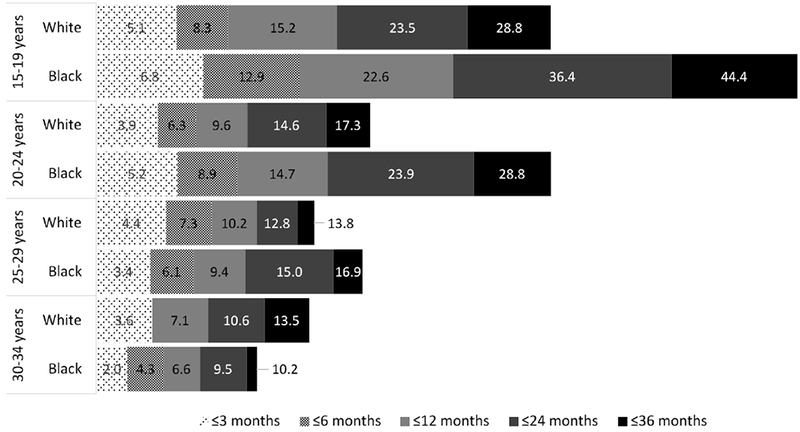
Cumulative risk of repeat chlamydial infection among women 15-34 years at first infection – Louisiana. 2012
Note: no reinfections were reported between 3–6 months for white 30-34 year old women
DISCUSSION
Young women diagnosed with chlamydia in Louisiana were at high risk for having another reported chlamydial infection. Among 15–34 year old women, one in four had a repeat infection in three years or less. Repeat infection rates were highest among young 15–19 year old black women of which 44.4% had a repeat chlamydial infection within three years. Repeat chlamydial infections continued to be diagnosed throughout the three year follow-up periods. This is consistent with findings from another study that estimated the cumulative risk of chlamydial infection among young women in Florida.13 Based on surveillance data, approximately 25% of 15–20 year old women who were initially infected in 2000–2003 had a repeat chlamydial infection within three years. It is notable that these and current study findings underestimate the true risk of repeat infection because retesting rates are often low.14,16,18 Only about a quarter of women who test positive for chlamydia are retested at three months, as recommended, and many are not tested annually.24 Furthermore, asymptomatic chlamydial infections can clear within months without treatment and could be easily missed without timely and frequent testing.25
Previous studies have reported repeat infection rates as high as 26.5% over a 12 month period in diverse populations although not all removed chlamydial infections reported less than 30 days after a previous positive test, and the proportion of women who were retested ranged from 26% to 93% or was not reported.14 In a U.S.-based, multicenter, randomized controlled trial of patient-delivered partner treatment among women aged 14–34 years (1996–2000), the cumulative rate of repeat chlamydial infection was 12% (87/728 intervention group) and 15% (108/726 control group) four months after treatment.26 More recently, a retrospective cohort study from New Zealand reported 5.9% of 4,139 women infected with chlamydia at baseline had a repeat infection within 6 months; however, positivity increased to 17.5% when restricted to only women who were retested (n=1,454).16 Similarly, a randomized controlled trial of retesting intervals in the Netherlands found 69 people assigned to be retested 26 weeks after treatment for urogenital chlamydia had a repeat infection, which was 14.4% of 478 retested or 9.3% of 745 assigned to be retested.17 Thus, additional and frequent testing beyond the currently recommended three-month rescreening may be warranted for women with chlamydia.
