Abstract
Fetal erythropoietin (EPO), in addition to regulating erythropoiesis, has also tissue protective properties based on its anti-inflammatory, anti-apoptotic, antioxidant and neurotrophic effects. Notably, EPO concentrations needed for tissue protection are 100–1 000 times higher than concentrations needed for regulating erythropoiesis. This dual effect of EPO is based on EPOC receptor (EPO-R) isoforms, which differ structurally and functionally. We hypothesize in this Integrated Mechanism Review that during severe fetal hypoxia the observed, but poorly understood, marked increases of fetal plasma EPO concentrations occur to protect the brain, heart and other vital fetal organs. We further hypothesize that the concurrent marked increases of EPO in the amniotic fluid during fetal hypoxia, occur to protect newborn infants from necrotizing enterocolitis. This review presents experimental and clinical evidence in support of these hypotheses and points out unknown or poorly understood functions of EPO in the fetus. If these novel hypotheses are correct, the importance of fetal EPO as an antenatal hypoxia biomarker will become apparent. It will also likely point the way to important diagnostic and therapeutic fetal and neonatal interventions.
Keywords: Erythropoietin, Fetus, Newborn, Necrotizing Enterocolitis
BACKGROUND
Erythropoietin (EPO), a glycoprotein hormone with a molecular weight of 30.4 kDa, is primarily responsible for regulating erythrocyte production in adults, newborn infants and fetuses (1). During pregnancy, maternal serum EPO levels increase linearly from 16 to 65 mU/ml at term to meet the hematopoietic needs of pregnancy (2). Prior to 30 weeks of gestation in the fetus, the liver is the main organ of EPO production, after which a gradual transition to renal EPO production occurs (3,4). During normal oxygenation at term, fetal EPO production takes place mainly in the kidneys (4). Because EPO is not stored in tissue and does not cross the placenta (Figure 1) (5), steady-state fetal plasma EPO concentration reflects equal, opposing rates of EPO synthesis and elimination. During the last trimester of normal pregnancy, normoxemic human fetal plasma EPO hormone concentrations range from 10 to 50 mU/ml prior to labor (6,7). Concurrent amniotic fluid EPO concentrations during normal fetal oxygenation range from 2 to 20 mU/ml (6). Fetal plasma EPO concentrations under both normal and fetal hypoxemic conditions average 2.6 times higher than concurrent amniotic fluid EPO levels (6).
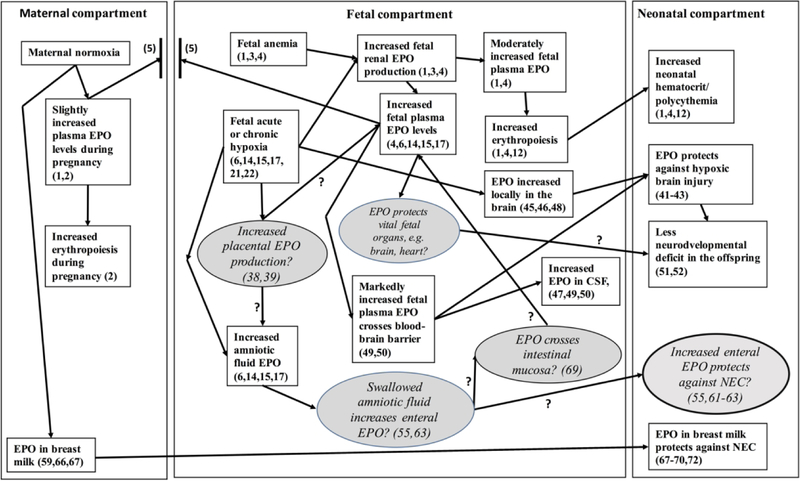
Figure 1.
Proposed physiological and pathophysiological mechanistic framework of erythropoietin (EPO) production and actions during fetal anemia and during acute and chronic hypoxia. Illustrated are EPO’s effects in the maternal, fetal, and neonatal compartments based on referenced supporting evidence from both basic research and clinical/epidemiologic studies. Unknown or poorly understood mechanisms are indicated in italics with question marks within ovals with grey background and question marks included between the ovals and rectangles indicating uncertain relationships. The protective effect of breast milk EPO on the neonatal intestine for avoiding necrotizing enterocolitis (NEC) is also indicated. The maternal, fetal and neonatal compartments are separated by the lines between. The two bold, vertical short lines between the maternal and fetal compartments depicts the placenta.
This Integrated Mechanism Review (8) focuses on important non-hematological actions of erythropoietin (EPO) that are being increasingly recognized in the fetus and the newborn infant (9,10). Our central hypothesis is that the observed marked increases of fetal EPO during severe fetal hypoxia occurs in order to protect its brain, heart, and other vital organs from the deleterious effects of severe hypoxia. We further hypothesize that the purpose of the concurrent increase in amniotic fluid EPO concentration during severe fetal hypoxia is to protect the intestine from necrotizing enterocolitis (NEC). In this review, the known effects of EPO on fetal erythropoiesis and its tissue protective properties are described to support these hypotheses based on existing experimental and clinical evidence. If our hypotheses are correct, they will further emphasize the importance of fetal EPO as an important antenatal hypoxia biomarker, and suggest potential new therapeutic approaches.
