Abstract
To characterize the patterns of ABR waves in tree shrews, we must understand the hearing sensitivity and auditory function of healthy adult tree shrews. Fifteen tree shrews (30 ears) were stimulated with clicks and tone-pips at 11 different frequencies from 1 to 60 kHz. The ABR waves were recorded and analyzed. The ABR consisted of five to seven positive waves in the first 10 ms after a click stimulus, and the average hearing threshold of component III was 27.86 ± 3.78 dB SPL. Wave III was the largest and most clear. The ABR threshold was related to the tone-pip sitmulus by a “U” shaped curve. The sensitive frequency was approximately 8 kHz in tree shrews. The latencies systematically decreased with increasing stimulus frequencies. The ABR amplitudes of wave III increased as the sound pressure level increased. All of these results provide an empirical basis for future studies of hearing diseases in tree shrews.
Keywords: Tree shrew (Tupaia belangeri), Ear, Auditory brainstem response, Primates
1. Introduction
The auditory brainstem response (ABR) is an evoked potential that is commonly recorded to study auditory function in either normal or pathological conditions. ABRs are recorded far-field from the scalp and are typically composed of five to seven vertex positive waves within the first 10 ms after an auditory stimulus. This technique is non-invasive, objective and easy to use and is extensively employed in both clinical and experimental studies (Parham et al., 2001; Schopf et al., 2014). The localization of the sources of each wave remains unclear. However, for most mammalian animals and humans, the responses of the activity in the auditory nerve, cochlear nucleus, superior olivary complex, lateral lemniscus and inferior colliculus are generally accepted to correspond to waves I, II, III, IV, and V, respectively (Alvarado et al., 2012; Chiappa, 2007; Parkkonen et al., 2009; Reichmuth et al., 2007). Several studies have reported noticeable differences between rodent ABRs and the ABRs of other mammals. Differences in the localization of the source of ABR waves are believed to occur due to differences in species. In humans, waves I, III, and V are the most common and are frequently used to measure ABRs. Waves III and V, and especially wave V are the largest and therefore are often used to determine the hearing thresholds and to identify the remaining waves. Wave III could be generated in the cochlear nucleus complex and possibly the trapezoid body (Moller and Jannetta, 1983). However, in mice, wave II has been suggested to be generated by the posterior ventral cochlear nucleus and wave V by the lateral lemniscus and inferior colliculus. In rats, wave II is the largest, and wave III is the smallest. Wave V is not commonly used for the evaluation of ABR hearing thresholds (Overbeck and Church, 1992). Thus, there are important implications for studying ABR waveform characteristics in hearing research.
Many studies have reported that the tree shrew (Tupaia belangeri) is a primitive primate mammal and has a wide distribution in Asia and Southwest China. Yan Fan et al. presented a genome sequence for the Chinese tree shrew (Fan et al., 2013) and showed that the tree shrew has close affinity to primates by characterizing key factors and signaling pathways in the nervous system and immune system. Compared to other large non-human primates such as monkeys or orangutans, the tree shrew has several characteristics or advantages more suitable for use as experimental animals, such as its small body, easy ability to feed, and short reproductive cycle and lifespan (Fuchs, 2015; Xu et al., 2012). The tree shrew has been proposed to be a viable and alternative animal model to primates in disease studies and drug safety testing. Many researchers have recently used the tree shrew to create animal models for studying hepatitis B or C virus infections and myopia as well as social stress and depression (Baldivia et al., 2016; Fuchs, 2005; Norton et al., 2006; Wang et al., 2012; Zhao et al., 2002). Nevertheless, there have been few studies on the otology of tree shrews. In our previous studies, we found that the morphology of ear such as the number of cochlear circles and ear ossicles of tree shrews is closer to that of humans than guinea pigs and rats. In this paper, we further examined hearing function by using ABRs on the basis of our original study of the temporal bone anatomy in tree shrews (Lihong et al., 2016; Xu et al., 2012). The goal of the current study was to evaluate the patterns of ABR waves in healthy adult tree shrews by click and tone-pips stimuli and to examine the auditory sensitivity and hearing range. These findings can provide experimental data for studying ears in tree shrews in the future.
