Abstract
Background & Aims
Patients with bile acid diarrhea (BAD) are identified based on increased levels of BAs in fecal samples collected over a 48-hr period while on a 100-gram fat diet (48-hr BA), retention of 75Se-labeled homocholic acid taurine, or serum levels of C4 or FGF19. BAD increases fecal weight and colonic transit. We investigated whether results of tests for BAD associate with increased fecal weight and more rapid colonic transit over a 24- or 48-hr period in patients with irritable bowel syndrome with diarrhea (IBS-D). We also estimated the prevalence of increased 48-hr fecal BAs in patients with chronic diarrhea.
Methods
We performed a retrospective study of 64 patients with IBS-D, 30 patients with IBS-constipation, 30 healthy volunteers (controls). We collected data on fecal weights (measured over a 48-hr period), colonic transit over a 24-hr period (measured by scintigraphy), and percentages of different BAs in stool samples. Colonic transit was measured as the geometric center (weighted average) of colonic counts on a scale of 1 (100% in ascending colon) to 5 (100% in stool). We performed area under the curve (AUC) analyses to assess the association between result of serum and stool tests and high fecal weight (>400g/48 hrs) or rapid colonic transit (>3.34, corresponding to isotope geometric center in sigmoid colon). We estimated the prevalence of increased 48-hr fecal BAs among 938 patients with chronic diarrhea.
Results
Total fecal 48-hr BA alone, or in combination with percentage of primary fecal BAs, identified patients with increased fecal weight with an AUROC of 0.86. Percentage of primary fecal BA alone identified patients with increased fecal weight with an AUROC of 0.73. Total fecal 48-hr BA alone identified patients with increased colonic transit with an AUROC of 0.65 and percentage of primary fecal BA alone identified patients with increased colonic transit with an AUROC of 0.69; combined data on these features identified patients with increased colonic transit with an AUROC of 0.70. Serum level of C4 identified patients with increased colonic transit with an AUROC of 0.60. Primary BAs >10% identified patients with increased fecal weight (sensitivity 49% and specificity 91%) and rapid colonic transit (sensitivity 48% and specificity 87%). Among the patients with chronic diarrhea, 45.6% had fecal primary BAs >10% and 27% had increased total fecal BAs (>2337 μmol/48 hrs).
Conclusion
In a retrospective analysis of patients with IBS-D, we found percentage of primary BAs in fecal samples to provide an alternative to total fecal BAs in identification of patients with BAD or chronic diarrhea.
Keywords: cholic acid, chenodeoxycholic, diagnostic, CDCA
INTRODUCTION
Bile acid diarrhea (BAD) is recognized as the cause of about 1 in 4 cases of functional diarrhea or irritable bowel syndrome with diarrhea (IBS-D).1–4 In the United States, BAD is currently diagnosed indirectly by response to trial with BA sequestrants or by elevated total fecal bile acids (BAs) (>2337μmol/48h), elevated fasting serum 7α-hydroxy-4-cholesten-3-one (C4 ≥52.5ng/mL), or reduced fasting fibroblast growth factor 19 (FGF19 ≤61.7pg/mL).5,6 BAD should be considered in the diagnostic algorithm for chronic diarrhea.7
Chenodeoxycholic acid (CDCA) stimulates colonic transit (CT) and secretion,8–11 and cholic acid (CA) undergoes 7α dehydroxylation and deconjugation in the colon to the secretory secondary BA, deoxycholic acid (DCA). CDCA and DCA have two α-hydroxyl groups which are key to inducing secretion.12 In 90 healthy volunteers (HVs), fecal CDCA and CA constituted 0.2% to 9.4% of total fecal BAs (unpublished data: Leslie Donato, PhD, Mayo Clinic), and the 95th %ile of the main secretory BAs (CDCA and DCA) in 30 HVs was 77.5%.13
The aims of this study were: First, to compare associations of available tests for BAD with increased fecal weight and more rapid CT at 24h (GC24) in patients with IBS-D and calculate a cut-off for fecal primary and secretory BAs to identify BAD with ~90% specificity. Our second aim was to estimate prevalence of abnormal total and % primary BAs in 48h fecal collection in 938 patients who underwent fecal BA measurement in evaluation for chronic diarrhea. Thus, our strategy involved the development of cut-offs for primary fecal BA excretion associated with biomarkers of diarrhea in the IBS-D testing group and to use the clinical cohort data as a validation set to assess utility of the cut-off values.
METHODS
Participants and Study Design
This was a single-center, retrospective study of two patient cohorts evaluated at Mayo Clinic, Rochester, Minnesota.