Several unknown or poorly understood EPO effects in the fetus include: 1) what is the contribution and the role of the placenta in increasing fetal EPO production during hypoxia?; 2) how and through what mechanism is amniotic fluid EPO concentration also increased during fetal hypoxia?; 3) how and through what mechanism does fetal EPO reach the intestine?; and 4) through what mechanism and in which fetal organs does EPO exert protective effects during tissue hypoxia? As a basis for clarifying these uncertainties, Figure 1 depicts maternal EPO production during normal pregnancy and the postpartum period. It also depicts fetal EPO production during anemia and chronic hypoxia in the context of previously documented or hypothetical effects of EPO on the fetus and the newborn infant.
Role of erythropoietin in fetal red cell production
Fetal red cell production is regulated by EPO to maintain normal steady-state erythropoiesis and blood hemoglobin levels during normoxemic conditions and to accelerate erythropoiesis during tissue hypoxia due to anemia and other causes (1,3,4) (Figure 1). EPO synthesis is regulated by the partial pressure of oxygen (pO2) of blood perfusing the oxygen sensitive renal peritubular fibroblasts where it is mediated by the hypoxia-inducible transcription factor (HIF) complex (11–13).
EPO in amniotic fluid
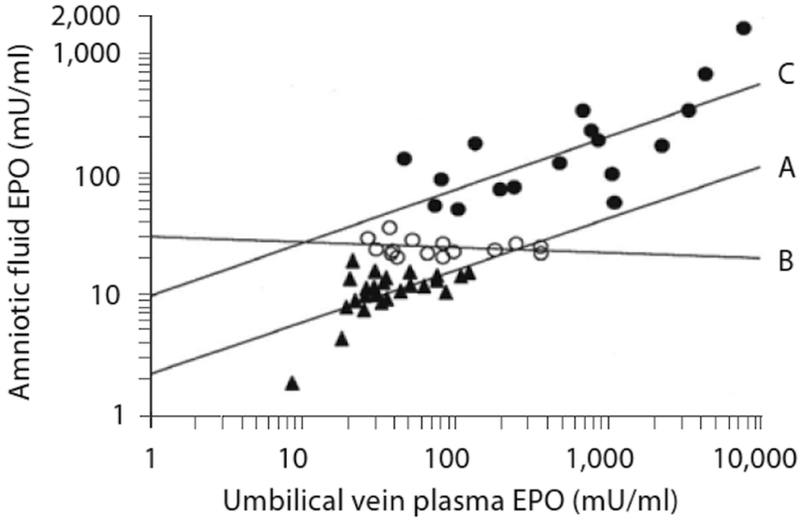
Amniotic fluid EPO concentrations correlate highly with fetal plasma EPO concentrations both in normal and abnormal pregnancies (6). In pregnancies complicated by type 1 diabetes or hypertension, amniotic fluid EPO concentrations obtained within 2 days before birth and prior to labor are highly correlated with fetal plasma EPO concentrations, r=0.86 and r=0.89, respectively (14,15). In these pregnancies, both normal amniotic fluid EPO levels (i.e., <20 mU/ml) and pathologically elevated amniotic fluid EPO levels (i.e., >50 mU/ml) correlate highly significantly with fetal plasma EPO levels (Figure 2). In contrast, slightly elevated (i.e., 20–50 mU/ml) do not. The latter may be explained by a delay in the increase in amniotic fluid EPO concentration relative to the rate of increase in fetal plasma EPO concentration during fetal hypoxia (16).
Figure 2.
Relationship of fetal plasma and amniotic fluid EPO in pregnancies complicated by preeclampsia or hypertension grouped by amniotic fluid EPO concentration: A) low EPO<20 mU/ml, r=0.87, p<0.0001, n=28; B) slightly elevated EPO 20–50 mU/ml, r=−0.10, p=NS; n=13; and C) high EPO >50 mU/ml, r=0.86, p<0.0001, n=17. (Reproduced with permission from Neonatology 2009; 95:105–116.)
Serial measurements of amniotic fluid EPO levels in individual pregnancies demonstrate that once the fetus becomes hypoxic, the amniotic fluid EPO concentration starts to increase exponentially (14,15,17). It is noteworthy from serial amniotic fluid EPO measurements in individual cases that once amniotic fluid EPO levels reach pathological levels (i.e., >50 mU/ml), amniotic fluid concentrations increase exponentially (14,15). Calculated from these exponential amniotic fluid EPO increases, the daily increase in amniotic fluid EPO levels can be as much as 30–40 mU/ml (15). This is equivalent to daily increases of approximately 80–100 mU/ml in plasma during fetal hypoxia. Once fetal hypoxia triggers increased fetal EPO production, pathological amniotic fluid EPO levels do not level off or decrease. Instead, they continue to increase (14,15,17).
