Abstract
Prenatal exposure to alcohol causes a wide range of deficits known as Fetal Alcohol Spectrum Disorders (FASDs). Many factors determine vulnerability to developmental alcohol exposure including timing and pattern of exposure, nutrition, and genetics. Here, we characterized how a prevalent single nucleotide polymorphism in the human brain-derived neurotrophic factor (BDNF) gene (val66met) modulates FASDs severity. This polymorphism disrupts BDNF’s intracellular trafficking and activity dependent secretion, and has been linked to increased incidence of neuropsychiatric disorders such as depression and anxiety. We hypothesized that developmental ethanol (EtOH) exposure more severely affects mice carrying this polymorphism. We used transgenic mice homozygous for either valine (BDNFval/val) or methionine (BDNFmet/met) in residue 68, equivalent to residue 66 in humans. To model EtOH exposure during the 2nd and 3rd trimesters of human pregnancy, we exposed mice to EtOH in vapor chambers during gestational days 12–19 and postnatal days 2–9. We found that EtOH exposure reduces cell layer volume in the dentate gyrus and the CA1 hippocampal regions of BDNFmet/met but not BDNFval/val mice during the juvenile period (postnatal day 15). During adulthood, EtOH exposure reduced anxiety-like behavior and disrupted trace fear conditioning in BDNFmet/met mice, with most effects observed in males. EtOH exposure reduced adult neurogenesis only in the ventral hippocampus of BDNFval/val male mice. These studies demonstrate that the BDNF val66met polymorphism modulates, in a complex manner, the effects of developmental EtOH exposure, and identify a novel genetic risk factor that may regulate FASDs severity in humans.
Keywords: Brain derived neurotrophic factor, fetal alcohol, ethanol, development, hippocampus, behavior, trace fear conditioning, elevated zero maze, anxiety, hippocampal volume
INTRODUCTION
Alcohol exposure during embryonic development causes a series of deficits (ranging from mild to severe) on multiple organs and systems, a condition known as Fetal Alcohol Spectrum Disorders (FASDs). The most severe manifestation of FASDs is Fetal Alcohol Syndrome, characterized by growth retardation, facial abnormalities, structural or functional brain deficiencies, and neurobehavioral impairments (Hoyme et al., 2016). The latter include learning disabilities, motor coordination deficits, executive function alterations, and speech-language delays. Studies indicate that a number of factors determine the severity of FASDs, including the pattern of ethanol (EtOH) consumption (e.g., amount, frequency, duration, binge drinking), nutritional factors, socio-economic and marital status, maternal education level, access to medical care, and co-exposure to other substances of abuse or environmental pollutants (Guerri et al., 2009). In addition, genetic factors modulate the impact of developmental EtOH exposure on craniofacial development (Reviewed in Eberhart and Parnell, 2016). Alcohol dehydrogenase gene variants that encode for enzymes with higher efficiency to oxidize EtOH to acetaldehyde (an agent that causes a number of unpleasant effects such as flushing) are associated with reduced alcohol consumption and a lower risk for developing FASDs (Warren & Li, 2005). The presence of a serotonin transporter promoter gene polymorphism (long vs. short) is associated with neonatal irritability and higher stress reactivity in offspring of primates exposed to moderate EtOH exposure during pregnancy (Schneider et al., 2011). However, the role of gene-EtOH interactions in determining the severity of the neurobehavioral effects of developmental EtOH exposure has not been fully elucidated.
Brain-derived neurotrophic factor (BDNF) plays a central role in the development and maturation of neuronal circuits and alterations in its function contribute to the pathophysiology of FASDs (Reviewed in Boschen and Klintsova, 2017). Studies suggest that BDNF is protective against some of the effects of developmental EtOH exposure (Reviewed in Boschen and Klintsova, 2017). Therefore, we hypothesized that factors that alter BDNF function could increase the severity of FASDs. One such factor is a single nucleotide polymorphism in the coding sequence for BDNF that impairs its intracellular trafficking and activity-dependent secretion (Egan et al, 2003; reviewed in Notaras et al, 2015). A guanine to adenine nucleotide change at position 196 in the human BDNF coding sequence leads to a missense amino acid substitution from valine to methionine at position 66 (val66met) in the BDNF protein. This polymorphism has been associated with increased incidence of a number of neurological and psychological deficits, including disrupted episodic memory (Dempster et al., 2005, Egan et al., 2003, Hariri et al., 2003), reduced hippocampal volume (Bueller et al., 2006, Hajek et al., 2012), and increased incidence of anxiety, depression, and post-traumatic stress disorder (Frielingsdorf et al., 2010, Hosang et al., 2014, Montag et al., 2010, Zhang et al., 2014). Because these deficits overlap with those commonly found in individuals with FASDs, we investigated whether this BDNF polymorphism modulates the impact of developmental alcohol exposure. To test this hypothesis, we utilized a BDNF knock-in transgenic mouse that expresses the mouse homolog of the human BDNF val66met mutation, which was recently shown to increase compulsive alcohol drinking (Warnault et al., 2016).
MATERIALS AND METHODS
All experimental procedures described in this manuscript adhered to the U.S. Public Health Service policy on humane care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee of the University of New Mexico Health Sciences Center. For all the experiments described below, the experimenters were blinded to the treatment group assignment.
Subjects
Wild-type Val68BDNF (BDNFval/val) and mutant Met68BDNF (BDNFmet/met) mice were generated and validated in Dr. Ron’s laboratory at the University of California San Francisco, where they did not show alterations in gross development, basal locomotion and sensorimotor coordination (Warnault et al., 2016). A breeding colony was established at the University of New Mexico Health Sciences Center Animal Resource Facility. Mice were bred as homozygous (BDNFval/val × BDNFval/val and BDNFmet/met × BDNFmet/met). The integrity of mouse lines was confirmed in randomly selected mice, as described below (latest round of genotyping completed in March, 2018). Offspring were weaned at approximately 25 days of age, ear tagged, and group-housed with littermates of the same sex at 22°C on a reverse 12-hr light/dark cycle (lights on at 8 PM) with standard chow and water available ad libitum. Behavioral experiments were performed between 9 AM to 3 PM in a room illuminated by red lights. Detailed information on the number of subjects and litters used for individual experiments can be found in Supplementary Table 1. In this study, we focused on the impact of developmental EtOH exposure on homozygous mice; however, BDNFval/met heterozygous mice and humans have also been shown to have a variety of behavioral deficits and it is important to determine in the future if these are modulated by developmental EtOH exposure (Chen et al., 2006, Notaras et al., 2015).
Genotyping
Tails were digested in 200 μl of DirectPCR Lysis Reagent (Viagen Biotech, Los Angeles, CA) and 2 μl of Proteinase K (Sigma-Aldrich, St. Louis, MO) for 55°C overnight followed by incubation at 85°C for 45 min to inactivate Proteinase K. Polymerase chain reaction (PCR) amplification of the BDNF transgene was carried out using ChromaTaq DNA polymerase (Denville Scientific Inc., Metuchen, NJ) with the following primers: CGTGAATGGGCCCAGGGCAG (forward) and ATGTCTATGAGGGTTCGGCGCCACTC (reverse) (Thermo Fisher, Waltham, MA). The reaction mixture (50 μl total volume) contained water (20.1 μl), 1X reaction buffer (10 μl of 5X stock; Denville Scientific Inc.), 1.5 mM MgCl2 (1.5 μl of 50 mM stock; Denville Scientific Inc.), 400 μM dNTP mix (2 μl of 10 mM stock; Thermo Fisher) 0.5 M betaine (5 μl of 5M stock; Sigma-Aldrich), 0.3 μM forward and reverse primers (1.5 μl/each; stock 10 μM), 2 U ChromaTaq DNA polymerase (0.4 μl of 5U/μl stock) and 8 μl of genomic DNA from tail digestion. The following PCR program was used (C1000 Touch Thermal Cycler, Bio-Rad, Hercules, CA): 1 cycle of 94°C for 1 min; 36 cycles of 94°C for 30 s, 57°C (40 s), 72°C (30 s); and 1 cycle of 72°C (10 min). Samples were then cleaned up using the PureLink Quick PCR Purification Kit (Thermo Fisher). Sanger DNA sequencing of purified PCR products was performed by Genewiz (South Plainfield, NJ).