The first cohort identified previous research participants with IBS-D (n=64), IBS with constipation [IBS-C (n=30)], and HVs (n=30). Additional details regarding recruitment, eligibility criteria, and data collection are available in previous publications.13, 14 Demographic data were collected at the time of enrollment, including previously obtained fasting serum FGF19 and C4, 48-hour primary (CDCA and CA), secretory (CDCA and DCA), individual (CDCA, DCA, CA, and lithocholic acid) and total unconjugated fecal BAs, 48-hour stool weight, and 24-hour CT.
The second patient cohort was 986 patients who presented to the clinical practice with chronic diarrhea and underwent 48-hour fecal BA measurements. Further details regarding patient search is available in Supplemental Materials.
This study was approved by Mayo Clinic Institutional Review Board (IRB #17-009774).
Diagnostic Methods for BAD
Serum C4 and FGF19 are validated biomarkers of BAD,14 and total fecal BAs are diagnostic for BAD. BAD was defined as total fecal BAs >2,337 μmol/48 hours. Individual fecal BAs were measured from the entire 48-hour sample. Additional details regarding each test method are provided in Supplemental Materials.
Markers for Diarrhea
In our current practice, the gold standard to diagnose BAD is the 48-hour total fecal BAs measurement. Fasting serum C4 and FGF19 are good screening tools, and 75SeHCAT is not available. We utilized two surrogate markers of diarrhea (fecal weight and CT) to identify manifestations of BAD in patients with IBS-D, since BAs cause both increased colonic secretion and motility.15,16 In the research cohort (testing group), we assessed which measurements of fecal BAs (total and primary) or available serological tests (fasting C4 and FGF-19) predicted pathophysiological features of BAD (increased 48h fecal weight and more rapid 24h CT).
Additional information about measurements and cut-off selection is available in Supplemental Materials.
Statistical Analysis
Demographics, fasting serum FGF19 and C4, primary and total fecal BAs, fecal weight, and CT were reported as median and interquartile range (IQR).
We used analysis of variance (ANOVA) on ranks test, followed by Dunn’s test corrected for multiple comparisons (2-sided α=0.05) to compare demographics (age and BMI), BAD diagnostic tests, fecal weight, and CT at 24 hours between the 4 different groups. When the overall ANOVA on ranks was borderline (<0.12), we performed Mann Whitney rank sum tests to compare the BAD group with the three other groups, with Bonferroni correction (p<0.0168) to correct for three comparisons (SigmaPlot, Systat Software, San Jose, CA).
SAS/STAT® [Version (9.4) of the SAS System for (Unix) Copyright© (2017) SAS Institute, Inc.] and JMP (JMP®, Version 13, SAS Institute, Inc., Cary, NC, 1989–2017) software were utilized to create receiver operating curves (ROC) to determine the associations of the BAD diagnostic tests with fecal weight >400 grams/48h and CT at 24 hours >3.34. Logistic regression and odds ratios were calculated to compare the differences in the demographic data (age, sex and BMI) in patients with elevated percentage of primary BAs alone versus those with increased total fecal BAs.
Supplemental Materials include details of patient selection and all measurements, including relevant references.18–39
RESULTS
Demographics
We classified IBS-D with fecal BA >2,337 μmol/48h as BAD, and those with fecal BA ≤2,337 μmol/48h as IBS-D. Twenty-three patients with BAD, 41 with IBS-D, 30 with IBS-C, and 30 HVs were included. Demographic data (Table 1) showed no significant group differences other than BMI, which was significantly higher in BAD compared to the HV and IBS-C groups (Table 1).
Table 1.