2. Materials and methods
2.1. Subjects
Fifteen healthy adult tree shrews (30 ears) were provided by the Experimental Animal Center, Kunming Medical College in China to be studied and bred in this study. The tree shrews were of a Western Yunnan subspecies with ages ranging from 2 to 3 months and weights varing from 120 to 150 g. In this study, we selected tree shrews with sensitivity for the following: the Preyer response, no cerumen in the external auditory canal, and no middle ear infection. All of the procedures were approved by the institutional ethics committees and conformed to Chinese regulations for the use and care of animals in research.
2.2. ABR recordings
The tree shrews were anesthetized by intraperitoneal injection with 1% sodium pentobarbital (4.0 ml/kg). After loss of the righting reflex, the animals were positioned on an electric warming pad at 37–38 °C located inside a sound attenuating room.
A silver recording electrode (positive) was placed subdermally in the midpoint of the parietal periosteal surface. A silver reference electrode (negative) was placed in the test ear, and a silver ground electrode was placed at the tip of the nose.
Clicks and tone-pips were generated by TDT BioSigRP software being run on a computer connected with a TDT System III evoked potential system (Tucker Davis Technologies, USA). Sound stimuli were delivered via ear plugs inserted one ear, and the rate of stimulus delivery was 21.0/s. The presented tone-pips ranged from 1 kHz to 60 kHz with 11 frequencies of 1, 2, 4, 8, 12, 16, 20, 24, 32, 48 and 60 kHz. The tone-pip stimuli were delivered in 2 ms stimulus blocks for each frequency. The scan time was 10 ms with a bandpass filter of 300–3000 Hz, and the number of superpositions was 1024.
Auditory thresholds were defined as the lowest stimulus level with at least 2 repeated responses. Auditory thresholds were obtained for each tested frequency in the present study. To determine the threshold, the sound pressure level was varied from 100 dB to 10 dB SPL and was decreased in 5–10 dB steps during the measurements. The mean value of the ABR amplitude was measured as the peak-to-baseline amplitude of the positive wave. The ABR latency was defined as the time from the stimulus onset to the starting point of the potential. All of these values were measured through the TDT system using the BioSigRP software interface. After the measurements, the animals recovered from anesthesia in their nest box and were returned to their home cage.
2.3. Statistical analysis
Data were measured as the mean ± standard deviation and analyzed using SPSS17.01 statistical software. A correlation analysis was performed, and statistical significance was determined at a level of p < 0.05.
3. Results
3.1. ABR waveforms and the thresholds in tree shrews with the click stimulus
ABRs were recorded and consisted of five positive waves in the first 10 ms after a click stimulus reached the tympanic membrane, and six to seven positive waves could be detected at high sound pressure levels from 70 to 100 dB SPL. The positive components were numbered with Roman numerals I-VI (Fig. 1). However, not all of the components could be detected in all animals. Components I, III and V were easily detected,whereas component II was often unclear. Wave II sometimes occurred, and at other times, there was no wave II. Wave III was the largest, clearest and most stable wave, and the amplitude of wave V was less than that of wave III. Therefore, wave III was used to identify the hearing thresholds and to determine the remaining waves in this study. The average hearing threshold of component III was 27.86 ± 3.78 dB SPL (n = 30).
Fig. 1.
The ABR waveform series for tree shrew using click, the stimulus level is dB SPL.
3.1.1. The latencies and amplitudes of waves with clicks
The latencies and inter-peak latencies of waves I, III, and V were analyzed in the present study (Fig. 2, Table 1). The stimulus intensity ranged from 20 to 100 dB SPL. The latencies were decreased with increasing intensities (wave I, r = −0.998, p < 0.01; wave III, r = −0.973,p < 0.01; wave V, r = −0.988, p < 0.01). Similar to the results observed with the latencies, the amplitudes showed a positive relationship with stimulus intensity, and the amplitudes increased with increasing intensity (wave I, r = 0.987 p < 0.01; wave III, r = 0.985, p < 0.01; wave V, r = 0.988, p < 0.01) (Fig. 3, Table 2).
