Abstract
A commonly used anesthetic, isoflurane, can impair auditory function in a dose-dependent manner. However, in rats, isoflurane-induced auditory impairments have only been assessed with auditory brainstem responses; a measure which is unable to distinguish if changes originate from the central or peripheral auditory system. Studies performed in other species, such as mice and guinea-pigs, suggests auditory impairment stems from disrupted OHC amplification. Despite the wide use of the rat in auditory research, these observations have yet to be replicated in the rat animal model. This study used distortion product otoacoustic emissions to assess outer hair cell function in rats that were anesthetized with either isoflurane or a ketamine/xylazine cocktail for approximately 45 min. Results indicate that isoflurane can significantly reduce DPOAE amplitudes compared to ketamine/xylazine, and that responses were more variable with isoflurane than ketamine/xylazine over the 45-min test period. Based on these observations, isoflurane should be used with caution when assessing peripheral auditory function to avoid potentially confounding effects.
Keywords: Isoflurane, Ketamine, Distortion product otoacoustic emissions
Abbreviations: OHC, Outer Hair Cell; DPOAE, Distortion Product Otoacoustic Emissions; ABR, Auditory Brainstem Response
1. Introduction
Isoflurane is a fast-acting anesthetic that is commonly used in auditory research. Recent studies have indicated that isoflurane can have deleterious effects on both the peripheral and central auditory systems (Bielefeld, 2014; Cederholm et al., 2012; Ruebhausen et al., 2012; Santarelli et al., 2003a; Santarelli et al., 2003b; Stronks et al., 2010). In contrast, others have suggested isoflurane may improve auditory function by enhancing outer hair cell (OHC) amplification (Drexl et al., 2004) or by protecting against noise-induced hearing loss (Chung et al., 2007; Kim et al., 2005). These conflicting reports warrant the further investigation of isoflurane's effect on auditory function.
In C57B1/6J and C129/SvEv mice, isoflurane initially results in poorer auditory brainstem response (ABR) and DPOAE thresholds than those obtained with a ketamine/xylazine (k/x) cocktail. Although, after 1 h, k/x demonstrated poorer DPOAE thresholds than isoflurane (Cederholm et al., 2012). This report highlights the importance of understanding temporal changes in auditory function while under anesthesia. Other studies monitoring ABR thresholds support the notion that isoflurane may initially induce greater hearing loss than k/x (Ruebhausen et al., 2012). In guinea-pigs, isoflurane significantly reduces compound action potentials (CAPs), the cochlear microphonic (CM), and ABR thresholds in a dose-dependent manner (Stronks et al., 2010). Therefore, it's probable that isoflurane-induced changes in auditory function originate at the level of OHCs. In contrast, isoflurane has also been reported to enhance DPOAE amplitudes in the mustache bat (Drexl et al., 2004), suggesting anesthetics may have species-specific interactions on auditory function. Similar to results obtained in guinea-pigs and mice, ABR thresholds are poorer with isoflurane than k/x in the rat (Ruebhausen et al., 2012).
This study aimed to develop a more thorough understanding of how two commonly used anesthetics in auditory research, k/x and isoflurane, effect the peripheral auditory system of the rat. Based on previous reports, we hypothesized that OHC amplification would be significantly poorer during the use of isoflurane compared to k/x. Distortion product (DP) amplitudes were measured with isoflurane or k/x and analyzed for changes over time or for differences between the anesthetics.
2. Materials & methods
2.1. Subjects
Six male Sprague–Dawley rats (3–4 months old, 300–400 g, Charles River) were used in the study. Animals were housed two per cage, provided free access to food and water, and kept on a 12/12 h light–dark cycle. Each animal underwent DPOAE measures with both a k/x cocktail and isoflurane inhaled anesthetic, with a one-week washout period in between. Previous studies performed by our lab suggest there is no long-lasting effect of either k/x or isoflurane that would confound the presented results.