In clinical intrauterine red cell transfusion studies of Rh-immunized pregnancies, fetal hemoglobin concentrations ranging from 11 to 159 g/l obtained at the first cordocentesis—and thus prior to fetal red cell transfusion—correlate inversely with concurrent amniotic fluid EPO levels that range from 2 to 42 mU/ml (16). This correlation and the slopes of this relationship are similar before and after 27 weeks of gestation (r= −0.57 and r= −0.49, respectively). This observation is consistent with the observation that only low circulating EPO concentrations are required for red cell production even when fetal hemoglobin levels are extremely low (18). Despite this, even anemic fetuses markedly increase their EPO production as indicated by exponential increases in amniotic fluid EPO levels when already anemic fetuses become severely hypoxic (17). This suggests a different function for EPO under these circumstances.
It is unknown how EPO reaches amniotic fluid in human pregnancy (Figure 1). In the ovine pregnancy EPO mRNA and EPO hormone are both present in the fetal amnion and chorion membranes during the last trimester of pregnancy (19). This indicates that EPO is functionally active in the ovine fetal membranes and that the membranes could be one of the sources of EPO in the amniotic fluid (19). The relative contributions of potential sources of EPO and the transport mechanisms of fetal EPO into the amniotic fluid in human pregnancy is currently unknown. To address this potentially clinically important issue, there is an urgent need for this to be studied both in animal models and human pregnancies.
Chronic fetal hypoxia and EPO
As in adults, tissue hypoxia in the fetus is the major stimulus of EPO production (3). In adults, plasma EPO levels start to increase 90 minutes after the beginning of acute hypoxia, with the magnitude of the increase correlating directly with the duration and severity of hypoxia (20). In the fetal sheep, plasma EPO levels begin to increase 3–4 hours after a massive acute hemorrhage or after induction of moderate to severe fetal hypoxia (21,22). In human pregnancy both umbilical cord plasma and amniotic fluid EPO levels correlate inversely with umbilical artery pH, base excess and pO2, and directly with pCO2 and lactate in cases of fetal chronic hypoxia (6,14,15,17,23–25). In the fetal sheep, plasma EPO levels start to increase exponentially when the arterial oxygen content falls below 60% of normal (26). A similar exponential increase in amniotic fluid EPO levels has been observed in human type 1 diabetic pregnancies when umbilical artery pO2 levels decrease below 2.0 kPa (15.0 mmHg) (14).
Hypoxia increases both reactive oxygen species (ROS) (27,28) and reactive nitrogen species (RNS) (27). The degree of fetal oxidative stress can be quantitated by measuring the concentration of specific oxidative and nitrosative stress biomarkers in amniotic fluid (29,30). In support of this we have reported in diabetic pregnancies that amniotic fluid EPO levels correlate highly significantly with two amniotic fluid oxidative stress biomarkers (meta-L-tyrosine/Phe and 8-oxodG/2dG ratios) and one nitrosative stress biomarker (3Cnitro-LCtyrosine/Phe ratio) (r=0.59, r=0.93 and r=0.90, all p<0.001) (27). While this association further emphasizes the importance of fetal EPO level as a marker of intrauterine hypoxia, the nature of and the possible mechanisms responsible for this association are unknown. Once discovered, new therapeutic approaches might become apparent.
Increased fetal umbilical cord blood or amniotic fluid EPO levels have been reported in a broad spectrum of pregnancies in which fetal hypoxia occurs. These include prolonged pregnancies (31) and pregnancies complicated by meconium stained amniotic fluid (32,33), growth restricted fetuses (15,34,35), abnormal fetal heart rate recordings (36,37), type 1 diabetes (14), hypertension (15), and severe fetal anemia (17). While clinical evidence of increased hypoxia in fetuses or asphyxia in newborn infants are common among all these abnormal antepartum situations, the pathogenic mechanisms leading to fetal hypoxia differ among these conditions.
Placental EPO production
In the ovine fetus, a switch of EPO synthesis from the kidneys to the placenta occurs during hypoxia (38). In the study by Davis et al. (38), placental production of EPO was not observed during normal fetal oxygenation. However, after inducing fetal hypoxia, umbilical vein (UV) EPO concentrations increased to levels 13% higher than umbilical artery (UA) concentrations, thereby suggesting increased placental EPO production. The investigators calculated that the placenta produced 1.1 million mU of EPO per hour during hypoxia. This value is 15 times greater than simultaneously measured EPO production by the fetal kidneys (38). Similarly, when we measured UV and UA EPO plasma concentrations separately at birth in type 1 diabetic pregnancies delivered during elective cesarean section before labor, UV EPO levels were significantly higher than UA levels in hypoxic fetuses with UA pO2 below 2 kPa (15 mmHg) at delivery (39). In addition, our observation that the UV/UA plasma EPO ratio correlated inversely with UA pO2 (39) further indicates that placental EPO production increases during fetal hypoxia. We therefore speculate that the observed increased fetal plasma EPO concentrations in human pregnancy is at least partially a function of the increased placental EPO synthesis during fetal hypoxia in order to protect the fetal brain and other vital organs (Figure 1).
Although EPO protein, mRNA and receptors are all present in both the human and ovine placenta (40,41), their functional importance for the fetus is not well understood. Clearly, the transport mechanisms of placental EPO hormone into the fetal circulation needs to be delineated during normal and hypoxic conditions in human pregnancies.
