Abstract
Peripheral blood C-reactive protein (CRP) is a biomarker used clinically to measure systemic inflammation and is reproducibly increased in a subset of patients with major depressive disorder (MDD). Furthermore, increased peripheral blood CRP in MDD has been associated with altered reward circuitry and increased brain glutamate in relation with symptoms of anhedonia. Nevertheless, the relationship between peripheral CRP and other peripheral and central markers of inflammation in depressed patients has not been established. Plasma (n=89) and CSF (n=73) was collected from medically-stable, currently-unmedicated adult outpatients with MDD. Associations among plasma and CSF CRP and plasma and CSF inflammatory cytokines (interleukin [IL]-6, tumor necrosis factor [TNF] and IL-1beta) and their soluble receptors/antagonists were examined. Relationships between plasma and CSF inflammatory markers and depressive symptoms including anhedonia and reduced motivation (RM) were also explored. Plasma CRP was correlated with multiple plasma inflammatory markers (all p<0.05), and a strong correlation was found between plasma and CSF CRP (r=0.855, p<0.001). CSF CRP in turn correlated with CSF cytokine receptors/antagonists (all p<0.05). Principal component analyses revealed clusters of CSF inflammatory markers that were associated with high plasma CRP (>3mg/L) and correlated with depressive symptom severity. These findings were driven by CSF TNF, which correlated with RM (r=0.236, p=0.045), and CSF IL-6 soluble receptor, which correlated with anhedonia (r=0.301, p=0.010) in the sample as a whole and particularly females. CRP appears to be a peripheral biomarker that reflects peripheral and central inflammation and seems well-suited for guiding immunotherapies targeting TNF and IL-6 in patients with MDD.
Introduction
There has been a relative explosion of studies investigating the role of inflammation in psychiatric illnesses, and especially major depressive disorder (MDD).1,2 Patients with MDD reliably exhibit increased peripheral blood concentrations of the acute phase reactant C-reactive protein (CRP) and other markers of inflammation including the innate immune cytokines interleukin (IL)-1, IL-6 and tumor necrosis factor (TNF) and their soluble receptors/antagonists.1,3,4 Elevated CRP and other peripheral blood markers of inflammation have been found to predict future development of depression,5–7 as well as resistance to standard antidepressant therapies.8–10 Moreover, clinical trials using anti-cytokine therapies for MDD have targeted patients based on elevated peripheral blood CRP concentrations.2
Peripheral blood CRP is an acute phase reactant produced by the liver in response to innate immune cytokines, notably IL-6 and TNF, and is involved in the inflammatory response.11,12 CRP is also used in clinical practice as a biomarker of systemic inflammation and is routinely measured across medical centers and research laboratories.13,14 A multitude of epidemiologic studies in large populations have shown that increased concentrations of peripheral blood CRP are associated with greater risk for a number of medical illnesses that have been associated with MDD such as cardiovascular disease, metabolic disorders and diabetes. In addition, a recent clinical trial found that anti-IL-1beta treatment was successful in reducing cardiac complications in patients with a previous myocardial infarction and elevated CRP.15 Despite the prominence of peripheral CRP as a viable marker of peripheral inflammation, little is known about how increased concentrations of CRP in the blood relate to inflammation in the brain.16,17
The American Heart Association considers peripheral CRP >3mg/L, e.g. ‘high CRP,’ to be associated with the greatest risk for development of cardiovascular disease relative to concentrations considered low (<1mg/L) and moderate (1–3mg/L).18 These guidelines for risk of heart disease are consistent with emerging evidence that inflammation serves as a common mechanism of disease affecting multiple bodily systems, including the brain.19 Although not every patient with MDD exhibits high inflammatory markers, evidence suggests that ‘high’ CRP (>3mg/L) is detected in a substantial proportion of patients (~25–45% depending on the sample).20–24 Recent data indicate that increasing plasma CRP concentrations in patients with MDD is also associated with decreased functional connectivity within reward circuitry and with high CNS glutamate, which correlated with symptoms of anhedonia.25,26 These findings in MDD patients with high plasma CRP are strikingly similar to the effects of peripheral administration of inflammatory stimuli on neural activity in reward circuitry and CNS neurotransmitters such as glutamate and dopamine, and may reflect activity of inflammatory mediators in the CNS.27–31 Although peripheral blood CRP serves as an excellent marker of peripheral inflammation with potential implications for both research and clinical care, critical knowledge is lacking regarding relationships between peripheral CRP and other inflammatory markers in both the periphery and CNS of patients with MDD.
Several studies have observed increased inflammatory cytokines in cerebrospinal fluid (CSF) of MDD patients and presumably depressed suicide attempters,32–34 yet relationships between CRP and other inflammatory markers in the periphery and CNS of patients with MDD remain unclear. CRP and cytokines are large molecules (~15–25kd) that do not freely cross the blood-brain barrier (BBB), but can access the CNS and activate local inflammatory pathways through several routes: 1) passage through leaky regions of the BBB; 2) active uptake; 3) activation of endothelial cells and immune cells in cerebral vasculature that release inflammatory mediators into brain parenchyma; and 4) binding to cytokine receptors on peripheral nerve afferents (e.g., vagus nerve), which then relay cytokine signals to brain.35–37 Due to the complexity of transmission of inflammatory signals from periphery to brain, it is unknown whether elevated CRP and other inflammatory biomarkers in plasma are mirrored in CSF, or whether peripheral inflammatory signals in MDD drive a unique profile of inflammatory activity in the CNS.
Given the paucity of data on the relationship between peripheral blood CRP and other markers of inflammation in the periphery and CNS, we explored relationships between plasma and CSF concentrations of CRP and inflammatory cytokines and their receptors/antagonists in medically-stable, currently-unmedicated outpatients with MDD, and examined their relationship with depressive symptoms, particularly those related to anhedonia and reduced motivation (RM).25,26,38 The ratio of albumin in CSF to plasma was also examined as a marker of BBB integrity.32,39
Methods
Participants and Eligibility Criteria
Eighty-nine male and female participants (aged 21–65) recruited through local media outlets and mental health providers had a primary diagnosis of MDD, or bipolar disorder-current episode depressed (n=6), determined by Structured Clinical Interview for Diagnostic and Statistical Manual-IV (SCID-IV).40 Subjects had a 17-Item Hamilton Rating Scale for Depression41 score ≥18 and were currently free of psychotropic medications, medications that affect the immune system and drugs of abuse. Other medications were allowed per patients’ treating physicians. Medical illness affecting the immune system were exclusionary, but other disorders were allowed as long as patients were medically-stable, as determined by medical history, physical exam and laboratory testing. All patients were carefully monitored for safety (e.g. suicidality, significant worsening) during the course of the study. See Supplemental Methods for details on psychiatric and medical exclusions. Screening peripheral blood CRP was assessed by Emory University Hospital Clinical Laboratory over two visits spaced 1–4 weeks apart. Measurements >10mg/L were repeated at ~2-week intervals to ensure stability (within 25%). Participants in this sample overlap with those of published studies on inflammation effects on cognition or neuroimaging outcomes.25,26,42 All procedures were a priori approved by Emory University Institutional Review Board, and all participants provided written informed consent.