Fig. 2.
Latency-level of component (I, III, V) for tree shrew in response to click stimuli (for sample sizes, see Table 1). Mean amplitudes and standard deviations. Abscissa: dB SPL; ordinate latency in milliseconds. The latency showed an inverse relationship with stimulus intensity (p < 0.01).
Table 1.
Latency-level functions of component (I, III, V) and inter-peak latency for tree shrew in response to click stimuli (n = 30) (‾x ± S).
| Sound intensity (dB SPL) | Peak Latency (ms) |
Inter-peak Latency (ms) |
||||
|---|---|---|---|---|---|---|
| I | III | Ⅴ | Ⅰ–Ⅲ | Ⅲ–Ⅴ | Ⅰ–Ⅴ | |
| 100 | 1.35 ± 0.26 | 2.13 ± 0.15 | 3.63 ± 0.25 | 0.80 ± 0.21 | 1.46 ± 0.30 | 2.22 ± 0.33 |
| 90 | 1.45 ± 0.24 | 2.16 ± 0.18 | 3.75 ± 0.18 | 0.73 ± 0.19 | 1.58 ± 0.11 | 2.27 ± 0.22 |
| 80 | 1.56 ± 0.25 | 2.21 ± 0.23 | 3.81 ± 0.19 | 0.67 ± 0.23 | 1.58 ± 0.13 | 2.20 ± 0.24 |
| 70 | 1.70 ± 0.24 | 2.35 ± 0.26 | 3.94 ± 0.23 | 0.68 ± 0.21 | 1.56 ± 0.12 | 2.20 ± 0.25 |
| 60 | 1.78 ± 0.23 | 2.47 ± 0.32 | 4.01 ± 0.25 | 0.72 ± 0.22 | 1.52 ± 0.14 | 2.19 ± 0.21 |
| 50 | 1.88 ± 0.25 | 2.63 ± 0.35 | 4.14 ± 0.28 | 0.78 ± 0.21 | 1.46 ± 0.20 | 2.17 ± 0.23 |
| 40 | 1.98 ± 0.26 | 2.63 ± 0.30 | 4.24 ± 0.29 | 0.68 ± 0.21 | 1.55 ± 0.22 | 2.19 ± 0.29 |
| 30 | 2.07 ± 0.26 | 2.65 ± 0.29 | 4.46 ± 0.18 | 0.59 ± 0.16 | 1.77 ± 0.23 | 2.34 ± 0.25 |
| 25 | – | 2.66 ± 0.31 | 4.53 ± 0.19 | – | 1.83 ± 0.25 | – |
Fig. 3.
Amplitude-level functions of component (I, III, V) for tree shrew in response to click stimuli, Abscissa: dB SPL; ordinate component (I, III, V) amplitude in microvolts. The amplitudes showed a positive relationship with stimulus intensity (p < 0.05).
Table 2.
Amplitude-level functions of component III for tree shrew in response to click stimuli (n = 30) (‾x ± S).
| Sound pressure level [dB SPL] | Amplitude [μV] |
||
|---|---|---|---|
| Ⅰ | Ⅲ | Ⅴ | |
| 100 | 1.19 ± 0.25 | 2.84 ± 0.54 | 2.17 ± 0.54 |
| 90 | 1.05 ± 0.35 | 2.18 ± 0.53 | 1.76 ± 0.65 |
| 80 | 0.74 ± 0.33 | 1.86 ± 0.45 | 1.52 ± 0.58 |
| 70 | 0.67 ± 0.30 | 1.79 ± 0.43 | 1.26 ± 0.51 |
| 60 | 0.53 ± 0.21 | 1.01 ± 0.39 | 0.97 ± 0.50 |
| 50 | 0.39 ± 0.18 | 0.76 ± 0.40 | 0.75 ± 0.36 |
| 40 | 0.25 ± 0.13 | 0.51 ± 0.25 | 0.59 ± 0.26 |
| 30 | 0.17 ± 0.10 | 0.25 ± 0.10 | 0.40 ± 0.23 |
| 25 | 0.19 ± 0.07 | 0.26 ± 0.12 | |
3.2. The ABR results of tree shrews with a tone-pip stimulus
3.2.1. ABR waveforms
ABRs consist of five typical waves at 85 dB SPL, and all waves were clearly detected. The related data could be easily measured from the waves. Therefore, we selected 85 dB SPL as the intensity of the tone-pip stimulus. The ABR waveforms were detected from tree shrews at 1, 2, 4, 8, 12, 16, 20, 24, 32, 48, and 60 kHz with the 85 dB SPL intensity tone-pip stimulus (Fig. 4). Four to five distinct components were detectable at 8 kHz and 12 kHz with a higher stimulus level. At 85 dB SPL, the latencies of component III decreased with increasing frequency. The ABR waveforms at 4 kHz could be seen with different stimulus intensities (Fig. 5). The latencies of component III decreased with increasing intensity at 4 kHz.