EtOH vapor chamber exposure
Timed-pregnant dams were exposed to increasing concentrations of vaporized EtOH from gestational day (G) 12 to 19, and following parturition pups and dams were exposed to increasing concentrations of vaporized EtOH from postnatal day (P) 2 to 9. EtOH vapor exposures took place in custom-built EtOH vapor chambers described previously (Morton 2014). EtOH vapor concentrations were measured using a breathalyzer (Intoximeters, St. Louis, MO). EtOH vapor chamber levels were 4–5 g/dl at G12–14, 6–7 g/dl at G15–17, 7–8 g/dl at G18–19, 3–4 g/dl at P2–3, 5–6 g/dl at P4–5 and 7–8 g/dl at P6–9. To measure blood EtOH concentrations (BECs) in dams, blood was collected from the tail vein at G17–18 and at P7 immediately following vapor chamber exposure. To measure BECs in pups, P7 animals were anesthetized with isoflurane immediately after vapor chamber exposure, decapitated, and trunk blood was collected. BECs were measured using an alcohol dehydrogenase-based assay as previously described (Galindo & Valenzuela, 2006). None of the animals from whom blood was collected were used in any subsequent experiments. To assess acquisition of developmental milestones, pups from each BDNF genotype and vapor chamber exposure condition were monitored daily to determine at what postnatal day they acquired a righting reflex, opened their eyes, displayed an auditory startle reflex, or displayed an ear twitch response to tactile stimulation (Chi et al., 2016).
Volume measurement of hippocampal cell layers
On P15 and P50, 8 mice from each sex, BDNF genotype, and EtOH vapor chamber exposure condition were deeply anesthetized with ketamine (250 mg/kg i.p.), and perfused transcardially with 32°C phosphate buffered saline (PBS) pH 7.4 containing procaine hydrochloride (1 g/L; Sigma-Aldrich) and heparin (1 USP unit/mL; Sagent Pharmaceuticals, Schaumburg, IL) for 2 min, followed by room-temperature 4% paraformaldehyde (PFA; Sigma-Aldrich) in PBS for 2 min, then with ice cold 4% PFA in PBS for 5 minutes. Brains were extracted and maintained in 4% PFA in PBS for 48 h at 4°C, then cryoprotected in 30% sucrose in PBS for 48 h. Brains were then embedded in Optimal Cutting Temperature compound (Fisher Healthcare, Houston, TX) and flash frozen in isopentane (Avantor Performance Materials, Center Valley, PA) cooled with a slurry of dry ice and 95% EtOH. Brains were kept frozen at −80°C until sectioned in the parasagittal plane on a cryostat (Microm model# HM 505E, Walldorf, Germany) at 50 μm. Floating sections were maintained at −20°C in freezing medium (0.05 M phosphate buffer pH 7.4, 25% glycerol and 25% ethylene glycol).
Every 8th parasagittal section containing the dorsal hippocampus (lateral 0.24 mm to 2.52 mm according to the Paxinos mouse brain atlas (Paxinos & Franklin, 2013) was stained with 4’,6-diamidino-2-phenylindole hydrochloride (DAPI, Sigma-Aldrich). If any section was torn or damaged, the closest intact section was used instead. Sections were washed 4 times for 5 min with PBS, and then incubated with 600 nM DAPI in PBS. Sections were again washed 4X in PBS, before being mounted on Superfrost plus slides (VWR, Radnor, PA) and coverslipped with Vectashield mounting media (Vector Laboratories, Burlingame, CA). Coverslips were sealed with clear nail polish. The volumes of the pyramidal cell layers of the CA1 and CA3 regions, as well as the granule cell layer of the dentate gyrus (DG) were assessed according to the Cavalieri principle (Gundersen et al., 1988) using Stereoinvestigator software (Microbrightfield Bioscience, Williston, VT). Sections were imaged using an Olympus IX-81 DSU spinning disk confocal microscope. Hippocampal region of interest contours were outlined manually in each section using a 10X objective. Volumes were estimated using a 25 × 25 μm point grid.
Zero maze
To assess anxiety-like behavior, we used the elevated zero maze, a modification of the elevated plus maze (Shepherd et al., 1994). The zero maze (Catalog number 2325–0231; San Diego Instruments, San Diego, CA) consists of an elevated circular platform (51.4 cm high; 50.8 cm min diameter and 60.96 cm max diameter) with two opposing enclosed sections enclosed by a wall (15.24 cm high) and two opposing open sections with curbs (1.27 cm high). Animal home cages were moved into the testing room illuminated at 90 lux approximately 2 h before testing. A white noise-generating system was present in the room. Each mouse was placed on one of the enclosed areas and allowed to explore for 5 min. During this time, the researcher stayed in the same area of the room. An overhead camera (ICD-49 B/W digital video camera, Ikegami Electronics, Maywood, NJ) and Ethovision X-T video-tracking software (Noldus, Leesburg, VA) were used to monitor the position of the central point of the mouse body. Between trials, the apparatus was cleaned with 70% (v/v) isopropyl alcohol solution (Sigma-Aldrich) and thoroughly dried.
Trace fear conditioning
Trace fear conditioning was conducted with adult mice (approximately 90 days old), using methods adapted from previous publications (Brady et al., 2012, Seo et al., 2015). Trace fear conditioning experiments took place in a dedicated room illuminated with red lights. On the training day of the trace fear conditioning protocol, animals were placed into a Coulbourn Instruments (Allentown, PA) Habitest® System for 180 s to habituate to the apparatus. They were then exposed to the conditioned stimulus (CS), an 80 dB, 6 Hz clicker for 10 s. After a 30 s trace period the unconditioned stimulus (US) was delivered, which consisted of a 2 s, 0.8 mA scrambled foot shock. The CS, trace, and US sequence was repeated for a total of 7 trials with a 180 s inter-trial interval. The mouse was removed from the chamber 60 s after the final US was delivered. Between each subject, the apparatus was thoroughly cleaned with a 70% (v/v) isopropyl alcohol solution. Twenty-four hours after the training session, freezing to the CS in a novel context was measured. One hundred and eighty seconds after being placed in a novel environment (a clean, standard mouse cage with bedding) the 10 s CS was delivered. One hundred and twenty seconds later, another 10 s CS was delivered. The animal was returned to its home cage 120 seconds after the second CS. Animals were video recorded during both the training and testing sessions, and the amount of time spent freezing during the entire fear conditioning session was measured. Videos were coded using the Simple Video Coder software (Barto et al., 2016). Sensitivity to the foot shock was analyzed during the training day by analyzing the occurrence of two common responses to the shock: jumping and running behavior (Seo et al., 2015).
To minimize the aversive component of trace fear conditioning, a separate cohort of animals was assessed in a modified trace fear-conditioning paradigm adapted from a recent publication (Seo et al., 2015). Animals were handled for 2 min per day for 5 days before conditioning. The shock during the training day was reduced to 0.5 mA, the inter-trial interval was increased (tones delivered at 180, 370, 620, 900, and 1060s), and the total number of CS-trace-US trials was reduced from 7 to 5. On the test day, the CS was delivered after a 180 second habituation period to the novel testing cage. The CS delivery was repeated for a total of 5 tone tests (tones delivered at 180, 280, 390, 510, and 620s). Animals were returned to their home cage ~2 min after the final CS presentation. Freezing behavior was again measured using the Simple Video Coder software.
Doublecortin positive (DCX+) cell counting
Twenty-four hours after the test day of the modified trace fear conditioning protocol, brains were collected, perfused with 4%PFA, and sectioned to 50 μm thickness as described above. Immunohistochemistry was performed as described previously (Topper et al., 2015), with some modifications. Briefly, floating parasagittal sections from the dorsal hippocampus (lateral 0.48 mm to lateral 2.52 mm) and ventral hippocampus (lateral 2.04 mm to 2.88 mm) were incubated with PBS containing 1% bovine serum albumin (Sigma-Aldrich), 0.2% Triton X-100 (Sigma-Aldrich), and 5% donkey serum (Jackson ImmunoResearch West Grove, PA) for 2 h. Sections were then incubated with a 1:500 dilution primary anti-DCX antibody (Cell Signaling, Danvers, MA; cat# 4640S) for 72 h on an orbital shaker at 4°C. Sections were rinsed and then incubated for 30 min in PBS containing 1% bovine serum albumin and 5% donkey serum. Donkey anti-rabbit IgG Alexa Fluor 555 antibody (cat# A-31572, Thermo Fisher) was then applied to the tissue sections at a 1:1000 dilution for 1–2 hours, then rinsed with PBS. Tissue sections were incubated with 600 nM DAPI solution for 20 minutes, rinsed with PBS, then mounted on Superfrost plus slides and coverslipped with Vectashield mounting media.
Slides containing brain sections stained for DCX were examined on the same microscope used for hippocampal volume measurements. Three sections through the dorsal hippocampus and 3 sections through the ventral hippocampus per brain were randomly selected for DCX+ cell density analysis. In each tissue section, a contour was drawn around the entire DG granule cell layer, and DCX+ cells were exhaustively counted. The area of the contour was used to estimate the volume of the granule cell layer, and the number of DCX+ cells per granule cell layer volume was averaged across the three tissue sections.