In addition to these uncertainties of placental EPO production and transport, very little is known about how factors such as chorioamnionitis or drugs might interfere with, or otherwise modulate fetal EPO production and transport. If our hypothesis that fetal EPO synthesis is increased during fetal hypoxia to protect its brain and other vital organs is correct, it will emphasize the clinical importance and utility of antenatal EPO measurement in amniotic fluid and fetal plasma at birth. This is especially important in pregnancies at an increased risk of fetal hypoxic sequelae. Finally, the use of amniotic fluid EPO levels as an antenatal hypoxia marker and predictor of poor neurodevelopmental outcome needs to be addressed in adequately powered single and multi-center randomized clinical trials. It is imperative that such randomized clinical trials include follow-up studies of subsequent cognitive functions.
Role of erythropoietin in tissue protection
In addition to its role in hematopoietic regulation, EPO also has tissue protective properties (41–43). This is the result of EPO receptor (EPO-R) isoforms, which differ structurally and functionally from the hematopoietic EPO-R isoform. Different EPO-Rs exert both short- and long-term tissue protective effects (41). Acute, short-term EPO effects are mediated by its anti-inflammatory, anti-apoptotic and antioxidant properties (41–43). Long-term EPO effects are those promoting more gradual processes, including erythropoiesis, angiogenesis and neurogenesis (42). In vivo and in vitro studies indicate that high EPO concentrations and short receptor occupancy are required to activate the tissue-protective isoform of EPO-R with a relatively low EPO affinity (41). In contrast, much lower but sustained concentrations of EPO are needed for the regulation of erythropoiesis as the high-affinity EPOCR isoform is expressed by proerythrocytes during erythrocyte maturation (11,13,41).
EPO and the fetal brain
During acute and chronic hypoxia, the fetus redistributes cardiac output to maintain adequate oxygen supply to the brain. This brain-sparing redistribution of cardiac output is a well known fetal adaptive mechanism to counteract deleterious effects of chronic hypoxia (44). In addition, local EPO production has been shown to take place in the fetal brain (45). This is based on EPO and EPOCR gene expression in astrocytes, glial cells, neurons and brain endothelial cells (46). In asphyxiated newborn infants in the first two days of life, cerebrospinal fluid (CSF) EPO concentrations correlate highly significantly with concurrent plasma EPO concentrations (r=0.99, p<0.0001) (47). Hence, local endogenous EPO production in CNS tissue is another adaptive mechanism to attenuate the harmful effects of hypoxia on the brain (48).
Numerous preclinical, and more recently, clinical studies in preterm newborn infants have shown that exogenously administered recombinant human EPO (rhEPO) has neuroprotective effects in the brain (for review see reference 42). As proof that EPO can cross the blood-brain barrier, marked increases in CSF EPO levels are observed in fetal sheep within 2–4 hours after a high dose of parenteral rhEPO to levels shown to offer tissue protection from hypoxia (49,50). What remains uncertain is whether the high EPO levels in the CSF are caused by high plasma EPO levels crossing the blood-brain barrier during hypoxia or the result of local EPO production by brain tissue (Figure 1). This missing information has important therapeutic implications. It is noteworthy that EPO concentrations of over 10 000 mU/ml have been measured in cord blood plasma at birth of asphyxiated fetuses (14,15). Hence, we infer that EPO can cross the fetal blood-brain barrier in human pregnancies.
There is growing evidence that preterm infants weighing <1 000 g at birth experience neuroprotective benefit from rhEPO treatment given to stimulate erythropoiesis (51). Newborn infants with severe intraventricular hemorrhage receiving rhEPO had better neurodevelopmental outcome at the age of 10 to 13 years than those not receiving rhEPO during the neonatal period (51). Although therapeutic body cooling is presently the method of choice of term newborn infants suffering from hypoxic-ischemic encephalopathy (HIE), almost half of HIE survivors have severe neurologic sequelae. High dose rhEPO parenteral treatment added to cooling treatment of newborn infants with HIE resulted in smaller brain injury volume than cooling treatment alone as detected by MRI (52). As expected, the neurodevelopmental outcome was poorer at 12 months of age among the newborn infants receiving cooling treatment alone compared to those receiving both EPO and cooling treatment (52).
Amniotic fluid and the neonatal intestine
Amniotic fluid contains a large number of cytokines, including EPO, and antimicrobial peptides known to influence the development of fetal gastrointestinal tract (53,54). It is noteworthy that necrotizing enterocolitis (NEC) does not exists in the fetus, which has led to the speculation that amniotic fluid has protective effects against NEC (54). Although the possible protective functions of amniotic fluid against NEC has recently been reviewed (55), a plausible explanation of how amniotic fluid exerts its protective effects against NEC in the newborn infant has not been given (55).
It is well known that perinatal asphyxia is associated with neonatal gut injury, impaired intestinal motility, and NEC (56–58). Notably, EPO receptors are present both in human and rat enterocytes (59). Parenteral rhEPO given to newborn infants weighing ≤1 250 g at birth has been associated with a decrease in the incidence of NEC (60). Moreover, in a neonatal rat model of NEC, exogenous EPO has been shown to protect intestinal cells both in vitro and in vivo (61) and to stimulate vasculogenesis of microvascular endothelial intestine cells (62). In a recent randomized controlled study in preterm newborn infants both recombinant human granulocyte colony-stimulating factor (rhG-CSF) and rhEPO, administered enterally separately or in combination during the first days of life, decreased the risk of NEC and improved feeding outcomes (63) (Figure 1).