Study procedures
To minimize circadian variations, blood was collected Day 1 at 0900±1h and behavior was collected at 1100±1h. Plasma was isolated from EDTA-blood (collected by indwelling catheter after ≥30 minutes of rest) by centrifugation (1000g for 15-minutes at 4°C) and stored at −80°C until batched assay. Seventy-three of 89 patients agreed to undergo lumbar puncture, conducted on Day 2 between 1200 and 1600h by trained physicians. After discarding initial 1cc to avoid blood contamination, 10cc of CSF was collected into chilled tubes, aliquoted at 1cc and frozen at -80°C until batched assay.
Behavioral assessments
Symptoms of depression, including anhedonia and RM, were measured by the Inventory of Depressive Symptomatology-Self-Reported (IDS-SR)43 and the 20-item Multidimensional Fatigue Inventory (MFI) self-report.44 Anhedonia was assessed as previously reported using a 3-item subscale of the IDS-SR25,45 and RM was assessed by the RM subscale of MFI.
Measurement of inflammatory biomarkers
Plasma concentrations of high sensitivity CRP, and plasma and CSF albumin, were determined by immunoturbidometric method (Sekisui Diagnostics, Lexington, MA) on a Beckman AU480 automatic analyzer.21,25,26 Due to enhanced sensitivity compared to the immunoturbidometric method, CRP in CSF was quantified by electrochemiluminescence-based immunoassay (MesoScale Discovery, Gaithersburg, MD).46 CRP in plasma was also measured on the this platform to confirm consistency with the immunoturbidometric method. Plasma and CSF cytokines (IL-6, TNF, IL-1beta) and their soluble receptors/antagonists (soluble TNF receptor 2 [sTNFR2], IL-1 receptor antagonist [IL-1ra] and IL-6 soluble receptor [IL-6sr]), which are reliably associated with depression and also fatigue in breast cancer,1,3,47–49 were assessed in duplicate (plasma) and triplicate (CSF) using multiplex bead-based assays (R&D Systems, Minneapolis, MN) analyzed with MAGPIX CCD imaging system (Luminex, Austin, TX).25,26,50–53. Mean inter- and intra-assay coefficients of variation were ≤10%. See Supplementary Table 1 for detection limits.
Statistics
Clinical characteristics were summarized using mean and standard deviation (SD) for continuous variables and percent for categorical variables. Although the sample size exceeded criteria for central limit theorem,54 non-parametric statistics were reported for any correlation where at least one (y) variable was not normally distributed. To improve normality for parametric statistical modeling and consistent with previous analyses, immune markers were natural log (ln) transformed and extreme values (mean +/-3 SDs) were excluded.23,55–57 Associations between CRP, inflammatory cytokines and their receptors/antagonists in plasma and CSF as well as the CSF to plasma albumin ratio and behavior were assessed using Pearson’s r or Spearman correlation coefficient where indicated. The Benjamini–Hochberg step-up procedure was used to control for multiple comparisons. Significant relationships between plasma and CSF CRP and other inflammatory markers were assessed in linear regression models with clinical covariates that may modify relationships between central and peripheral inflammation, including CSF/plasma albumin, age, sex, race, and body mass index (BMI). To determine whether patients with high versus low plasma CRP (>versus≤3mg/L) exhibited an increase in other inflammatory markers in plasma or CSF, Z scores for cytokines and their soluble receptors/antagonists were compared between groups by general linear model, with and without covariates. Principal components analysis (PCA) was used to identify clusters of CSF inflammatory markers that were associated with high plasma CRP (>3mg/L). Varimax rotation with Kaiser Normalization was used to simplify factor structure, as determined by Eigen values >1, and only individual variable contributions of >0.3 qualified for loading components.58 In exploratory analyses, resulting Bartlett factor scores were correlated with IDS-SR and MFI scores. Individual inflammatory markers from components that significantly predicted behavioral scores were entered into backward and forward linear regression models using the same criteria for entry and removal that included clinical covariates to identify markers that were most significantly associated with IDS-SR and MFI scores, and subsequently their subscales of anhedonia and RM. Finally, whether CRP in plasma and CSF associated with scores over the median (≥6) on the anhedonia subscale was explored by linear and logistic regression. Tests of significance were two-tailed, conducted in IBM SPSS Statistics 24, and medium effect sizes (r=~0.3) could be detected with β=0.80 and α<0.05.
Results
Patient characteristics and biomarker concentrations
Demographic and clinical characteristics and biomarker data of the study sample are presented in Table 1. No differences were observed between patients providing both plasma and CSF samples (n=73) and those providing plasma only (n=89) (see Supplementary Table 2). BMI was the only clinical or demographic variable associated with plasma CRP (r=0.428, p<0.001), consistent with relationships between adiposity, inflammation and depression.59 To achieve normality for parametric analyses, in plasma one extreme value (mean +/-3 SDs) was excluded for CRP (immunoturbidometric), TNF, IL-1beta and IL-6sr, whereas two extreme values for IL-1ra and CRP (electrochemilumiminescence) and three values for IL-6 were excluded. In CSF, one value each was excluded for IL-6, IL-1beta, IL-6sr and sTNFR2, and two values were excluded for CRP (electrochemilumiminescence) and IL-1ra. IL-1beta was not detected in one CSF sample. Of note, plasma CRP measured by immunoturbidometric and electrochemiluminescence methods were highly correlated (r=0.950, df=84, p<0.001). Because the immunoturbidometric method is the clinical standard for measurement of CRP that is routinely used in CLIA-certified laboratories across the United States, it was chosen for subsequent statistical analysis.13,14
Table 1.