Demographics, BAD diagnostic tests, and surrogate markers for the research cohort compared using ANOVA
| Median (IQR) | BAD | IBS-D | IBS-C | Healthy | Overall ANOVA p value | Multi-group p value vs BAD group* |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| N (F/M) | 23 (19/4) | 41 (40/1) | 30 (30/0) | 30 (22/8) | ||
| Age (years) | 45 (32–51) | 41 (32–51) | 49 (39–51) | 41 (32–48) | 0.4 | |
| BMI (kg/cm2) | 29 (27–35) | 28 (23–32) | 26 (24–29) | 26 (22–28) | 0.004 | |
| BAD Diagnostic Tests | ||||||
| C4 (ng/mL) | 33 (19–41) | 21 (15–45) | 15 (8.6–25)* | 22 (13–33) | 0.03 | <0.05 |
| FGF19 (pg/mL) | 94 (59–159) | 98 (51–158) | 100 (64–183) | 120 (89–216) | 0.113 | <0.05 |
| Fecal primary BAs (%) | 13 (8–25) | 5.8 (1.3–16) | 0.5 (0.3–0.8)* | 0.6 (0.4–1.2)* | <0.001 | <0.05 |
| Fecal secretory BAs (%) | 61 (56–64) | 62 (53–66) | 51 (39–62) | 62 (51–69) | 0.049 | |
| Total fecal BAs (μmol/48h) | 3442 (2727–5237) | 998 (479–1623)* | 317 (168–762)* | 512 (189–1606)* | <0.001 | <0.05 |
| Surrogate Markers of Diarrhea | ||||||
| Fecal weight (g)/48h | 516 (434–689) | 322 (165–404)* | 159 (85–243)* | 250 (175–371)* | <0.001 | <0.05 |
| 24h colonic transit (GC24) | 3.3 (2–4) | 2.3 (1.7–3.6) | 2 (1.6–2.8)# | 2.2 (1.9–3.1) | 0.066 | p=0.013# |
BAs=bile acids; BAD=bile acid diarrhea
significant p value compared to BAD
significant difference between IBS-C and BAD by Mann Whitney rank sum test (p<0.017 corrected for 3 comparisons vs. BAD group)
Diagnostic Tests of BAD in Patients with IBS-D, IBS-C and Healthy Volunteers
Serum C4 and FGF19
Fasting serum C4 and FGF19 measurements were available for all participants (Table 1). There were overall differences in serum C4 (p=0.031), with significantly higher serum C4 in the BAD group compared to the IBS-C group. There was no overall significant difference in FGF19 among the patient groups (p=0.113), with nonsignificant differences between BAD and HV groups (p=0.054).
Fecal bile acids
Fecal BA measurements were missing from 7 patients: 3 patients with IBS-D, 2 with IBS-C, and 2 HVs. Details of the fecal BA results are shown in Table 1.
By definition, there was significantly higher total fecal BAs in the BAD group, but no significant differences among the other groups (Table 1). Percentages of primary fecal BAs were higher in both BAD and IBS-D groups compared to IBS-C and HVs, but not different between BAD and IBS-D or between IBS-C and HVs (Table 1).
There were significant overall differences in the percentages of secretory BAs (p=0.049), but no differences among the individual groups.
Surrogate Markers of Diarrhea
Fecal weight
Fecal weight was missing in 4 participants: 3 with IBS-D and 1 HV. Fecal weight >400 grams/48h was documented in 83% (19/23) of BAD, 28% (11/39) of IBS-D, 14% (4/29) of IBS-C, and 17% (5/29) of HVs. There was an overall significant difference in 48-hour fecal weight among the 4 groups, with higher fecal weight in BAD compared to three other groups (Tables 1 and 2).
Table 2.
Sensitivity and specificity for the optimal cut-offs determined by ROC curves for colonic transit GC >3.34 and fecal weight >400g/48h for primary BAs and total fecal BAs, primary BAs alone, and secretory BAs.
| Sensitivity | Specificity | |
|---|---|---|
| Fecal weight > 400g/48h | ||
| Primary BA >4% + total fecal BA >1,000 μmol/48h | 46% | 97% |
| Primary BA > 10% | 49% | 91% |
| Secretory BA > 77.5% | 5% | 96% |
| Colonic Transit 24 hrs, GC > 3.34 | ||
| Primary BA >4% + total fecal BA >1,000 μmol/48h | 36% | 90% |
| Primary BA > 10% | 48% | 87% |
| Secretory BA > 77.5% | 9% | 98% |
Colonic transit
One CT measurement was missing in the IBS-D group. The proportions of IBS-D participants with GC24 >3.34 were 48% (11/23) in BAD and 35% (14/40) in the IBS-D groups. There was borderline overall difference in GC24 (p=0.066, Table 1), with more rapid GC24 in BAD compared to IBS-C (p=0.013) and to HVs (p=0.024, Table 1).
