Abstract
Background
Point of Care Bedside Ultrasound is widely utilized as a rapid technique to evaluate patients with acute pulmonary emergencies, including acute pneumothorax. The presence of a pneumothorax is a known cause of loss of lung sliding by ultrasound exam, but no other risk factors have been clearly identified. We attempted to identify demographic and patient characteristics that are risk factors for loss of ultrasonographic lung sliding in the absence of a pneumothorax.
Methods
Data was collected on 159 patients admitted to the Medical Intensive Care Unit with Acute Respiratory Failure, undergoing routine admission lung ultrasound. The lung ultrasound exam consisted of 3 views of each hemithorax using a phased array abdominal probe.
Results
There were four confirmed pneumothoraces out of 20 patients with loss of lung sliding at one or more ultrasound interrogation points on either hemithorax. Hypercarbic respiratory failure (OR 5.59) and low body mass index (OR 0.88) were statistically significant risk factors for the loss of lung sliding in the absence of pneumothorax. There was a trend towards significance in patients with a known history of a decreased FEV1/FVC ratio (OR 0.02), COPD/Asthma exacerbation as the cause of their respiratory failure (OR 4.52) and prior pneumothorax (OR 11.53).
Conclusions
Common diagnoses and co-morbidities are associated with the loss of ultrasonographic lung sliding, in the absence of pneumothorax.
Keywords: ultrasound, lung sliding, pneumothorax
INTRODUCTION
Bedside critical care ultrasound is an evolving field within the practice of critical care medicine and encompasses the use of ultrasound for diagnosis, titration of therapeutic interventions and procedural guidance1. One component of bedside ultrasound is the ability of the intensivist to rapidly detect the presence or absence of a number of life threatening conditions, including acute pneumothorax and the etiology of shock2,3. There has been a dramatic adoption and acceptance of this bedside technology due to the ease of use and diagnostic accuracy.
The bedside detection of a pneumothorax utilizing lung ultrasound is heavily dependent on an ultrasound finding called lung sliding, which was first described in the early 1980s4. The designation of lung sliding refers to a to-and-fro movement of the visceral pleura in contact with the parietal pleura and has been variably described as a shimmering/glimmering (or twinkling) of the pleural line on 2-Dimensional ultrasound5. The absence of lung sliding on a lung ultrasound exam could indicate pneumothorax but it could also be absent with severe acute lung injury, prior thoracic surgery, atelectasis, lobar consolidation, a large parenchymal tumor or an examination performed on the opposite side of a mainstem intubation6. In addition, there are questions regarding these ultrasound findings in patients with Chronic Obstructive Pulmonary Disease (COPD) and hyper-inflated lungs and in patients with bullous disease7,8. There are additional confirmatory ultrasound findings that help rule out a pneumothorax (the presence of B lines and lung pulse) or rule in a pneumothorax (presence of lung point) 9–11.
A recent meta-analysis of lung ultrasound versus chest radiography for detecting pneumothorax found that lung ultrasound was superior to chest radiography, in the hands of a skilled operator (ultrasound sensitivity 78.6%, specificity 98.4%, chest x-ray sensitivity 39.8%, specificity 99.3%)12. In this study, we attempted to define the prevalence of absent lung sliding in all patients admitted to the MICU with acute respiratory failure, and determine the risk factors for this. It is important to recognize that we are looking for risk factors for isolated loss of lung sliding only, not for other findings associated with pneumothorax on lung ultrasound, such as lung point on M mode, lung pulse or the presence of B lines.
MATERIALS AND METHODS
This study was a prospective data collection study, designed to assess risk factors for the loss of lung sliding in a MICU who presented with acute respiratory failure (defined as the use of invasive or non-invasive mechanical ventilation). This study was performed in the MICU of Barnes Jewish Hospital, affiliated with Washington University in St Louis, a large academic referral hospital in St Louis, Missouri, USA. Patients consecutively admitted to the MICU with acute respiratory failure from August 2013 through November 2013 who underwent a routine admission screening ultrasound were included. The study was approved by the Institutional Review Board of Washington University in St Louis (IRB ID #201305023). Individual patient consent was waived for the study as the use of bedside ultrasound is considered standard practice in the MICU. Every patient with shock and respiratory failure, on admission, undergoes lung ultrasound, bedside echocardiogram and, if deemed necessary by the attending physician, lower extremity venous ultrasound to aid in clinical decision-making.
