Abstract
Information that is the most salient and important for future use is preferentially preserved through active processing during sleep. Emotional salience is a biologically adaptive cue that influences episodic memory processing through interactions between amygdalar and hippocampal activity. However, other cues that influence the importance of information, such as the explicit direction to remember or forget, interact with the inherent salience of information to determine its fate in memory. It is unknown how sleep-based processes selectively consolidate this complex information. The current study examined the development of memory for emotional and neutral information that was either cued to-be-remembered (TBR) or to-be-forgotten (TBF) across a daytime period including either napping or wakefulness. Baseline memory revealed dominance of the TBR cue, regardless of emotional salience. As anticipated, napping was found to preserve memory overall significantly better than remaining awake. Furthermore, we observed a trending interaction indicating that napping specifically enhanced the discrimination between the most salient information (negative TBR items) over other information. We found that memory for negative items was positively associated with the percentage of SWS obtained during a nap. Furthermore, the magnitude of the difference in memory between negative TBR items and negative TBF items increased with greater sleep spindle activity. Taken together, our results suggest that although the cue to actively remember or intentionally forget initially wins out, active processes during sleep facilitate the competition between salience cues to promote the most salient information in memory.
Keywords: Memory, Emotion, Directed Forgetting, Napping, Selective Consolidation, Hippocampus
1. Introduction
Recent studies suggest that sleep preferentially preserves aspects of memory that are most important and valuable to remember – that is to say most salient -- over less relevant details (Payne, 2011; Stickgold & Walker, 2013). Emotional salience is one type of relevance cue used by our memory system to prioritize information and is, therefore, better remembered than neutral information. Sleep has repeatedly been shown to magnify this emotional memory benefit (Hu et al., 2006; Payne et al., 2008a). Emotional salience has biological importance for adaptive survival, by sustaining memories of situations that are negative and highly arousing in order for us to avoid similar situations in the future. However, the emotional salience of stimuli is not the only determining factor that assigns importance to information (Saletin et al., 2011; Murty et al., 2017). Although it is certainly important to remember certain information, it is also important to forget for the sake of updating knowledge, creating space for new memories, and to erase unwanted information (Anderson et al., 2004, Wylie et al., 2007). Thus, both the affective tone of a memory and the intention to remember or forget a memory are factors that may influence selective consolidation (Nowicka et al., 2010). Given that emotionally salient information is so often prioritized by our memory systems, is it more difficult to intentionally forget this information? How does sleep influence this question, especially given its tendency to selectively benefit memory for emotional information (Payne & Kensinger, 2010, 2018, Alger et al., 2018)? The manner in which these salience cues interact and/or compete to determine the fate of information in memory, and the role of sleep in preferentially promoting this information, is understudied. The current study aimed to address this gap in knowledge.
The hippocampus is a critical brain region that is highly involved in processing memories for experiences and events in life and storing them in long-term memory. Activity in brain areas that functionally connect to the hippocampus, such as the amygdala and the prefrontal cortex (PFC), can modulate activity within the hippocampus to modify the strength of the memory during encoding and consolidation. The neural patterns underlying a memory representation that are actively inhibited via hippocampal inhibition during encoding lead to that memory being more likely to be forgotten (Rizio & Dennis, 2013; Anderson & Hanslmayr, 2014). Conversely, those memories that are more strongly activated during wakefulness, which are perhaps tagged for further selective processing, are more likely to be remembered (Tucker & Fishbein, 2008; Payne & Kensinger, 2018 for review). Consistent with the general theory of systems consolidation, reactivation of the neural networks, especially in the hippocampus, that have been most strongly potentiated during wakeful learning leads to the strengthening of synaptic connections and a gradual redistribution of memory from the hippocampus into long-term cortical storage (see Diekelmann & Born, 2010; Payne et al., 2008b). This reactivation optimally occurs during sleep, specifically during slow wave sleep (SWS) for hippocampally-based, episodic memories. The reactivation process involves the collaboration of slow oscillations (0.5–1 Hz), hippocampal sharp wave ripples (SPW-Rs, 150–250 Hz), and sleep spindles (11–15 Hz), each of which is a unique plasticity signal that occurs in the sleeping brain (Born et al., 2006). In this complex mechanism, SPW-Rs, thought to induce long-term potentiation (LTP) in the neural circuitry, and thalamically-generated sleep spindles (Pavlides & Winson, 1989; Buzsaki, 1989), are grouped by the up-states of slow oscillations. For each of the salience cues we mentioned above, and explored in our current study, there are separate key brain regions that modulate hippocampal strength and that may lead to preferential consolidation of selective information through this mechanism.
1.1. Sleep facilitates emotional memory consolidation
Emotionally salient information is typically better remembered than neutral, being more biologically useful to retain to inform future decisions (e.g., Kensinger, 2009; Mather & Sutherland, 2011; Payne & Kensinger, 2010, 2018). The intrinsic characteristics of an event evoke an emotional reaction generated by some level of physiological arousal, as well as a perception of the valence of the experience (i.e., negative, neutral, or positive). Neuropsychological evidence suggests that activation of the amygdala with physiological arousal in response to emotional information leads to modulation of activity in the medial temporal lobe (MTL) and hippocampal regions and an enhancement of emotional memory (Dolcos et al., 2004; Hamann, 2001; Labar & Phelps, 1998; Phelps, 2004, Ritchey et al., 2008). The involvement of the amygdala seems to determine what information is processed in interactions between the hippocampus and target regions in the neocortex (Hermans et al., 2014).
A wealth of evidence shows that sleep facilitates better retention, in some cases enhancement, of emotional memories compared to a period of wakefulness (Wagner et al., 2001; Hu et al., 2006; Payne et al., 2008a, 2015; Nishida et al., 2009). This preferential consolidation of emotionally salient information is biologically adaptive, further strengthening and stabilizing this type of biologically relevant information in long term memory. The specific sleep physiology behind the preferential consolidation of emotionally salient information is still unclear, but there is evidence of a role for both SWS (Groch et al., 2011; Payne et al., 2015) and rapid eye movement (REM) sleep (Wagner et al., 2001; Payne et al., 2012; Genzel et al., 2015). Neural connectivity between the amygdala and hippocampus has been shown to be strengthened during REM sleep, with increased theta activity during this stage related to subsequent performance (Nishida et al., 2009; Popa et al., 2010). However, during non-REM (NREM) sleep, coordinated reactivation of the hippocampus and amygdala has been observed post-training, peaking during hippocampal SPW-Rs, and is thought to consolidate aspects of emotional memory (Girardeau et al., 2017). Interestingly, it may be that nocturnal sleep contributes to preferential consolidation of emotional information differently than daytime napping. Memory for emotional components of events has been related to REM sleep overnight (Payne et al., 2012) but to SWS during a nap (Payne et al., 2015; Alger et al., 2018), using the same emotional memory task. One goal of the current study was to further uncover the specific role of sleep physiology in preferential consolidation of salient information to add to this literature. However, emotional salience is not the only salience cue under consideration here.
1.2. The directed forgetting effect
The ability to overtly control what information is remembered and forgotten is much like controlling physical behavior in that it requires intention. The directed forgetting effect has been examined in laboratory studies using two methods – the item-method and the list-method. In the item method directed forgetting task, which the current study employed, items are presented one at a time, with each item followed by a direction to remember or forget. The list-method involves presenting a complete list of items, such as words, followed by instructions that one does not need to remember those items in the future. As these methods are thought to involve different brain processes (Anderson & Hanslmayr, 2014), we will focus only on the item-method used here. For this method, there are two key processes behind the effect. First is the idea of selective rehearsal during encoding. As each item is presented, the stimulus is temporarily maintained in working or short-term memory stores until the direction to remember or forget that item is delivered. Items that are instructed to-be-forgotten (TBF) are then dropped from rehearsal while to-be-remembered (TBR) items are further elaborated through selective rehearsal for eventual long-term storage (Yang et al., 2012).
Accompanying selective rehearsal is an active inhibitory control mechanism that suppresses or interrupts deeper encoding of the TBF items through attentional inhibition (Anderson et al., 2004, Mecklinger et al., 2009). Using fMRI, the instruction to forget, contrasted with a remember cue, has been seen to elicit activation in lateral prefrontal and parietal cortices, consistent with an inhibitory process acting on TBF items (Rizio & Dennis, 2013) during encoding. TBF items led to increased activation of dorsolateral PFC (DLPFC) as well as decreased activation of left and right hippocampal areas. Both of these activation alterations were found to predict the magnitude of forgetting (Yang et al., 2012). These results suggest that the DLPFC imposes cognitive control over the hippocampus to keep the unwanted memory from being attended to and stored for future reference.