Repeat chlamydial infection rates were especially high among young black women in our analysis. One study that assessed repeat infections among women aged 16–24 years entering the National Job Training Program found little difference between blacks (6.1% of 3,210 retested) and whites (4.9% of 1,006 retested) who retested positive for chlamydia 1–2 months after treatment (OR=1.3, 95% CI=0.9–1.8). However, black women were significantly less likely to get retested compared to their white counterparts (OR=0.4, 95% CI=0.4–0.5).27 Low retesting among black women (39.4% vs. 59.4% whites) could have underestimated the true repeat infection rate and attenuated disparities by race. Racial disparities in chlamydia and other sexually transmitted infections (STIs) have been well documented, and are attributed to a wide range of sociodemographic, environmental, behavioral and social factors.28–30 However, pronounced racial disparities have persisted even after controlling for risk behaviors and other individual level factors.28,29 In a recent study of the Longitudinal Survey of Adolescent Health, race remained significantly associated with chlamydia independent of sociodemographic characteristics, access to health care, and risky sexual behavior (OR=5.2, 95% CI=3.8–7.2 for non-Hispanic blacks)29 In addition, the structure of local sexual networks like partner concurrency and assortative mixing by race disproportionately affects populations with high underlying prevalence of STIs from which sexual partners are selected.29 Repeat testing is necessary for all women positive for chlamydia, who are at increased risk of repeat infection and adverse health sequelae.2
In the current study, chlamydia repeat infection rates gradually decreased from 2000 to 2004, increased in 2004–2008 then remained stable through 2012. Increasing number of reported chlamydial cases1 are influenced by widespread screening31 and more sensitive diagnostic tests.3 Our trends in repeat chlamydial infection mirror findings for all infections in the general population.11,12 One study using population-based data from the National Health and Nutrition Examination Survey (1999–2008) found decreased chlamydia prevalence among black persons 14–19 years old from the 1999–2000 cycle (13%) to 2003–2004 (6%), which slightly increased then stabilized through the last cycle in 2007–2008 (8%).11 Though reasons for persistent disparities in positivity and leveled trends in recent years are not clear, the high proportion of repeat infections underscore the need for better targeted screening and primary prevention efforts, particularly for women at high-risk for having repeat chlamydial infections.
There is low adherence to repeat testing in various clinical settings,24,27 however, little is known about the impact of provider characteristics or sources of care on repeat infections. One study that assessed reported STIs (i.e., chlamydial, gonorrhea, syphilis) in Massachusetts during 2014–2016 found 44% of cases with five or more STIs were diagnosed within the same health care system with shared access to clinical and laboratory information.32 This highlights potential opportunities for providers to retest women for repeat infections, get their partners tested and treated, and discuss primary prevention strategies (e.g., condoms and other barrier contraceptive methods) tailored to women’s preferences to prevent subsequent repeat infections.2 Partner dynamics should be considered and is particularly relevant to women unable to negotiate male condom use. The impact of provider communication on sexual health outcomes is not well understood but a qualitative study of contraceptive counseling in family planning clinics found attention to sexual behavior patterns and STI risk was largely absent during patient-provider interactions.33 Future studies could evaluate how content and quality of provider communication reduces repeat chlamydial infections and other STIs among women. Provider characteristics associated with repeat screening could also be assessed.
This study had several limitations. We examined population-based trends in reported chlamydia in Louisiana that spanned almost two decades; however, chlamydial repeat infection rates were likely underestimated. We had only positive test results so could not measure rescreening rates. Although improved provider knowledge of screening guidelines could potentially lead to increased detection of repeat infections, studies have shown people are not retested frequently enough to catch all chlamydial infections.14,16,18 Repeat infection rates can be underestimated if women who are not retested are considered uninfected. Thus, the true repeat infection rates could have been substantially higher. Data entry errors of patient information (e.g., name, date of birth, profile number) could have also underestimated the number of repeat infections if records for the same person were counted as infections from different individuals. It was assumed that all reported cases were treated and infections occurring more than 30 days after the first infection were incident events; however, untreated cases or treatment failures may have led to overestimation of the number of repeat infections. Repeat chlamydial infection trends in the beginning of the study period (e.g., 2000) could have included women with one or more infections prior to 2000 particularly among older age groups. When women with previous infections were included in recent years (i.e., any person with a reported infection in a year), repeat infection rates increased among older women (data not shown). Thus, the overall increase in repeat chlamydial infections from 2000 to 2012 would have been greater than we estimated. Chlamydia testing technology also changed during this time resulting in more sensitive tests, so early incidence rates are likely to underestimate true rates.
High rates of repeat chlamydia were reported among young women in Louisiana. The true number of repeat infections is likely higher because surveillance data only count infections that were detected and reported. Current recommendations state that women who test positive for chlamydia should be rescreened three months after treatment to detect new repeat infections. However, we found the majority of new repeat infections occurred after three months. Our findings suggest that additional testing after three months may be helpful. Routine visits for sexual or reproductive health services provide apt opportunities for clinicians and other health workers to detect asymptomatic infections and discuss risk reduction strategies (e.g., expedited partner therapy, barrier contraceptive methods).7,9,34 Strategies to improve adherence to repeat testing could include standing orders, phone reminders, and mailed screening test kits.35–37 Low condom use among young women at risk for STIs highlights the importance for health promotion and program activities to emphasize dual prevention of STI and unintended pregnancy.38 A flexible and comprehensive approach to STI prevention is needed to address high rates of repeat infections among women.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflict of interest: None declared.