Data Collection
Data was prospectively collected on 159 consecutive patients admitted to the MICU with Acute Respiratory Failure who were undergoing routine admission lung ultrasound. The lung ultrasound exam consisted of three views of each hemithorax per the Bedside Lung Ultrasound in Emergency (BLUE) Protocol, originally described by Lichtenstein, et al.4 A Sonosite M-Turbo Ultrasound machine was utilized with a p21x transducer (5.1 MHz) set in abdominal preset mode to acquire the images of lung sliding, as this probe allows for an efficient admission exam. Gain and depth were adjusted by the ultrasonographer to facilitate observation of the pleural line. M mode was utilized if there was no lung sliding present on exam. Four-second clips at each location on the hemithorax were stored for review per the MICU protocol. These clips were de-identified and reviewed by an expert ICU ultrasonographer who was blinded to the patient’s demographics and clinical scenario, who independently interpreted each four-second clip. The expert ICU ultrasonographer received formal ultrasound training, and certification, through the American College of Chest Physicians. Their assessment was used for the final data analyses.
Patients who were identified to not have lung sliding were ruled out for a pneumothorax by other more specific ultrasonographic findings. These include the presence/absence of B-lines, and lung pulse. Other imaging modalities that were used to aid in the diagnosis of pneumothorax included standard chest x-rays and occasionally CT scans.
Patient characteristics were recorded to identify risk factors for loss of lung sliding in the absence of a pneumothorax, which was also evaluated by computed tomography (CT) scan when available for review. Baseline demographics, type of respiratory failure, cause of respiratory failure and past medical history were identified by chart review and recorded (Table 1).
Table 1.
Baseline Characteristics of All Participants
| Demographics | |
| Age (years), mean | 57.7 +/− 15.8 |
| Male, % (n) | 55.4 (88) |
| Caucasian, % (n) | 67.9 (108) |
| African American, %, (n) | 30.8 (49) |
| BMI, median (range) | 29.8 (10.54, 69.19) |
| Clinical Characteristics | |
| Mechanism of Respiratory Failure, % (n) | |
| Hypoxemic | 56.6 (90) |
| Hypercarbic | 10.1 (16) |
| Hypoxemic/Hypercarbic | 20.8 (33) |
| Other* | 13.9 (22) |
| Principle ICU Diagnoses as cause of Respiratory Failure, % (n) | |
| ARDS | 5.0 (8) |
| Pneumonia | 45.3 (72) |
| COPD/Asthma Exacerbation | 6.9 (11) |
| Acute Pulmonary Edema | 13.8 (22) |
| Interstitial Lung Disease Exacerbation | 5.0 (8) |
| Progressive Malignancy | 3.1 (5) |
| Other* | 20.7 (33) |
| Past Medical History, % (n) | |
| Lung Transplant | 4.4 (7) |
| Cancer | 29.8 (47) |
| Pulmonary Hypertension | 3.8 (6) |
| Coronary Artery Disease | 21.5 (34) |
| COPD/Asthma | 30.4 (48) |
| Deep Vein Thrombosis/Pulmonary Embolism(DVT/PE) | 15.8 (25) |
| Interstitial Lung Disease (ILD) | 7.0 (11) |
| Gastroesophageal Reflux Disease | 15.2 (24) |
| Diabetes Mellitus | 38.0 (60) |
| Prior Chest Tube Placement | 3.80 (6) |
| Prior Pneumothorax | 1.9 (3) |
| Prior Thoracic Surgery | 11.4 (18) |
Other causes include miscellaneous diagnoses (intubation for airway protection or acute drug overdose, etc.)
Data Analysis
Summary statistics were used to describe data, including means, standard deviations and ranges. Logistic regression analysis was used to determine risk factors for the loss of lung sliding, removing all cases that were due to pneumothorax. The final data utilized for determination of lung sliding was that determined by the expert ultrasonographer. Statistics were conducted using STATA 14.2 statistical software (College Station, TX).