2.2. Anesthetics
K/x was administered at a dose of 60 mg/kg ketamine, and 6 mg/kg xylazine via an intraperitoneal injection. DPOAEs were subsequently collected for three consecutive trials (15 min each). Animals were allowed a one-week washout period prior to collecting DPOAEs with isoflurane. Isoflurane was administered by placing the rats in an induction chamber with 4% isoflurane in O2 with a flow rate of 0.5 L/minute until they were in a stable anesthetic plane (plane III using Guedel's classification). They were subsequently maintained under anesthesia with 1.5–2% isoflurane for the duration of three consecutive DPOAE trials. With both anesthetic procedures, body temperature was maintained at 37 °C with a homoeothermic blanket (Harvard Apparatus) throughout the testing procedure. If animals awoke from anesthesia during data acquisition, subsequent trials were not completed and the animal was removed from the study to avoid confounding effects associated with multiple anesthesia inductions.
2.3. Distortion product otoacoustic emissions
Using a commercial instrument (Intelligent Hearing Systems; Miami, FL), DPOAEs were obtained with two primary tones (f1 and f2) presented at an f2/f1 ratio of 1.2 to each animal's right ear using an insert probe. The probe was not moved during the three DPOAE trials. The intensity of f1 (L1) was presented 10 dB higher than the intensity of f2 (L2). Input/output (I/O) functions were plotted for the 2f1-f2 distortion product signal to noise ratios. The f2 frequencies were 4, 6, 8, 6, 13.2, 24, and 30 kHz and L1 intensities were presented from 80 to 25 dB SPL in 5 dB steps. I/O functions were plotted and analyzed for significance used Prism Graph Pad v5. The changes in DPOAE amplitudes over time and differences between k/x and isoflurane were analyzed for statistical significance using a repeated measures two-way ANOVA with Bonferroni post-hoc analysis.
3. Results
3.1. DPOAE over time with ketamine
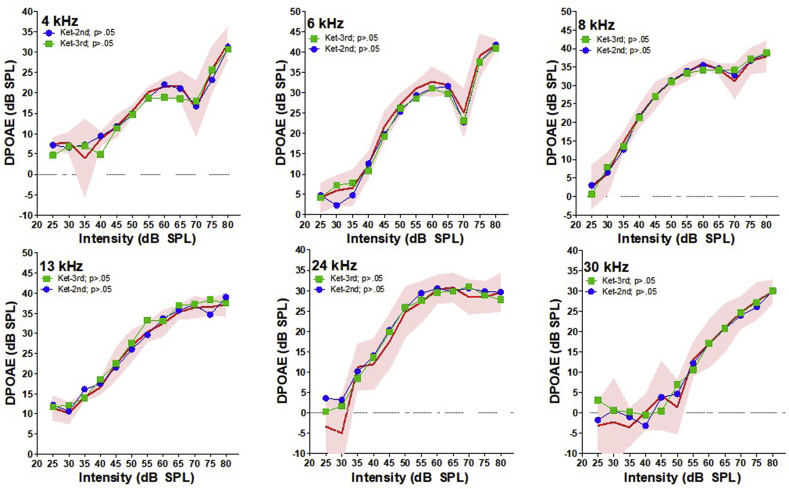
To determine if k/x had an effect on DP amplitudes over time, we plotted I/O functions for each trial and compared the amplitudes for statistical significance (Fig. 1). For visual comparison, the first trial is plotted as the mean with 95% confidence interval (CI), i.e., the red shaded area. There was no significant changes in DP amplitudes over the three measured trials across all measured frequency (repeated two-way ANOVA, p > .05). This observation indicates that DPOAEs can be repeatedly measured under k/x anesthesia without significant changes in DP amplitudes. It should also be noted that the overall variability of DPOAE amplitudes that were measured was relatively small, with the exception of the DPs obtained at very low L1 intensities where the SNR is inherently poor. This is visually represented by the red shaded 95% CI of the first trial in each I/O function.