Statistical Analysis
Statistical analyses were performed using Prism version 7.03 (GraphPad Software, San Diego, CA) and SPSS version 24 (IBM, Armonk, NY). Because there is significant variation in the level of EtOH exposure of individual pups in vapor chambers, the unit of determination for statistical analyses was defined as an animal, unless indicated (litter numbers provided in Supplementary Table 1) (Baculis et al., 2015). For dam BEC measurements, two-way ANOVA was performed using genotype and time of measurement (i.e., gestation vs. lactation) as the independent variables. Pup BEC measurements were analyzed with an unpaired t-test. Mortality rates were analyzed using a Fisher’s exact test. For all data analyzed by ANOVA, multiple comparisons using the Sidak test were planned in advance of performing the experiments (i.e., planned comparisons) to assess the effect of genotype in air exposed animals and EtOH exposure within genotypes for both male and female mice. Animal weights during development were analyzed with separate two-way ANOVAs within age. Fear conditioning data were first analyzed using a repeated measures three-way ANOVA within level of sex using genotype and exposure condition as between-subjects factors, and time point as the within-subjects factor. Fear-conditioning data were then analyzed using repeated measures two-way ANOVAs within level of sex and genotype, using exposure condition as the between-subjects factor and time bin as the within-subjects factor. Planned multiple comparisons were performed to explore the effects of exposure condition within individual time bins, and Sidak multiplicity adjusted p values are reported. For repeated measures that violated assumptions of sphericity, Greenhouse-Geisser corrected F ratios and p values are reported. All other data were analyzed using two-way ANOVAs, with vapor chamber exposure condition and BDNF genotype as fixed factors, followed by planned multiple comparison analysis of exposure effects within genotype with reported Sidak multiplicity adjusted p values. Statistical values from ANOVAs include F ratio, p value, and effect size reported as partial eta squared (ηp2). Effect sizes from planned comparisons and t-tests are reported as Hedges’ g. Hedges’ g effect size is similar to Cohen’s d (Cohen, 1988), but corrects for bias resulting from using smaller sample sizes (Cumming, 2012, Lakens, 2013). Established benchmarks for small (ηp2 = 0.01; g = 0.2), medium (ηp2 = 0.06; g = 0.5); and large (ηp2 = 0.14; g = 0.8) effect sizes are based on the work of Cohen (1988). Collected results from ANOVAs, including 95% confidence intervals from multiple comparison analyses, appear in Supplemental Tables 2–6. Due to the fact that there were effects of sex on many of the measures examined in this study, data are presented separately within sex. To determine sex effects for fear-conditioning data, data were analyzed using repeated measures ANOVA with exposure condition, genotype, and sex as between-subjects factors and time point as the within-subjects factor. For all other experiments sex effects were analyzed using a univariate ANOVA with exposure condition, genotype, and sex as fixed factors. A detailed list of significant sex effects, as well as significant interactions of sex with other experimental factors is presented in Supplementary Table 7.
RESULTS
The characterization of the vapor chamber exposure paradigm (including BECs, pup weights, and acquisition of developmental milestones) is presented in the Supplementary Results Section.
Volumes of Hippocampal Cell Layers
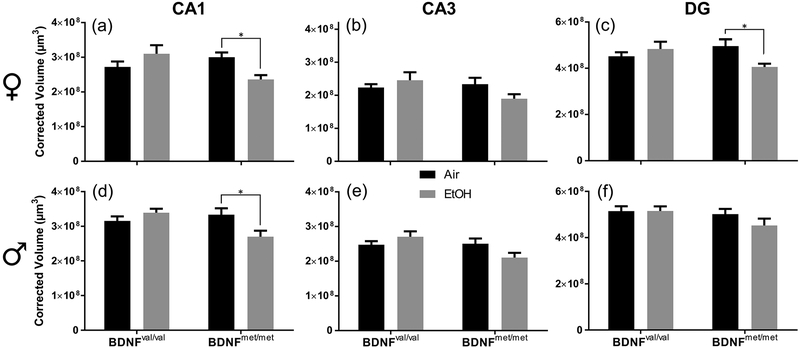
Both developmental EtOH exposure (Berman & Hannigan, 2000) and the BDNF valine to methionine mutation can significantly reduce hippocampal volume in humans and mice (Reviewed in Notaras et al, 2015). We predicted that the BDNF val68met mutation would make animals more vulnerable to the effects of developmental EtOH exposure and alter hippocampal structure to a greater degree than in wild-type animals. Following our EtOH exposure paradigm, brains were collected from animals at P15 and P50 and the volumes of hippocampal cell layers were measured. Two-way ANOVAs were performed within each sex and hippocampal region (CA1, CA3 and DG) to detect differences in cell layer volumes between experimental groups caused by the BDNF val68met polymorphism and developmental EtOH exposure. In P15 females (Fig 1a–c), there was a significant interaction between BDNF genotype and exposure condition on the volume of both the CA1 pyramidal cell layer (F(1,28) = 8.622; p = 0.007; ηp2 = 0.235) and DG granule cell layer (F(1,28) = 6.356; p = 0.018; ηp2 = 0.185). Planned comparisons revealed that these differences were due to a reduction in CA1 pyramidal cell layer volume in EtOH-exposed BDNFmet/met females compared to the air exposure condition (p = 0.029; g = 1.62) and a reduction in DG granule cell layer volume in EtOH BDNFmet/met females compared to air-exposed BDNFmet/met females (p = 0.027; g = 1.33). There were no significant effects in BDNFval/val females. There was a significant interaction of BDNF genotype and exposure condition in male P15 animals in the CA1 pyramidal cell layer (F(1,28) = 8.153; p = 0.008; ηp2 = 0.226) and the CA3 pyramidal cell layer (F(1,28) = 5.200, p = 0.030; ηp2 = 0.157), with no effect observed in the DG granule cell layer (Fig 1d–f). Planned comparison analysis determined that the volume of the CA1 pyramidal cell layer in EtOH-exposed BDNFmet/met P15 males was reduced compared to air-exposed BDNFmet/met males (p = 0.013; g = 1.19). There was no effect of exposure condition in BDNFval/val males. In the CA3 pyramidal and DG granule cell layers, planned comparisons did not find significant differences in volume between exposure conditions in either BDNFval/val or BDNFmet/met male mice. The volume of the CA1 pyramidal (F(1,56) = 9.103, p = 0.004, ηp2 = 0.230) and DG granule cell F(1,56) = 4.886, p = 0.031, ηp2 = 0.080) layers were significantly lower in female mice compared to male mice, regardless of genotype or exposure condition (Fig 1 and Supplementary Table 7).
Figure 1.
The BDNF val68met polymorphism interacts with developmental EtOH exposure at P15 to affect volumes of hippocampal cell layers. Black bars represent air-exposed animals; gray bars represent EtOH-exposed animals. Corrected cell layer volumes in μm3 are presented for: (a) female CA1, (b) female CA3, (c) female DG, (d) male CA1, (e) male CA3, and (f) male DG (n = 8 mice per group). *p < 0.05 from planned comparison of vapor chamber exposure effect within genotype.
Later in development at P50 (Supplementary Fig 3), there was a significant interaction of vapor chamber exposure condition and BDNF genotype on the volume of the DG granule cell layer in males (F(1,28) = 4.818; p = 0.037; ηp2 = 0.147); however, there was no effect of exposure condition within BDNF genotype. There were no significant interactions of exposure condition and BDNF genotype in other regions examined in either males or females. Independent of sex, genotype, or exposure condition there was a significant decrease in the volumes of hippocampal cell layers (CA1: F(1,112) = 87.043, p < 0.001, ηp2 = 0.437; CA3: F(1,112) = 10.176, p = 0.002, ηp2 = 0.083; DG: F(1,112) = 60.867, p < 0.001, ηp2 = 0.352) between P15 and P50 mice. These results are in general agreement with a recent report, which found decreases in soma area in hippocampal subfields in rats between P10 and P30/P60 (Jakubowska-Dogru et al., 2017). Taken together with the P15 data, these findings demonstrate that early in development vapor chamber EtOH exposure interacts with the BDNF val68met polymorphism to reduce the volume of cell layers in some hippocampal regions, but that these effects are not static, disappearing as the animal ages. At P50, there were no significant differences in the volume of any of the hippocampal cell layers between male and female mice (Supplementary Fig 3 and Supplementary Table 7).