RESULTS
There were 159 patients who met inclusion criteria for enrollment into this study. The performing ultrasonographer and expert sonographer had high agreement in image interpretation (Kappa 0.84, 95.5% agreement). In total, there were four right sided pneumothoraces, confirmed by ultrasound, out of the 13 patients with loss of lung sliding at one or more ultrasound interrogation points on the right hemithorax. There were no left sided pneumothoraces and seven patients with loss of lung sliding on the left hemithorax at one or more ultrasound interrogation points. The majority of locations for absent lung sliding in the absence of a pneumothorax were noted to be at the apical interrogation position. Lung sliding often became more pronounced as the probe was moved from the apex to the lung base.
Among patient demographics, increasing BMI (OR 0.88, 95% CI 0.80, 0.96; p = 0.048) was the only statistically significant risk factor for being protective of the loss of lung sliding in a patient without a pneumothorax. With the use of splines, we identified a BMI of 31 to be the point where elevated BMI becomes protective against the loss of lung slide. Other demographics and baseline characteristics of the patients enrolled can be found in Table 1. Of the patients enrolled, 86.1% were on invasive mechanical ventilation and 13.9% were on non-invasive mechanical ventilation.
Hypercarbic respiratory failure was a risk factor for the loss of lung sliding, in the absence of a pneumothorax (OR 5.59; 95% CI 1.34, 23.32; p = 0.02). Table 2 outlines past medical history as well as causes of respiratory failure and their association with loss of lung sliding. Odds ratios controlled for both age and BMI can be found in Table 3. Increasing FEV1/FVC trended towards significance (OR 0.02; 95% CI 0.0001, 3.32; p = 0.14) for being protective against the loss of lung sliding (Figure 1). There was no association between the loss of sliding lung and tidal volume (OR 1.00; 95% CI 0.99, 1.01; p = 0.49), respiratory rate (OR 1.03; 95% CI 0.94, 1.12; p = 0.53) or positive end-expiratory pressure (OR 0.87, 95% CI 0.66, 1.15; p = 0.34).
Table 2.
Odds Ratio, univariate analysis of covariates, for having loss of lung sliding.
| Covariates (n) | Odds Ratio | 95% CI | P-value |
|---|---|---|---|
| Age | 0.99 | 0.96, 1.02 | 0.53 |
| BMI | 0.88 | 0.80, 0.96 | 0.01 |
| Acute Pulmonary Edema* (21) | 2.03 | 0.51, 8.1 | 0.31 |
| Hypercarbic Respiratory Failure (15) | 5.21 | 1.38, 19.69 | 0.02 |
| Hypoxemic Respiratory Failure (86) | 0.46 | 0.14, 1.46 | 0.19 |
| Mixed Respiratory Failure (32) | 1.15 | 0.30, 4.44 | 0.84 |
| Prior Pneumothorax (2) | 11.50 | 0.68, 195.63 | 0.09 |
| History of Thoracic Surgery (15) | 1.32 | 0.37, 4.74 | 0.67 |
| History of Lung Transplant (7) | 1.19 | 0.24, 5.83 | 0.83 |
| ARDS* (7) | 1.86 | 0.21, 16.76 | 0.58 |
| COPD/Asthma Exacerbation* (10) | 3.00 | 0.56, 15.89 | 0.20 |
| Pneumonia* (69) | 0.74 | 0.23, 2.38 | 0.62 |
| DVT/PE (23) | 1.02 | 0.21, 4.94 | 0.98 |
| ILD (11) | 1.58 | 0.18, 13.96 | 0.68 |
| COPD (36) | 3.00 | 0.57, 15.89 | 0.20 |
| Prior Chest Tube (5) | 3.09 | 0.32, 30.09 | 0.33 |
Indicates Cause of Respiratory Failure
Table 3.
Odds Ratio for having loss of lung sliding, multivariable analysis adjusted for Age and BMI.