References
- 1.CDC. 2016. Sexually Transmitted Disease Surveillance. 2017; https://www.cdc.gov/std/stats16/Syphilis.htm. Accessed January 8, 2018.
- 2.Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(3):1–137. [PMC free article] [PubMed] [Google Scholar]
- 3.CDC. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae−-2014. MMWR Recomm Rep. 2014;63(RR-02):1–19. [PMC free article] [PubMed] [Google Scholar]
- 4.Tsevat DG, Wiesenfeld HC, Parks C, et al. Sexually transmitted diseases and infertility. Am J Obstet Gynecol. 2017;216(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hay PE, Kerry SR, Normansell R, et al. Which sexually active young female students are most at risk of pelvic inflammatory disease? A prospective study. Sex Transm Infect. 2016;92(1):63–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med. 2003;36(4):502–509. [DOI] [PubMed] [Google Scholar]
- 7.Gannon-Loew KE, Holland-Hall C, Bonny AE. A Review of Expedited Partner Therapy for the Management of Sexually Transmitted Infections in Adolescents. J Pediatr Adolesc Gynecol. 2017;30(3):341–348. [DOI] [PubMed] [Google Scholar]
- 8.Kamb ML, Fishbein M, Douglas JM, Jr., et al. Efficacy of risk-reduction counseling to prevent human immunodeficiency virus and sexually transmitted diseases: a randomized controlled trial. Project RESPECT Study Group. JAMA. 1998;280(13):1161–1167. [DOI] [PubMed] [Google Scholar]
- 9.Kissinger P, Brown R, Reed K, et al. Effectiveness of patient delivered partner medication for preventing recurrent Chlamydia trachomatis. Sex Transm Infect. 1998;74(5):331–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee KC, Ngo-Metzger Q, Wolff T, et al. Sexually transmitted infections: recommendations from the U.S. Preventive Services Task Force. Am Fam Physician. 2016;94(11):907–915. [PubMed] [Google Scholar]
- 11.Datta SD, Torrone E, Kruszon-Moran D, et al. Chlamydia trachomatis trends in the United States among persons 14 to 39 years of age, 1999–2008. Sex Transm Dis. 2012;39(2):92–96. [DOI] [PubMed] [Google Scholar]
- 12.Learner ER, Torrone EA, Fine JP, et al. Chlamydia prevalence trends among women and men entering the National Job Training Program from 1990 through 2012. Sex Transm Dis. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterman TA, Newman DR, Torrone E, et al. Cumulative risk of chlamydial infection among young women in Florida, 2000–2011. J Adolesc Health. 2014;55(2):241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosenfeld CB, Workowski KA, Berman S, et al. Repeat infection with Chlamydia and gonorrhea among females: a systematic review of the literature. Sex Transm Dis. 2009;36(8):478–489. [DOI] [PubMed] [Google Scholar]
- 15.Wiesenfeld HC. Screening for Chlamydia trachomatis Infections in Women. N Engl J Med. 2017;376(22):2198. [DOI] [PubMed] [Google Scholar]
- 16.Rose SB, Garrett SM, Stanley J, et al. Retesting and repeat positivity following diagnosis of Chlamydia trachomatis and Neisseria gonorrhoea in New Zealand: a retrospective cohort study. BMC Infect Dis. 2017;17(1):526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Helm JJ, Koekenbier RH, van Rooijen MS, et al. What is the optimal time to retest patients with a urogenital chlamydia infection? A randomized controlled trial. Sex Transm Dis. 2018;45(2):132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Visser M, van Aar F, Koedijk FDH, et al. Repeat Chlamydia trachomatis testing among heterosexual STI outpatient clinic visitors in the Netherlands: a longitudinal study. BMC Infect Dis. 2017;17(782):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.U.S. Census Bureau. Annual estimates of the resident population by sex, race alone or in combination, and Hispanic origin for the United States, States, and Counties: April 1, 2010 to July 1, 2015: 2015 population estimates. 2016; https://factfinder.census.gov/bkmk/table/1.0/en/PEP/2015/PEPSR5H?slice=Sex∼totsex!Year∼est72015. Accessed April 3, 2018.
- 20.CDC, National Center for HIV, STD, and TB Prevention (NCHSTP), Division of STD/HIV Prevention. Selected STDs by Age, Race/Ethnicity, and Gender, 1996–2014 Results, CDC WONDER Online Database. 2014; https://wonder.cdc.gov/controller/saved/D128/D33F501. Accessed March 29, 2018.