Zero maze
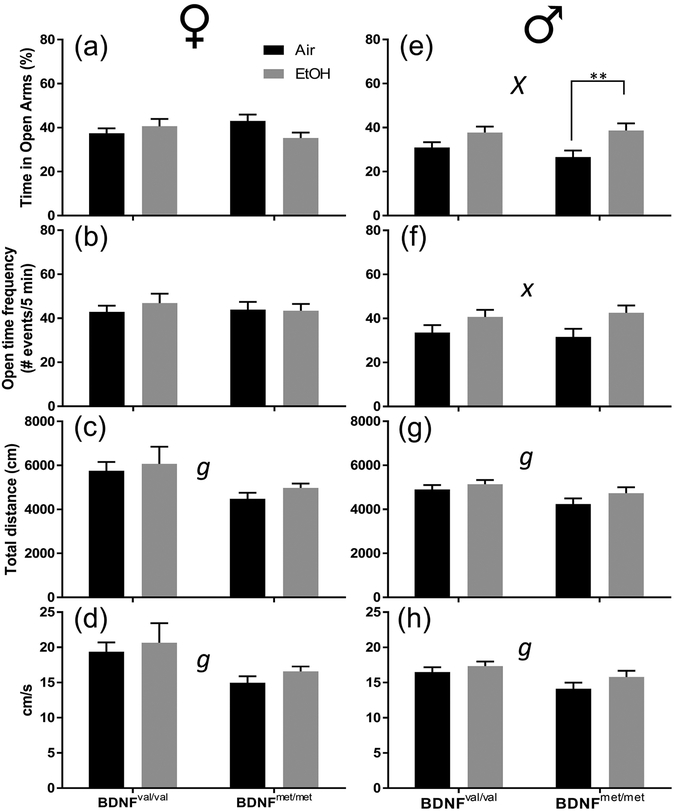
We next investigated if there were behavioral alterations caused by the interaction of the BDNF val68met polymorphism and developmental EtOH exposure. To examine anxiety like behavior, we measured performance on the elevated zero maze at approximately P80 (Fig 2). We expected to observe that developmental alcohol exposure would interact with the BDNF val68met polymorphism to increase anxiety. Contrary to our expectations, we observed the opposite phenomenon in male mice (Fig 2e), in which there was a significant effect of vapor chamber exposure condition (F(1,69) = 11.36; p = 0.001; ηp2 = 0.141). Planned comparison analysis determined that this was due to a significant effect in male BDNFmet/met mice, as EtOH exposure increased the percent time the animals spent in the open quadrants (p = 0.096; g = 0.94). There was no effect of EtOH exposure in BDNFval/val males. Although there were no significant effects of genotype on % time in open arms (Supplementary Table 2), an alternative interpretation of the results shown in Fig 2 is that the % time in open arms was reduced in air-exposed BDNFmet/met mice and that EtOH exposure increased values to levels comparable to those observed in EtOH-exposed BDNFval/val mice.
Figure 2.
The BDNF val68met polymorphism affects how developmental EtOH exposure reduces anxiety-like behavior measured by the zero maze in male BDNFmet/met mice at P80. Black bars represent air-exposed animals; gray bars represent EtOH-exposed animals. (a & e) Percent of time spent in open arms for female (a) and male (e) mice. (b & f) Number of open quadrant entries for female (b) and male (f) mice. (c & g) Total distance traveled for female (c) and male (g) mice. (d & h) Average velocity in cm/s for female (d) and male (h) mice. Sample sizes: female air BDNFval/val n = 26, female EtOH BDNFval/val n = 13, female air BDNFmet/met n = 8, female EtOH BDNFmet/met n = 10, male air BDNFval/val n = 22, male EtOH BDNFval/val n = 18, male air BDNFmet/met n = 16, male EtOH BDNFmet/met n = 17. Lowercase x indicates a significant effect of vapor chamber exposure condition at p < 0.05, uppercase X at p < 0.01. Lowercase g indicates a significant effect of BDNF genotype at p < 0.05. **p < 0.01 from planned comparison of vapor chamber exposure effect within genotype.
In females, there were no significant effects (Fig 2a). EtOH exposure also increased the open time frequency in males (F(1,69) = 6.877; p = 0.011; ηp2 = 0.091), but planned comparison analysis did not show any significant effect of EtOH exposure within BDNF genotype. In both female and male mice, the BDNFmet/met genotype reduced the total distance travelled (female: F(1,53) = 4.226, p = 0.045, ηp2 = 0.074; male: F(1,69) = 5.439, p = 0.023, ηp2 = 0.073) (Fig 2c,g) and the animals’ velocity (female: F(1,53) = 4.655, p = 0.036, ηp2 = 0.081; male: F(1,69) = 6.488, p = 0.013, ηp2 = 0.086) (Fig 2 d,h).
The percent time spent in the open arms (F(1,122) = 6.902, p = 0.010, ηp2 = 0.054), open time frequency (F(1,122) = 7.424, p = 0.007, ηp2 = 0.057), total distance traveled (F(1,122) = 4.047, p = 0.046, ηp2 = 0.032) and velocity (F(1,122) = 4.201, p = 0.043, ηp2 = 0.033) were significantly lower in male mice compared to female mice regardless of genotype or exposure condition (Fig 2 and Supplementary Table 7). There was also significant interaction between vapor chamber exposure condition and sex on the time spent in the open arms (F(1,122) = 7.580, p = 0.007, ηp2 = 0.058) (Fig 2 and Supplementary Table 7).
Trace fear conditioning
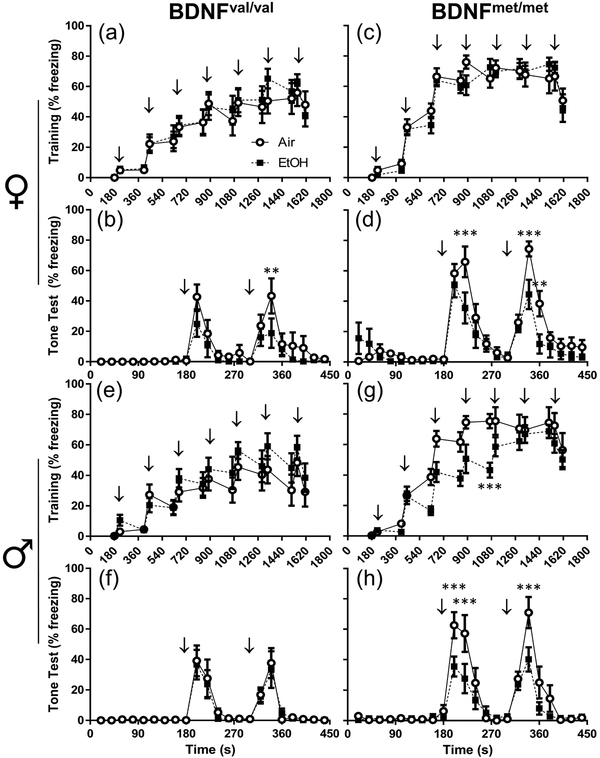
One of the most common negative outcomes resulting from developmental EtOH exposure concerns deficits in learning and memory processes. These deficiencies are observed in humans diagnosed with FASDs (Hamilton et al., 2003, Mattson & Riley, 1999, Mattson et al., 1996), as well as in rodent models of FASDs (Brady et al., 2012, Savage et al., 2010). Further, the BDNF valine to methionine polymorphism is associated with deficits in episodic memory in humans (Egan et al., 2003, Hariri et al., 2003) and animals (Dincheva et al., 2014, Yu et al., 2009). Therefore, we used a trace fear-conditioning paradigm to assess learning and memory in val68met mice. P90 animals learned to associate a tone (conditioned stimulus, CS) with a shock (unconditioned stimulus, US) on the training day by receiving seven CS, trace, and US pairings (Fig 3a, c, e, and g). Twenty-four hours later, memory of the pairings was tested by exposing animals to the CS and measuring time spent freezing (Fig 3b, d, f, and h). Detailed statistics for fear-conditioning experiments appear in Supplemental Tables 3–5. On the training day, three-way repeated measures ANOVA revealed a significant genotype by exposure interaction on freezing behavior in male mice (F(1,36) = 4.740; p = 0.036; ηp2 = 0.116). This interaction was driven by a significant effect of vapor chamber exposure condition in BDNFmet/met males (Fig 3g). In BDNFmet/met male mice, EtOH exposure decreased overall freezing behavior (F(1,28) = 7.42; p = 0.014; ηp2 = 0.292). Planned comparison analysis determined that there was a significant difference caused by exposure condition at t = 1068s, as EtOH-exposed BDNFmet/met males froze less during this period than their air-exposed counterparts (p = 0.003; g = 2.05). No effects of exposure condition were observed in BDNFval/val males and females, or in BDNFmet/met females.