Behaviorally, studies typically find that TBR items are better remembered than TBF items, whether testing via free recall or choosing from available options during recognition testing. However, the majority of studies examining this effect assess memory after only a short period of time. How the consolidation of TBR material relative to TBF is altered over longer time intervals, especially across retention periods that either contain sleep versus wakefulness, is relatively unstudied. There is evidence that the information you strive to remember is associated with stronger hippocampal activity and is subsequently consolidated to a greater extent than items that you do not attempt to learn (Rauchs et al., 2011). In one notable study, Saletin and colleagues (2011) examined the memory for neutral words that were either TBR or TBF across a retention period containing daytime wakefulness or a 100-minute nap opportunity. For both conditions, immediate memory was better for the TBR items over the TBF items, as assessed during a baseline recognition test. The nap, relative to the wake control condition, resulted in preferential enhancement of recall of the TBR items, while not providing any facilitation of TBF items. The difference in TBR and TBF memory at retest strongly correlated with sleep spindle activity. Furthermore, source analysis of the neural sources of sleep spindles revealed a repeating loop of activity through a network including the superior parietal, medial temporal, and right prefrontal cortices, areas involved with the selective remembering and forgetting of information. Interestingly, spindles had an inverse relationship with TBR and TBF items, positively predicting TBR items while being negatively associated with memory for TBF items. The current study aimed to extend the ideas within the Saletin et al. (2011) study by examining the influence of napping on the directed forgetting effect with the added dimension of emotion as an additional salience cue that might interact with or compete with the directed forgetting cue.
1.3. The interaction of emotional salience and intention to forget
Few studies have looked at the interaction of emotion and the intention to remember or forget. Moreover, no research, to our knowledge, has examined how sleep might modulate this interaction. The central question in this line of research is whether people can intentionally forget emotionally salient memories. The answer to this thus far is equivocal. Several studies have found robust directed forgetting effects for both negative and neutral (Yang et al., 2012; Marchewka et al., 2016) or pleasant and unpleasant (Tolin et al., 2002) stimuli. A few studies demonstrated that TBF items elicited greater inhibitory activity, compared to TBR items, and that the direction to forget negative items resulted in enhanced frontal activity compared to neutral items (Nowicka et al., 2010; Yang et al., 2012). This suggests that it is more cognitively demanding to inhibit the negatively salient items. However, others have shown less successful intentional forgetting of emotional compared to neutral information (Hauswald et al., 2010; Otani et al., 2012; Yang et al., 2016).
The current study builds on this prior work by investigating the interaction of two cues that can modulate the salience of information, inherent emotional salience and the task-related direction that items should be remembered or forgotten. We assessed baseline memory after encoding to examine how information was initially prioritized. We then investigated how memory fared across a period of wakefulness, compared to either an immediate or a delayed daytime nap. We chose this design to examine whether sleep immediately after learning stabilized and protected memory from subsequent interference compared to a period of interference prior to a nap (i.e., delayed nap). We also wanted to determine if differing sleep stage architecture within the nap times influenced how information was consolidated (Alger et al., 2010). If properties of SWS during a nap underlie selective consolidation of salient information as previously reported (Payne et al., 2015), it is possible that naps later in the day, with theoretically more SWS due to increased homeostatic sleep pressure, would reveal superior preferential consolidation.
Based on the prior work reviewed above, we hypothesized that baseline performance would reveal a hierarchy of memory, with emotional TBR items being remembered the best and neutral TBF items the worst. We hypothesized that a period of sleep, compared to a matched time spent awake, would facilitate the preservation of information, which also follows prior findings of how sleep impacts hippocampally-based memories. Specifically, we hypothesized that sleep would preferentially consolidate information that is the most salient (emotional TBR items) above and beyond other information, resulting in better retention of this material compared to those who remained awake. This prediction follows the findings of studies demonstrating preferential consolidation of salient information. We anticipated that napping immediately after encoding would result in greater preferential consolidation compared to delaying sleep, although we anticipated both nap conditions to preserve memory more than wakefulness. We based this prediction from literature demonstrating that sleeping soon after learning leads to stabilization and protection of the memory from subsequent interference (Talamini et al., 2008; Van der Werf et al., 2009), including preferential preservation of emotional information (Payne et al., 2012). Finally, we hypothesized that sleep physiology, specifically SWS and/or sleep spindles, would actively promote consolidation of salient information, whether that salience is defined as a directed forgetting cue or an emotional salience cue, as seen in previous studies (Saletin et al., 2011; Payne et al., 2015).
2. Methods
2.1. Participants
Fifty participants (32 females) were recruited from the Northern Indiana Community through advertisements in local online publications (e.g., Craigslist) and flyers around the University of Notre Dame campus. Undergraduate students from the university were not recruited. Though many studies typically sample from undergraduate populations as they are “captive audiences”, the reduced variability in such a sample may not be reflective of the general population. Subjects recruited were 18–39 years of age (mean ± SEM, 28.46 ± 0.97 years) and in good health as assessed by an in-depth screening self-report, with no history of sleep disturbance, learning disorders, or mental/emotional problems. They were free from all medications known to impair or facilitate sleep, mood, and attention.
Prior to arrival to the lab, participants were contacted via email to ensure eligibility by administering the screening form. Participants were instructed to get no less than 6 hours of sleep the night before the experimental day and refrain from caffeine, alcohol, nicotine, and unnecessary medication 24 hours before and for the duration of the study. In accordance with these restrictions, they kept a one-week sleep log to track their sleep/wake times, napping habits, and alcohol and caffeine intake prior to the experimental day. Upon arrival to the lab, participants were asked to sign a consent form explaining the nature of the research. All subjects received monetary compensation at the completion of their participation. This study was conducted according to the principles expressed in the University of Notre Dame Human Subjects Institutional Review Board, with all subjects providing written informed consent.
2.2. Materials
2.2.1. Subjective Questionnaires
Throughout the experimental day, subjects completed a demographics questionnaire as well as several other questionnaires to assess sleep habits and rule out psychopathology. These included the Pittsburg Sleep Quality Index (PSQI; Buysse, Reynolds, Monk, Berman, & Kupfer, 1989) to assess general sleep quality, the Morningness – Eveningness Questionnaire (MEQ; Horne & Ostberg, 1976) to assess morningness–eveningness tendencies, the Beck Anxiety Inventory (BAI; Beck, Epstein, Brown, & Steer, 1988) and State-Trait Anxiety Inventory (STAI-X1, Spielberger, 2010) to assess level of state anxiety, the Beck Depression Inventory – II (BDI; Beck, Ward, & Mendelson, 1961) to assess depressive symptoms, the Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988) to assess positive and negative affect, and the Stanford Sleepiness Scale (SSS; Hoddes, Zarcone, Smythe, Phillips, & Dement, 1973) to assess state sleepiness. These measures were used primarily to compare nap and wake groups to ensure they were similar to one another as well as to determine if any participant should be excluded because of scoring in elevated (i.e., clinical) ranges for depression or anxiety, which could impact both sleep and emotional memory formation.
2.2.2. Emotional directed forgetting task
During the encoding task, participants viewed a set of 120 scenes that were either negatively salient (60 images, e.g., an accident with a burning car) or neutral (60 images, e.g., a bird on a sidewalk). In order to ensure that the images used in the task had appropriate emotional salience, a pilot study (N = 45) was conducted to norm images for their valence and arousal ratings. Images were selected from two separate standardized image databases, the International Affective Picture System (IAPS; Lang, Bradley & Cuthbert, 1997) and the Nencki Affective Picture System (NAPS; Marchewka, Zurawski, Jednorog & Grabowska, 2014). The pilot study allowed us to select appropriate images from both databases and confirm within our population that the ratings were consistent with the reported normative ratings. The images used in the pilot experiment were rated on a 1–7 scale for valence (1 = most negative, 7 = most positive) and arousal (1 = not arousing at all, 7 = highly arousing). Although not pertinent to the main task, positive images were used to ensure a full spectrum of emotional images were presented to participants to avoid neutral images being rated as positive in contrast to negative. Of the images used in the pilot, 120 images were classified as negative if they had low valence scores (M = 2.29, SD = 0.57) and high arousal scores (M = 6.10, SD = 0.32). Similarly, 120 images were classified as neutral if they had moderate valence scores (M = 4.94, SD = 0.32) and low arousal scores (M = 2.22, SD = 0.38). Independent samples t-test revealed significant differences in valence, t238 = 44.45, p < .001 and arousal, t238 = 38.66, p < .001 by emotion.