Fig. 1.
K/x DPOAE I/O functions plotted over time. Red shaded area represents the 95% CI for the first obtained trial at each frequency. The mean DP amplitudes are plotted for the second trial in green, and the third trial in blue. There was no significant change in DP amplitudes for any frequency over the three measured trials.
3.2. DPOAE over time with isoflurane
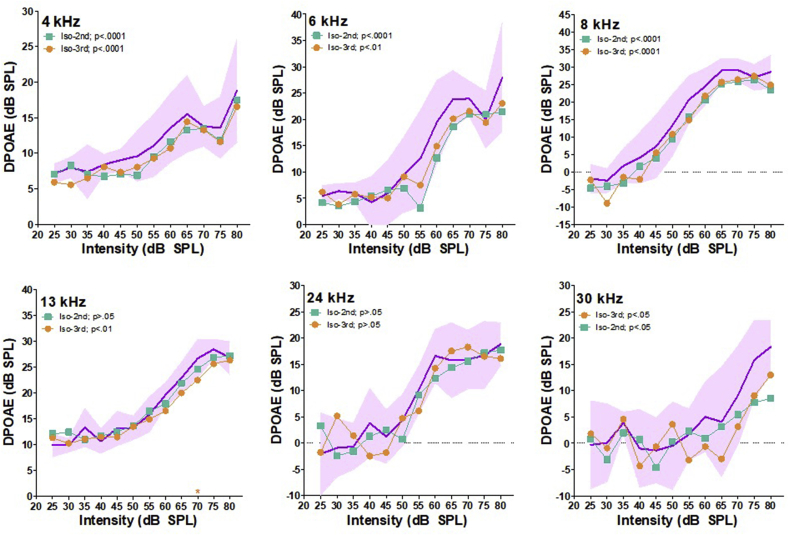
To determine if isoflurane influenced DPOAEs over time, we plotted I/O functions for each trial and compared the amplitudes for statistical significance (Fig. 2). For visual comparison, the initial trial is plotted as the mean with 95% CI, i.e., the purple shaded area. When analyzing DP I/O slopes as a whole, significant reductions were present during the second trial (15–30 min) at 4 kHz (p<.0001), 6 kHz (p<.0001) and 8 kHz (p<.05). When comparing DP amplitudes from the third trial (30–45 min) to the first trial amplitudes, significant reductions were present at 4 kHz (p<.0001), 6 kHz (p<.0001), 8 kHz (p<.0001), 13 kHz (p<.01) and 30 kHz (p<.05). Using Bonferonni post-hoc analysis to test for L1 intensity specific DP reductions, only one intensity in the third trial (13 kHz at 70 dB) was significantly reduced. These observations indicate that over time isoflurane results in a progressive reduction of DPOAE amplitudes that begins at lower frequencies and later expands to higher frequencies. Finally, it should also be noted that the variability of DP amplitudes measured within each trial was greater than those obtained with k/x. This is visually represented by the 95% CI spread of the first trial.
Fig. 2.
Isoflurane DPOAE I/O functions plotted over time. Purple shaded area represents the 95% CI for the first obtained trial at each frequency. The mean DP amplitudes are plotted for the second trial in green, and the third trial in orange. Significant reductions in DP amplitudes as a whole are indicated by p-values in each graphs legend. Post-hoc analysis significance for each L1 intensity is indicated by stars at along each X-axis. * = p<.05, ** = p<.001, *** = p<.0001.