| Covariates (n) | Odds Ratio | 95% CI | P-value |
|---|---|---|---|
| Age | 0.99 | 0.95, 1.02 | 0.42 |
| BMI | 0.88 | 0.80, 0.96 | 0.01 |
| Acute Pulmonary Edema* (21) | 2.38 | 0.56, 10.18 | 0.24 |
| Hypercarbic Respiratory Failure (15) | 5.59 | 1.34, 23.32 | 0.02 |
| Hypoxemic Respiratory Failure (86) | 0.43 | 0.13, 1.47 | 0.18 |
| Mixed Respiratory Failure (32) | 1.14 | 0.27, 4.85 | 0.85 |
| Prior Pneumothorax (2) | 11.53 | 0.63, 212.17 | 0.10 |
| History of Thoracic Surgery (15) | 1.55 | 0.30, 7.95 | 0.60 |
| History of Lung Transplant (7) | 1.35 | 0.20, 8.45 | 0.75 |
| ARDS* (7) | 2.01 | 0.19, 21.25 | 0.56 |
| COPD/Asthma Exacerbation* (10) | 4.52 | 0.72, 28.31 | 0.11 |
| Pneumonia* (69) | 0.52 | 0.15, 1.77 | 0.29 |
| DVT/PE (23) | 0.81 | 0.16, 4.13 | 0.80 |
| ILD (11) | 3.08 | 0.55, 17.39 | 0.20 |
| COPD (36) | 2.03 | 0.53, 7.74 | 0.30 |
| Prior Chest Tube (5) | 4.98 | 0.43, 58.23 | 0.20 |
Indicates Cause of Respiratory Failure
Figure 1.
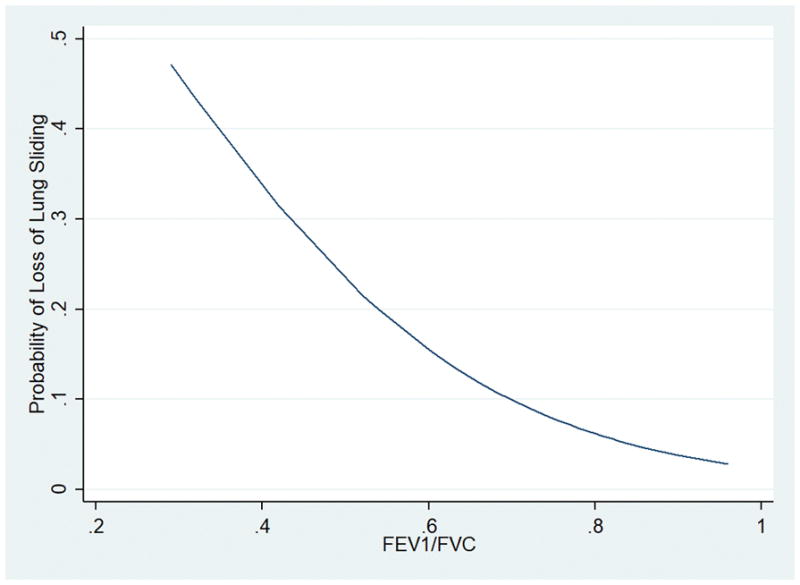
FEV1/FVC versus The Probability of Loss of Lung Sliding.
DISCUSSION
Our study confirms that different disease processes can mimic the presence of a pneumothorax. Overall, we noted that lung sliding was absent more frequently at the apex of the lung compared to lower thoracic interrogation points, a finding which has previously been noted13. It is also well known that patients with COPD or bullous lung disease can have ultrasound findings that could be misinterpreted as a pneumothorax7,8. With this in mind, we showed that patients with hypercarbic respiratory failure and low BMI had a statistically significant increase in the odds of loss of lung sliding. There was also a trend toward significance for patients with a decreased FEV1/FVC ratio, cause of respiratory failure being COPD/asthma exacerbation and prior pneumothorax.
The association of hypercarbic respiratory failure with loss of lung sliding likely reflects a large proportion of patients in our study with underlying obstructive lung disease. Interestingly, a prior chart history of COPD did not associate with an increased risk of absent lung sliding, likely because many patients are misdiagnosed with COPD in our experience and many of these “COPD” patients had never had prior pulmonary function testing performed when the medical record was reviewed. Obstructive lung diseases resulting in hypercarbia likely makes lung sliding more difficult to detect at the apex due to the combination of air trapping, hyperinflation and less actual movement of the parietal pleura on the visceral pleura in these patients. Our findings that a decreased FEV1/FVC ratio is also associated with loss of lung sliding, supports our theory that many of these patients with hypercarbia, likely had obstructive lung disease. In summary, the prior literature as well as our combined findings of hypercarbia and a low FEV1/FVC ratio as risk factors for loss of lung sliding are important as many MICU patients have obstructive lung disease either as a primary reason for ICU admission or a comorbid illness.