We created two sets of images, set A and B, in which half the images were neutral and half were negative. Within each set, half of the negative and neutral images were assigned as to-be-remembered (TBR) and the other half to-be-forgotten (TBF). In order to counterbalance, image sets were used both at encoding and as foils at recognition tests across subjects, in that one subject would encode set A, with set B used as foils during recognition testing, whereas the next subject would encode set B with set A used at testing as foils. Furthermore, each set of images had 2 versions in which the direction to remember or forget was switched for each image so that every image was presented with both instructions across the two versions.
2.3. Procedures
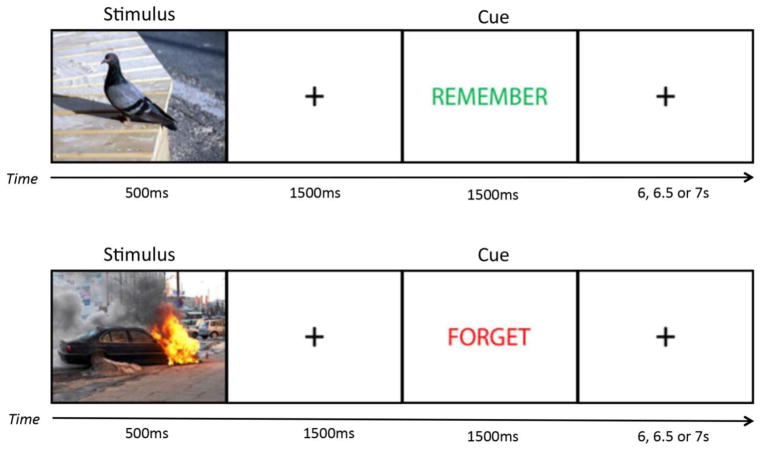
At approximately 9:30am, all participants arrived in the Sleep, Stress and Memory Lab, signed informed consent, and completed a set of questionnaires including the MEQ, PSQI, BAI, STAI-X1, PANAS-Now and SSS. At approximately 10:00am participants encoded all 120 images. Similar to the methods of previous work (Nowicka et al., 2010), each image was displayed for 500ms, followed by a 1500ms crosshair, followed either by the word ‘REMEMBER’ in green print or the word ‘FORGET’ in red print for 1500ms to cue participants to respectively remember or forget the image, and a post-cue crosshair that lasted 6, 6.5 or 7s (Figure 1). Participants were instructed to pay close attention to each image but that they would not need to remember any of the cued TBF images in the future.
Figure 1. Emotional Directed Forgetting Task.
During the encoding phase, participants encoded 60 negative and 60 neutral images, with half of each valence cued as to-be-remembered (TBR) or to-be-forgotten (TBF). Each image was displayed for 500ms, followed by a 1500ms fixation crosshair, followed by the direction to REMEMBER in green or FORGET in red, presented for 1500ms. The post-cue fixation crosshair is presented pseudo-randomly with equal probability across all trials for 6000, 6500, or 7000ms.
A surprise baseline test was administered ten minutes after encoding consisting of 60 old images (15 from each combination of valence and task direction) from the encoded stimuli, intermixed with 60 foils that were similar in valence and arousal to the encoded images. Participants viewed each image and were asked whether the image was old or completely new. The test was self-paced and contained no time-restraints for responses. However, this test was framed as an evaluation of how they experienced the images, not a test, in order to minimize expectations of a later test in which they would need to remember TBF items.
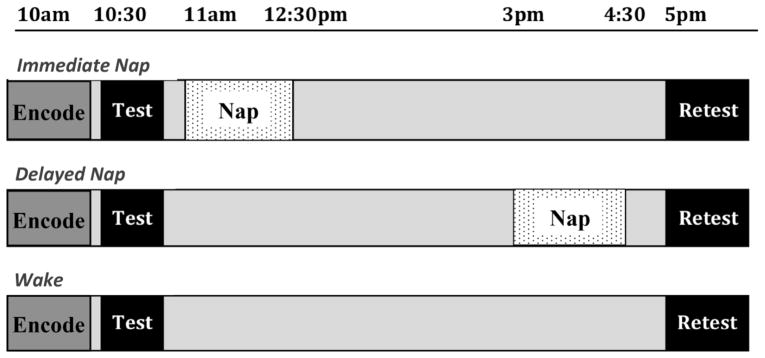
The current study was designed to have a wake control condition compared to two nap conditions in which participants obtained an equal amount of sleep and wake as one another, with the primary difference being the positioning of the nap in time. Prior to the participation date, subjects were provided with the choice of two potential schedules to which they would need to commit based on their schedules (i.e., 9:30am–1pm and 5–5:30pm or 9:30–10:30am and 2:30–5:30pm) in order to participate, which corresponded to either the immediate nap or delayed nap conditions. Once they indicated the schedule that fit within their availability, they were then randomly assigned into either a nap condition or wake control condition within those timeframes. Wake and delayed nap participants were allowed to leave the lab following the baseline test with the instruction not to nap or consume caffeine, alcohol, nicotine, or unnecessary medication during the retention period. Those in the immediate nap group were prepared for polysomnography (PSG). At around 11:00am, immediate nap subjects were given a 90-minute PSG-recorded sleep opportunity lasting until about 12:30pm. If participants obtained SWS or REM sleep during this time, they were allowed to sleep until they transitioned out of these stages in order to reduce sleep inertia and disorientation. After awakening, electrodes were removed and participants were allowed to leave the lab until the second session, approximately 4 hours later. Delayed nap participants returned to the lab at 2:30pm and were prepared in the same manner for a 90-minute PSG-monitored nap opportunity, beginning at 3pm. They were awakened at approximately 4:30pm and allowed 30 minutes to recover for sleep inertia before beginning questionnaires and the retest portion of the session (see Figure 2 for timeline of protocol).
Figure 2. Protocol.
All participants encoded the stimuli and were tested at the same time of day as one another, with encoding of the images at 10am, followed by baseline testing, and retest occurring at 5pm. Nap groups had a 90-minute nap opportunity either at 11am (immediate nap condition) or 3pm (delayed nap condition).
All participants returned to the lab at 5pm and began by completing a second set of questionnaires similar to the previous session (BDI, STAI-XI, PANAS, and SSS). Following these questionnaires, participants completed a psychomotor vigilance task (PVT, Dinges & Powell, 1985) to obtain objective measures of alertness and address the possible confound of an overall vigilance difference impacting task performance, rather than specifically sleep or lack of sleep accounting for performance differences. This cognitive test is a sustained reaction-time task that measures the average speed of a participants’ response to visual stimulus and demonstrates high test-retest reliability (median intraclass correlation coefficients/ICC > 0.8) and sensitivity (Dorrian et al. 2005, Basner & Dinges, 2012) to the effects of sleep deprivation (sensitivity = 93.7%), suggesting high convergent validity as well. After completing the PVT, participants were again tested via recognition on their memory for the remaining half of encoded material using the same method described above. However, this time, we emphasized that this test was a memory test and that they should judge whether they thought the image was old or new regardless of the earlier direction to remember or forget the pictures. Following the completion of this task, participants were debriefed and given an exit interview to determine whether they had anticipated being tested on their memory for the images during either session and, if so, whether they anticipated that they would be tested on their memory for the TBF images.
2.4. Polysomnography recordings
Nap participants were monitored online using digital EEG acquisition software (Comet System-Grass/Twin PSG Clinical Software) at a 200 Hz sampling rate using a standard polysomnographic montage, which included electroencephalography (EEG; F3, F4, C3, C4, Cz), electrooculography (EOG), and chin electromyography (EMG) channels, each referenced to contralateral mastoids. EEG data were filtered at 0.3–35 Hz (with a 60 Hz notch filter) and all impedances were kept at or below 10kΩ throughout recording periods. Each 30-s epoch of recorded sleep was scored blind to participants’ behavioral task performance according to the standards of Rechtschaffen and Kales (1968). The PSG recording was scored visually as NREM Stages 1, 2, SWS, and REM sleep, or wakefulness. Artifacts were then visually identified and removed using the EEGLAB 13.4 (Delorme & Makeig, 2004) toolbox for MATLAB 9.1 (The MathWorks Inc., Natick, MA, 2012). Spectral analysis was then applied to all artifact-free epochs of NREM and REM sleep during the nap. Mean absolute power spectral density (μV2/Hz) was calculated by Fast Fourier transform, applying a Hanning window to successive 3s epochs of sleep with 50% overlap. These calculations were carried out in MATLAB 9.1. Slow oscillation (0.5–1 Hz), delta (1–4 Hz), theta (4–7 Hz), and sigma (11–15 Hz) frequency bands were examined in order to investigate the frequencies associated with SWS, NREM sleep spindles, and REM sleep. Sleep spindle counts were obtained by further band-pass filtering all artifact-free epochs of Stage 2 sleep and SWS at 11–15 Hz and then applying the established automatic detection algorithm of Ferrarelli et al. (2007) using MATLAB 9.1. Automated detection derived discrete spindle events for each channel, with amplitude fluctuations in the filtered time-series exceeding a pre-determined threshold counted as spindles. Thresholds were calculated relative to the mean channel amplitude (8 times average amplitude). Sleep spindle density (number of spindles/total analyzed time in Stage 2 or SWS), duration and amplitude of the spindle events were calculated for frontal and central scalp electrodes (F3, F4, C3, C4) as well as the average across electrodes. The spindle range of 11–15 Hz was chosen based on prior research using this definition (Schabus et al., 2007; Tamminen et al., 2010; Lewis & Durrant, 2011).