3.3. Ketamine versus isoflurane DPOAEs
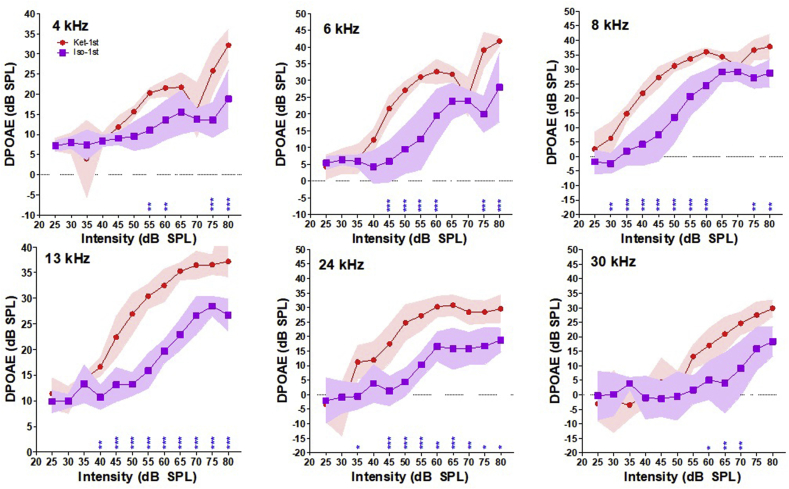
To determine which anesthetic provides better DP amplitudes for a single DPOAE measurement, we analyzed the difference between DP amplitudes obtained during the first trial with either k/x or isoflurane. For visual comparison, we plotted DP I/O functions with mean and 95% CI for each anesthetic (Fig. 3). The red shaded area represents the 95% CI for DP amplitudes measured with k/x, and the purple shaded areas represents the 95% CI for DP amplitudes measured with isoflurane. DP amplitudes measured with isoflurane were significantly lower than those obtained with k/x at every tested frequency (two-way repeated ANOVA, 4 kHz p<.0001, 6 kHz p<.0001, 8 kHz p<.0001, 13 kHz p<.0001, 24 kHz p<.0001 & 30 kHz p<.0001). Results of the post-hoc analysis testing for L1 specific changes are illustrated by blue stars on x-axis of I/O functions for each frequency (Fig. 3). At 4 kHz, isoflurane amplitudes were significantly reduced at L1 intensities from 55 to 60 dB (p<.01) and 75–80 dB (p<.0001). At 6 kHz, significant reductions were present from 45 to 60 dB and from 75 to 80 dB (p<.0001). At 8 kHz, significant reductions were present at 30 dB (p<.001), 35–60 dB (p<.0001), and 75–80 dB (p<.0001). At 13 kHz, significant reductions were present at 40 dB (p<.001), and 45–80 dB (p<.0001). At 24 kHz, significant reductions were present at 35 dB (p<.05), 45–50 dB (p<.0001), 60 dB (p<.05), 65 dB (p<.0001), 70 dB (p<.001), and 75–80 dB (p<.05). At 30 kHz, significant reductions were seen at 60 dB (p<.05) and 65–70 dB (p<.001). The results of this analysis demonstrate dramatically lower DP amplitudes obtained with isoflurane compared to k/x. Furthermore, post-hoc analyses indicated that the degree of DP amplitude reductions occur relatively equal across the measured frequency range.
Fig. 3.
Comparison of DP amplitudes under k/x and isoflurane. Red symbols and shaded area represents the mean and 95% CI of DP amplitudes under k/x. Purple symbols and shaded area represents the mean and 95% CI of DP amplitudes under isoflurane. Significant post-hoc differences for each L1 intensity is indicated by stars along each X-axis. * = p<.05, ** = p<.001, *** = p<.0001.
4. Discussion
Many reports have indicated that isoflurane can impair auditory function (Bielefeld, 2014; Cederholm et al., 2012; Ruebhausen et al., 2012; Stronks et al., 2010). Studies in guinea-pigs point to impaired OHC amplification as a likely mechanism for isoflurane-induced impairment (Stronks et al., 2010), while results in the mustache bat show OHC amplification can actually improve under isoflurane (Drexl et al., 2004). These conflicting reports suggests there may be an important species-specific interaction. Furthermore, despite the wide use of rats in auditory research, no one has reported on isoflurane-induced changes in DP amplitudes in the rat animal model. To address this gap in knowledge, we monitored DPOAE amplitudes in rats anesthetized with isoflurane or k/x over 45 min. Our results indicate that isoflurane significantly reduces DPOAE amplitudes compared to k/x, and the deleterious effects of isoflurane progressively worsen over time. In contrast, k/x appears to have very little influence of DPOAE amplitudes. Below we discuss our results in the context of other possible contributing variables.