Figure 3.
Increased freezing behavior primarily in BDNFmet/met animals during the regular trace fear-conditioning paradigm. The percentage of time freezing for individual time bins is presented for: (a) female BDNFval/val training, (b) female BDNFval/val test, (c) female BDNFmet/met training, female BDNFmet/met test, (e) male BDNFval/val training, (f) male BDNFval/val test, (g) male BDNFmet/met training, and (h) male BDNFmet/met test. Arrows indicate when the tone (CS) was played. Open circles represent air-exposed animals; black squares represent EtOH-exposed animals. n = 10 for all experimental groups. **p < 0.01, ***p < 0.001 from planned comparison of vapor chamber exposure effect within genotype at individual time bins.
Next, we determined if there was a simple effect of BDNF genotype within sex in air-exposed animals that affected freezing behavior during training days. In both male and female mice, analysis revealed significant differences in freezing behavior caused by BDNF genotype on the training days in both female (F(1,18) = 5.554, p = 0.030, ηp2 = 0.236) and male mice (F(1,18) = 12.30, p = 0.003, ηp2 = 0.406). BDNFmet/met females froze more than BDNFval/val females on the training day at t = 666s (p = 0.010; g = 1.74). BDNFmet/met males froze more than BDNFval/val males on the training day at t = 666s (p = 0.010; g = 1.82), t = 846s (p = 0.046; g = 1.40), t = 888s (p = 0.004; g = 1.83), t = 1068s (p = 0.0002; g = 2.02), t = 1110s (p = 0.044; g = 1.04), t = 1290s (p = 0.044; g = 0.94), and at t = 1512s (p = 0.0003; g = 1.31).
On the test day, analysis of freezing behavior elicited by exposure to the CS revealed that EtOH exposure significantly reduced total freezing behavior in a sex- and genotype-dependent manner. In all female animals, there was a significant effect of vapor chamber exposure condition on freezing behavior (F(1,36) = 4.915; p = 0.033; ηp2 = 0.120). In BDNFval/val females, EtOH-exposed animals froze less compared to air-exposed BDNFval/val females at t = 340s (p = 0.003; g = 0.69) (Fig 3b). In BDNFmet/met females, EtOH exposed animals froze less than air-exposed animals at t = 220s (p = 0.001; g = 0.91), t = 340s (p = 0.001; g = 1.18) and at t = 360s (p = 0.008; g = 1.08) (Fig 3d). BDNFmet/met EtOH-exposed males froze more overall than air controls (F(1,18) = 4.638; p = 0.045; ηp2 = 0.205) (Fig 3h) but there was no effect of exposure in BDNFval/val males (Fig 3f). EtOH exposure caused a decrease in freezing in BDNFmet/met males at t = 200s (p = 0.001; g = 1.07), t = 220s (p = 0.0002; g = 0.82), and at t = 340s (p < 0.0001; g = 1.02) (Fig 3h).
We also examined if there was a simple effect of BDNF genotype within sex that affected freezing behavior on the test day in air-exposed animals. In both male and female mice exposed to air, analysis revealed significant differences in freezing behavior caused by BDNF genotype (female genotype: F(1,18) = 7.595, p = 0.013, ηp2 =. 0.297; male genotype: F(1,18) = 8.837, p = 0.008, ηp2 = 0.329). BDNFmet/met females froze more than BDNFval/val females on the test day at t = 220s (p < 0.0001; g = 1.50), t = 240s (p = 0.009; g = 1.13), t = 340s (p = 0.0002; g = 1.06), and at t = 360s (p = 0.003; g = 1.05). BDNFmet/met males froze more than BDNFval/val males on the test day at t = 200s (p = 0.015; g = 0.76), at t = 240s (p = 0.0005; g = 0.83), at t = 340s (p < 0.0001; g = 1.00), and at t = 360s (p = 0.009; g = 1.00).
To assess sensitivity to foot shock, we analyzed the occurrence of two common behaviors elicited by the shock during the training session: jumping and running (Seo et al., 2015). In the regular trace fear conditioning paradigm, BDNFmet/met animals exhibited more jumping (female genotype: F(1,36) = 16.20, p = 0.0003, ηp2 = 0.310; male genotype F(1,36) = 11.34, p = 0.002, ηp2 = 0.239) and running (female genotype F(1,36) = 9.684, p = 0.004, ηp2 = 0.212; male genotype F(1,36) = 16.23, p = 0.0003; ηp2 = 0.311) behaviors in response to the foot shock (Supplementary Figure 4a–d).
Modified trace fear conditioning
Because trace fear conditioning is a stressful, aversive procedure, a generalized anxiety phenotype could have elicited non-associative fear in the BDNFmet/met animals, masking hippocampal-dependent deficits caused by developmental alcohol exposure. This generalized anxiety may be due, in part, to an increased sensitivity to the foot shock caused by the BDNF polymorphism. To reduce the aversive component of trace fear-conditioning, we utilized a modified trace fear-conditioning protocol, which minimizes non-associative fear (Seo et al., 2015). Using the modified trace fear conditioning protocol, effects of genotype on shock sensitivity were eliminated (female jump genotype: F(1,48) = 1.290, p = 0.262, ηp2 = 0.026; male jump genotype: F(1,46) = 1.688, p = 0.200; ηp2 = 0.035; female run genotype: F(1,48) = 2.160, p = 0.148; ηp2 = 0.043; male run genotype: F(1,46) = 1.858, p = 0.179, ηp2 = 0.039) (Supplementary Figure 4e–h). Because the modified trace fear conditioning protocol eliminated any effect of genotype on sensitivity to the foot shock, it is unlikely that significant outcomes from the modified trace fear paradigm were confounded by differential sensitivity to shock caused by the BDNF polymorphism.
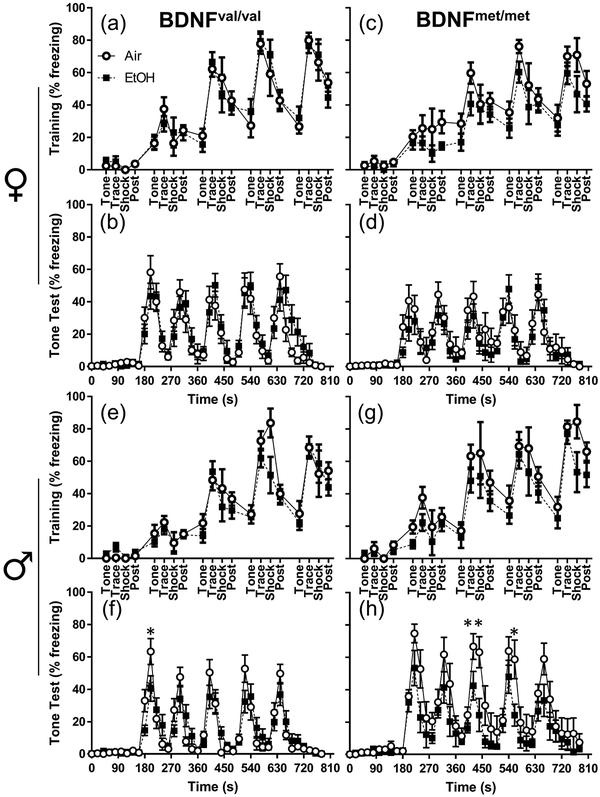
On the training day of the modified trace fear-conditioning paradigm, we observed a decrease in overall freezing time caused by developmental EtOH exposure in female BDNFmet/met mice (F(1,25) = 4.531; p = 0.043; ηp2 = 0.153) (Fig 4c). However, planned comparison analysis did not reveal significant differences between treatment groups at any individual time points. There were no effects of treatment in female or male BDNFval/val mice (Figure 4a,e). There was no effect of genotype on air-exposed female mice; however, air-exposed male BDNFmet/met mice froze more than air-exposed male BDNFval/val mice for the last shock of the training day (p = 0.036; g = 0.76).
Figure 4.
Increased freezing behavior primarily in BDNFmet/met males during the test day from the modified trace fear-conditioning protocol. The percentage of time freezing for individual time bins is presented for: (a) female BDNFval/val training, (b) female BDNFval/val test, (c) female BDNFmet/met training, (d) female BDNFmet/met test, (e) male BDNFval/val training, (f) male BDNFval/val test, (g) male BDNFmet/met training, and (h) male BDNFmet/met test. Open circles represent air-exposed animals; black squares represent EtOH-exposed animals. (abcd) female air BDNFval/val n = 11, female EtOH BDNFval/val n = 15, female air BDNFmet/met n = 12, female EtOH BDNFmet/met n = 15. (efgh) male air BDNFval/val n = 11, male EtOH BDNFval/val n = 17, male air BDNFmet/met n = 10, male EtOH BDNFmet/met n = 12. *p < 0.05, **p < 0.01 from planned comparison of vapor chamber exposure effect within genotype at individual time bins.