Demographic, clinical and biomarker variables of the study sample.
| Variable | Mean, SD |
|---|---|
| Demographic and Clinical | |
| Age (years) | 42.1 (11.1) |
| Sex, Male (n, %) | 31 (35) |
| Race | |
| Caucasian (n, %) | 38 (43) |
| African American (n, %) | 51 (57) |
| BMI (kg/m2) | 27.5 (7.0) |
| ATQR - previous medication in current episode (n, %) | 33 (37.1) |
| IDS-SR Score | 39.2 (9.1) |
| Plasma Biomarkers | |
| CRP (mg/L) | 2.191 ± 2.408 |
| IL-6 (pg/ml) | 1.466 ± 0.782 |
| TNF (pg/ml) | 5.363 ± 2.049 |
| IL-1β (pg/ml) | 0.406 ± 0.167 |
| IL-6sr (ng/ml) | 15.682 ± 2.781 |
| sTNFR2, plasma (ng/ml) | 2.417 ± 0.779 |
| IL-1ra, plasma (ng/ml) | 1.491 ± 1.620 |
| Albumin, plasma (g/dl) | 3.328 ± 0.242 |
| CSF Biomarkers | |
| CRP (mg/L) | 0.013 ± 0.017 |
| IL-6 (pg/ml) | 2.593 ± 1.340 |
| TNF (pg/ml) | 1.203 ± 0.404 |
| L-1β (pg/ml) | 0.317 ± 0.073 |
| IL-6sr (ng/ml) | 0.690 ± 0.328 |
| sTNFR2 (ng/ml) | 0.119 ± 0.597 |
| IL-1ra (ng/ml) | 0.006 ± 0.003 |
| Albumin (g/dl) | 0.031 ± 0.012 |
a - antagonist; ATRQ - Antidepressant Treatment Response Questionnaire; BMI - body mass index; CRP - high sensitivity C-reactive protein; CSF - cerebrospinal fluid; IDS-SR - Inventory of Depressive Symptomatology-Self Report; IL - interleukin; r - receptor; s - soluble; SD - standard deviation; TNF - tumor necrosis factor
Correlations of CRP and other inflammatory markers in plasma and CSF
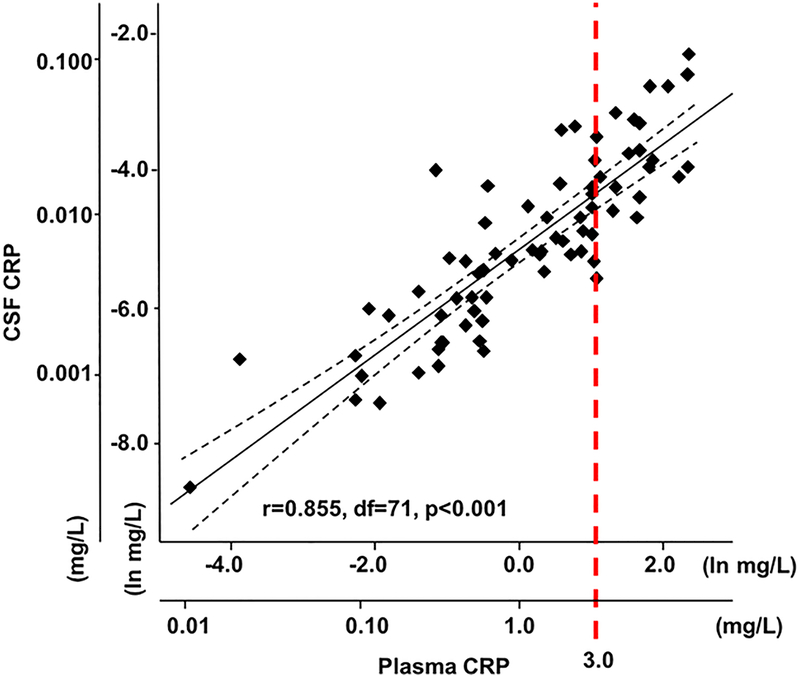
Plasma CRP (n=89) was significantly correlated with numerous other plasma inflammatory markers including IL-6, TNF, sTNFR2 and IL-1ra (r=0.232 to 0.537, all p<0.05 after correction for multiple comparisons) (Table 2). In addition, plasma TNF and IL-1beta were correlated with their respective receptors/antagonists, while TNF and IL-1ra correlated with all other markers in plasma (all r=0.232 to 0.522, all p<0.05), except for IL-1ra to IL-6sr. In terms of relationships between plasma and CSF inflammatory markers (n=73), there was a strong correlation between plasma CRP and CSF CRP (r=0.855, df=71, p<0.001) (Figure 1). Interestingly, CRP in CSF was further correlated with every other marker in plasma except for IL-1beta (r=0.297 to 0.442, all p<0.01) as well as with CSF concentrations of IL-6sr, sTNFR2 and IL-1ra (r=0.261 to 0.366, all p<0.05), with a trend for correlation with CSF IL-6 (r=0.205, p=0.081) (Table 2). After correction for multiple comparisons, plasma CRP remained significantly associated with CSF CRP, and CSF CRP remained significantly associated with CSF IL-6sr and sTNFR2 (adjusted p<0.05).
Table 2.
Correlations (r, p) between inflammatory markers in plasma vs. plasma (n=89), and plasma and CSF vs. CSF (n=73), in medically-stable, currently-unmedicated MDD patients.