4.1. Anesthesia dosage and temporal aspects
As previously noted, there are some conflicting reports on the direction DP amplitude change (i.e., enhanced or reduced) while under isoflurane anesthesia. Contrasting reports may result from a difference in the dose of isoflurane used between studies. In guinea-pigs, DP amplitudes are enhanced with 1.15% isoflurane, but reduced at dosages greater than 2% (Stronks et al., 2010; Xiao et al., 2014). Similarly, DP amplitudes were enhanced in bats with 1.5% isoflurane (Drexl et al., 2004), but are reduced in mice at 1.5% (Cederholm et al., 2012). The data we present here in rats used 1.5–2% isoflurane, which resulted in significantly lower DP amplitudes compared to k/x. Taken together, it's conceivable that lower dosages of isoflurane may enhance DP amplitudes, while dosages greater than approximately 1.6% would dramatically lower DP amplitudes.
To our knowledge, there is only one other study that has monitored the stability of DP amplitudes over time with isoflurane and k/x (Cederholm et al., 2012). Our study failed to demonstrate any change in DP amplitudes with k/x for the duration of 45 min. However, previous reports in mice report k/x anesthesia lowered DP amplitudes and thresholds even more than isoflurane after 1 h (Cederholm et al., 2012). The differences between our results in rats versus those in mice suggests again that there may be species-specific differences in regards to ketamine's temporal influence on OHC function, or that k/x has a slow activating component that influence DPOAE amplitudes after approximately 1 h.
4.2. Noise induced hearing loss protection from isoflurane
Traumatic noise exposure occurring under isoflurane causes less hearing loss and less sensory cell damage compared to awake animals (Chung et al., 2007; Kim et al., 2005). One explanation for this effect is isoflurane's antagonistic interaction at the site of N-methyl-d-aspartate (NMDA) receptors, which theoretically reduces the production of reactive oxygen species (ROS) known to cause hearing loss (Chung et al., 2007; Kim et al., 2005). Other NMDA antagonist drugs have also demonstrated similar protection from traumatic noise exposure (Chen et al., 2001; Ohinata et al., 2003), lending some credit to this theory. However, ketamine also acts as an NMDA receptor antagonist (Harada et al., 1999; Zorumski et al., 2016), and has not shown similar protective effects against traumatic noise exposure. An alternative explanation for why isoflurane protects against traumatic noise exposures could be the temporary reduction in OHC amplification, resulting in less damaging cochlear mechanics during the exposure, and subsequently less hearing loss and sensory cell damage. The results presented here support this explanation. The exact mechanism by which isoflurane is capable of reducing OHC amplification remains unknown, but some plausible mechanisms are discussed below.
4.3. Cochlear blood flow changes
One possible mechanism responsible for isoflurane-induced DPOAE reductions is changes in cochlear blood flow (CBF). Impaired CBF is thought to be involved in a plethora of hearing disorders (Nakashima et al., 2003). In guinea-pigs, isoflurane can either enhance or reduce DPOAE amplitudes depending on the dosage, and these changes are positively correlated with CBF (Xiao et al., 2014). At low dosages, isoflurane enhanced CBF and DPOAE amplitudes, whereas at higher dosages (>2.3 vol%) isoflurane reduced CBF and DPOAE amplitudes and damages OHC stereocilia (Xiao et al., 2014). This latter pattern of DPOAE reduction closely mirrors the observations in the present study, suggesting that impaired CBF may be partially responsible for isoflurane-induced reductions in DPOAEs.