Association of BAD Tests with Surrogates of Diarrhea
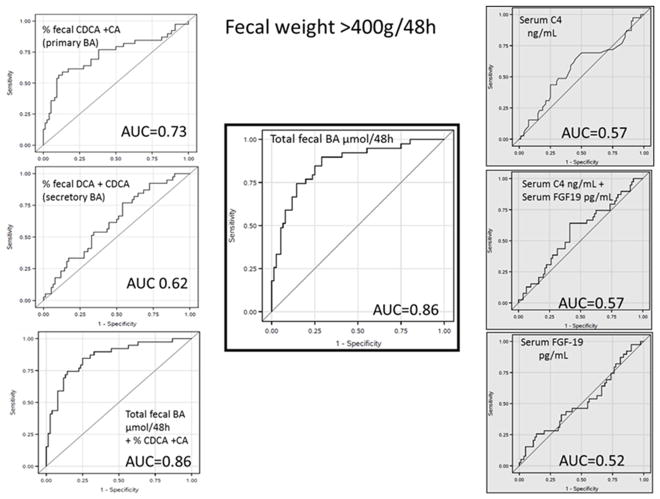
Association with fecal weight >400g/48h
The highest AUCs were for total fecal BAs 0.86, fecal primary BAs 0.73, fecal secretory BAs 0.62, serum C4 0.57, and serum FGF19 0.52 (Figure 1).
Figure 1.
ROC curves evaluating each BAD diagnostic test and the ability to predict increased fecal weight. Stool tests have white background and serum tests have gray background.
The optimal cut-offs based on ROC curves were observed (sensitivity and specificities in Table 2) with combined primary BAs >4% and total fecal BAs >1,000 μmol/48h, or primary BAs >10% or secretory BAs >77.5%. Individual and total fecal BAs had >90% specificity, with higher sensitivity noted for primary BAs alone or for primary BAs with total fecal BAs.
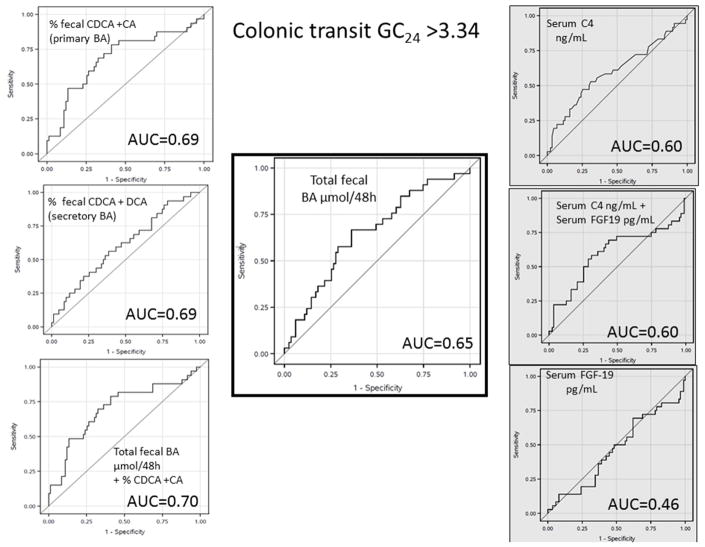
Association with colonic transit >3.34
The gold standard, total 48-hour fecal BAs (middle panel, Figure 2), had an AUC of 0.65. The AUCs for other measurements were: 0.69 for primary BAs alone, 0.70 for combined primary BAs with total fecal BAs, 0.61 for .secretory BAs (CDCA + DCA), 0.60 for serum C4, with no improvement when combining C4 with FGF19. Individual BAs had AUCs <0.60.
Figure 2.
ROC curves evaluating each BAD diagnostic test and the ability to predict more rapid colonic transit. Stool tests have the white background, and C4 and FGF19 have gray backgrounds.
Table 2 has the corresponding sensitivity and specificity for the CT >3.34 using the same cut-offs identified for fecal weight >400g. All markers had specificities >85%, with higher sensitivity for primary fecal BAs alone or combined primary fecal BAs with total fecal BAs compared to the secretory BAs.
Prevalence of Elevated Primary Bile Acids in Research Cohort and Validation Set of Clinical Patients Presenting with Chronic Diarrhea
Research cohort of patients with IBS-D
In the patients with IBS-D and normal 48h total fecal BAs, 29% (11/38) had primary fecal BAs >10%. Demographic details and diagnostic tests and surrogate markers are shown in Table 3. In this cohort, 55% (6/11) had increased fecal weight (>400 g/48h) and 45% (5/11) had a CT GC24 >3.34.
Table 3.
Demographics, BAD diagnostic tests, and surrogate markers in patients with IBS-D with elevated primary bile acids (BAs, >10%) and normal 48h total fecal BAs.