An important unanswered question with potential therapeutic implications for NEC is why amniotic fluid EPO concentration increases exponentially during fetal hypoxia (14,15,17) (Figure 1). We hypothesize that high amniotic fluid EPO concentrations associated with fetal hypoxia exert beneficial effects on the fetal and neonatal intestine as a result of fetal swallowing of amniotic fluid with high EPO concentration (Figure 1). It is well known that the near-term fetus swallows up to 700 ml of amniotic fluid daily (64,65). In this regard, it is noteworthy that EPO in milk resists degradation in the intestine of the newborn infant (66,67). Although EPO concentrations greater than 1 500 mU/ml have been measured in the amniotic fluid during fetal hypoxia (14,15), the potential beneficial effects of these markedly increased levels of amniotic fluid EPO in reducing neonatal intestine hypoxic tissue injury has yet to be determined (Figure 1). If these beneficial effects are shown, therapeutic EPO given enterally or parenterally would be the next important step to study.
The current widely accepted practice of human milk feeding as an important approach to NEC prevention in preterm newborn infants (66) lends further support to our hypothesis that increased amniotic fluid EPO levels exert beneficial effects on the fetal and neonatal intestine. The beneficial effects of human milk on the intestine of preterm newborn infants has been shown at least in part to be due to high levels of EPO and other growth factors in the breast milk (67). EPO levels in human milk are similar to maternal plasma EPO levels in the early postpartum period. EPO levels in breast milk increase above maternal plasma EPO levels during ongoing nursing, thus suggesting active secretion of EPO by the mammary gland (68) (Figure 1).
Presently there is controversy whether enteral EPO is absorbed intact into the circulation of the newborn offspring (69,70). In a rat model, parenterally increased plasma EPO in dams did not increase plasma EPO levels or hematocrit in suckling rats compared with controls (70). However, enterally administered 125I-rhEPO did show enteral absorption in suckling rats with 5% of the total EPO dose administered being found in the plasma and another 8 to 10% localized in the bone marrow (69). Radiolabeled rhEPO was also found localized in liver and other solid tissues. In suckling rats, a high dose of EPO (1 700 U × kg−1 × day−1) fed artificially together with iron sulfate was shown to increase hemoglobin levels (71). In another study, enteral administration of EPO to preterm infants was shown to stimulate erythropoiesis (72). It can be concluded from these studies that enteral absorption of EPO into the circulation seems possible. Nonetheless, it is clear that more studies are needed to delineate the circumstances that determine enteral absorption of EPO in newborns. Whether enteral absorption of EPO into the fetal circulation also occurs with swallowed amniotic fluid EPO in human pregnancies is unknown (Figure 1).
In conclusion, in addition to the role of EPO as a regulator of erythropoiesis, EPO also exerts both short- and long-term tissue protective effects. This dual role of EPO is based on different EPO receptor isoforms. Notably, EPO’s tissue protective effect requires markedly higher EPO concentrations than its hematopoietic effects. In human pregnancy, we and others have measured extremely high levels of EPO in fetal plasma and in the amniotic fluid. The functional significance of this is not well understood. Here we hypothesize that the fetus increases its EPO production in order to protect its brain and other vital organs. We further hypothesize, based on fetal swallowing of amniotic fluid, that the fetus increases amniotic fluid EPO levels during fetal hypoxia to protect the intestine during the neonatal period. In this Mechanistic Integrated Review, we suggest a new way of looking at the effects of EPO on fetal erythropoiesis as well as EPO’s central nervous and gut tissue protective properties. Our hypotheses are based on a review the literature of both experimental and clinical studies. If our hypotheses are correct, the importance of fetal EPO as an antenatal hypoxia biomarker and therapeutic agent will become apparent and provide an urgently needed impetus for confirming our hypotheses and exploring fetal EPO’s diagnostic and therapeutic potential.
Acknowledgement
We acknowledge and appreciate the valuable editorial and secretarial assistance provided by Mark Hart.
Statement of Financial Support: JAW received support for this work from NIH NHLBI Program Project Grant P01 HL046925.
Footnotes
Disclosure: The authors have no conflict of interest to declare.