2.5. Data analyses
To address our research hypotheses, for both sessions, memory for the images was calculated as the number of items accurately remembered (i.e. hits) divided by the number of items originally viewed. To correct for response bias, we calculated corrected memory by subtracting the proportion of false alarms (‘old’ judgments to new pictures) from the proportion of hits (Snodgrass and Corwin, 1988). This yielded corrected hit rates as the dependent variable in a 2 (session: baseline, retest) x 2 (valence: negative, neutral) x 2 (direction: TBR, TBF) x 3 (condition: wake, immediate nap, delayed nap) repeated measures ANOVA. Post hoc analyses of significant effects were conducted. Correlations were conducted between sleep variables and behavior to investigate the nature of the role of sleep in performance benefits.
3. Results
One participant who did not report a history of depression on the pre-screening form scored in the range of ‘moderately depressed’ on the Beck’s Depression Inventory (BDI) and was removed from further analysis. Three participants were identified as outliers whose memory performance at baseline testing was no better than random guessing (corrected hit rate < 50%) with statistical average memory scores greater than two standard deviations from the norm. These participants were removed from further analyses. The remaining sample comprised of 46 participants in the wake (n = 15), immediate nap (n = 16), and delayed nap (n = 15) conditions.
3.1. Questionnaires and sleepiness measures
All analyses to compare questionnaire data were conducted between the three groups (i.e., wake, immediate nap, delayed nap). A One-Way ANOVA was conducted to verify that there were no differences between conditions on any questionnaire responses or sleep log data, such as amount of sleep achieved the night before the experimental day. There were no significant differences between groups on any measure of anxiety, depression, positive or negative affect, sleep quality, or morningness/eveningness tendencies. When analyzing the sleep and wake activity as recorded on the sleep logs, all subjects were compliant in getting more than 6 hours of sleep the night prior to the experimental day (mean ± SEM, wake 7.97 ± 0.20, immediate nap 7.44 ± 0.35, delayed nap 8.20 ± 0.24) and groups were not significantly different from one another (F2,43 = 2.07, p = 0.14). For the week prior to participation, the groups obtained similar amounts of nightly sleep, on average (wake 7.67 ± 0.18, immediate nap 7.49 ± 0.26, delayed nap 8.05 ± 0.17; F2,43 = 1.86, p = 0.17). Subjects awoke, on average, between 6:45–8:00 AM the morning of the experiment. Additionally, we found no difference in average number of daytime naps taken over the week prior to participation between experimental conditions.
In order to verify that there were no group differences in sleepiness that could explain performance variations, we compared scores on the Stanford Sleepiness Scale and found no difference between groups either at the first session (wake 2.40 ± 0.23, immediate nap 2.44 ± 0.30, delayed nap 2.27 ± 0.18; F2,43 = 0.13, p = 0.88) or second session (wake 1.87 ± 0.22, immediate nap 2.44 ± 0.27, delayed nap 2.20 ± 0.22; F2,43 = 1.43, p = 0.25). In addition, comparisons of mean response time on the PVT revealed no significant differences between groups at session 2 in basic vigilance and alertness (wake 291.80 ± 14.10, immediate 316.81 ± 10.84, delayed 282.47 ± 13.89; F2,43 = 1.92, p = 0.16), although the difference was trending toward significance between immediate and delayed nap conditions (t29 = 1.96, p = 0.06).
3.2. Performance on the Emotional Directed Forgetting Task
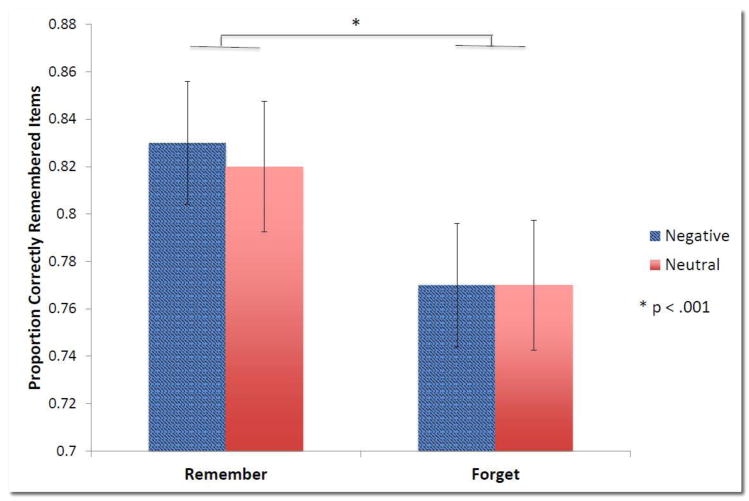
Prior to examining the change in performance across the retention period, we first analyzed baseline performance in order to elucidate the way in which the salience cues influenced initial memory of the material. We conducted a 2 (valence) x 2 (direction) x 3 (condition) repeated measures ANOVA, which revealed a significant main effect of direction (F1,43 = 15.42, p < 0.001) indicating that items cued TBR (mean ± SEM; 0.83 ± 0.03) were better remembered than items cued TBF (0.77 ± 0.02). Negative (0.80 ± 0.03) and neutral (0.79 ± 0.03) images, regardless of direction, were remembered similarly (F1, 43 = 0.33, p = 0.57). There were no significant interactions at baseline. Figure 3 shows the hierarchy that emerged in this immediate test, demonstrating a clear priority of memory for cued TBR items, regardless of valence, over TBF items across participants.
Figure 3. Hierarchy of memory at baseline.
Baseline performance shows the hierarchy of remembering based on salience cues. The cue to remember or forget clearly took precedence over the emotional salience cue, with negative and neutral items being remembered similarly within each task cue to remember or forget. To-be-remembered (TBR) items are remembered significantly better than to-be-forgotten (TBF) items (p < 0.001). Error bars reflect SEM.
Next, we examined the consolidation of information over time with either a nap or wake in the intervening retention period, with a 2 × 2 × 2 × 3 mixed ANOVA with between-subject variable of condition and within-subject variables of session, valence, and direction to remember or forget. Table 1 describes performance measures at and across these time points. We found a highly significant main effect of session (F1,43 = 100.65, p < 0.001), indicating that memory, overall, degrades over time. A significant interaction between session x condition (F2,43 = 10.08, p < 0.001) further revealed that the wake condition showed significantly more decay in memory than both the immediate nap (p < 0.001) and the delayed nap (p = 0.002) conditions. We also found significant main effects of valence (F1,43 = 6.27, p =0.02) and direction (F1,43 = 33.28, p < 0.001), with negative and TBR items being remembered significantly more than neutral and TBF items, overall. These main effects were further qualified by significant interactions with session (session x valence: F1,43 = 4.49, p = 0.04; session x direction: F1,43 = 4.21 p = 0.05), indicating that, across conditions, negative and TBR items are better retained across the retention period compared to neutral and TBF items. No 3-way interactions were significant (all p-values > 0.33). Analyses also revealed a trending 4-way interaction (F2,43 = 2.78, p = 0.07).
Table 1.
Behavioral Performance
| Condition | Baseline (B) | Retest (R) | Change (R-B) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Remember | Forget | Remember | Forget | Remember | Forget | |
| Neutral Performance | ||||||
| Wake | 0.77 ± 0.04 | 0.73 ± 0.05 | 0.52 ± 0.05 | 0.40 ± 0.05 | −0.25 ± 0.03 | −0.33 ± 0.04 |
| Immediate | 0.81 ± 0.06 | 0.76 ± 0.05 | 0.71 ± 0.06* | 0.64 ± 0.06*** | −0.10 ± 0.04** | −0.12 ± 0.05*** |
| Delayed | 0.86 ± 0.05 | 0.82 ± 0.04 | 0.74 ± 0.07* | 0.68 ± 0.07*** | −0.12 ± 0.03** | −0.14 ± 0.05*** |
|
| ||||||
| Negative Performance | ||||||
| Wake | 0.82 ± 0.03 | 0.73 ± 0.05 | 0.57 ± 0.06 | 0.54 ± 0.06 | −0.25 ± 0.03 | −0.19 ± 0.04 |
| Immediate | 0.83 ± 0.04 | 0.75 ± 0.05 | 0.78 ± 0.05** | 0.68 ± 0.05 | −0.05 ± 0.03*** | −0.08 ± 0.02* |
| Delayed | 0.85 ± 0.05 | 0.83 ± 0.05 | 0.78 ± 0.06* | 0.67 ± 0.07 | −0.08 ± 0.03*** | −0.16 ± 0.04 |
p < 0.05
p ≤ 0.01
p < 0.005
Mean ± SEM
All noted significant comparisons are with the wake condition, with notation of significance adjacent to the nap condition to which the wake group is being compared.