On the test day of the modified trace fear conditioning paradigm, all significant effects were observed exclusively in male mice. There was a three-way interaction of time point by exposure by genotype on freezing behavior in males (F(7.526,346.2 = 3.054, p = 0.003; ηp2 = 0.062). There was also a significant effect of genotype on freezing behavior in male animals on the test day of the modified trace fear conditioning paradigm (F(1,46) = 5.666, p = 0.021; ηp2 = 0.110). Analysis of individual time bins from the test day of the modified trace fear-conditioning paradigm found significant effects of EtOH exposure only in male mice (Fig 4f,h). EtOH exposure reduced freezing in male BDNFval/val mice at a single time point (t = 200s; p = 0.017; g = 0.75)(Fig 4f). In BDNFmet/met males, EtOH exposure significantly reduced freezing at t = 440s (p = 0.002; g = 1.13), as well as at t = 560s (p = 0.014; g = 1.05) (Fig 4h). Genotype did not significantly affect freezing in air-exposed females; however, air-exposed BDNFmet/met males froze significantly more than air-exposed BDNFval/val males (p = 0.013 at t = 180s; p<0.0001 at t = 220s, 240s, 320s, 340s, 440s, 560s, 660s; p = 0.0017 at t = 420s; p = 0.009 at t = 520s; p = 0.002 at t = 540s; p = 0.013 at t = 680s; effect sizes appear in Supplementary Table 4). On the test day of the modified trace fear conditioning paradigm, there were two significant effects on time freezing when sex was included as a factor: an interaction between BDNF genotype and sex (F(1,95) = 6.391, p = 0.013, ηp2 = 0.063), and a 3-way interaction between BDNF genotype, sex, and time-point (F(8.170,776.165) = 5.282, p < 0.001, ηp2 = 0.053) (Fig 4 and Supplementary Table 7).
DCX+ cell counting
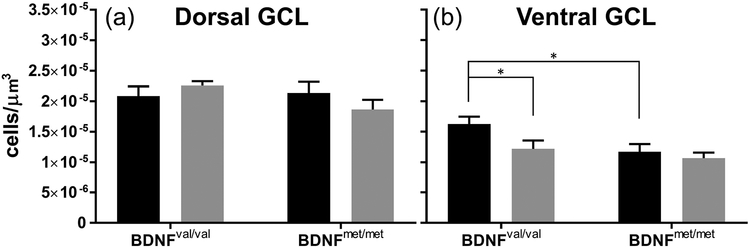
Data shown above suggest that the BDNF val68met polymorphism, in combination with developmental EtOH exposure, impairs associative trace conditioning in male mice. Neurogenesis in the DG of the hippocampus modulates fear learning (Seo et al., 2015). Both developmental EtOH exposure (Gil-Mohapel et al., 2014, Hamilton et al., 2011, Ieraci & Herrera, 2007, Kajimoto et al., 2013, Klintsova et al., 2007) and the BDNF valine to methionine polymorphism (Bath et al., 2012, Ieraci et al., 2016) affect adult hippocampal neurogenesis. Therefore, we assessed levels of adult hippocampal neurogenesis in BDNFval/val and BDNFmet/met male mice (Fig 5). Parasagittal sections were collected through the dorsal and ventral hippocampus, and levels of neurogenesis were inferred by counting DCX+ cells. DCX is expressed in migrating neuronal progenitors, and data has shown that alterations in the number of DCX+ cells are an index of changes in adult hippocampal neurogenesis (Couillard-Despres et al., 2005). In the dorsal hippocampus of male animals, there were no significant effects of either vapor chamber exposure condition or BDNF genotype. In the ventral hippocampus, EtOH exposure reduced DCX+ cell density in BDNFval/val animals (p = 0.042; g = 0.96), with no effect in BDNFmet/met mice. There was also an effect of BDNF genotype in air-exposed animals, as the BDNFmet/met genotype caused a reduction in DCX+ cell density (p = 0.022; g = 1.12).
Figure 5.
Reduced DCX+ cell density in the ventral hippocampus of BDNFmet/met male mice. Black bars represent air-exposed animals, gray bars represent EtOH-exposed animals. DCX+ cell density in the granule cell layer of the DG for the (a) dorsal and (b) ventral hippocampi are presented. n = 10 per group. *p < 0.05 for planned comparison of vapor chamber exposure effect within genotype, or for comparison of BDNF genotype in air-exposed animals.
DISCUSSION
To our knowledge, this is the first report of how the BDNF valine to methionine polymorphism can influence the severity of FASDs. Binge-like EtOH exposure during the 2nd and 3rd trimester-equivalent developmental periods significantly reduced hippocampal cell layer volumes only in BDNFmet/met mice. This effect was observed at P15 in both males and females in the CA1 region but only in females in the DG. These differences in hippocampal cell layer volumes disappeared by P50, suggesting that compensatory mechanisms are able to correct these deficits in EtOH-exposed BDNFmet/met mice. Alternatively, it is possible that EtOH exposure caused a delay in the development of the hippocampal cell layers that was apparent at P15 but not P50. It is not surprising that our exposure paradigm did not have a significant effect on hippocampal cell layer volumes in wild-type mice (i.e, BDNFval/val mice) because the effects of developmental EtOH exposure on hippocampal morphology are highly dependent on the dose, timing, EtOH delivery method, and animal model used for the experiments (Berman & Hannigan, 2000). Future studies should investigate whether the BDNF polymorphism modulates the impact of other exposure paradigms on the hippocampal formation.
Genotype alone did not have an effect on hippocampal cell layer volumes in air-exposed mice at either P15 or P50. These findings are in general agreement with human studies indicating that the val66met polymorphism is not associated with decreases in hippocampal volume or has a small effect on this parameter (Reviewed in Notaras et al, 2015 and Harrisberger et al, 2016). Our results are also consistent with those of Chen et al., (2006) who found no significant effect of genotype on DG soma area of P60 mice. However, further characterization of the effect of genotype on hippocampal morphology in our mouse model is warranted considering that our volume measurements only focused on the cell layers and it is possible that the polymorphism affects volume of other layers (i.e., dendritic). Indeed, Chen et al., (2006) detected a decrease in total hippocampal volume and DG dendritic complexity in BDNFmet/met transgenic mice expressing the human BDNF coding sequence.
In adult mice, the zero maze task revealed a decrease in anxiety-like behavior only in EtOH-exposed BDNFmet/met male mice. These findings could be interpreted to indicate that the BDNF val68met polymorphism has a beneficial effect in EtOH-exposed male mice. It is important to determine if lower levels of anxiety are also observed in prenatally EtOH-exposed human carriers of the 66met allele and if this is associated with an increase in risk-taking behavior. It is common that individuals with FASDs have difficulties connecting actions and consequences, as well as learning from experiences; they often exhibit social deficits that encourage them to engage in risky behaviors (Rasmussen et al., 2008, Schonfeld et al., 2005). In agreement with a previous report (Warnault et al., 2016), we did not observe a significant effect of the BDNF val68met polymorphism alone on anxiety-like behavior. These findings are in contrast to those of Chen et al (2006) who found that BDNFmet knock-in mice exhibit a decrease in the percentage of time spent in the open arms and the percentage of open arm entries in the elevated plus maze. A potential reason for the discrepancy between results is that the two mouse strains were developed using different approaches; Chen et al replaced the mouse BDNF coding sequence with the human BDNF sequence containing a single point mutation, whereas the mouse model we used was generated by introducing two point mutations in the mouse BDNF sequence (Chen et al., 2006, Warnault et al., 2016). In addition, Chen et al. inserted a carboxy-terminal histidine tag that could have impaired BDNF function, perhaps contributing to the differences detected between the mouse models.
In contrast to the results from the zero maze, we did find significant effects of genotype in the trace fear-conditioning test. During training and test days, air-exposed BDNFmet/met male and female mice froze significantly more than BDNFval/val mice. Importantly, this genotype effect was not observed during the training day when using the milder trace fear-conditioning paradigm that reduces the contribution of non-associative mechanisms to tone-induced fear (Seo et al., 2015). These findings suggest that BDNFmet/met mice perform better on the trace fear-conditioning test because they are sensitized to the stressful effects (i.e., generalized anxiety and fear) of this paradigm. Interestingly, Notaras et al (2016) recently reported that adult knock-in mice expressing the human BDNFmet/met polymorphism freeze less than BDNFval/val mice in a contextual fear-conditioning paradigm. However, when these mice were chronically exposed to corticosterone during adolescence, BDNFmet/met mice performed better than BDNFval/val mice. Future studies should investigate if our BDNFmet/met mice have abnormalities in the hypothalamic-pituitary-adrenal axis that could explain their enhanced ability to form memories of fear-related events. Moreover, it is important to characterize further the effects of EtOH and genotype on other fear learning paradigms. Expression of the 66Met genotype significantly reduced contextual fear conditioning responses in adult mice (Chen et al., 2006, Dincheva et al., 2014).