REFERENCES
- 1.Jelkmann W Physiology and pharmacology of erythropoietin. Transfusion Med Hemother 2013;40:302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cotes PM, Canning CE, Lind T. Changes in serum immunoreactive erythropoietin during the menstrual cycle and normal pregnancy. BJOG 1983;90:304–11. [DOI] [PubMed] [Google Scholar]
- 3.Dame C, Fahnenstich H, Freitag P, et al. Erythropoietin mRNA expression in human fetal and neonatal tissue. Blood 1998;92:3218–25. [PubMed] [Google Scholar]
- 4.Moritz KM, Lim GB, Wintour EM. Developmental regulation of erythropoietin and erythropoiesis. Am J Physiol 1997;272:R1829–44. [DOI] [PubMed] [Google Scholar]
- 5.Widness JA, Schmidt RL, Sawyer ST. Erythropoietin transplacental passage – review of animal studies. J Perinat Med 1995;23:61–70. [DOI] [PubMed] [Google Scholar]
- 6.Teramo KA, Widness JA, Clemons GK, Voutilainen P, McKinlay S, Schwartz R. Amniotic fluid erythropoietin correlates with umbilical plasma erythropoietin in normal and abnormal pregnancy. Obstet Gynecol 1987;69:710–6. [PubMed] [Google Scholar]
- 7.Forestier F, Daffos F, Catherine N, Renard M, Adreux JP. Developmental hematopiesis in normal human fetal blood. Blood 1991;77:2360–3. [PubMed] [Google Scholar]
- 8.Dammann O, Gressens P. Integrated mechanism reviews. Pediatr Res 2012;71:530–1. [DOI] [PubMed] [Google Scholar]
- 9.Arcasoy MO. Non-erythroid effects of erythropoietin. Haematologica 2010;95:1803–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nekoui A, Blaise G. Erythropoietin and nonhematopoietic effects. Am J Med Sci 2017;353:76–81. [DOI] [PubMed] [Google Scholar]
- 11.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annual Rev Cell Dev Biol 1999;15:551–78. [DOI] [PubMed] [Google Scholar]
- 12.Jelkmann W Erythropoietin after a century of research: younger than ever. Eur J Haematol 2007;78:183–205. [DOI] [PubMed] [Google Scholar]
- 13.Brines M, Cerami A. Erythropoietin-mediated tissue protection: reducing collateral damage from the primary injury response. J Intern Med 2008;264:405–32. [DOI] [PubMed] [Google Scholar]
- 14.Teramo K, Kari MA, Eronen M, Markkanen H, Hiilesmaa V. High amniotic fluid erythropoietin levels are associated with an increased frequency of fetal and neonatal morbidity in type 1 diabetic pregnancy. Diabetologia 2004; 47:1695–703. [DOI] [PubMed] [Google Scholar]
- 15.Teramo KA, Hiilesmaa VK, Schwartz R, Clemons GK, Widness JA. Amniotic fluid and cord blood plasma erythropoietin levels in pregnancies complicated by preeclampsia, pregnancy-induced hypertension and chronic hypertension. J Perinat Med 2004; 32:240–7. [DOI] [PubMed] [Google Scholar]
- 16.Teramo KA, Widness JA. Increased fetal plasma and amniotic fluid erythropoietin concentrations: Markers of intrauterine hypoxia. Neonatology 2009;95:105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voutilainen PJ, Widness JA, Clemons GK, Schwartz R, Teramo K. Amniotic fluid erythropoietin predicts fetal distress in Rh-immunized pregnancies. Am J Obstet Gynecol 1989;160:429–34. [DOI] [PubMed] [Google Scholar]
- 18.Freise KJ, Widness JA, Veng-Pedersen P. Erythropoietic response to endogenous erythropoietin in premature very low birth weight infants. J Pharmacol Exp Ther 2010;332−229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim MJ, Bogic L, Cheung CY, Brace RA. Expression of erythropoietin mRNA, protein and receptor in ovine fetal membranes. Placenta 2001;22:846–51. [DOI] [PubMed] [Google Scholar]
- 20.Eckardt KU, Boutellier U, Kurtz M, Schopen M, Koller EA, Bauer C. Rate of erythropoietin formation formation in humans in response to acute hypobaric hypoxia. J Appl Physiol 1989;66:1785–8. [DOI] [PubMed] [Google Scholar]
- 21.Widness JA, Teramo KA, Clemons GK, et al. Temporal response of immunoreactive erythropoietin to acute hypoxemia in fetal sheep. Pediatr Res 1986;20:15–9. [DOI] [PubMed] [Google Scholar]
- 22.Kim SJ, Cheung CY, Widness JA, Brace RA. Temporal response of plasma erythropoietin to hemorrhage in the ovine fetus. J Soc Gynecol Invest 2002;9:75–9. [DOI] [PubMed] [Google Scholar]
- 23.Rollins MD, Maxwell AP, Afrasiabi M, Halliday HL, Lappin TRJ. Cord blood erythropoietin, pH, PaO2, and haemocrit following caesarean section before labour. Biol Neonate 1993;63:147–52. [DOI] [PubMed] [Google Scholar]
- 24.Stangenberg M, Legarth J, Hong-Lie C, et al. Erythropoietin concentrations in amniotic fluid and umbilical venous blood from Rh-immunized pregnancies. J Perinat Med 1993:21:225–34. [DOI] [PubMed] [Google Scholar]