The association between elevated BMI and protection from loss of lung sliding is another interesting finding in our study. We are not aware of this finding of elevated BMI previously being reported as a risk factor for protection of absent lung sliding. Conceptually, we hypothesize that lung sliding could be harder to see in patients with less extrapleural fat and soft tissue, and this may be related to the difficulty of performing ultrasound with an inadequate air/soft tissue interface to adequately delineate the pleural line. This finding requires a further dedicated study of lung ultrasound in patients with elevated BMI in the ICU.
It is reassuring that in our study, some of the previously recognized risk factors for absence of lung sliding were again apparent, namely history of a prior pneumothorax. This is likely due to the pleurodesis that occurs after a prior pneumothorax treated by a thoracostomy tube. This study included one patient with known history of prior pleurodesis, and the lung ultrasound findings in the patient revealed total absence of lung sliding. Pleurodesis has been a known risk factor for the loss of lung sliding14. Interestingly, we have noted many lung transplant recipients in our institution to have preserved lung sliding, and the individual risk factors (e.g. coexistent obstructive lung disease) within a thoracic surgery population for future loss of lung sliding have not been previously defined. These individual patient factors should be studied and perhaps a prospective study looking at loss of lung sliding in lung transplant patients with evolving chronic rejection and the development of obstructive lung disease may be helpful to answer the question.
Perhaps the most compelling result of our study is that patients were more likely to not have a pneumothorax, than to have a pneumothorax, when found to have absent lung sliding. All patients who did not have sliding lung, in the absence of a pneumothorax, were ruled out for pneumothorax with the presence of b-lines or lung pulse. The high false positive rate implies that lung sliding alone should not be used to determine the presence of a pneumothorax, and further finding (i.e. B-lines, lung pulse) need to be assessed at the time of ultrasonography.
There were several limitations to this study. First, we did not use M mode on all patients. It was only used if the patient did not have visible lung sliding. The objective of this study was to look for participants that seemingly had a pneumothorax by one parameter, lung sliding, which is the most widely obtainable variable by ultrasound. Patients who had loss of sliding were further assessed with M mode, as well as other ultrasonographic findings such as b-lines and lung pulse to identify whether there was a pneumothorax present. Second, all final diagnoses we used were based off chart review. Therefore, we may have had multiple patients that may have been misdiagnosed for the cause or type of respiratory failure (ARDS may have been under represented due to this. The findings may also be different if the study population was different. If the patients did not have any underlying lung disease, we may have had more true positive rather than false positive events. It also may have been beneficial to have a pool of trained ICU ultrasonographers to interpret the images, but after the obtaining and expert ultrasonographer had high agreement, it was deemed unnecessary by the statistical team. We also did not differentiate between times of prior thoracic surgery, type of surgery, and how recent drug use was. There may be a difference in patients who had a surgery 30 years ago versus 30 days ago. Last, this study was done in a single MICU at a tertiary care center, which may limit the generalizability.
An important difference between this study, and prior investigations, is that we used an ultrasound probe which serves as a mixed thoracic and abdominal probe in our MICU and is the “work-horse” probe of most intensivists (frequency of 5.1MHz and selectable abdominal or cardiac preset modes). This type of probe is used in most protocols for assessing the lung, heart and thoracic structures. We recognize that a linear, higher frequency probe would likely have provided better resolution of the pleural line to assess pleural sliding. However, our study examines our current practice of performing lung ultrasound in critically ill patients, to allow for an efficient bedside admission exam.
It is important to understand that our study was not designed to assess the accuracy of ultrasound to detect pneumothorax. Instead, the study focused on one ultrasonographic finding seen in the presence of a pneumothorax but also possibly seen in other conditions frequently encountered in MICU patients. In clinical practice, trained bedside practitioners who routinely utilize lung ultrasound to rule out pneumothorax (including ourselves), combine multiple, dynamic, ultrasonographic findings (lung sliding, lung pulse, lung point and B lines) to confirm the diagnosis of a pneumothorax prior to intervening. However, with the rapid adoption of this technology and the concerns about competence and credentialing of practitioners, our study findings merit precaution when expanding this technology on a wider scale without appropriate education.