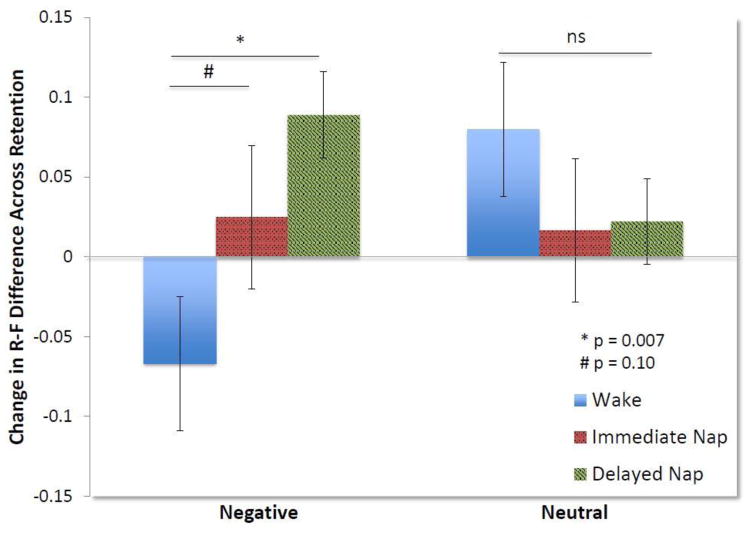
As clear from baseline performance, the direction to remember or forget appeared to be the dominant salience cue. In order to capture efficiency of the forgetting effect, as established in prior research (Saletin et al., 2011), as well as to clarify and interpret the trending 4-way interaction above, we calculated an R-F difference score, subtracting the proportion of TBF images correctly remembered from the proportion of TBR images correctly remembered. This R-F difference score allowed us to compare the magnitude of the difference between memory for to-be-remembered and to-be-forgotten items between conditions to determine how napping or remaining awake modulated this relationship. We calculated this score for both negative and neutral images. Groups were not significantly different from one another in terms of the R-F difference scores at baseline (F2,43 = 0.98, p = 0.39). A 2 (valence) x 3 (condition) ANOVA using the change in R-F difference scores across the retention period yielded a trending interaction (F2,43 = 2.78, p = 0.07) reflective of the trending 4-way interaction above. Although these results were trending, yet non-significant, we conducted post hoc analyses to explore our a priori hypotheses. We caution the interpretation of these post hoc analyses as they stem from this trending interaction. Using one-way ANOVAs, we examined the differences between conditions in the change in the magnitude of the R-F difference across the retention period. These analyses revealed that for neutral items, there was no significant difference between conditions (F2,43 = 0.62, p = 0.54). However, for negative items, the conditions were significantly different (F2,43 = 4.70, p = 0.01). While both immediate and delayed nap conditions showed an increase in the magnitude of the R-F difference for negative images over time (0.03 ± 0.03; 0.09 ± 0.03, respectively), the wake condition had a decrease in the R-F difference (−0.07 ± 0.05). Further t-tests showed that the wake condition was significantly different from the delayed nap group (t28 = −.293, p = 0.007) and trending when compared to the immediate nap group (t29 = −1.66, p = 0.10). This suggests that napping facilitated the selective retention of negative TBR items and further forgetting of TBF items. This led to an increase in the magnitude of the difference in memory between negative TBR and TBF items. Conversely, the wake condition demonstrated greater forgetting of negative TBR items compared to negative TBF items (see Figure 4). Comparison of performance between the nap conditions showed no significant differences (negative t29 = −1.51, p = 0.14; neutral t29 = −0.08, p = 0.93).
Figure 4. Change in the difference between memory for TBR and TBF items across the retention period.
The R-F difference was calculated as a measure of the efficiency of the forgetting effect. A positive change in the magnitude of this difference across the nap/wake retention period indicates better retention of the TBR items and more decay of TBF items. For neutral items, on the right of the figure, there was no significant difference between conditions in increase of the magnitude of the R-F difference over time. However, for negative items, napping facilitated a greater increase in the R-F difference, while the wake condition showed reduced R-F difference, indicating more decay of negative TBR compared to TBF items. The wake condition was significantly, or nearly significantly, different from both the delayed (p = 0.007) and immediate nap conditions (p = 0.10). Error bars reflect SEM.
As mentioned in the Methods, we asked participants during an exit interview whether they had expected either test and, if so, whether they expected that their memory for TBF items would be assessed. Of the 46 subjects, nobody expected the initial baseline test. As the subjects were told during the initial instructions during encoding that they would not need to remember any cued TBF images, only the ones they were told to remember, most subjects did anticipate a test during session 2. However, only 6 of the 46 subjects suspected they may be tested on memory for the TBF items. Excluding these subjects from the above analyses did not change any significant, non-significant, or trending effect, nor the interpretation of any of these effects.
3.3. Sleep stages, EEG spectral power, and sleep spindles
One of our goals in using this dual-nap design was to examine the sleep stage composition of the immediate and delayed naps. We first examined whether there was a shift in the relative amounts of Stage 2, SWS, and REM sleep, as a function of increased sleep pressure throughout the waking day (Alger et al., 2010). Table 2 summarizes sleep parameters between the two naps. We found no significant differences between immediate and delayed nap architecture. Of particular interest, total sleep time (TST, t29 = −0.85, p = 0.41) and the percentages of Stage 2 (p = 0.60), SWS (p = 0.48), and REM sleep (p = 0.27) were all similar between naps. Therefore, we collapsed across the two nap times in order to examine the relationship between sleep physiology and memory.
Table 2.
Sleep Parameters Between Nap Conditions
| Immediate Nap | Delayed Nap | p-value | |
|---|---|---|---|
| TST | 74.78 ± 5.38 | 68.83 ± 4.46 | 0.40 |
| Onset Latency | 6.91 ± 1.49 | 6.80 ± 2.26 | 0.97 |
| REM latency | 47.55 ± 6.37 | 56.09 ± 5.50 | 0.32 |
| WASO | 14.81 ± 3.20 | 16.87 ± 3.31 | 0.66 |
| Efficiency | 76.47 ± 4.34 | 74.05 ± 4.26 | 0.70 |
| Stage 1 % | 19.15 ± 2.93 | 15.74 ± 3.12 | 0.43 |
| Stage 2 % | 44.20 ± 4.17 | 47.32 ± 4.06 | 0.60 |
| SWS % | 19.00 ± 4.88 | 24.54 ± 5.99 | 0.48 |
| REM % | 17.64 ± 3.90 | 12.41 ± 2.36 | 0.27 |
A limited number of comparisons were planned between sleep and memory measures. Based on our a priori hypotheses and the previous literature on emotional memory (Nishida et al., 2009; Payne et al., 2015) and directed forgetting (Saletin et al., 2011), we focused our correlational sleep stage analyses on percentages of Stage 2, SWS, and REM sleep. Looking across all nappers, we found a significant positive correlation between the percentage of SWS and memory for the negative objects at retest, both for TBR (r31 = 0.39, p = 0.03) and TBF (r31 = 0.38, p = 0.04) items. Of the total number of subjects in the napping conditions, 23 subjects obtained SWS. When we removed those who did not reach SWS from the analyses, the correlations remained significant (negative TBR, r23 = 0.48, p = 0.02; negative TBF, r23 = 0.51, p = 0.01). No other significant correlations were found.
We also examined mean absolute spectral power (μV2/Hz) as well as relative power in order to standardize absolute power across subjects. We specifically looked at slow oscillations (.5–1 Hz), delta (1–4 Hz), theta (4–7 Hz), and sigma activity (11–15 Hz) to reflect activity in NREM Stage 2 sleep and SWS and REM sleep. We did not find any significant relationships between spectral power and memory performance.