- 21.CDC. Program operations guidelines for STD prevention: surveillance and data management. https://www.cdc.gov/std/program/surveillance.pdf. Accessed June 16, 2017.
- 22.Kissinger P, Schmidt N, Sanders C, et al. The effect of the hurricane Katrina disaster on sexual behavior and access to reproductive care for young women in New Orleans. Sex Transm Dis. 2007;34(11):883–886. [DOI] [PubMed] [Google Scholar]
- 23.CDC. Public health response to Hurricanes Katrina and Rita--Louisiana, 2005. MMWR Morb Mortal Wkly Rep. 2006;55(2):29–30. [PubMed] [Google Scholar]
- 24.Hoover KW, Tao G, Nye MB, et al. Suboptimal adherence to repeat testing recommendations for men and women with positive Chlamydia tests in the United States, 2008–2010. Clin Infect Dis. 2013;56(1):51–57. [DOI] [PubMed] [Google Scholar]
- 25.Geisler WM. Duration of untreated, uncomplicated Chlamydia trachomatis genital infection and factors associated with chlamydia resolution: a review of human studies. J Infect Dis. 2010;201 Suppl 2:S104–113. [DOI] [PubMed] [Google Scholar]
- 26.Schillinger JA, Kissinger P, Calvet H, et al. Patient-delivered partner treatment with azithromycin to prevent repeated Chlamydia trachomatis infection among women: a randomized, controlled trial. Sex Transm Dis. 2003;30(1):49–56. [DOI] [PubMed] [Google Scholar]
- 27.Joesoef MR, Weinstock HS, Johnson RE. Factors associated with recurrent chlamydial infection and failure to return for retesting in young women entering national job training program, 1998−-2005. Sex Transm Dis. 2008;35(4):368–371. [DOI] [PubMed] [Google Scholar]
- 28.Fine D, Thomas KK, Nakatsukasa-Ono W, et al. Chlamydia positivity in women screened in family planning clinics: racial/ethnic differences and trends in the northwest U.S., 1997–2006. Public Health Rep. 2012;127(1):38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamilton DT, Morris M. The racial disparities in STI in the U.S.: Concurrency, STI prevalence, and heterogeneity in partner selection. Epidemics. 2015;11:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sales JM, Smearman EL, Swartzendruber A, et al. Socioeconomic-related risk and sexually transmitted infection among African-American adolescent females. J Adolesc Health. 2014;55(5):698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Satterwhite CL, Chow JM, Bernstein KT, et al. Opportunities for chlamydia control in the era of healthcare reform: lessons from two decades of innovative family planning care. Womens Health (Lond). 2013;9(1):25–38. [DOI] [PubMed] [Google Scholar]
- 32.Hsu KK, Molotnikov LE, Roosevelt KA, et al. Characteristics of Cases with Repeated Sexually Transmitted Infections, Massachusetts, 2014–2016. Clin Infect Dis. 2018. [DOI] [PubMed] [Google Scholar]
- 33.Minnis AM, Mavedzenge SN, Luecke E, et al. Provider counseling to young women seeking family planning services. Perspect Sex Reprod Health. 2014;46(4):223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marrazzo JM, Cates W. Interventions to prevent sexually transmitted infections, including HIV infection. Clin Infect Dis. 2011;53 Suppl 3:S64–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gift TL, Malotte CK, Ledsky R, et al. A cost-effectiveness analysis of interventions to increase repeat testing in patients treated for gonorrhea or chlamydia at public sexually transmitted disease clinics. Sex Transm Dis. 2005;32(9):542–549. [DOI] [PubMed] [Google Scholar]
- 36.Guy R, Hocking J, Low N, et al. Interventions to increase rescreening for repeat chlamydial infection. Sex Transm Dis. 2012;39(2):136–146. [DOI] [PubMed] [Google Scholar]
- 37.Park IU, Amey A, Creegan L, et al. Retesting for repeat chlamydial infection: family planning provider knowledge, attitudes, and practices. J Womens Health (Larchmt). 2010;19(6):1139–1144. [DOI] [PubMed] [Google Scholar]
- 38.Tyler CP, Whiteman MK, Kraft JM, et al. Dual use of condoms with other contraceptive methods among adolescents and young women in the United States. J Adolesc Health. 2014;54(2):169–175. [DOI] [PubMed] [Google Scholar]