The units for N (total number of people) and sex are the number of females and males. Median (IQR) is the unit for the remaining continuous variables.
| IBS-D with Primary BAs >10% | |
|---|---|
| N (F/M) | 11 (11/0) |
| Age (years) | 35 (31–48) |
| BMI (kg/cm2) | 23.0 (21 – 29.2) |
| C4 (ng/mL) | 23 (14 – 50) |
| FGF19 (pg/mL) | 131.4 (51.9 – 221.9) |
| Primary fecal BAs (%) | 20.4 (16.1 – 28.1) |
| Total fecal BAs (μmol/48h) | 1099 (484.5 – 1829.5) |
| Fecal weight (g)/48h | 404 (322 – 538) |
| 24 hour colonic transit (GC24) | 2.9 (1.6–4.0) |
Clinical patients who underwent bile acid evaluation for chronic diarrhea or IBS-D
There were 986 patients who underwent evaluation for chronic diarrhea or IBS-D over the time period of 2015–2017 since the launch of the fecal BA test at Mayo Clinic. There were 9 incomplete collections and 39 duplicates, of which the first test completed was included in the analysis. In the remaining 938 patients, 47% had normal primary (≤10%) and total fecal BAs (≤2,337 μmol/48h). However, 19.6% had elevations of both primary (>10%) and total fecal BAs (>2,337 μmol/48h), 7.4% had elevated total fecal BAs alone, and 26% had increased percentage of primary BAs alone; thus 45.6% patients with chronic diarrhea had elevated % primary fecal BAs.
Table 4 shows the demographics and primary and total fecal BA measurements for each group. There were differences in age and BMI, but not sex between the clinical groups. Patients with elevated total fecal BAs alone were older, and those with elevated primary BAs alone were younger than patients with normal total and primary fecal BAs. Patients with both elevated total and primary fecal BAs had higher BMI.
Table 4.
Demographics of the clinical cohort of 938 patients who underwent evaluation for chronic diarrhea or IBS-D
The units for N (total number of participants), sex, and race are represented as the number of participants in each category. Age and BMI are reported as median (IQR).
| Normal % primary and total fecal BAs | Elevated % primary and total fecal BAs | Elevated total fecal BAs (>2337 μmol/48h) | Elevated % primary fecal BAs (>10%) | Overall ANOVA p value | |
|---|---|---|---|---|---|
| N (F/M) | 441 (299/142) | 184 (116/68) | 69 (42/27) | 244 (174/70) | 0.24 |
| White/other | 410/31 | 178/6 | 67/1 | 222/22 | |
| Age (years) | 52 (36–67) | 53 (38–64) | 61 (51–70) | 47 (32 – 61.5) | < 0.001 |
| BMI (kg/cm2) | 25.5 (21–30) | 28 (23.5 – 34) | 26.2 (23 – 32) | 26.2 (21 – 32) | < 0.001 |
BAs=bile acids
Logistic regression to identify demographic data in patients with elevated BAs
The odds ratios for age (per year), BMI (per kg/cm2), and sex were significant (p<0.0001, p=0.016, and p=0.021, respectively) in patients with elevated primary BAs alone compared to those with elevated total fecal BAs. The odds ratios for age (per year) was 0.98 (95% CI 0.97 – 0.99), for BMI was 0.97 (95% CI 0.95 – 0.99), and for females was 1.57 (95% CI 1.07 – 2.32), indicating that females had 57% higher odds of having elevated primary fecal BAs alone over the likelihood of having increased total 48-hour fecal BAs. Conversely, for every decrease in age of 10 years or every decrease in BMI of 10 kg/cm2, there were respectively 24% and 34% increases in the odds of patients with BAD presenting with increased primary BAs alone.
DISCUSSION
Our study shows that, among research participants with IBS-D or functional diarrhea, there is a subset of patients (36%) who has evidence of BA malabsorption (based on total fecal BA >2,337 μmol/48h), similar to a previously reported prevalence of 25–33%.1–3 Patients who present with chronic diarrhea >400 g/48h have a higher likelihood of having BAD rather than IBS-D alone. Our work has developed biochemical tests to detect BAD, in the absence of 75SeHCAT retention test in the United States. Final validation of the biochemical measurements of stool and serum will require prospective, placebo-controlled trials with BA sequestrants in order to confirm observations from our prior open-label study of colesevelam in patients with elevated 48-hour stool total BAs (11). In the current study, we show that, with a 48-hour fecal collection, primary BAs >10% provide the same degree of association as total fecal BAs with increased fecal weight and CT in patients presenting with IBS-D. The data show high specificity (excluding false negatives) to predict increased stool weight and CT.