- 25.Buescher U, Hertwig K, Wolf C, Dudenhausen JW. Erythropoietin in amniotic fluid as a marker of chronic fetal hypoxia. Int J Gynecol Obstet 1998;60:257–63. [DOI] [PubMed] [Google Scholar]
- 26.Philipps AF, Widness JA, Garcia JF, Raye JR, Schwartz R. Erythropoietin in the chronically hyperglycemic fetal lamb. Proc Soc Exp Biol Med 1982;170:42–7. [DOI] [PubMed] [Google Scholar]
- 27.Escobar J, Teramo K, Stefanovic V, Andersson S, et al. Amniotic fluid oxidative and nitrosative stree biomarkers correlate with fetal chronic hypoxia in diabetic pregnancies. Neonatology 2013;103:181–6. [DOI] [PubMed] [Google Scholar]
- 28.Song J, Sundar K, Gangaraju R, Prchal JT. Regulation of erythropoiesis after normoxic return from chronic sustained and intermittent hypoxia. J Appl Physiol 2017;123:1671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 2007;87:315–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Underwood MA, Gilbert WM, Sherman MP. Amniotic fluid: not just fetal urine anymore. J Perinatol 2005;25:341–8. [DOI] [PubMed] [Google Scholar]
- 31.Jazayeri A, Tsibris JC, Spellacy WN. Elevated umbilical cord plasma erythropoietin levels in prolonged pregnancies. Obstet Gynecol 1998;92:61–3. [DOI] [PubMed] [Google Scholar]
- 32.Richey SD, Ramin SM, Bawdon RE, et al. Markers of acute and chronic asphyxia in infants with meconium-stained amniotic fluid. Am J Obstet Gynecol 1995;172:1212–5. [DOI] [PubMed] [Google Scholar]
- 33.Jazayeri A, Politz L, Tsibris JC, Queen T, Spellacy WM. Fetal erythropoietin levels in pregnancies complicated by meonium passage: does meconium suggest fetal hypoxia? Am J Obstet Gynecol 2000;183:188–90. [DOI] [PubMed] [Google Scholar]
- 34.Morley R, Moore VM, Dwyer T, Owens JA, Umstad MP, Carlin JB. Association between erythropoietin in cord blood of twins and size at birth: does it relate to gestational factors or to factors during labor or delivery. Pediatr Res 2005;57:680–4. [DOI] [PubMed] [Google Scholar]
- 35.Girsen A, Mäkikallio K, Hiilesmaa V, Hämäläinen E, Teramo K, Räsänen J. The relationship between human fetal cardiovascular hemodynamics and serum erythropoietin levels in growth-restricted fetuses. Am J Obstet Gynecol 2007;196:467.e1–e6. [DOI] [PubMed] [Google Scholar]
- 36.Widness JA, Teramo KA, Clemons GK, et al. Correlation of the interpretation of fetal heart rate recordsa with cord plasma erythropoietin levels. Br J Obstet Gynaecol 1985;92:326–32. [DOI] [PubMed] [Google Scholar]
- 37.Kakuya F, Shirai M, Takase M, et al. Relationship between erythropoietin levels both in cord serum and amniotic fluid at birth and abnormal fetal heart rate records. Pediatr Int 2002;44:414–9. [DOI] [PubMed] [Google Scholar]
- 38.Davis LE, Widness JA, Brace RA. Renal and placental secretion of erythropoietin during anemia or hypoxia in the ovine fetus. Am J Obstet Gynecol 2003; 189:1764–70. [DOI] [PubMed] [Google Scholar]
- 39.Teramo K, Loukovaara M, Stefanovic V, Hämäläinen E, Andersson S. The placenta increases its erythropoietin synthesis during fetal hypoxia in diabetic pregnancies. Reprod Sci 2012;19:258A–9A. [Google Scholar]
- 40.Conrad KP, Benyo DF, Westerhausen-Larsen A, Miles TM. Expression of erythropoietin by the human placenta. FASEB J 1996;10:760–6. [DOI] [PubMed] [Google Scholar]
- 41.Brines M, Cerami A. The Receptor that tames the innate immune response. Mol Med 2012; 18:486–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Juul SE, Pet GC. Erythropoietin and neonatal neuroprotection. Clin Perinatol 2015:42:469–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rabie T, Marti HH. Brain protection by erythropoietin: a manifold task. Physiology 2008;23:263–74. [DOI] [PubMed] [Google Scholar]
- 44.Gruslin A, Lemyre B. Pre-eclampsia: fetal assessment and neonatal outcomes. Best Pract Res Clin Obstet Gynecol 2011;25:491–507. [DOI] [PubMed] [Google Scholar]
- 45.Juul SE, Anderson SK, Christensen RD. Erythropoietin and erythropoietin receptor in the developing human central nervous system. Pediatr Res 1998;43:40–49. [DOI] [PubMed] [Google Scholar]
- 46.Marti HH. Erythropoietin and the hypoxic brain. J Exp Biology 2004;207:3233–42. [DOI] [PubMed] [Google Scholar]
- 47.Juul SE, Stallings SA, Christensen RD. Erythropoietin in the cerebrospinal fluid of neonates who sustained CNS injury. Pediatr Res 1999;46:543. [DOI] [PubMed] [Google Scholar]