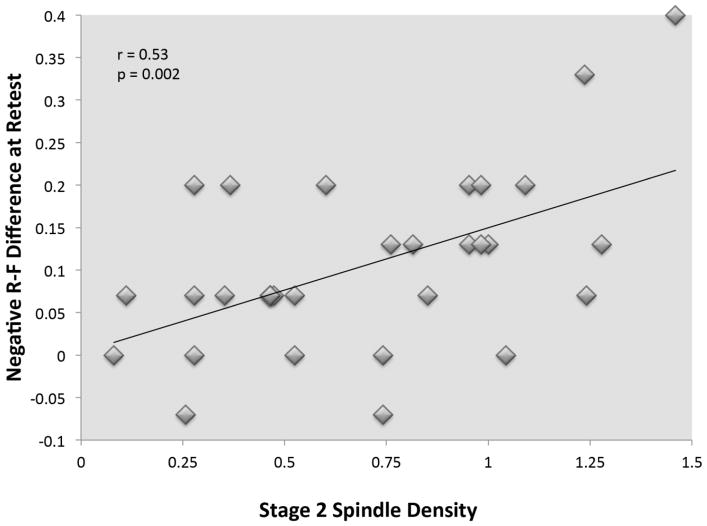
Finally, based on our a priori hypotheses regarding a possible role for spindles, we examined sleep spindle activity within Stage 2 and SWS, looking specifically at the main features of spindle density, duration, and amplitude averaged across scalp electrodes. Although we did not find correlations between spindles and memory for performance on individual salience cue combinations (i.e. negative TBR, neutral TBF), we did find that sleep spindles were related to the difference in memory between TBR and TBF negative items. A greater increase in the R-F difference score (i.e., memory difference between TBR and TBF items) for negative items was associated with greater spindle density (r31 = 0.53, p = 0.002), and duration (r31 = 0.51, p = 0.004) during Stage 2 sleep, although not spindle amplitude (see Figure 5). In order to determine if related spindle activity was located more frontal or central, we conducted exploratory correlational analyses. Spindle density and duration was significantly related to the negative R-F difference more strongly over frontal sites (density: F3 r31 = 0.55, p = 0.002; F4 r31 = 0.59, p = 0.001; duration: F3 r31 = 0.49, p = 0.006; F4 r31 = 0.57, p = 0.001), but not over central locations (density: r31 = 0.34, C3 p = 0.07; C4 r31 = 0.31, p = 0.09; duration C3 r31 = 0.25, p = 0.18; C4 r31 = 0.30, p = 0.10). No other correlations between performance and spindles were found.
Figure 5. Relationship between sleep spindle density and the negative R-F difference at retest.
The magnitude of the difference between memory for TBR and TBF negative scenes increased relative to the density of sleep spindle activity in Stage 2 sleep. This indicates that sleep spindles are associated with the discrimination of memory for salient items, specifically the preferential preservation of items cued TBR over those cued TBF.
Discussion
The present study examined the interaction and competition of salience cues through consolidation of information across a daytime nap. Previous studies have investigated whether emotional information would be able to be intentionally forgotten, given its inherent emotional salience, although findings have been equivocal. The majority of these studies have only examined memory shortly after learning. Our study is the first to explore the effect of sleep-based consolidation processes on the fate of this information in memory. We predicted that the salience cues of emotion and the task-related cue to intentionally remember or forget would result in a hierarchy of memory, with the most salient items (i.e. negative TBR) being remembered the best and the least salient (i.e., neutral TBF), the poorest. We found, however, that initially (at baseline test), the cue to remember or forget seemed to “win out”, being prioritized in memory as the more important cue to guide how the information was retained. Negative and neutral items were found to be equally recognized within TBR and TBF designations. Therefore, we can confirm that, at least at baseline performance immediately after encoding, we observed the ability to intentionally forget emotional information.
When tracking the consolidation of this material across the retention period across nap and wake conditions, we found that in addition to memory for TBR items being preferentially preserved, emotional salience also emerged as a cue that influenced consolidation processes. Previous research has demonstrated that the impact of emotion on memory consolidation evolves over time. While the initial effects of arousal in response to emotional salience may be minimal, these effects often become more apparent when memory is tested after longer delays (for review, see Kensinger, 2009). Ritchey and colleagues (2008) examined brain activity during encoding of emotionally negative and neutral images and assessed performance after 20 minutes and again after 1 week. They determined that the persistence of negative memories over time was greater than neutral memories, and that amygdala activity predicted this negative memory better than neutral. Importantly, the contribution of the connectivity between the amygdala and the MTL to subsequent negatively salient memories increased over time. The refinement of this interaction between the amygdala, MTL, as well as the PFC representing the emotional memory system has been shown to benefit from sleep (Payne & Kensinger, 2011). While we did not see significant differences between wake and nap conditions in the gradual enhancement of emotional memories over neutral one in the current nap study, it may have become evident with more time and more (perhaps nocturnal) sleep.
As predicted and supported by a wealth of prior research, the results demonstrated that napping facilitated the preservation of information overall, with significantly greater decay in memory across wakefulness. Beyond merely observing an overarching benefit for memory, there was also a more nuanced benefit of napping. We hypothesized that sleep would preferentially consolidate the most salient information (i.e., emotional TBR item memory) to a greater extent than other information. Similar to Saletin et al. (2011), we calculated a measure of the difference between TBR and TBF items for each valence, called the R-F difference score. We anticipated that preferential consolidation during sleep would essentially increase the magnitude of this R-F score, selectively preserving TBR memories while furthering the forgetting of TBF items. For neutral items, the R-F difference increased incrementally and similarly between all conditions. However, for negative memory, both the immediate and delayed naps produced greater increases in the R-F difference, while this difference appeared to diminish over a period of wake. These findings indicate that memory for negative TBR items, the most salient information as a function of the additive salience cues of emotion and an instruction to remember, is prioritized during sleep. With wakefulness, however, there appears to be more forgetting of these more salient items as well as less ability to intentionally forget the negative items, which led to the decrease in R-F difference over time. We do caution, though, that these results, while interesting and based on our a priori hypotheses, were found in post hoc analyses on a trending, yet not significant, interaction.
This particular interaction of emotional salience and intentional forgetting has not previously been examined over a sleep. However, the sleep-based effects are similar conceptually to literature regarding the emotional memory trade-off effect. This type of emotion-driven memory trade-off occurs when memory for the emotionally salient focus of an experience is preferentially preserved while memory for neutral, contextual detail is forgotten or suppressed (Kensinger et al., 2007). Importantly, the magnitude of the emotional memory trade-off has been shown to increase over a period of sleep through selective consolidation of salient components (Payne et al., 2008a; Payne & Kensinger, 2011; Payne et al., 2015, Alger et al., 2018). This effect is much like the one we observed in which there is a trade-off in memory for negative cued TBR items at the expense of memory for TBF items, which increases across sleep compared to wakefulness. This, in turn, effectively increased the signal (TBR memory) to noise (TBF memory) retention ratio.
We also predicted that napping immediately after encoding would result in greater preferential consolidation compared to delaying sleep due to memory decline from interference, although both nap conditions were predicted to preserve memory more than wakefulness. This hypothesis was partially supported in that napping, overall, did indeed lead to superior preservation, particularly of the more salient information, compared to wakefulness. However, we did not see any significant differences between our nap conditions, despite the altered proximity of the naps to learning. The delayed nap condition showed numerically more forgetting across all stimuli types, but this was not significantly different from the amount of forgetting in the immediate nap condition.
Previous research using immediate and delayed naps have also found similar performance between naps (Lau et al., 2011), and even increased memory with a delayed nap later in the day that contained more SWS due to increased homeostatic sleep pressure. This increased amount of SWS in the delayed nap was thought to actively facilitate declarative memory consolidation (Alger et al., 2010). Unexpectedly, in the present study, comparison of nap architecture in two nap conditions did not reveal any significant differences. While participants in the delayed nap did spend greater than 5% more of the nap in SWS than the earlier immediate nap, this difference was not significant and cannot explain why we did not see more of a decrease in memory in the late nap condition. One potential reason we did not see increased SWS in the late nap is because we sampled from a wider age range from the greater community, not focusing on the commonly used narrow population of young undergraduate university students. Previous research has demonstrated that intensive learning, such as that experienced daily by a typical undergraduate student, results in a more pronounced increase in SWS over time (Tononi & Cirelli, 2003, Huber et al., 2004; Eschenko et al., 2008). This increased slow wave activity satisfies a homeostatic need to downscale membrane potentials to baseline levels and increase synaptic plasticity. We may not have seen a significant difference in sleep architecture in the current study as we sampled from a non-student population who likely has more stable sleep habits and does not typically experience intensive learning. Future studies will need to replicate the lack of this shift in sleep architecture we observed in the current study to delve into the possible reasons behind it.