Data obtained from the research participants (testing set) showed that percent fecal primary BAs was almost equivalent to total fecal BAs excretion in predicting markers of chronic diarrhea. In addition, the combination of >4% fecal primary and >1,000 μmol total BAs/48h or fecal primary BAs >10% alone are associated with similar specificity and sensitivity for identifying BAD as total fecal BAs >2,337 μmol/48h.
The utility of a fecal BA test in clinical diagnosis is illustrated by the evaluation of the results from 938 patients who underwent the fecal BA excretion test clinically. In this validation cohort, using the current diagnostic criteria, 27% had BAD (19.6% with both elevated primary and total fecal BAs and 7.4% with elevated total fecal BAs alone). However, there was 26% more patients who only presented with elevated primary BAs (without elevated total 48h fecal BAs). Additionally, >10% fecal primary BAs was present in 45.6% of patients of the 938 “validation” patient cohort: 19.6% with elevated primary and total fecal BAs as well as 26% with elevated primary BAs alone. In summary, we note that there are three groups of patients in the research and clinical cohorts with chronic diarrhea: BAD with elevated total fecal BAs; IBS-D with >10% primary BAs and features consistent with the BAD group; and IBS-D with normal primary BAs who typically have normal stool weight and CT. The patients with IBS-D and >10% primary fecal BAs alone tend to be younger and have lower BMI compared to patients with elevated total fecal BAs.
The validation of % primary fecal BAs as a diagnostic test simplifies the reporting and diagnostic interpretation of the test in clinical practice, and identifies more patients who might be candidates for treatment, e.g. with BA sequestrants, based on combination of total fecal BAs >1000 μmol/48h with >4% primary BAs, or the presence of >10% primary BAs (irrespective of the 48h total fecal BAs). Based on the ROC curves, we believe fasting serum C4 is a useful, convenient screening test (AUCs for stool weight and CT 0.57 and 0.6, respectively). Given the high concordance correlation coefficients (all ~0.80) in the percentages of CA, CDCA and DCA between individual stool samples, and the entire fecal 48-hour collection in our prior studies,17 we anticipate that the 10% primary BAs cut-off in a single, random stool sample should have similar biological relevance (e.g. association with CT GC24 >3.34) and serve as a more convenient diagnostic marker for BAD.
We are currently conducting prospective studies to confirm this hypothesis. If future studies show validity, the diagnosis of BAD could be accomplished on a single, random stool sample, instead of the cumbersome 48-hour collection with high fat diet that can aggravate baseline abdominal symptoms in these patients. Moreover, given the relatively high prevalence of BAD in almost 50% of the sample of clinical patients presenting with chronic non-bloody diarrhea, the introduction of the single stool sample for measurement of primary BAs could decrease need for expensive endoscopic and imaging tests. However, we appreciate that the high prevalence of BAD in this study may reflect the secondary or tertiary care patients being investigated for chronic diarrhea.
Previous literature has demonstrated a similar increase in primary BAs in patients with IBS-D and noted differences in the various bacterial species responsible for colonic deconjugation. 18 The leptum group of bacterial species, which transform BAs within the colon, was decreased in patients with IBS-D compared to HVs, resulting in less deconjugation and more primary BAs in the stool. Clostridium is a well-recognized bacterium in this group.19 Interestingly, ursodeoxycholic acid inhibited germination and vegetative growth in 11 clinical isolates of Clostridium difficile,20 demonstrating effect of BAs on colonic bacteria and the effect of colonic bacteria on BA moieties. Further evaluation of the microbiome and colonic BAs will provide greater insight to variations in individual BAs, and patient presentation and treatment options.
Limitations
We utilized surrogate biomarkers, fecal weight and CT to assess BAD diagnostic tests in the absence of 75SeHCAT. A concern could be that more rapid CT could result in decreased secondary transformation of the primary BAs and increased stool weight. However, previous studies have demonstrated that the colon has a significant capacitance to reabsorb fluid.21, 22 Additionally, from our own prior studies, six of 10 IBS-D patients had accelerated proximal colonic emptying, but only two patients had stool weight >200 g/day.23 Lastly, we evaluated previous data from our lab13 and found five HVs with GC >3.3; in these HVs, mean primary fecal BAs was 2.63% and secondary fecal BAs was 96.14%, indicating that more rapid CT is not sufficient to explain the increase in primary BAs.
The clinical cohort may reflect referral bias, as illustrated by 53% having extended criteria consistent with BAD, greater than the reported prevalence rate of 25–33% among patients with functional diarrhea.2 In the future, the true prevalence of BAD, using these more sensitive cut-offs, should be studied in patients who are not referred for tertiary level care.