EtOH exposure had a complex interaction with sex and genotype in the trace fear-conditioning paradigm. EtOH exposure significantly reduced freezing during the tone test day in both BDNFval/val and BDNFmet/met female mice, with the latter being affected to a greater extent. These effects were not due to differences in performance during the training day. In male mice, EtOH exposure caused significant effects only in BDNFmet/met mice, which displayed reduced performance during both training and tone test days. These findings suggest that EtOH-exposed BDNFmet/met mice were less susceptible to conditioning than air-exposed BDNFmet/met mice during the training phase of the paradigm, resulting in decreased freezing during the tone test phase. Perhaps the EtOH-exposed BDNFmet/met male mice experienced less stress during the training phase of trace fear-conditioning. These results could be interpreted to indicate that EtOH has a protective effect by normalizing fear learning in BDNFmet/met mice. It is noteworthy, however, that EtOH-exposed BDNFmet/met male mice exhibited a significantly reduced freezing response during the tone test day even in the milder trace fear-conditioning paradigm that reduces the influence of non-associative tone-induced fear during conditioning (Seo et al., 2015). This paradigm eliminated genotype- and EtOH-induced differences during the training day of the trace fear-conditioning paradigm. These findings suggest that EtOH impairs associative fear learning in BDNFmet/met male mice independently of non-associative mechanisms.
Recent work has shown that adult neurogenesis in the DG plays a critical role in trace fear-conditioning by facilitating associative conditioning while reducing the influence of non-associative mechanisms (Seo et al., 2015). In general agreement with the results of this study, we found that air-exposed BDNFmet/met male mice had lower numbers of DCX+ cells than air-exposed BDNFval/val mice. Importantly, this effect was only observed in the ventral DG, where neurogenesis-mediated modulation of DG granule cell excitability is thought to control the formation of emotional memories (Anacker & Hen, 2017). Therefore, it is possible that the lower levels of neurogenesis in the ventral DG of BDNFmet/met mice are responsible for the increased freezing of these animals during both the training and testing phases of the trace fear-conditioning paradigm. The findings of Seo et al. (2015) support this possibility, as they determined that arrest of hippocampal neurogenesis in mice produces a similar pattern of performance in the trace fear-conditioning paradigm to that of BDNFmet/met mice. In contrast to the effect of genotype, EtOH exposure only reduced DCX+ cell number in the ventral hippocampus of BDNFval/val mice, with no effect observed in BDNFmet/met animals. It is interesting that EtOH reduced DCX+ cell numbers in the ventral hippocampus of BDNFval/val male mice, which exhibit little deficits on trace fear conditioning. This finding suggests that larger effects on neurogenesis would be required to cause significant impairments in this behavioral paradigm in BDNFval/val male mice, a possibility that is supported by the findings of Seo et al., (2015). Conversely, the lack of an effect of EtOH on DCX+ cell numbers in BDNFmet/met animals could be due to a BDNFmet/met genotype-induced reduction of DCX+ cell number to such a degree that precluded EtOH exposure from having an additional effect. Another possible explanation for our findings is that neurogenesis does not play a critical role in trace fear conditioning in our transgenic mouse model. These results indicate that other mechanisms mediate the effects of EtOH exposure on BDNFmet/met animals during trace fear-conditioning performance. Future studies should investigate the potential role of alterations in the function of neuronal circuits involved in trace fear-conditioning, including those located in the CA1 and CA3 hippocampal fields, entorhinal cortex, perirhinal cortex, medial prefrontal cortex, anterior cingulate cortex, and the amygdala (reviewed in Raybuck and Lattal, 2014). In addition, future studies should also examine the expression of other markers to further characterize neurogenesis in these mice. It is also possible that EtOH-induced alterations in trace-fear performance are independent of adult hippocampal neurogenesis, and are instead due to changes in dendritic complexity (Chen et al., 2006) and synaptic plasticity (Bath et al., 2012) caused by the BDNF polymorphism.
In conclusion, the results reported here provide evidence for a novel sex-dependent effect of a common BDNF genetic variant that modulates the effects of developmental EtOH exposure. Our findings underscore that interactions between genes and the environment play an important role in determining the behavioral phenotype of individuals with FASDs. We hope that this study encourages future clinical research on whether screening for the BDNF val66met polymorphism could be useful in estimating the risk of developing more severe forms of FASDs, allowing for the early implementation of better-targeted interventions against this devastating, highly prevalent condition.
Supplementary Material
Acknowledgements:
Author contributions: CWB and CFV designed experiments, analyzed data, interpreted findings and wrote the paper; BCB, JJM, GJC, TO, DJP, AJM, and ALG performed experiments and analyzed data; DR generated and provided transgenic mice, interpreted findings and edited the paper. We thank Drs. Kevin Caldwell and Jonathan Brigman for assistance with the behavioral studies.
Funding and Disclosure:
The authors declare no competing financial interests or other conflicts of interest. Supported by NIH grants R37 AA015614 and P50 AA022534 (CFV); UNM’s Post-Baccalaureate Research Education Program (TO) and Undergraduate Pipeline Network Program (DJP); R37 AA016848, P50 AA017072, and the State of California (DR). Unbiased stereology studies were carried out at the University of New Mexico & Cancer Center Fluorescence Microscopy Shared Resource, funded as detailed on: http://hsc.unm.edu/crtc/microscopy/acknowledgement.shtml.
REFERENCES
- Anacker C & Hen R (2017) Adult hippocampal neurogenesis and cognitive flexibility - linking memory and mood. Nat Rev Neurosci, 18, 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baculis BC, Diaz MR & Valenzuela CF (2015) Third trimester-equivalent ethanol exposure increases anxiety-like behavior and glutamatergic transmission in the basolateral amygdala. Pharmacol Biochem Behav, 137, 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barto D, Bird CW, Hamilton DA & Fink BC (2016) The Simple Video Coder: A free tool for efficiently coding social video data. Behav Res Methods. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath KG, Jing DQ, Dincheva I, Neeb CC, Pattwell SS, Chao MV, Lee FS & Ninan I (2012) BDNF Val66Met impairs fluoxetine-induced enhancement of adult hippocampus plasticity. Neuropsychopharmacology, 37, 1297–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RF & Hannigan JH (2000) Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology, and neuroanatomy. Hippocampus, 10, 94–110. [DOI] [PubMed] [Google Scholar]
- Brady ML, Allan AM & Caldwell KK (2012) A limited access mouse model of prenatal alcohol exposure that produces long-lasting deficits in hippocampal-dependent learning and memory. Alcohol Clin Exp Res, 36, 457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueller JA, Aftab M, Sen S, Gomez-Hassan D, Burmeister M & Zubieta JK (2006) BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol Psychiatry, 59, 812–815. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL & Lee FS (2006) Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science, 314, 140–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi P, Aras R, Martin K & Favero C (2016) Using Swiss Webster mice to model Fetal Alcohol Spectrum Disorders (FASD): An analysis of multilevel time-to-event data through mixed-effects Cox proportional hazards models. Behav Brain Res, 305, 1–7. [DOI] [PubMed] [Google Scholar]
- Cohen J (1988) Statistical Power Analysis for the Behavioral Sciences, Routledge Academic, New York, NY. [Google Scholar]
- Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG & Aigner L (2005) Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci, 21, 1–14. [DOI] [PubMed] [Google Scholar]
- Cumming G (2012) Understanding the new statistics : effect sizes, confidence intervals, and meta-analysis, Routledge, Taylor & Francis Group, New York. [Google Scholar]
- Dempster E, Toulopoulou T, McDonald C, Bramon E, Walshe M, Filbey F, Wickham H, Sham PC, Murray RM & Collier DA (2005) Association between BDNF val66 met genotype and episodic memory. Am J Med Genet B Neuropsychiatr Genet, 134B, 73–75. [DOI] [PubMed] [Google Scholar]