The findings of this study make it clear that napping played a critical role in preserving information and that particular sleep physiology during the naps actively facilitated selective consolidation of different aspects of memory. We hypothesized that sleep physiology, specifically sleep spindle activity and SWS, would actively promote consolidation of salient information. Similar in concept to our previous research (Payne et al., 2015; Alger et al., 2018), we found that the percentage of time spent in SWS during both naps was associated with greater selective consolidation of negatively salient information. This relationship between SWS and negative memory consolidation may be due to transient firing of neurons in the locus coeruleus during SWS (Eschenko & Sara, 2008), and to interactions between the hippocampus and amygdala during this sleep stage. This activity leads to the release of norepinephrine (NE), which can lead to concurrent activation of the amygdala and hippocampus through NE pathways (Strange & Dolan, 2004). The amygdala modulates activity in the hippocampus, leading to stronger activation of the emotional memories and preferential consolidation of this information during SWS.
Although emotional memory has been thought to benefit largely from REM sleep (Hu et al., 2006; Nishida et al., 2009), recent evidence contradicts this idea (Groch et al., 2011), demonstrating that emotional memory consolidation is not limited to processing during REM sleep. In our previous studies (Payne et al., 2015; Alger et al., 2018) exploring consolidation of emotional components of complex information in the emotional memory trade-off paradigm, we found that memory for negative objects was related to the amount of SWS in the nap. Interestingly, REM sleep was found to be related to the same information in an overnight study. Our current findings support the idea that perhaps daytime sleep contributes to emotional memory consolidation differently than nocturnal sleep, leading us to believe that other factors, such as neurohormones and circadian rhythms may interact with sleep physiology to influence consolidation. While we have replicated the relationship between SWS and selective consolidation of emotional memory, more research is needed to further explore this relationship.
Furthermore, we found that sleep spindle activity played a role in discriminately consolidating the most salient information at the expense of other information. Spindle density and average duration of spindles within Stage 2 sleep were related to an increased magnitude of the difference in memory between items that were cued TBR over those cued TBF. Interestingly, this was only found for the negative items, thus related to preferentially preserving negative TBR items while increasing the forgetting of TBF items. We did not find a significant inverse relationship between sleep spindles and TBR/TBF items, such that spindles were associated with increased TBR and decreased TBF items independently, as was found by Saletin et al. (2011). They, however, did find a similar relationship as we did between spindles and the R-F difference. We note that we cannot directly compare our results to those of Saletin et al., as they found a significant relationship between memory and fast spindles (13.5–15 Hz) at parietal locations. We did not record sleep from parietal leads and we analyzed the full range of spindle activity rather than fast versus slow activity. In doing so, the relationship between spindles and memory appears to be stronger over the frontal cortex, rather than parietal cortex. As this type of selective consolidation of information involving the interaction between salience cues is novel, further research will need to confirm the associations that were found in the current study.
In conclusion, the present study is novel in examining the individual contributions and interaction of emotional salience and task-related salience imposed on information through the explicit direction to either remember or forget information across a daytime nap. Intentional forgetting of both emotional and neutral information was revealed immediately post-encoding, with the task direction to actively remember or forget dominating over inherent cues to preferentially remember emotional information. Emotional salience perhaps acted more slowly in influencing amygdala activity, which, in turn, may have modulated hippocampal activity, leading to less forgetting of negative information across time compared to neutral information. While memory declined significantly across the board in those who remained awake, sleeping led to discriminatory consolidation, with selective preservation of negative TBR item memory at the expense of other information. Non-REM sleep physiology, specifically sleep spindles during Stage 2 sleep and the amount of SWS obtained in the nap, actively contributed to preferential consolidation of different aspects of the salient information.
Highlights.
Intention to forget was prioritized as a cue for memory formation, regardless of emotional salience.
Napping preferentially preserved the most salient information over less important information.
Slow wave sleep was associated with better memory for negative scenes.
Sleep spindles were related to greater discrimination between negative to-be-remembered and -forgotten items.
Acknowledgments
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Number F32AG047807. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Internal funding from Notre Dame was also provided by the Institute for Scholarship in the Liberal Arts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alger SE, Lau H, Fishbein W. Delayed onset of a daytime nap facilitates retention of declarative memory. PLoS One. 2010;5(8):e12131. doi: 10.1371/journal.pone.0012131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger SE, Kensinger EA, Payne JD. Preferential consolidation of emotionally salient information during a nap is preserved in middle Age. Neurobiology of Aging. 2018 doi: 10.1016/j.neurobiolaging.2018.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MC, Hanslmayr S. Neural mechanisms of motivated forgetting. Trends in cognitive sciences. 2014;18(6):279–292. doi: 10.1016/j.tics.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MC, Ochsner KN, Kuhl B, Cooper J, Robertson E, Gabrieli SW, … Gabrieli JD. Neural systems underlying the suppression of unwanted memories. Science. 2004;303(5655):232–235. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- Basner M, Dinges DF. An adaptive-duration version of the PVT accurately tracks changes in psychomotor vigilance induced by sleep restriction. Sleep. 2012;35(2):193–202. doi: 10.5665/sleep.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. Journal of consulting and clinical psychology. 1988;56(6):893. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring. Archives of general psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Born J, Rasch B, Gais S. Sleep to remember. The Neuroscientist. 2006;12(5):410–424. doi: 10.1177/1073858406292647. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience. 1989;31(3):551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of neuroscience methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nature Reviews Neuroscience. 2010;11(2):114. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behavior research methods, instruments, & computers. 1985;17(6):652–655. [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42(5):855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Dorrian J, Rogers NL, Dinges DF. Doctoral dissertation. Marcel Dekker; 2005. Psychomotor vigilance performance: Neurocognitive assay sensitive to sleep loss. [Google Scholar]
- Eschenko O, Sara SJ. Learning-dependent, transient increase of activity in noradrenergic neurons of locus coeruleus during slow wave sleep in the rat: Brain stem–cortex interplay for memory consolidation? Cerebral Cortex. 2008;18(11):2596–2603. doi: 10.1093/cercor/bhn020. [DOI] [PubMed] [Google Scholar]
- Eschenko O, Ramadan W, Mölle M, Born J, Sara SJ. Sustained increase in hippocampal sharp-wave ripple activity during slow-wave sleep after learning. Learning & Memory. 2008;15(4):222–228. doi: 10.1101/lm.726008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarelli F, Huber R, Peterson MJ, Massimini M, Murphy M, Riedner BA, … Tononi G. Reduced sleep spindle activity in schizophrenia patients. American Journal of Psychiatry. 2007;164(3):483–492. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- Genzel L, Spoormaker VI, Konrad BN, Dresler M. The role of rapid eye movement sleep for amygdala-related memory processing. Neurobiology of learning and memory. 2015;122:110–121. doi: 10.1016/j.nlm.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Girardeau G, Inema I, Buzsáki G. Reactivations of emotional memory in the hippocampus–amygdala system during sleep. Nature neuroscience. 2017;20(11):1634. doi: 10.1038/nn.4637. [DOI] [PubMed] [Google Scholar]
- Groch S, Wilhelm I, Diekelmann S, Sayk F, Gais S, Born J. Contribution of norepinephrine to emotional memory consolidation during sleep. Psychoneuroendocrinology. 2011;36(9):1342–1350. doi: 10.1016/j.psyneuen.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Hamann S. Cognitive and neural mechanisms of emotional memory. Trends in cognitive sciences. 2001;5(9):394–400. doi: 10.1016/s1364-6613(00)01707-1. [DOI] [PubMed] [Google Scholar]
- Hauswald A, Schulz H, Iordanov T, Kissler J. ERP dynamics underlying successful directed forgetting of neutral but not negative pictures. Social Cognitive and Affective Neuroscience. 2010;6(4):450–459. doi: 10.1093/scan/nsq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans EJ, Battaglia FP, Atsak P, de Voogd LD, Fernández G, Roozendaal B. How the amygdala affects emotional memory by altering brain network properties. Neurobiology of Learning and Memory. 2014;112:2–16. doi: 10.1016/j.nlm.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10(4):431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Horne JA, Östberg O. A self-assessment questionnaire to determine morningness- eveningness in human circadian rhythms. International journal of chronobiology. 1976 [PubMed] [Google Scholar]