Fig. 4.
The ABR waveform series for tree shrew using 85 dB SPL tone-pip stimuli.
Fig. 5.
The ABR waveform series for Tree shrew using 4 kHz tone-pip stimuli. The stimulus level is dB SPL.
3.2.2. The ABR thresholds for different frequencies of the tone-pips
The mean thresholds were demonstrated here in the tone-pip stimulus by using different frequencies from 1 kHz to 60 kHz at 1, 2, 4, 8, 12, 16, 20, 24, 32, 48, and 60 kHz. In healthy adult tree shrews, the ABR thresholds were 20.00 ± 6.67 dB SPL at an 8 kHz frequency. The results showed that the ABR thresholds decreased from 1 kHz to 8 kHz and then increased from 8 kHz to 60 kHz (Fig. 6, Table 3). The threshold at 8 kHz was the lowest. The sensitive frequency was approximately 8 kHz in the tree shrews. The ABR threshold exhibited a “U”-shaped curve with stimulus frequency.
Fig. 6.
Mean hearing threshold with standard deviation of the measured thresholds as determined from tone-pip evoked ABRs for the tree shrew.
Table 3.
Hearing threshold as determined from tone-evoked ABRs (n = 30) (‾x ± S).
| Frequency (kHz) | Mean hearing threshold (dB SPL) | SD (dB SPL) |
|---|---|---|
| 1 | 55.00 | 4.63 |
| 2 | 40.67 | 5.94 |
| 4 | 30.67 | 5.94 |
| 8 | 20.00 | 6.67 |
| 12 | 33.33 | 6.99 |
| 16 | 31.00 | 6.32 |
| 20 | 36.33 | 6.94 |
| 24 | 39.67 | 7.67 |
| 32 | 37.67 | 5.30 |
| 48 | 46.67 | 6.46 |
| 60 | 38.00 | 7.75 |
3.2.3. The ABR latencies for different frequencies of tone-pips at 85 dB SPL
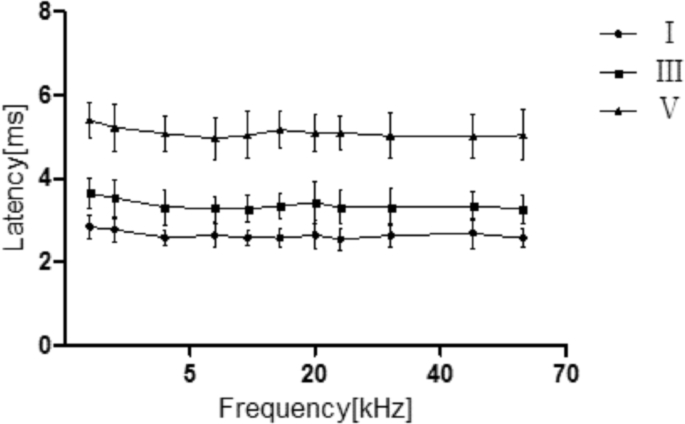
In this study, we estimated the latencies of waves Ⅰ, Ⅲ, and Ⅴ at 1, 2, 4, 8, 12, 16, 20, 24, 32, 48, and 60 kHz frequencies. For all components, the decrements were in the submillisecond range (Fig. 7, Table 4). There was no relationship between latency and frequency. The results are as follows: wave I (r = −0.123, p = 0.115), wave Ⅲ (r = −0.115, p = 0.135) and wave Ⅴ (r = −0.083, p = 0.291).