4.4. Cochlear efferents
Another mechanism that could play a role in isoflurane-induced DPOAE reductions is the centrally mediated cochlear efferent system. Cochlear efferents are subdivided into two functionally distinct tracks, the lateral olivocochlear (LOC) and the medial olivocochlear (MOC). The MOC system is known to suppress OHC amplification via cholinergic or GABAergic mediated interactions (Wersinger and Fuchs, 2011). Isoflurane can block acetylcholine (Ach) receptors (Dilger et al., 1992) and enhance GABA-gated channel currents (Ming et al., 2001), which could enhance inhibition mediated by MOC efferent, resulting in less OHC amplification and therefore smaller DP amplitudes. Furthermore, dopamine is known to mediate IHC activity via LOC efferent tracks (Le Prell et al., 2005), and isoflurane can impair dopamine re-uptake (Votaw et al., 2003), which may be capable of indirectly modifying the inhibitory actions of MOC efferents (Maison et al., 2012). The complex cochlear efferent network is not fully understood, but could conceivably contribute to isoflurane-induced DP amplitude reductions if OHC amplification were impaired through isoflurane's interactions on cochlear MOC efferents.
4.5. Middle ear mechanics
Finally, isoflurane-induced reductions in DPOAE amplitudes may not solely represent changes in OHC amplification. DP amplitude measures are reliant on normal middle ear mechanics, any impairment in sound transduction through the middle ear space could dramatically alter the ability to measure DOPAEs. The middle ear reflex (MER) is activated bilaterally in response to intense sound, which leads to the constriction of the stapedius muscle, and subsequent tensioning of the ossicular chain (Møller, 1974). Both isoflurane and k/x can suppress the MER (Campo et al., 2013; Chambers et al., 2012). However, the rat MER is not activated until approximately 75 dB after a presentation duration of 10–20 ms (Pilz et al., 1997), and therefore is unlikely to have a significant impact on the majority of DP amplitudes in the present study. However, impedance changes in middle ear mechanics cannot be completely ruled out; others have reported halogenated inhalants and nitrous oxide can diffuse into the middle ears space thereby changing equivalent pressure and impairing sound transduction bilaterally (Chinn et al., 1997; Doyle and Banks, 2003; Perreault et al., 1982). Therefore, it may be possible that the observed changes in DP amplitudes in the present study reflect middle ear changes, which influence DPOAEs measurements.
5. Conclusion
In conclusion, our results show that isoflurane significantly impairs DPOAEs compared to k/x in a rat animal model. The results are similar to those previously observed in mice (Cederholm et al., 2012), but contradict other observations in bats (Drexl et al., 2004). We also observed progressively worsening DPOAE amplitudes when animals were anesthetized with isoflurane over the 45 min monitoring, whereas k/x showed no change. The results reported here highlight the significant variability in auditory function induced by isoflurane. Nevertheless, isoflurane continues to be a commonly used anesthetic in animal research due to its fast acting properties. However, this study, along with many others have raised a number of concerns regarding its confounding influence on the auditory system. Therefore, caution should be used while employing isoflurane in auditory research in order to avoid confounding variables, especially if studies are analyzing the effects of intense noise exposure or investigating small fluctuations in hearing acuity.
Conflicts of interest
The authors declare that there is no conflict of interests regarding the publication of this article.