| Matrix | Marker | Plasma (n=89) | ||||||
| CRP | IL-6 | TNF | IL-1β | IL-6sr | sTNFR2 | IL-1ra | ||
|
Plasma (n=89) |
CRP | - |
0.537† (<0.001) |
0.232† (0.029) |
0.150 (0.160) |
0.251† (0.018) |
0.354† (0.001) |
0.522† (<0.001) |
| IL-6 | - | - |
0.267† (0.011) |
−0.075 (0.485) |
0.258† (0.015) |
0.249† (0.019) |
0.410† (<0.001) |
|
| TNF | - | - | - | 0.217 (0.041) |
0.240† (0.023) |
0.554† (<0.001) |
0.316† (0.003) |
|
| IL-1β | - | - | - | - | −0.098† (0.359) |
0.060 (0.579) |
0.251† (0.018) |
|
| IL-6sr | - | - | - | - | - |
0.252† (0.017) |
0.158† (0.140) |
|
| TNFR2 | - | - | - | - | - | - |
0.354† (0.001) |
|
| Plasma (n=73) | ||||||||
|
CSF (n=73) |
CRP |
0.855 (<0.001) |
0.442 (<0.001) |
0.360 (0.002) |
0.202 (0.087) |
0.328 (0.005) |
0.297 (0.011) |
0.429 (<0.001) |
| IL-6 | 0.231† (0.050) |
0.175† (0.138) |
0.334† (0.004) |
0.118 (0.321) |
0.262† (0.025) |
0.180† (0.127) |
0.067† 0.574 |
|
| TNF | 0.025 (0.831) |
0.173 (0.144) |
−0.209 (0.076) |
−0.209 (0.076) |
0.141 (0.233) |
0.068 (0.567) |
0.028 (0.815) |
|
| IL-1β | 0.003† (0.878) |
−0.056† (0.640) |
0.041† (0.733) |
0.057 (0.632) |
−0.041† (0.729) |
−0.007† (0.953) |
−0.045† (0.704) |
|
| IL-6sr | 0.232 (0.048) |
0.273 (0.019) |
0.143 (0.229) |
−0.108 (0.365) |
0.418 (<0.001) |
0.154 (0.194) |
0.048 (0.689) |
|
| TNFR2 | 0.134 (0.258) |
0.119 (0.317) |
0.135 (0.257) |
−0.151 (0.201) |
0.238 (0.043) |
0.270† (0.021) |
−0.018 (0.881) |
|
| IL-1ra | 0.183† (0.122) |
0.181† (0.125) |
0.230† (0.051) |
−0.081 (0.496) |
0.273† (0.019) |
0.235† (0.056) |
0.298† (0.010) |
|
| Matrix | Marker | CSF (n=73) | ||||||
| CRP | IL-6 | TNF | IL-1β | IL-6sr | sTNFR2 | |||
|
CSF (n=73) |
IL-6 | 0.205 (0.081) |
- | - | - | - | - | |
| TNF | −0.023 (0.849) |
0.271 (0.021) |
- | - | - | - | ||
| IL-1β | −0.047 (0.690) |
0.225† (0.055) |
0.306† (0.008) |
- | - | - | ||
| IL-6sr |
0.366 (0.001) |
0.218 (0.070) |
0.131 (0.274) |
−0.182 (0.124) |
- | - | ||
| TNFR2 |
0.330 (0.004) |
0.249 (0.038) |
0.233 (0.049) |
−0.138 (0.244) |
0.800 (<0.001) |
- | ||
| IL-1ra | 0.261† (0.026) |
0.242† (0.039) |
0.111 (0.358) |
−0.009† (0.938) |
0.535 (<0.001) |
0.462 (<0.001) |
||
Data are presented as Pearson’s correlation coefficient (r) unless otherwise indicated, followed by p-value in parentheses. Relationships that remained significant after correction for multiple comparisons (p<0.05) appear in bold.
Spearman’s rho. a - antagonist; CRP - high sensitivity C-reactive protein; CSF - cerebrospinal fluid; IL - interleukin; MDD - major depressive disorder; r - receptor; s - soluble; TNF - tumor necrosis factor; vs. - versus
Figure 1. Correlation between CRP in plasma and CRP in CSF of MDD patients.
The concentrations of high sensitivity CRP in plasma were highly correlated with concentrations of CRP in the CSF of currently-unmedicated, medically-stable MDD patients. Please note that this relationship between plasma and CSF concentrations of CRP is graphed on the natural log scale. Respective concentrations of plasma and CSF CRP (mg/L) are also shown as multiples of 10. The dashed line (red) indicates plasma CRP >3 mg/L. CRP in plasma was measured by immunoturbidometric method and CRP in CSF was measured by electrochemilumiminescence. Dashed lines (black) indicate the 95% confidence interval of the best fit line. CRP - C-reactive protein; CSF - cerebrospinal fluid; MDD - major depressive disorder
Relationship between CRP and inflammatory markers in plasma and CSF with CSF/plasma albumin ratio and clinical covariates
The ratio of albumin in CSF to albumin in plasma (CSF/plasma albumin) did not correlate with plasma CRP (r=-0.009, p=0.942), and did not differ between patients with high versus low plasma CRP (>versus≤3mg/L; t=-0.216, df=71, p=0.829). Correlations between plasma and CSF CRP with each other and other inflammatory markers that passed correction for multiple comparisons remained significant when controlling for CSF/albumin ratio (r=0.289 to 0.881, p<0.05), as well as covariates relevant to BBB integrity (age, sex, race and BMI; r=0.293 to 0.842, p<0.05) except for CSF CRP to plasma IL-6sr (p=0.101).
Inflammatory markers in plasma and CSF of MDD patients with high versus low plasma CRP
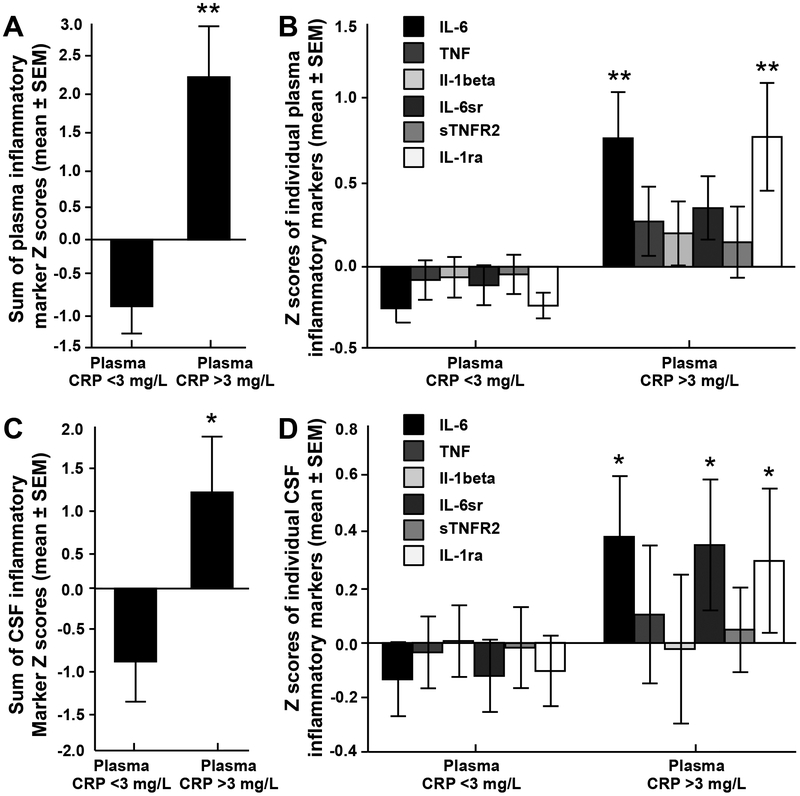
Comparison of Z scores for cytokines and their receptors/antagonists in plasma and CSF revealed that patients with high (n=18) versus low (n=55) plasma CRP (>3versus≤3mg/L) exhibited an overall increase in these markers in both plasma (F(1,82)=7.10, p<0.001) and CSF (F(1,64)=2.87, p=0.016; Figure 2a,c), which remained significant when controlling for clinical covariates (p<0.05). These relationships were driven primarily by significant higher concentrations of IL-6 and IL-1ra in plasma (p<0.001), and IL-6, IL-6sr and IL-1ra in CSF (p<0.05; Figure 2b,d). Additionally, three clusters of inflammatory markers in CSF that were significantly associated with high plasma CRP (>3mg/L) were revealed by PCA. We identified three factors, which together explained 77.02% of the total variance (Component 1=31.86%, Component 2=26.42%, Component 3=18.74%). Factor loadings of each biomarker are shown in Supplementary Table 3. Component 1 was comprised of IL-6, IL-6sr and sTNFR2, Component 2 of TNF and IL-1beta, and Component 3 was comprised of IL-6sr and sTNFR2 with a negative loading for IL-1ra (Supplementary Figure 1).