Fig. 7.
Latency-level functions for tree shrew in response to tone-pip stimuli Mean latencies and standard deviations of the three measured waves (I, III, V). There were no relationship between the latency and frequency (p > 0.05).
Table 4.
Latency-level functions for tree shrew in response to tone-pip stimuli Mean latencies (n = 30) (‾x ± S).
| Frequency (kHz) | I | III | V |
|---|---|---|---|
| 1 | 2.84 ± 0.28 | 3.65 ± 0.37 | 5.40 ± 0.44 |
| 2 | 2.77 ± 0.28 | 3.54 ± 0.44 | 5.23 ± 0.56 |
| 4 | 2.59 ± 0.19 | 3.31 ± 0.43 | 5.08 ± 0.43 |
| 8 | 2.64 ± 0.29 | 3.28 ± 0.30 | 4.96 ± 0.50 |
| 12 | 2.59 ± 0.18 | 3.27 ± 0.32 | 5.05 ± 0.57 |
| 16 | 2.57 ± 0.23 | 3.34 ± 0.30 | 5.17 ± 0.46 |
| 20 | 2.65 ± 0.34 | 3.43 ± 0.50 | 5.10 ± 0.45 |
| 24 | 2.54 ± 0.27 | 3.31 ± 0.40 | 5.10 ± 0.40 |
| 32 | 2.64 ± 0.27 | 3.32 ± 0.44 | 5.03 ± 0.55 |
| 48 | 2.68 ± 0.36 | 3.33 ± 0.35 | 5.01 ± 0.53 |
| 60 | 2.57 ± 0.22 | 3.28 ± 0.34 | 5.04 ± 0.60 |
3.2.4. The ABR amplitudes for the tone-pip stimuli 1, 4, 8, and 16 kHz
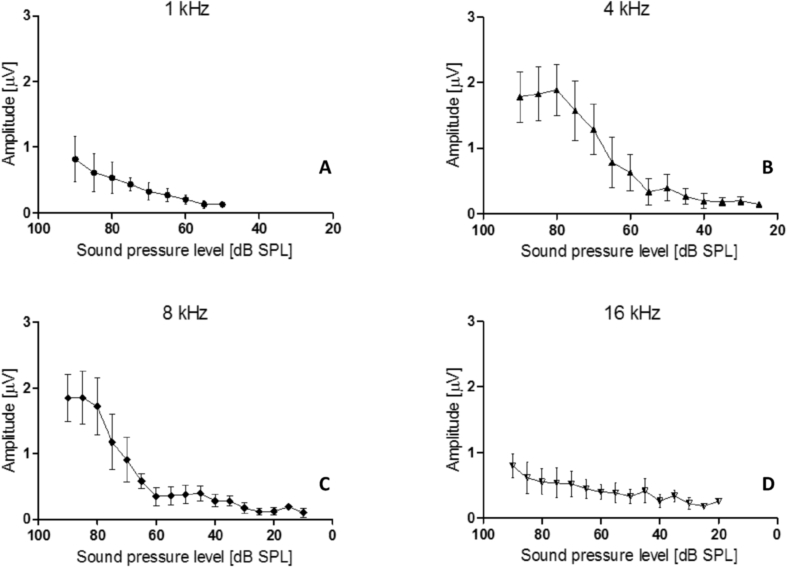
The ABR amplitudes of wave III were under 3 μV and increased with the sound pressure level at 1, 4, 8, and 16 kHz (Fig. 8, Table 5). The largest amplitudes were observed for a tone-pip of 8 kHz. As shown in the figure, wave III exhibit a lower intensity at 8 kHz. The amplitudes showed a relationship with stimulus intensity, as the amplitudes increased with increasing intensity of the tone-pip at different frequencies. The results are as follows: 1 kHz (r = 0.688, p < 0.01), 4 kHz (r = 0.863, p < 0.01), 8 kHz (r = 0.837, p < 0.01) and16 kHz (r = 0.638, p < 0.01) (See Fig. 8, Table 4).