Acknowledgments
Research supported in part by grants from the National Institutes of Health to AS (F31DC015933) and RS (R01DC014452), from the American Academy of Audiology to AS, and from the China Scholarship Council (No. 201606095027) to DL.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
References
- Bielefeld E.C. Influence of dose and duration of isoflurane anesthesia on the auditory brainstem response in the rat. Int. J. Audiol. 2014;53(4):250–258. doi: 10.3109/14992027.2013.858280. [DOI] [PubMed] [Google Scholar]
- Campo P., Venet T., Thomas A., Cour C., Castel B., Nunge H., Cosnier F. Inhaled toluene can modulate the effects of anesthetics on the middle-ear acoustic reflex. Neurotoxicol. Teratol. 2013;35:1–6. doi: 10.1016/j.ntt.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Cederholm J.M., Froud K.E., Wong A.C., Ko M., Ryan A.F., Housley G.D. Differential actions of isoflurane and ketamine-based anaesthetics on cochlear function in the mouse. Hear. Res. 2012;292(1–2):71–79. doi: 10.1016/j.heares.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers A.R., Hancock K.E., Maison S.F., Liberman M.C., Polley D.B. Sound-evoked olivocochlear activation in unanesthetized mice. J. Assoc. Res. Otolaryngol. 2012;13(2):209–217. doi: 10.1007/s10162-011-0306-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G.-D., Kong J., Reinhard K., Fechter L.D. NMDA receptor blockage protects against permanent noise-induced hearing loss but not its potentiation by carbon monoxide. Hear. Res. 2001;154(1–2):108–115. doi: 10.1016/s0378-5955(01)00228-3. [DOI] [PubMed] [Google Scholar]
- Chinn K., Brown O.E., Manning S.C., Crandell C.C. Middle ear pressure variation: effect of nitrous oxide. Laryngoscope. 1997;107(3):357–363. doi: 10.1097/00005537-199703000-00015. [DOI] [PubMed] [Google Scholar]
- Chung J.W., Ahn J.H., Kim J.Y., Lee H.J., Kang H.H., Lee Y.K. The effect of isoflurane, halothane and pentobarbital on noise-induced hearing loss in mice. Anesth. Analg. 2007;104(6):1404–1408. doi: 10.1213/01.ane.0000261508.24083.6c. table of contents. [DOI] [PubMed] [Google Scholar]
- Dilger J., Brett R., Lesko L. Effects of isoflurane on acetylcholine receptor channels. 1. Single-channel currents. Mol. Pharmacol. 1992;41(1):127–133. [PubMed] [Google Scholar]
- Doyle W.J., Banks J.M. Middle ear pressure change during controlled breathing with gas mixtures containing nitrous oxide. J. Appl. Physiol. 2003;94(1):199–204. doi: 10.1152/japplphysiol.00634.2002. 1985. [DOI] [PubMed] [Google Scholar]
- Drexl M., Henke J., Kossl M. Isoflurane increases amplitude and incidence of evoked and spontaneous otoacoustic emissions. Hear. Res. 2004;194(1–2):135–142. doi: 10.1016/j.heares.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Harada H., Kelly P.J., Cole D.J., Drummond J.C., Patel P.M. Isoflurane reduces N-methyl-D-aspartate toxicity in vivo in the rat cerebral cortex. Anesth. Analg. 1999;89(6):1442–1447. doi: 10.1097/00000539-199912000-00022. [DOI] [PubMed] [Google Scholar]
- Kim J.U., Lee H.J., Kang H.H., Shin J.W., Ku S.W., Ahn J.H. Protective effect of isoflurane anesthesia on noise-induced hearing loss in mice. Laryngoscope. 2005;115(11):1996–1999. doi: 10.1097/01.mlg.0000180173.81034.4d. [DOI] [PubMed] [Google Scholar]
- Maison S.F., Liu X.-P., Eatock R.A., Sibley D.R., Grandy D.K., Liberman M.C. Dopaminergic signaling in the cochlea: receptor expression patterns and deletion phenotypes. J. Neurosci. 2012;32(1):344–355. doi: 10.1523/JNEUROSCI.4720-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming Z., Knapp D.J., Mueller R.A., Breese G.R., Criswell H.E. Differential modulation of GABA-and NMDA-gated currents by ethanol and isoflurane in cultured rat cerebral cortical neurons. Brain Res. 2001;920(1–2):117–124. doi: 10.1016/s0006-8993(01)03044-x. [DOI] [PubMed] [Google Scholar]