Figure 2. Inflammatory cytokines and their soluble receptors and antagonists were increased in plasma and CSF of MDD patients with high plasma CRP.
The sum of Z scores for concentrations of the inflammatory cytokines IL-6, TNF, IL-1beta and their soluble receptors were increased in plasma (A) and CSF (C) of patients with high (>3 mg/L) versus low (≤3 mg/L) plasma CRP. Z scores for concentrations of individual inflammatory cytokines and their soluble receptors in plasma (B) and CSF (C) that contributed to the overall increase in patients with plasma CRP >3 versus ≤3 mg/L. Data are presented as mean ± standard error. *p <0.05; **p<0.001. a - antagonist; CRP - C-reactive protein; CSF - cerebrospinal fluid; IL - interleukin; MDD - major depressive disorder; r - receptor; s - soluble; TNF - tumor necrosis factor
Correlations between inflammatory markers in CSF that were associated with high plasma CRP and symptoms of depression and anhedonia
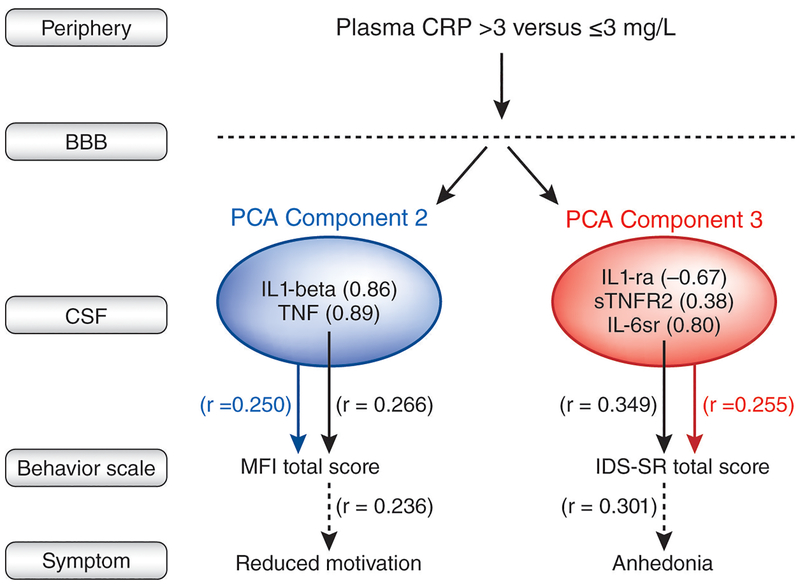
In exploratory analyses, components scores from PCA of inflammatory markers in CSF that were associated with high plasma CRP (>3mg/L) were correlated with total IDS-SR and MFI scores (Figure 3). Component 3, containing CSF IL-6sr, sTNFR2 and IL-1ra was significantly correlated with total IDS-SR scores (r=0.255, p=0.033), and Component 2, containing CSF TNF and IL-1beta, was significantly correlated with total MFI scores (r=0.250, p=0.037). Component 1 was not significantly correlated with behavior (p>0.80). Backward and forward linear regression models using the same criteria for entry and removal that included individual inflammatory markers from Components 2 or 3 and clinical covariates (age, sex, race and BMI) revealed that of those included in Component 2, CSF TNF was most significantly predictive of total MFI scores (r=0.266, p=0.026), and of Component 3, CSF IL-6sr was most significantly positively associated with total IDS-SR (r=0.349, p=0.002; Figure 3 and Supplemental Figure 2). Based on our previous studies showing relations between plasma CRP and anhedonia (also seen in this sample, although only significant in women [rs=0.265, df=56, p=0.044]), relationships between individual inflammatory markers from Components 2 and 3 and symptoms of anhedonia and RM, respectively, were examined. CSF TNF correlated with the RM subscale of MFI (r=0.236, p=0.045), and CSF IL-6sr correlated with the anhedonia subscale of ISD-SR (r=0.301, p=0.010; Figure 3).
Figure 3. PCA revealed clusters of CSF inflammatory markers that were associated with high plasma CRP (>3mg/L) and that correlated with behavior.
Three clusters of CSF inflammatory markers that were significantly associated with high versus low plasma CRP (> vs ≤ 3mg/L) in patients with MDD were revealed by PCA. Of these clusters, Bartlett factor scores for Component 2 were correlated with total MFI scores (blue arrow and text), and those of Component 3 were correlated with total IDS-SR scores (red arrow and text). Multivariate regression including clinical covariates (black arrows and text) revealed that of Component 2, CSF TNF was most significantly associated with MFI scores, and then with the MFI subscale of reduced motivation (RM). Of Component 3, CSF IL-6sr was most significantly associated with IDS-SR scores, and then with the IDS-SR subscale of anhedonia. a - antagonist; BBB - blood brain barrier; CRP - C-reactive protein; CSF - cerebrospinal fluid; IDS-SR - inventory of depressive symptomatology-self report; IL - interleukin; MDD - major depressive disorder; MFI - multidimensional fatigue inventory; PCA - principal component analysis; r - receptor; s - soluble; TNF - tumor necrosis factor
Exploratory analyses of relationships between other inflammatory markers and anhedonia: role of sex differences
As with CRP (see above), correlations between TNF and IL-6sr and anhedonia (but not RM) were significant only in females (rs=0.284–0.344, p<0.05) (see Supplemental Table 4 for inflammatory markers stratified by sex). Accordingly, potential sex differences in the above described relationships between CSF markers and behavior were explored and showed that the correlation between CSF IL-6sr and anhedonia (but not CSF TNF and RM) was significant only in females (r=0.40, df=44, p=0.006; Supplemental Figure 2). Further exploration of relationships between CRP in plasma and CSF and anhedonia showed that patients with anhedonia scores above the median (≥6) had higher concentrations of plasma (F[1,72]=4.34, p=0.041) but not CSF (p=0.12) CRP compared to those with low (<6) anhedonia (Supplemental Figure 3). Interestingly, higher plasma CRP also predicted high anhedonia by logistic regression (OR=1.49; 95%CI=1.07,2.87; p=0.044). Nevertheless, increased CSF CRP was observed in females with high anhedonia (F[1,45]=4.90, p=0.032; Supplemental Figure 3), and CSF CRP predicted high anhedonia in females (OR=1.75; 95%CI=1.03,2.98; p=0.040).