Fig. 8.
Amplitude-level functions of component III for tree shrew in response to tone-pip stimuli (for sample sizes, see Table 1). Mean amplitudes and standard deviations for component III. A.1 kHz Tone pip. B. 2 kHz tone pip. C. 8 kHz tone pip. D 16 kHz tone pip. Abscissa: dB SPL; ordinate component III amplitude in microvolts. The amplitudes showed a positive relationship with stimulus intensity at 1 KHz, 4 KHz, 8 KHz, and 16 KHz (p < 0.01).
Table 5.
Amplitude-level functions of component III for tree shrew in response to tone-pip stimuli (n = 30) (‾x ± S).
| Amplitude (μV) | Frequency (kHz) |
|||
|---|---|---|---|---|
| 1 | 4 | 8 | 16 | |
| 90 dB SPL | 0.81 ± 0.35 | 1.78 ± 0.39 | 1.85 ± 0.36 | 0.80 ± 0.18 |
| 85 dB SPL | 0.61 ± 0.29 | 1.83 ± 0.41 | 1.85 ± 0.40 | 0.62 ± 0.24 |
| 80 dB SPL | 0.53 ± 0.24 | 1.87 ± 0.39 | 1.72 ± 0.44 | 0.55 ± 0.20 |
| 75 dB SPL | 0.43 ± 0.10 | 1.57 ± 0.45 | 1.74 ± 0.42 | 0.54 ± 0.23 |
| 70 dB SPL | 0.32 ± 0.13 | 1.28 ± 0.38 | 0.91 ± 0.34 | 0.52 ± 0.19 |
| 65 dB SPL | 0.27 ± 0.10 | 0.78 ± 0.38 | 0.58 ± 0.11 | 0.45 ± 0.15 |
| 60 dB SPL | 0.20 ± 0.07 | 0.63 ± 0.28 | 0.35 ± 0.14 | 0.40 ± 0.12 |
| 55 dB SPL | 0.13 ± 0.06 | 0.33 ± 0.20 | 0.36 ± 0.14 | 0.39 ± 0.15 |
| 50 dB SPL | 0.13 ± 0.03 | 0.39 ± 0.21 | 0.38 ± 0.14 | 0.33 ± 0.11 |
| 45 dB SPL | 0.26 ± 0.12 | 0.39 ± 0.11 | 0.42 ± 0.18 | |
| 40 dB SPL | 0.19 ± 0.12 | 0.28 ± 0.09 | 0.27 ± 0.10 | |
| 35 dB SPL | 0.18 ± 0.06 | 0.28 ± 0.08 | 0.34 ± 0.09 | |
| 30 dB SPL | 0.19 ± 0.07 | 0.12 ± 0.05 | 0.23 ± 0.09 | |
| 25 dB SPL | 0.14 ± 0.04 | 0.12 ± 0.07 | 0.18 ± 0.03 | |
| 20 dB SPL | 0.12 ± 0.06 | 0.26 | ||
| 15 dB SPL | 0.19 ± 0.02 | |||
| 10 dB SPL | 0.1 ± 0.07 | |||
4. Discussion
The ABR is effective for checking hearing sensitivity and brain function and is a short-latency, far-field evoked potential (Parham et al., 2001). The ABR waves are stable and easy to record. In this study, our goal was to evaluate the hearing function in normal adult tree shrews by using this simple and non-invasion electrophysiological technique. The stimuli included clicks and tone-pips. The frequency of the tone-pip stimulus ranged from 1 kHz to 60 kHz and included 11 frequencies. The ABR waveform pattern of the tree shrews was examined. Four to five vertex positive components in the recorded ABR waveforms of the tree shrews could be observed for the click and tone-pip stimuli, similar to the results of preview studies in mammals, including humans, rats, and gray mouse lemurs (M., 2009; Ramsier et al., 2012b; S et al., 2010). However, the waveform patterns recorded in this study differed from those reported in human and rats: The previous studies indicated that wave II was the largest in rats, while wave III was the smallest. In humans, wave V was the most obvious (Chiappa, 2007; Ingham et al., 1998; Ramsier and Dominy, 2010; Schopf et al., 2014). In this study of tree shrews, wave III was the largest, wave II was the smallest, and the complex of wave II and III complex could often could be seen. The difference in the ABR's maximum ABR waveform may be related to species and the development of the nucleus of the auditory pathway.