- Møller A.R. Springer; 1974. The Acoustic Middle Ear Muscle Reflex Auditory System; pp. 519–548. [Google Scholar]
- Nakashima T., Naganawa S., Sone M., Tominaga M., Hayashi H., Yamamoto H. Disorders of cochlear blood flow. Brain Res. Rev. 2003;43(1):17–28. doi: 10.1016/s0165-0173(03)00189-9. [DOI] [PubMed] [Google Scholar]
- Ohinata Y., Miller J.M., Schacht J. Protection from noise-induced lipid peroxidation and hair cell loss in the cochlea. Brain Res. 2003;966(2):265–273. doi: 10.1016/s0006-8993(02)04205-1. [DOI] [PubMed] [Google Scholar]
- Perreault L., Normandin N., Plamondon L., Blain R., Rousseau P., Girard M., Forget G. Middle ear pressure variations during nitrous oxide and oxygen anaesthesia. Can. Anaesth. Soc. J. 1982;29(5):428–434. doi: 10.1007/BF03009404. [DOI] [PubMed] [Google Scholar]
- Pilz P., Ostwald J., Kreiter A., Schnitzler H.-U. Effect of the middle ear reflex on sound transmission to the inner ear of rat. Hear. Res. 1997;105(1–2):171–182. doi: 10.1016/s0378-5955(96)00206-7. [DOI] [PubMed] [Google Scholar]
- Le Prell C.G., Halsey K., Hughes L.F., Dolan D.F., Bledsoe S.C. Disruption of lateral olivocochlear neurons via a dopaminergic neurotoxin depresses sound-evoked auditory nerve activity. J. Assoc. Res. Otolaryngol. 2005;6(1):48–62. doi: 10.1007/s10162-004-5009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruebhausen M.R., Brozoski T.J., Bauer C.A. A comparison of the effects of isoflurane and ketamine anesthesia on auditory brainstem response (ABR) thresholds in rats. Hear. Res. 2012;287(1–2):25–29. doi: 10.1016/j.heares.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Santarelli R., Arslan E., Carraro L., Conti G., Capello M., Plourde G. Effects of isoflurane on the auditory brainstem responses and middle latency responses of rats. Acta Otolaryngol. 2003;123(2):176–181. doi: 10.1080/0036554021000028108. [DOI] [PubMed] [Google Scholar]
- Santarelli R., Carraro L., Conti G., Capello M., Plourde G., Arslan E. Effects of isoflurane on auditory middle latency (MLRs) and steady-state (SSRs) responses recorded from the temporal cortex of the rat. Brain Res. 2003;973(2):240–251. doi: 10.1016/s0006-8993(03)02520-4. [DOI] [PubMed] [Google Scholar]
- Stronks H.C., Aarts M.C., Klis S.F. Effects of isoflurane on auditory evoked potentials in the cochlea and brainstem of Guinea pigs. Hear. Res. 2010;260(1–2):20–29. doi: 10.1016/j.heares.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Votaw J., Byas-Smith M., Hua J., Voll R., Martarello L., Levey A.I. Interaction of isoflurane with the dopamine transporter. Anesthesiol. J. Am. Soc. Anesthesiologists. 2003;98(2):404–411. doi: 10.1097/00000542-200302000-00021. [DOI] [PubMed] [Google Scholar]
- Wersinger E., Fuchs P.A. Modulation of hair cell efferents. Hear. Res. 2011;279(1–2):1–12. doi: 10.1016/j.heares.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Wen J., Bai Y., Duan N., Jing G.X. Different effects of propofol and isoflurane on cochlear blood flow and hearing function in Guinea pigs. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0096861. e96861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski C.F., Izumi Y., Mennerick S. Ketamine: NMDA receptors and beyond. J. Neurosci. 2016;36(44):11158–11164. doi: 10.1523/JNEUROSCI.1547-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]