Discussion
Plasma CRP in patients with MDD was not only positively associated with plasma inflammatory cytokines and their soluble receptors/antagonists, but also correlated with CSF CRP (r>0.8) and other CSF soluble receptors/antagonists. High compared to low plasma CRP (>versus≤3mg/L) was also associated with higher levels of inflammatory cytokines and/or their receptors/antagonists in both plasma and CSF. PCA revealed clusters of cytokines and their receptors/antagonists in CSF that were associated with high plasma CRP (>3mg/L), the component scores of which were correlated with increased IDS-SR and MFI total scores. These findings were driven by CSF IL-6sr and TNF, which in turn correlated with subscales of anhedonia and RM, respectively. Furthermore, high anhedonia was predicted by CRP in plasma (in the group as a whole) and CSF (in females). Together, these findings indicate that elevated plasma CRP is an excellent proxy for increased inflammatory markers in both the periphery and CNS, as well as clusters of cytokines in the CSF that are associated with specific behaviors, particularly in females. Moreover, the data support peripheral CRP as a marker to identify depressed patients appropriate for clinical trials testing immunotherapies targeting TNF and IL-6.2
A strong relationship between peripheral blood and CSF CRP has been previously reported in patients with Parkinson’s disease,46 who may display decreased BBB function.60,61 However, in our MDD patient sample, no relationship was found between plasma CRP and CSF/plasma albumin ratio, which is increased in individuals with reduced BBB integrity,62,63 suggesting that higher CRP is not associated with impaired BBB function. Moreover, these data suggest that high CSF CRP and other inflammatory markers were likely attributed to local CNS production rather than diffusion or active uptake from the periphery.39,64 The source of CRP in CSF of MDD patients is unknown but possibly explained by extra-hepatic sources of CRP that have been identified, including peripheral macrophages and microglia and astrocytes in the brain which may produce CRP in the CNS at low levels (ng/L versus mg/L in plasma).65–67 Interestingly, significant correlations between individual cytokines or their soluble receptors/antagonists in the plasma with their counterparts in CSF were not reliably detected, except for IL-6sr and IL-1ra. This is consistent with data from humans and non-human primates administered peripheral inflammatory stimuli whereby increased CSF IL-6 did not correlate with that of plasma,55,68 suggesting local CNS production of IL-6.
Distinct clusters of CNS cytokines were identified by PCA to be associated with high plasma CRP (>3mg/L) and correlated with depressive symptoms. Indeed, a cluster related to CSF concentrations of inflammatory cytokine receptors/antagonists correlated with increased IDS-SR scores, which was driven primarily by CSF IL-6sr. CSF IL-6sr in turn correlated with increased anhedonia, a symptom previously found to associate with peripheral inflammation-related alterations in reward circuitry and increased CNS glutamate.25,26 High plasma CRP was associated with a principal component containing CSF TNF and IL-beta that correlated with MFI scores, which was due to a relationship between CSF TNF and the RM subscale of the MFI. These findings of distinct clusters of cytokines in the brain that were associated with different symptom profiles are interesting in that they may originate from different tissue sources of inflammation, with IL-6 signaling pathways and elevated cytokine receptors more indicative of systemic inflammation driven by peripheral organs such as liver, whereas TNF and IL-1beta may be more related to local inflammatory pathways in peripheral and CNS tissues. Interestingly, however, the two divergent clusters of CSF inflammatory markers that were related to high plasma CRP were ultimately associated with either increased anhedonia or RM, indicating that both inflammatory signaling profiles may converge on common pathways in the brain.
The soluble cytokine receptors/antagonists are increased during inflammation,69,70 exist in plasma and CSF at higher concentrations than cytokines, and correlate with IFN-alpha-induced depressive symptoms.69–73 Unlike IL-1ra (which had a negative loading in PCA Component 3) and sTNFR2, which act as antagonists, IL-6sr forms a complex with IL-6 that readily binds gp130 to facilitate IL-6 signaling, even across the BBB.74 Direct inflammatory activity of IL-6sr may explain why it predicted behavior, particularly in PCA Component 3 where it accounted for 79.5% of the variability. Of note, IL-1beta did not correlated with CRP or predict behavior, yet its activity may be more reliably detected by gene expression.9,10
A limitation of this study is that it was cross-sectional. Longitudinal work will determine causal links between peripheral and central inflammation and behavior in MDD by examining change over time or following administration of anti-inflammatory medications. Another limitation includes lack of data from healthy controls, patients with other psychiatric disorders or with inflammatory illnesses, to determine whether relationships between blood versus CSF CRP and cytokines are generalizable or specific to MDD. Finally, not all cytokines were measured and other unmeasured markers may be more strongly associated with plasma CRP or behavior. However, these data support that CRP associates with a pattern of CNS inflammatory activity which, like CRP, is predictive of symptoms of anhedonia.
Conclusions
This study provides evidence that CRP is a marker of both peripheral and central immune activity involving inflammatory cytokines previously found to be elevated in MDD.3,47 Findings herein justify using plasma CRP as a marker of peripheral and CNS inflammation to further understand the role of inflammation in MDD and other psychiatric disorders, and as a clinical marker to identify and potentially inform treatment of patients.
Supplementary Material
Acknowledgments
This study was supported by grants R01MH087604, R25MH101079 (Dr. Miller), R01MH109637, R21MH106904 (Dr. Felger) and R01MH H107033 (Dr. Haroon) from the National Institute of Mental Health; and grants BBRF22296 from the Brain and Behavioral Research Foundation and CADF49143 from the Dana Foundation (Dr. Felger). In addition, the study was supported in part by PHS Grants UL1TR000454 and KL2TR000455 from the Clinical and Translational Science Award program, and by the NIH/NCI under award number P30CA138292. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. We would like to acknowledge the Atlanta Clinical and Translational Science Institute (ACSTI) Clinical Research Network (CRN) staff.
Footnotes
Conflict of Interest Statement
All authors declare no conflicts of interest and have nothing to disclose.