Our previous study demonstrated that ABR waveforms changed with damage to the medial geniculate body (Zhu et al., 2015). Similar to the ABR waveform in rats and humans (Tillein et al., 2012), the waveforms in tree shrews were intensity-dependent in tree shrews. The number of components is was likely to be dependent on the stimulus level and neuronal synchrony. The number of components increased when the stimulus intensity was high, especially when the intensity was greater than 80 dB SPL. The waves of the ABR are related to the auditory nerve, cochlear nucleus, superior olivary complex, lateral lemniscus and inferior colliculus (AR, 2006; Chen et al., 2010).
The ABR thresholds are often an estimation of hearing thresholds and do not reach behavioral thresholds (Kappel et al., 2011). In this study, we used click and tone-pips stimuli to examine ABR waveforms. The amplitudes of waves III and V were high, and the amplitudes gradually increased as the intensity of sound stimulation increased. Component III was the largest, most obvious and most stable pattern and disappeared last. Therefore, we used wave III to identify the hearing threshold and the remaining waves. The lowest ABR threshold measured with the click was 27.86 ± 3.78 dB SPL.
The standard mammalian audiogram of the ABR is characterized by a U shape, with the greatest sensitivity in the mid-frequency region (M et al., 2008; Ramsier and Dominy, 2012), and the ABR of many primate species and rats has this general shape. The ABR thresholds in the tree shrew with tone-pips at an 85 dB SPL intensity stimulus also fit this pattern. The most distinct ABR was measured at 8 kHz in the audiogram. Compared to 1 kHz, 4 kHz, and 16 kHz, the amplitude was largest at 8 kHz, and the amplitude gradually increased as the intensity of sound stimulation increased. Wave III can be seen at a lower intensity at a frequency of 8 kHz. Therefore, we believed that 8 kHz was the tree shrew's sensitive hearing frequency. Schopf C found that the gray mouse lemur had a distinct ABR appearance in the audiogram at 7.9 kHz. He also found that the ABR threshold was higher at low frequencies. The ABR decreased with an increase in frequency but then increased at higher frequencies. The frequency for hearing sensitivity may represent an adaptation to frequencies of sounds emitted by prey or predators and may be related to acoustic resonances. As gray mouse lemurs are also a small-bodied primate species, like tree shrews, they may use prey-generated sounds for prey detection and localization (BM et al., 2007; Ramsier et al., 2012a).
Several studies have examined auditory function in tree shrews by different methods, such as shock avoidance and conditioned suppression techniques. These studies have yielded different results because they used different methods. Petersen et al. (Peterson et al., 1968) recorded cochlear potentials from the round window in six animals and detected the highest sensitivity in the range of 7–20 kHz. Heffner et al. (Heffner and Ravizza, 1969) used shock avoidance conditioning and conditioned suppression in two animals and found the lowest thresholds in the range of 4–32 kHz and 16–32 kHz, respectively. See [Fig. 9.] (Zimmermann, 1993) However, the ABR measurements we used to assess hearing function were more objective, and therefore, the measured auditory thresholds, latencies and amplitudes were more accurate.
Fig. 9.
Comparison of auditory thresholds in adult tree shrew as revealed by Elke Zimmermanna et al. (1993) and by Heffner et al. (1969) by shock-avoidance and conditioned suppression techniques (1978).