- Dincheva I, Pattwell SS, Tessarollo L, Bath KG & Lee FS (2014) BDNF modulates contextual fear learning during adolescence. Dev Neurosci, 36, 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart JK & Parnell SE (2016) The Genetics of Fetal Alcohol Spectrum Disorders. Alcohol Clin Exp Res, 40, 1154–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B & Weinberger DR (2003) The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell, 112, 257–269. [DOI] [PubMed] [Google Scholar]
- Frielingsdorf H, Bath KG, Soliman F, Difede J, Casey BJ & Lee FS (2010) Variant brain-derived neurotrophic factor Val66Met endophenotypes: implications for posttraumatic stress disorder. Ann N Y Acad Sci, 1208, 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo R & Valenzuela CF (2006) Immature hippocampal neuronal networks do not develop tolerance to the excitatory actions of ethanol. Alcohol, 40, 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Mohapel J, Titterness AK, Patten AR, Taylor S, Ratzlaff A, Ratzlaff T, Helfer J & Christie BR (2014) Prenatal ethanol exposure differentially affects hippocampal neurogenesis in the adolescent and aged brain. Neuroscience, 273, 174–188. [DOI] [PubMed] [Google Scholar]
- Guerri C, Bazinet A & Riley EP (2009) Foetal Alcohol Spectrum Disorders and alterations in brain and behaviour. Alcohol Alcohol, 44, 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A & et al. (1988) Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS, 96, 379–394. [DOI] [PubMed] [Google Scholar]
- Hajek T, Kopecek M & Höschl C (2012) Reduced hippocampal volumes in healthy carriers of brain-derived neurotrophic factor Val66Met polymorphism: meta-analysis. World J Biol Psychiatry, 13, 178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DA, Kodituwakku P, Sutherland RJ & Savage DD (2003) Children with Fetal Alcohol Syndrome are impaired at place learning but not cued-navigation in a virtual Morris water task. Behav Brain Res, 143, 85–94. [DOI] [PubMed] [Google Scholar]
- Hamilton GF, Murawski NJ, St Cyr SA, Jablonski SA, Schiffino FL, Stanton ME & Klintsova AY (2011) Neonatal alcohol exposure disrupts hippocampal neurogenesis and contextual fear conditioning in adult rats. Brain Res, 1412, 88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF & Weinberger DR (2003) Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci, 23, 6690–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrisberger F, Smieskova R, Schmidt A, Lenz C, Walter A, Wittfeld K, Grabe HJ, Lang UE, Fusar-Poli P & Borgwardt S (2015) BDNF Val66Met polymorphism and hippocampal volume in neuropsychiatric disorders: A systematic review and meta-analysis. Neurosci Biobehav Rev, 55, 107–118. [DOI] [PubMed] [Google Scholar]
- Hosang GM, Shiles C, Tansey KE, McGuffin P & Uher R (2014) Interaction between stress and the BDNF Val66Met polymorphism in depression: a systematic review and meta-analysis. BMC Med, 12, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyme HE, Kalberg WO, Elliott AJ, Blankenship J, Buckley D, Marais AS, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Jewett T, Coles CD, Chambers C, Jones KL, Adnams CM, Shah PE, Riley EP, Charness ME, Warren KR & May PA (2016) Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieraci A & Herrera DG (2007) Single alcohol exposure in early life damages hippocampal stem/progenitor cells and reduces adult neurogenesis. Neurobiol Dis, 26, 597–605. [DOI] [PubMed] [Google Scholar]
- Ieraci A, Madaio AI, Mallei A, Lee FS & Popoli M (2016) Brain-Derived Neurotrophic Factor Val66Met Human Polymorphism Impairs the Beneficial Exercise-Induced Neurobiological Changes in Mice. Neuropsychopharmacology, 41, 3070–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowska-Dogru E, Elibol B, Dursun I & Yuruker S (2017) Effects of prenatal binge-like ethanol exposure and maternal stress on postnatal morphological development of hippocampal neurons in rats. Int J Dev Neurosci, 61, 40–50. [DOI] [PubMed] [Google Scholar]
- Kajimoto K, Allan A & Cunningham LA (2013) Fate analysis of adult hippocampal progenitors in a murine model of fetal alcohol spectrum disorder (FASD). PLoS One, 8, e73788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klintsova AY, Helfer JL, Calizo LH, Dong WK, Goodlett CR & Greenough WT (2007) Persistent impairment of hippocampal neurogenesis in young adult rats following early postnatal alcohol exposure. Alcohol Clin Exp Res, 31, 2073–2082. [DOI] [PubMed] [Google Scholar]
- Lakens D (2013) Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol, 4, 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN & Riley EP (1999) Implicit and explicit memory functioning in children with heavy prenatal alcohol exposure. J Int Neuropsychol Soc, 5, 462–471. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Delis DC, Stern C & Jones KL (1996) Verbal learning and memory in children with fetal alcohol syndrome. Alcohol Clin Exp Res, 20, 810–816. [DOI] [PubMed] [Google Scholar]
- Montag C, Basten U, Stelzel C, Fiebach CJ & Reuter M (2010) The BDNF Val66Met polymorphism and anxiety: support for animal knock-in studies from a genetic association study in humans. Psychiatry Res, 179, 86–90. [DOI] [PubMed] [Google Scholar]
- Notaras M, Hill R, Gogos JA & van den Buuse M (2016) BDNF Val66Met genotype determines hippocampus-dependent behavior via sensitivity to glucocorticoid signaling. Mol Psychiatry, 21, 730–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notaras M, Hill R & van den Buuse M (2015) The BDNF gene Val66Met polymorphism as a modifier of psychiatric disorder susceptibility: progress and controversy. Mol Psychiatry, 20, 916–930. [DOI] [PubMed] [Google Scholar]
- Paxinos G & Franklin KBJ (2013) Paxinos and Franklin’s The Mouse Brain in Stereotaxic Coordinates, Elsevier. [Google Scholar]
- Rasmussen C, Andrew G, Zwaigenbaum L & Tough S (2008) Neurobehavioural outcomes of children with fetal alcohol spectrum disorders: A Canadian perspective. Paediatr Child Health, 13, 185–191. [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD & Lattal KM (2014) Bridging the interval: theory and neurobiology of trace conditioning. Behav Processes, 101, 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage DD, Rosenberg MJ, Wolff CR, Akers KG, El-Emawy A, Staples MC, Varaschin RK, Wright CA, Seidel JL, Caldwell KK & Hamilton DA (2010) Effects of a novel cognition-enhancing agent on fetal ethanol-induced learning deficits. Alcohol Clin Exp Res, 34, 1793–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Barr CS, Larson JA & Kraemer GW (2011) Moderate prenatal alcohol exposure and serotonin genotype interact to alter CNS serotonin function in rhesus monkey offspring. Alcohol Clin Exp Res, 35, 912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonfeld AM, Mattson SN & Riley EP (2005) Moral maturity and delinquency after prenatal alcohol exposure. J Stud Alcohol, 66, 545–554. [DOI] [PubMed] [Google Scholar]
- Seo DO, Carillo MA, Chih-Hsiung Lim S, Tanaka KF & Drew MR (2015) Adult Hippocampal Neurogenesis Modulates Fear Learning through Associative and Nonassociative Mechanisms. J Neurosci, 35, 11330–11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JK, Grewal SS, Fletcher A, Bill DJ & Dourish CT (1994) Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology (Berl), 116, 56–64. [DOI] [PubMed] [Google Scholar]
- Topper LA, Baculis BC & Valenzuela CF (2015) Exposure of neonatal rats to alcohol has differential effects on neuroinflammation and neuronal survival in the cerebellum and hippocampus. J Neuroinflammation, 12, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnault V, Darcq E, Morisot N, Phamluong K, Wilbrecht L, Massa SM, Longo FM & Ron D (2016) The BDNF Valine 68 to Methionine Polymorphism Increases Compulsive Alcohol Drinking in Mice That Is Reversed by Tropomyosin Receptor Kinase B Activation. Biol Psychiatry, 79, 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren KR & Li TK (2005) Genetic polymorphisms: impact on the risk of fetal alcohol spectrum disorders. Birth Defects Res A Clin Mol Teratol, 73, 195–203. [DOI] [PubMed] [Google Scholar]
- Yu H, Wang Y, Pattwell S, Jing D, Liu T, Zhang Y, Bath KG, Lee FS & Chen ZY (2009) Variant BDNF Val66Met polymorphism affects extinction of conditioned aversive memory. J Neurosci, 29, 4056–4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Benedek DM, Fullerton CS, Forsten RD, Naifeh JA, Li XX, Hu XZ, Li H, Jia M, Xing GQ, Benevides KN & Ursano RJ (2014) PTSD risk is associated with BDNF Val66Met and BDNF overexpression. Mol Psychiatry, 19, 8–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.