References
- 1.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171–186. [DOI] [PubMed] [Google Scholar]
- 2.Miller AH, Haroon E, Felger JC. Therapeutic Implications of Brain-Immune Interactions: Treatment in Translation. Neuropsychopharmacology. 2017;42(1):334–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9(11):853–858. [DOI] [PubMed] [Google Scholar]
- 4.Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimaki M. Cumulative meta-analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav Immun. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wium-Andersen MK, Orsted DD, Nielsen SF, Nordestgaard BG. Elevated C-reactive protein levels, psychological distress, and depression in 73, 131 individuals. JAMA Psychiatry. 2013;70(2):176–184. [DOI] [PubMed] [Google Scholar]
- 6.Gimeno D, Kivimaki M, Brunner EJ, Elovainio M, De Vogli R, Steptoe A, et al. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol Med. 2009;39(3):413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Au B, Smith KJ, Gariepy G, Schmitz N. The longitudinal associations between C-reactive protein and depressive symptoms: evidence from the English Longitudinal Study of Ageing (ELSA). Int J Geriatr Psychiatry. 2015;30(9):976–984. [DOI] [PubMed] [Google Scholar]
- 8.Strawbridge R, Arnone D, Danese A, Papadopoulos A, Herane Vives A, Cleare AJ. Inflammation and clinical response to treatment in depression: A meta-analysis. Eur Neuropsychopharmacol. 2015;25(10):1532–1543. [DOI] [PubMed] [Google Scholar]
- 9.Cattaneo A, Gennarelli M, Uher R, Breen G, Farmer A, Aitchison KJ, et al. Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline ‘predictors’ and longitudinal ‘targets’. Neuropsychopharmacology. 2013;38(3):377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cattaneo A, Ferrari C, Uher R, Bocchio-Chiavetto L, Riva MA, Consortium MRCI, et al. Absolute Measurements of Macrophage Migration Inhibitory Factor and Interleukin-1-beta mRNA Levels Accurately Predict Treatment Response in Depressed Patients. Int J Neuropsychopharmacol. 2016;19(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhodes B, Furnrohr BG, Vyse TJ. C-reactive protein in rheumatology: biology and genetics. Nat Rev Rheumatol. 2011;7(5):282–289. [DOI] [PubMed] [Google Scholar]
- 12.Devaraj S, Yun JM, Duncan-Staley C, Jialal I. C-reactive protein induces M-CSF release and macrophage proliferation. J Leukoc Biol. 2009;85(2):262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aziz N, Fahey JL, Detels R, Butch AW. Analytical performance of a highly sensitive C-reactive protein-based immunoassay and the effects of laboratory variables on levels of protein in blood. Clin Diagn Lab Immunol. 2003;10(4):652–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coventry BJ, Ashdown ML, Quinn MA, Markovic SN, Yatomi-Clarke SL, Robinson AP. CRP identifies homeostatic immune oscillations in cancer patients: a potential treatment targeting tool? J Transl Med. 2009;7:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377(12):1119–1131. [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM. Inflammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality: implications for longevity. Nutr Rev. 2007;65(12 Pt 2):S253–259. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM. Cardiology Patient Page. C-reactive protein: a simple test to help predict risk of heart attack and stroke. Circulation. 2003;108(12):e81–85. [DOI] [PubMed] [Google Scholar]
- 18.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107(3):363–369. [DOI] [PubMed] [Google Scholar]
- 19.Couzin-Frankel J Inflammation bares a dark side. Science. 2010;330(6011):1621. [DOI] [PubMed] [Google Scholar]
- 20.Rapaport MH, Nierenberg AA, Schettler PJ, Kinkead B, Cardoos A, Walker R, et al. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Mol Psychiatry. 2016;21(1):71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70(1):31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, Miller AH Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Molecular Psychiatry. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haroon E, Fleischer CC, Felger JC, Chen X, Woolwine BJ, Patel T, et al. Conceptual convergence: increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Mol Psychiatry. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uher R, Tansey KE, Dew T, Maier W, Mors O, Hauser J, et al. An Inflammatory Biomarker as a Differential Predictor of Outcome of Depression Treatment With Escitalopram and Nortriptyline. Am J Psychiatry. 2014. [DOI] [PubMed] [Google Scholar]
- 25.Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry. 2016;21(10):1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haroon E, Fleischer CC, Felger JC, Chen X, Woolwine BJ, Patel T, et al. Conceptual convergence: increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Mol Psychiatry. 2016;21(10):1351–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Felger JC, Mun J, Kimmel HL, Nye JA, Drake DF, Hernandez CR, et al. Chronic interferon-alpha decreases dopamine 2 receptor binding and striatal dopamine release in association with anhedonia-like behavior in nonhuman primates. Neuropsychopharmacology. 2013;38(11):2179–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, et al. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch Gen Psychiatry. 2012;69(10):1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 2010;68(8):748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harrison NA, Voon V, Cercignani M, Cooper EA, Pessiglione M, Critchley HD. A Neurocomputational Account of How Inflammation Enhances Sensitivity to Punishments Versus Rewards. Biol Psychiatry. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haroon E, Felger JC, Woolwine BJ, Chen X, Parekh S, Spivey JR, et al. Age-related increases in basal ganglia glutamate are associated with TNF, reduced motivation and decreased psychomotor speed during IFN-alpha treatment: Preliminary findings. Brain Behav Immun. 2015;46:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, et al. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry. 2009;66(3):287–292. [DOI] [PubMed] [Google Scholar]
- 33.Levine J, Barak Y, Chengappa KN, Rapoport A, Rebey M, Barak V. Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology. 1999;40(4):171–176. [DOI] [PubMed] [Google Scholar]
- 34.Martinez JM, Garakani A, Yehuda R, Gorman JM. Proinflammatory and “resiliency” proteins in the CSF of patients with major depression. Depress Anxiety. 2012;29(1):32–38. [DOI] [PubMed] [Google Scholar]
- 35.Quan N, Banks WA. Brain-immune communication pathways. Brain Behav Immun. 2007;21(6):727–735. [DOI] [PubMed] [Google Scholar]
- 36.Goehler LE, Gaykema RP, Hansen MK, Anderson K, Maier SF, Watkins LR. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton Neurosci. 2000;85(1–3):49–59. [DOI] [PubMed] [Google Scholar]
- 37.D’Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J Neurosci. 2009;29(7):2089–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Felger JC, Treadway MT. Inflammation Effects on Motivation and Motor Activity: Role of Dopamine. Neuropsychopharmacology. 2017;42(1):216–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolkowitz OM, Papadopoulos NM, Costello R, Breier A, Doran AR, Pickar D, et al. Prednisone effects on blood-brain barrier permeability and CNS IgG synthesis in healthy humans. Psychoneuroendocrinology. 1990;15(2):155–158. [DOI] [PubMed] [Google Scholar]