- Hu P, Stylos-Allan M, Walker MP. Sleep facilitates consolidation of emotional declarative memory. Psychological Science. 2006;17(10):891–898. doi: 10.1111/j.1467-9280.2006.01799.x. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430(6995):78. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Kensinger EA. What factors need to be considered to understand emotional memories? Emotion Review. 2009;1(2):120–121. doi: 10.1177/1754073908100436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Phelps EA. Arousal-mediated memory consolidation: Role of the medial temporal lobe in humans. Psychological Science. 1998;9(6):490–493. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Technical manual and affective ratings. NIMH Center for the Study of Emotion and Attention. 1997:39–58. [Google Scholar]
- Lau H, Alger SE, Fishbein W. Relational memory: a daytime nap facilitates the abstraction of general concepts. PloS one. 2011;6(11):e27139. doi: 10.1371/journal.pone.0027139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PA, Durrant SJ. Overlapping memory replay during sleep builds cognitive schemata. Trends in cognitive sciences. 2011;15(8):343–351. doi: 10.1016/j.tics.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Marchewka A, Wypych M, Michałowski JM, Sińczuk M, Wordecha M, Jednoróg K, Nowicka A. What is the effect of basic emotions on directed forgetting? Investigating the role of basic emotions in memory. Frontiers in human neuroscience. 2016;10:378. doi: 10.3389/fnhum.2016.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchewka A, Żurawski Ł, Jednoróg K, Grabowska A. The Nencki Affective Picture System (NAPS): Introduction to a novel, standardized, wide-range, high-quality, realistic picture database. Behavior research methods. 2014;46(2):596–610. doi: 10.3758/s13428-013-0379-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Sutherland MR. Arousal-biased competition in perception and memory. Perspectives on psychological science. 2011;6(2):114–133. doi: 10.1177/1745691611400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecklinger A, Parra M, Waldhauser GT. ERP correlates of intentional forgetting. Brain research. 2009;1255:132–147. doi: 10.1016/j.brainres.2008.11.073. [DOI] [PubMed] [Google Scholar]
- Murty VP, Tompary A, Adcock RA, Davachi L. Selectivity in postencoding connectivity with high-level visual cortex is associated with reward-motivated memory. Journal of Neuroscience. 2017;37(3):537–545. doi: 10.1523/JNEUROSCI.4032-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M, Pearsall J, Buckner RL, Walker MP. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cerebral cortex. 2008;19(5):1158–1166. doi: 10.1093/cercor/bhn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicka A, Marchewka A, Jednorog K, Tacikowski P, Brechmann A. Forgetting of emotional information is hard: an fMRI study of directed forgetting. Cerebral Cortex. 2010;21(3):539–549. doi: 10.1093/cercor/bhq117. [DOI] [PubMed] [Google Scholar]
- Otani H, Libkuman TM, Goernert PN, Kato K, Migita M, Freehafer SE, Landow MP. Emotion, directed forgetting, and source memory. British Journal of Psychology. 2012;103(3):343–358. doi: 10.1111/j.2044-8295.2011.02078.x. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Winson J. Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes. Journal of Neuroscience. 1989;9(8):2907–2918. doi: 10.1523/JNEUROSCI.09-08-02907.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JD. Learning, memory, and sleep in humans. Sleep Medicine Clinics. 2011;6(1):15–30. [Google Scholar]
- Payne JD, Kensinger EA. Sleep’s role in the consolidation of emotional episodic memories. Current Directions in Psychological Science. 2010;19(5):290–295. [Google Scholar]
- Payne JD, Kensinger EA. Sleep leads to changes in the emotional memory trace: evidence from FMRI. Journal of Cognitive Neuroscience. 2011;23(6):1285–1297. doi: 10.1162/jocn.2010.21526. [DOI] [PubMed] [Google Scholar]
- Payne JD, Kensinger EA. Stress, sleep, and the selective consolidation of emotional memories. Current Opinion in Behavioral Sciences. 2018;19:36–43. [Google Scholar]
- Payne JD, Stickgold R, Swanberg K, Kensinger EA. Sleep preferentially enhances memory for emotional components of scenes. Psychological Science. 2008a;19(8):781–788. doi: 10.1111/j.1467-9280.2008.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JD, Ellenbogen JM, Walker MP, Stickgold R. Learning and Memory: A comprehensive reference. New York: Elsevier; 2008b. The role of sleep in memory consolidation. [Google Scholar]
- Payne JD, Chambers AM, Kensinger EA. Sleep promotes lasting changes in selective memory for emotional scenes. Frontiers in Integrative Neuroscience. 2012;6 doi: 10.3389/fnint.2012.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JD, Kensinger EA, Wamsley EJ, Spreng RN, Alger SE, Gibler K, … Stickgold R. Napping and the selective consolidation of negative aspects of scenes. Emotion. 2015;15(2):176. doi: 10.1037/a0038683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Current opinion in neurobiology. 2004;14(2):198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Popa D, Duvarci S, Popescu AT, Léna C, Paré D. Coherent amygdalocortical theta promotes fear memory consolidation during paradoxical sleep. Proceedings of the National Academy of Sciences. 2010;107(14):6516–6519. doi: 10.1073/pnas.0913016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauchs G, Feyers D, Landeau B, Bastin C, Luxen A, Maquet P, Collette F. Sleep contributes to the strengthening of some memories over others, depending on hippocampal activity at learning. Journal of Neuroscience. 2011;31(7):2563–2568. doi: 10.1523/JNEUROSCI.3972-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A manual of standardized terminology. Techniques and scoring system for sleep stages of human subjects. 1968:1–350. doi: 10.1046/j.1440-1819.2001.00810.x. [DOI] [PubMed] [Google Scholar]
- Ritchey M, Dolcos F, Cabeza R. Role of amygdala connectivity in the persistence of emotional memories over time: An event-related fMRI investigation. Cerebral Cortex. 2008;18(11):2494–2504. doi: 10.1093/cercor/bhm262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizio AA, Dennis NA. The neural correlates of cognitive control: successful remembering and intentional forgetting. Journal of Cognitive Neuroscience. 2013;25(2):297–312. doi: 10.1162/jocn_a_00310. [DOI] [PubMed] [Google Scholar]
- Saletin JM, Goldstein AN, Walker MP. The role of sleep in directed forgetting and remembering of human memories. Cerebral Cortex. 2011;21(11):2534–2541. doi: 10.1093/cercor/bhr034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabus M, Dang-Vu TT, Albouy G, Balteau E, Boly M, Carrier J, … Phillips C. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proceedings of the National Academy of Sciences. 2007;104(32):13164–13169. doi: 10.1073/pnas.0703084104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. Journal of Experimental Psychology: General. 1988;117(1):34. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. State-Trait anxiety inventory. John Wiley & Sons, Inc; 2010. [Google Scholar]
- Stickgold R, Walker MP. Sleep-dependent memory triage: evolving generalization through selective processing. Nature neuroscience. 2013;16(2):139. doi: 10.1038/nn.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Dolan RJ. β-Adrenergic modulation of emotional memory-evoked human amygdala and hippocampal responses. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(31):11454–11458. doi: 10.1073/pnas.0404282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talamini LM, Nieuwenhuis IL, Takashima A, Jensen O. Sleep directly following learning benefits consolidation of spatial associative memory. Learning & memory. 2008;15(4):233–237. doi: 10.1101/lm.771608. [DOI] [PubMed] [Google Scholar]
- Tamminen J, Payne JD, Stickgold R, Wamsley EJ, Gaskell MG. Sleep spindle activity is associated with the integration of new memories and existing knowledge. Journal of Neuroscience. 2010;30(43):14356–14360. doi: 10.1523/JNEUROSCI.3028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin DF, Abramowitz JS, Przeworski A, Foa EB. Thought suppression in obsessive-compulsive disorder. Behaviour Research and Therapy. 2002;40(11):1255–1274. doi: 10.1016/s0005-7967(01)00095-x. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain research bulletin. 2003;62(2):143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Tucker MA, Fishbein W. Enhancement of declarative memory performance following a daytime nap is contingent on strength of initial task acquisition. Sleep. 2008;31(2):197–203. doi: 10.1093/sleep/31.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Werf YD, Van Der Helm E, Schoonheim MM, Ridderikhoff A, Van Someren EJ. Learning by observation requires an early sleep window. Proceedings of the National Academy of Sciences. 2009;106(45):18926–18930. doi: 10.1073/pnas.0901320106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learning & Memory. 2001;8(2):112–119. doi: 10.1101/lm.36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of personality and social psychology. 1988;54(6):1063. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wylie GR, Foxe JJ, Taylor TL. Forgetting as an active process: an fMRI investigation of item-method–directed forgetting. Cerebral Cortex. 2007;18(3):670–682. doi: 10.1093/cercor/bhm101. [DOI] [PubMed] [Google Scholar]
- Yang W, Liu P, Xiao X, Li X, Zeng C, Qiu J, Zhang Q. Different neural substrates underlying directed forgetting for negative and neutral images: An event- related potential study. Brain research. 2012;1441:53–63. doi: 10.1016/j.brainres.2011.10.042. [DOI] [PubMed] [Google Scholar]
- Yang T, Lei X, Anderson M. Decreased inhibitory control of negative information in directed forgetting. International Journal of Psychophysiology. 2016;100:44–51. doi: 10.1016/j.ijpsycho.2015.09.007. [DOI] [PubMed] [Google Scholar]