CONCLUSION
In summary, our study identifies some of the potential risk factors associated with isolated loss of lung sliding in the absence of pneumothorax. Despite these potential risk factors, it is important to understand that patients with loss of lung sliding may have other ultrasound findings that could easily rule out a pneumothorax, such as the presence of B lines, or lung pulse. Patients with hypercarbic respiratory failure, advanced age, low BMI, pulmonary edema and prior pneumothorax were more likely to not exhibit lung sliding. Prior thoracic surgery and a reduced FEV1/FVC ratio as a marker of obstructive lung disease also trended towards statistical significance. Lung sliding is harder to detect in the apical position on a hemithorax than at the base and ICU practitioners should be educated about this. Decisions in the intensive care unit, regarding the diagnosis and treatment of a pneumothorax using ultrasound should utilize multiple ultrasonographic findings, not just absent lung sliding, and the populations at risk for erroneous diagnosis, as noted in our study, should be considered. It is our hope that our findings help reduce misdiagnoses of pneumothoraces diagnosed via ultrasound. A large scale, prospective study is needed to aid in identifying ultrasonographic findings associated with the numerous causes of acute respiratory failure.
Acknowledgments
This study was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number T32HL007534. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest: None declared.
References
- 1.Moore CL, Copel JA. Point-of-care ultrasonography. N Engl J Med. 2011;364(8):749–757. doi: 10.1056/NEJMra0909487. [DOI] [PubMed] [Google Scholar]
- 2.Volpicelli G. Sonographic diagnosis of pneumothorax. Intensive care medicine. 2011;37(2):224–232. doi: 10.1007/s00134-010-2079-y. [DOI] [PubMed] [Google Scholar]
- 3.Ding W, Shen Y, Yang J, He X, Zhang M. Diagnosis of pneumothorax by radiography and ultrasonography: a meta-analysis. Chest. 2011;140(4):859–866. doi: 10.1378/chest.10-2946. [DOI] [PubMed] [Google Scholar]
- 4.Lichtenstein DA, Meziere GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest. 2008;134(1):117–125. doi: 10.1378/chest.07-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest. 1995;108(5):1345–1348. doi: 10.1378/chest.108.5.1345. [DOI] [PubMed] [Google Scholar]
- 6.Lichtenstein DA. Lung ultrasound in the critically ill. Annals of intensive care. 2014;4(1):1. doi: 10.1186/2110-5820-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slater A, Goodwin M, Anderson KE, Gleeson FV. COPD can mimic the appearance of pneumothorax on thoracic ultrasound. Chest. 2006;129(3):545–550. doi: 10.1378/chest.129.3.545. [DOI] [PubMed] [Google Scholar]
- 8.Gelabert C, Nelson M. Bleb point: mimicker of pneumothorax in bullous lung disease. The western journal of emergency medicine. 2015;16(3):447–449. doi: 10.5811/westjem.2015.3.24809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lichtenstein D, Meziere G, Biderman P, Gepner A, Barre O. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med. 1997;156(5):1640–1646. doi: 10.1164/ajrccm.156.5.96-07096. [DOI] [PubMed] [Google Scholar]
- 10.Lichtenstein DA, Lascols N, Prin S, Meziere G. The “lung pulse”: an early ultrasound sign of complete atelectasis. Intensive care medicine. 2003;29(12):2187–2192. doi: 10.1007/s00134-003-1930-9. [DOI] [PubMed] [Google Scholar]
- 11.Lichtenstein D, Meziere G, Biderman P, Gepner A. The “lung point”: an ultrasound sign specific to pneumothorax. Intensive care medicine. 2000;26(10):1434–1440. doi: 10.1007/s001340000627. [DOI] [PubMed] [Google Scholar]
- 12.Alrajab S, Youssef AM, Akkus NI, Caldito G. Pleural ultrasonography versus chest radiography for the diagnosis of pneumothorax: review of the literature and meta-analysis. Critical care (London, England) 2013;17(5):R208. doi: 10.1186/cc13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lichtenstein DA. Ultrasound in the management of thoracic disease. Critical care medicine. 2007;35(5 Suppl):S250–261. doi: 10.1097/01.CCM.0000260674.60761.85. [DOI] [PubMed] [Google Scholar]
- 14.Bouhemad B, Zhang M, Lu Q, Rouby JJ. Clinical review: Bedside lung ultrasound in critical care practice. Critical care (London, England) 2007;11(1):205. doi: 10.1186/cc5668. [DOI] [PMC free article] [PubMed] [Google Scholar]