Other potential limitations include the retrospective nature of the study in clinical patients and the potentially increased pre-test probability based on the clinician’s assessment on non-bloody diarrhea, including variable other tests performed to exclude organic disease.
While these limitations are acknowledged, these data suggest that there is still a significant proportion of patients in secondary or tertiary referral practice who present with chronic diarrhea or IBS-D. The availability of simpler tests in reference laboratories would provide a convenient method to make a positive diagnosis of BAD, and overcome the uncertainties associated with interpreting a therapeutic trial with BA binders, the current indirect test for BAD.
Conclusion and Future Directions
This study demonstrates that fecal primary BAs >10% are equivalent to total fecal BAs excreted over 48h in predicting increased fecal weight and CT in patients presenting with chronic functional diarrhea. In a cohort of almost 1,000 secondary or tertiary referral patients, >10% fecal primary BAs alone was identified in ~26% of patients.
Future prospective studies are required to validate primary BAs >10% as a diagnostic test of BAD, based on a random, single sample of stool, and determine ability of the test result to predict response to BA binders. This would lead to an alternative, valid, and more efficient diagnostic test for BAD.
Supplementary Material
Acknowledgments
Grant Support: R01-DK92179 and R01-DK115950 (M. Camilleri) from National Institutes of Health
The authors thank Mrs. Cindy Stanislav for excellent secretarial assistance.
Abbreviations
- ANOVA
analysis of variance
- AUC
area under the curve
- BA
bile acid
- BAD
bile acid diarrhea
- BMI
body mass index
- C4
7α-hydroxy-4-cholesten-3-one
- CA
cholic acid
- CDCA
chenodeoxycholic acid
- CT
colonic transit
- DCA
deoxycholic acid
- ELISA
Enzyme-Linked Immunosorbent Assay
- FGF19
fibroblast growth factor 19
- GC
geometric center
- HV
healthy volunteers
- HPLC
high performance liquid chromatography
- IBS-C
irritable bowel syndrome with constipation
- IBS-D
irritable bowel syndrome with diarrhea
- In
Indium
- IQR
interquartile range
- ROC
receiver operating curve
Footnotes
Disclosures: The authors have no conflicts of interest.
Authors’ contributions:
Priya Vijayvargiya - research fellow, data analysis, authorship of manuscript
Victor Chedid - research fellow, data analysis, review manuscript
Irene Busciglio - study coordination and database management
Paula Carlson - serum FGF-19 analysis
Duane Burton - database management, colonic transit measurement
Michael Camilleri - principal investigator, study concept and design, analysis and interpretation of data, drafting and revising manuscript, study supervisor
Leslie Donato - biomarker analysis (serum C4 and stool bile acids) and reviewing manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wedlake L, A’Hern R, Russell D, et al. Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Alimentary pharmacology & therapeutics. 2009;30(7):707–17. doi: 10.1111/j.1365-2036.2009.04081.x. [DOI] [PubMed] [Google Scholar]
- 2.Valentin N, Camilleri M, Altayar O, et al. Biomarkers for bile acid diarrhoea in functional bowel disorder with diarrhoea: a systematic review and meta-analysis. Gut. 2016;65(12):1951–9. doi: 10.1136/gutjnl-2015-309889. [DOI] [PubMed] [Google Scholar]
- 3.Slattery SA, Niaz O, Aziz Q, et al. Systematic review with meta-analysis: the prevalence of bile acid malabsorption in the irritable bowel syndrome with diarrhoea. Alimentary pharmacology & therapeutics. 2015;42(1):3–11. doi: 10.1111/apt.13227. [DOI] [PubMed] [Google Scholar]
- 4.Peleman C, Camilleri M, Busciglio I, et al. Colonic Transit and Bile Acid Synthesis or Excretion in Patients With Irritable Bowel Syndrome–Diarrhea Without Bile Acid Malabsorption. Clinical Gastroenterology and Hepatology. 2017;15(5):720–7. e1. doi: 10.1016/j.cgh.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vijayvargiya P, Camilleri M, Shin A, et al. Methods for diagnosis of bile acid malabsorption in clinical practice. Clinical Gastroenterology and Hepatology. 2013;11(10):1232–9. doi: 10.1016/j.cgh.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vijayvargiya P, Camilleri M, Carlson P, et al. Performance characteristics of serum C4 and FGF19 measurements to exclude the diagnosis of bile acid diarrhoea in IBS-diarrhoea and functional diarrhoea. Alimentary pharmacology & therapeutics. 