- 40.Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Archives of General Psychiatry. 1988;45(8):742–747. [DOI] [PubMed] [Google Scholar]
- 41.Hamilton M A rating scale for depression. Journal of Neurology, Neurosurgery, Psychiatry. 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldsmith DR, Haroon E, Woolwine BJ, Jung MY, Wommack EC, Harvey PD, et al. Inflammatory markers are associated with decreased psychomotor speed in patients with major depressive disorder. Brain Behav Immun. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26(3):477–486. [DOI] [PubMed] [Google Scholar]
- 44.Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39(3):315–325. [DOI] [PubMed] [Google Scholar]
- 45.Ameli R, Luckenbaugh DA, Gould NF, Holmes MK, Lally N, Ballard ED, et al. SHAPS-C: the Snaith-Hamilton pleasure scale modified for clinician administration. PeerJ. 2014;2:e429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindqvist D, Hall S, Surova Y, Nielsen HM, Janelidze S, Brundin L, et al. Cerebrospinal fluid inflammatory markers in Parkinson’s disease--associations with depression, fatigue, and cognitive impairment. Brain Behav Immun. 2013;33:183–189. [DOI] [PubMed] [Google Scholar]
- 47.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. [DOI] [PubMed] [Google Scholar]
- 48.Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64(4):604–611. [DOI] [PubMed] [Google Scholar]
- 49.Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12(9):2759–2766. [DOI] [PubMed] [Google Scholar]
- 50.Breen EC, Perez C, Olmstead R, Eisenberger N, Irwin MR. Comparison of multiplex immunoassays and ELISAs for the determination of circulating levels of inflammatory cytokines. Brain, Behavior, and Immunity. 2014;40S(Abstract #135):e39. [Google Scholar]
- 51.Epstein MM, Breen EC, Magpantay L, Detels R, Lepone L, Penugonda S, et al. Temporal stability of serum concentrations of cytokines and soluble receptors measured across two years in low-risk HIV-seronegative men. Cancer Epidemiol Biomarkers Prev. 2013;22(11):2009–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, Eisenberger NI. Sex differences in depressive and socioemotional responses to an inflammatory challenge: implications for sex differences in depression. Neuropsychopharmacology. 2015;40(7):1709–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inagaki TK, Muscatell KA, Irwin MR, Moieni M, Dutcher JM, Jevtic I, et al. The role of the ventral striatum in inflammatory-induced approach toward support figures. Brain Behav Immun. 2015;44:247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kwak SG, Kim JH. Central limit theorem: the cornerstone of modern statistics. Korean J Anesthesiol. 2017;70(2):144–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ, et al. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry. 2009;65(4):296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Torres MA, Pace TW, Liu T, Felger JC, Mister D, Doho GH, et al. Predictors of depression in breast cancer patients treated with radiation: role of prior chemotherapy and nuclear factor kappa B. Cancer. 2013;119(11):1951–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haroon E, Felger JC, Woolwine BJ, Chen X, Parekh S, Spivey JR, et al. Age-related increases in basal ganglia glutamate are associated with TNF, reduced motivation and decreased psychomotor speed during IFN-alpha treatment: Preliminary findings. Brain Behav Immun. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grimsholm O, Rantapaa-Dahlqvist S, Forsgren S. Levels of gastrin-releasing peptide and substance P in synovial fluid and serum correlate with levels of cytokines in rheumatoid arthritis. Arthritis Res Ther. 2005;7(3):R416–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Capuron L, Lasselin J, Castanon N. Role of Adiposity-Driven Inflammation in Depressive Morbidity. Neuropsychopharmacology. 2017;42(1):115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gray MT, Woulfe JM. Striatal blood-brain barrier permeability in Parkinson’s disease. J Cereb Blood Flow Metab. 2015;35(5):747–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pisani V, Stefani A, Pierantozzi M, Natoli S, Stanzione P, Franciotta D, et al. Increased blood-cerebrospinal fluid transfer of albumin in advanced Parkinson’s disease. J Neuroinflammation. 2012;9:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Algotsson A, Winblad B. The integrity of the blood-brain barrier in Alzheimer’s disease. Acta Neurol Scand. 2007;115(6):403–408. [DOI] [PubMed] [Google Scholar]
- 63.Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease--systematic review and meta-analysis. Neurobiol Aging. 2009;30(3):337–352. [DOI] [PubMed] [Google Scholar]
- 64.Kirch DG, Alexander RC, Suddath RL, Papadopoulos NM, Kaufmann CA, Daniel DG, et al. Blood-CSF barrier permeability and central nervous system immunoglobulin G in schizophrenia. J Neural Transm Gen Sect. 1992;89(3):219–232. [DOI] [PubMed] [Google Scholar]
- 65.Juma WM, Lira A, Marzuk A, Marzuk Z, Hakim AM, Thompson CS. C-reactive protein expression in a rodent model of chronic cerebral hypoperfusion. Brain Res. 2011;1414:85–93. [DOI] [PubMed] [Google Scholar]
- 66.Wight RD, Tull CA, Deel MW, Stroope BL, Eubanks AG, Chavis JA, et al. Resveratrol effects on astrocyte function: relevance to neurodegenerative diseases. Biochem Biophys Res Commun. 2012;426(1):112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dong Q, Wright JR. Expression of C-reactive protein by alveolar macrophages. J Immunol. 1996;156(12):4815–4820. [PubMed] [Google Scholar]
- 68.Reyes TM, Coe CL. Interleukin-1 beta differentially affects interleukin-6 and soluble interleukin-6 receptor in the blood and central nervous system of the monkey. J Neuroimmunol. 1996;66(1–2):135–141. [DOI] [PubMed] [Google Scholar]
- 69.Re F, Mengozzi M, Muzio M, Dinarello CA, Mantovani A, Colotta F. Expression of interleukin-1 receptor antagonist (IL-1ra) by human circulating polymorphonuclear cells. Eur J Immunol. 1993;23(2):570–573. [DOI] [PubMed] [Google Scholar]
- 70.Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM. The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J. 2001;15(1):43–58. [DOI] [PubMed] [Google Scholar]
- 71.Raison CL, Borisov AS, Woolwine BJ, Massung B, Vogt G, Miller AH. Interferon-alpha effects on diurnal hypothalamic-pituitary-adrenal axis activity: relationship with proinflammatory cytokines and behavior. Mol Psychiatry. 2010;15(5):535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wichers MC, Kenis G, Koek GH, Robaeys G, Nicolson NA, Maes M. Interferon-alpha-induced depressive symptoms are related to changes in the cytokine network but not to cortisol. J Psychosom Res. 2007;62(2):207–214. [DOI] [PubMed] [Google Scholar]
- 73.Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, et al. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry. 2010;15(4):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rothaug M, Becker-Pauly C, Rose-John S. The role of interleukin-6 signaling in nervous tissue. Biochim Biophys Acta. 2016;1863(6 Pt A):1218–1227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.