- 48.Noguchi CT, Asavaritikrai P, Teng R, Jia Y. Role of erythropoietin in the brain. Oncol Hematol 2007;64:159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brines ML, Ghezzi P, Keenan S, et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci 2000;97:10526–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Juul SE, McPherson RJ, Farrell FX, Jolliffe L, Ness DJ, Gleason CA. Erythropoietin concentrations in cerebrospinal fluid of nonhuman primates and fetal sheep following high-dose recombinant erythropoietin. Biol Neonate 2004;85:138–44. [DOI] [PubMed] [Google Scholar]
- 51.Neubauer AP, Voss W, Wachtendorf M, Jungmann T. Erythropoietin improves neurodevelopmental outcome of extremely preterm infants. Ann Neurol 2010;67:657–66. [DOI] [PubMed] [Google Scholar]
- 52.Mulkey SB, Ramakrishnaiah RH, McKinstry RC, et al. Erythropoietin and brain magnetic resonance imaging findings in hypoxic-ischemic enecephalopathy: volume of acute brain injury and 1Cyear neurodevelopmental outcome. J Pediatr 2017;186:196–9. [DOI] [PubMed] [Google Scholar]
- 53.Maheshwari A Role of cytokines in human intestinal villous development. Clin Perinatol 2004;31:143–55. [DOI] [PubMed] [Google Scholar]
- 54.Espinoza J, Chaiworapongsa T, Romero T, et al. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonat Med 2003;13:2–21. [DOI] [PubMed] [Google Scholar]
- 55.Dasgupta S, Jain SK. Protective effects of amniotic fluid in the setting of necrotizing enterocolitis. Pediatr Res 2017;82:584–95. [DOI] [PubMed] [Google Scholar]
- 56.Kliegman RM, Fanaroff AA. Necrotizing enterocolitis. New Engl J Med 1984;310:1093–103. [DOI] [PubMed] [Google Scholar]
- 57.Berseth CL, McCoy HH. Birth asphyxia alters neonatal intestinal motility in term neonates. Pediatrics 1992;90:669–73. [PubMed] [Google Scholar]
- 58.Nikiforou M, Willburger C, de Jong AE, Global hypoxia-ischemia induced inflammation and structural changes in the preterm ovine gut which were not ameliorated by mesenchymal stem cell treatment. Mol Med 2016;22:244–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Juul SE, Joyce AE, Zhao Y, Ledbetter DJ. Why is erythropoietin present in human milk? Erythropoietin receptors in enterocytes of human and rat neonates. Pediatr Res 1999;46:263–8. [DOI] [PubMed] [Google Scholar]
- 60.Ledbetter DJ, Juul SE. Erythropoietin and the incidence of necrotizing enterocolitis in infants with very low birth weight. J Pediatr Surg 2000;35:178–82. [DOI] [PubMed] [Google Scholar]
- 61.Yu Y, Shiou SR, Guo Y, et al. Erythropoietin protects epithelial cells from excessive autophagy and apoptosis in experimental neonatal necrotizing enterocolitis. Plos One 2013;8:e69620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ashley RA, Dubuque SH, Dvorak B, Woodward SS, Williams SK. Kling PJ. Erythropoietin stimulates vasculogenesis in neonatal rat mesenteric microvascular endothelial cells. Pediatr Res 2002;51:472–8. [DOI] [PubMed] [Google Scholar]
- 63.El-Ganzoury M, Awad HA, El-Farrash R et al. Enteral granulozyte-colony stimulating factor and erythropoietin early in life improves feeding tolerance in preterm infants: A randomized controlled trial. J Pediatr 2014;165:1140–5.. [DOI] [PubMed] [Google Scholar]
- 64.Pritchard JA. Fetal swallowing and amniotic fluid volume. Obstet Gynecol 1966;28:606–10. [PubMed] [Google Scholar]
- 65.Beall MH, van den Wijngaard JP, van Gemert MJ, Ross MG. Amniotic fluid water dynamics. Placenta 2007;28:816–23. [DOI] [PubMed] [Google Scholar]
- 66.Neu J, Walker WA. Necrotizing enterocolitis. New Engl J Med 2011;364:255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kling PJ, Sullivan TM, Roberts RA, Philipps AF, Koldovsky O. Human milk as a potential enteral source of erythropoietin. Padiatr Res 1998;43:216–21. [DOI] [PubMed] [Google Scholar]
- 68.Juul SE, Zhao Y, Dame JB, Du Y, Hutson AD, Chrstensen RD. Origin and fate of erythropoietin in human milk. Pediatr Res 2000;48:660–667. [DOI] [PubMed] [Google Scholar]
- 69.Kling PJ, Willeitner A, Dvorak B, Blohowiak SE. Enteral erythropoietin and iron stimulate erythropoiesis in suckling rats. J Pediatr Gastroenterol Nutr 2008;46:202–7. [DOI] [PubMed] [Google Scholar]
- 70.Juul SE, Ledbetter DJ, Joyce AE, et al. Erythropoietin acts as a trophic factor in neonatal rat intestine. Gut 2001;49:182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miller-Gilbert AL, Dubuque SH, Dvorak B et al. Enteral absorption of erythropoietin in the suckling rat. Pediatr Res 2001;50:261–7. [DOI] [PubMed] [Google Scholar]
- 72.Britton JR, Christensen RD. Enteral administration of recombinant erythropoietin to preterm infants. J Perinatol 1995;15:281–3. [PubMed] [Google Scholar]