2017;46(6):581–8. doi: 10.1111/apt.14214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camilleri M, Sellin JH, Barrett KE. Pathophysiology, Evaluation, and Management of Chronic Watery Diarrhea. Gastroenterology. 2017;152(3):515–32. e2. doi: 10.1053/j.gastro.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oduyebo I, Camilleri M. Bile acid disease: the emerging epidemic. Current opinion in gastroenterology. 2017;33(3):189–95. doi: 10.1097/MOG.0000000000000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conley DR, Coyne MJ, Bonorris GG, et al. Bile acid stimulation of colonic adenylate cyclase and secretion in the rabbit. The American journal of digestive diseases. 1976;21(6):453–8. doi: 10.1007/BF01072128. [DOI] [PubMed] [Google Scholar]
- 10.Ao M, Sarathy J, Domingue J, et al. Chenodeoxycholic acid stimulates Cl(−) secretion via cAMP signaling and increases cystic fibrosis transmembrane conductance regulator phosphorylation in T84 cells. American journal of physiology Cell physiology. 2013;305(4):C447–56. doi: 10.1152/ajpcell.00416.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Domingue JC, Ao M, Sarathy J, et al. Chenodeoxycholic acid requires activation of EGFR, EPAC, and Ca2+ to stimulate CFTR-dependent Cl- secretion in human colonic T84 cells. American journal of physiology Cell physiology. 2016;311(5):C777–c92. doi: 10.1152/ajpcell.00168.2016. [DOI] [PubMed] [Google Scholar]
- 12.Chadwick VS, Gaginella TS, Carlson GL, et al. Effect of molecular structure on bile acid-induced alterations in absorptive function, permeability, and morphology in the perfused rabbit colon. The Journal of laboratory and clinical medicine. 1979;94(5):661–74. [PubMed] [Google Scholar]
- 13.Shin A, Camilleri M, Vijayvargiya P, et al. Bowel functions, fecal unconjugated primary and secondary bile acids, and colonic transit in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2013;11(10):1270–5. e1. doi: 10.1016/j.cgh.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong BS, Camilleri M, Carlson P, et al. Increased bile acid biosynthesis is associated with irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol. 2012;10(9):1009–15. e3. doi: 10.1016/j.cgh.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bampton PA, Dinning PG, Kennedy ML, et al. The proximal colonic motor response to rectal mechanical and chemical stimulation. American journal of physiology Gastrointestinal and liver physiology. 2002;282(3):G443–9. doi: 10.1152/ajpgi.00194.2001. [DOI] [PubMed] [Google Scholar]
- 16.Alemi F, Poole DP, Chiu J, et al. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology. 2013;144(1):145–54. doi: 10.1053/j.gastro.2012.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camilleri M, Acosta A, Busciglio I, et al. Effect of colesevelam on faecal bile acids and bowel functions in diarrhoea-predominant irritable bowel syndrome. Alimentary pharmacology & therapeutics. 2015;41(5):438–48. doi: 10.1111/apt.13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duboc H, Rainteau D, Rajca S, et al. Increase in fecal primary bile acids and dysbiosis in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterology & Motility. 2012;24(6):513–e247. doi: 10.1111/j.1365-2982.2012.01893.x. [DOI] [PubMed] [Google Scholar]
- 19.Duboc H, Rajca S, Rainteau D, et al. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut. 2013;62(4):531–9. doi: 10.1136/gutjnl-2012-302578. [DOI] [PubMed] [Google Scholar]
- 20.Weingarden AR, Chen C, Zhang N, et al. Ursodeoxycholic Acid Inhibits Clostridium difficile Spore Germination and Vegetative Growth, and Prevents the Recurrence of Ileal Pouchitis Associated With the Infection. Journal of clinical gastroenterology. 2016;50(8):624–30. doi: 10.1097/MCG.0000000000000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Debongnie JC, Phillips SF. Capacity of the human colon to absorb fluid. Gastroenterology. 1978;74(4):698–703. [Google Scholar]
- 22.Hammer J, Phillips SF. Fluid loading of the human colon: effects on segmental transit and stool composition. Gastroenterology. 1993;105(4):988–98. doi: 10.1016/0016-5085(93)90941-5. [DOI] [PubMed] [Google Scholar]
- 23.Vassallo M, Camilleri M, Phillips SF, et al. Transit through the proximal colon influences stool weight in the irritable bowel syndrome. Gastroenterology. 1992;102(1):102–8. doi: 10.1016/0016-5085(92)91789-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.