According to the World Health Organization (WHO), hearing loss is one of the three most common causes of disability that people live with (Chen et al., 2010; S et al., 2010), representing an important social and economic burden in family and countries. In the past decade, many researchers have studied hearing loss using different animal models (Alegre et al., 2001; Pleis and Lethbridge-Cejku, 2007), including rats and guinea pigs. In the coming years, tree shrews may become a useful animal for studying ear diseases and may be a viable and alternative animal model for studying diseases in primates. Therefore, our study provides experimental data for understanding the anatomical, molecular, genetic and pathophysiological mechanisms in the tree shrew.
Acknowledgements
This work was supported by Natural Science Foundation of China[grant numbers 81760188] and Scientific Research Fund of Guangxi Provincial Education Department [grant numbers 2017KY0103 (02601217023C)].
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
Reference
- Alegre M., Gurtubay I.G., Iriarte J. Brainstem auditory evoked potentials (BAEPs) in the cynomolgus macaque monkey. Equivalence with human BAEPs and proposal of a new nomenclature. Hear. Res. 2001;151(1–2):115–120. doi: 10.1016/s0378-5955(00)00215-x. [DOI] [PubMed] [Google Scholar]
- Alvarado J.C., Fuentes-Santamaria V., Jareno-Flores T. Normal variations in the morphology of auditory brainstem response (ABR) waveforms, morphology of auditory brainstem response (ABR) waveforms: a study in Wistar rats. Neurosci. Res. 2012;73(4):302–311. doi: 10.1016/j.neures.2012.05.001. [DOI] [PubMed] [Google Scholar]
- AR M. 2006. Hearing: Anatomy, Physiology, and Disorders of the Auditory System. San Diego. [Google Scholar]
- Baldivia S., Levy A., Hegde S. A novel organ culture model to quantify collagen remodeling in tree shrew sclera. PLoS One. 2016;11(11) doi: 10.1371/journal.pone.0166644. e0166644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BM S., HR G., E R. Sensory basis of food detection in wild Microcebus murinus. Int. J. Primatol. 2007;28:291–304. [Google Scholar]
- Chen B., Zhong Y., Peng W. Age-related changes in the central auditory system: comparison of D-galactose-induced aging rats and naturally aging rats, comparison of D-galactose-induced aging rats and naturally aging rats. Brain Res. 2010;1344:43–53. doi: 10.1016/j.brainres.2010.04.082. [DOI] [PubMed] [Google Scholar]
- Chiappa K.H. Lippincott-Raven; New York: 2007. Evoked Potential in Clinical Medicine. [Google Scholar]
- Fan Y., Huang Z.Y., Cao C.C. Genome of the Chinese tree shrew. Nat. Commun. 2013;4:1426. doi: 10.1038/ncomms2416. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Social stress in tree shrews as an animal model of depression: an example of a behavioral model of a CNS disorder. CNS Spectr. 2005;10(3):182–190. doi: 10.1017/s1092852900010038. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Tree shrews at the German primate center. Primate Biol. 2015;2:111–118. [Google Scholar]
- Heffner H.E., Ravizza R.J. Hearing in primitive mammals:Tree shrews. Aud. Res. 1969;9:12–18. [Google Scholar]
- Ingham N.J., Thornton S.K., Comis S.D. The auditory brainstem response of aged Guinea pigs. Acta Otolaryngol. 1998;118(5):673–680. doi: 10.1080/00016489850183160. [DOI] [PubMed] [Google Scholar]
- Kappel P., Hohenbrink S., Radespiel U. Experimental evidence for olfactory predator recognition in wild mouse lemurs. Am. J. Primatol. 2011;73(9):928–938. doi: 10.1002/ajp.20963. [DOI] [PubMed] [Google Scholar]
- Lihong X., Heng L., Gyanwali B. Micro-computed tomography and microdissection of the temporal bone of tree shrews. Ann. Anat. 2016;208:69–77. doi: 10.1016/j.aanat.2015.08.005. [DOI] [PubMed] [Google Scholar]
- M P., U R., E Z. The sensory basis of prey detection in captive-born grey mouse lemurs, Microcebus murinus. Anim. Behav. 2008;75:871–878. [Google Scholar]
- M C. What do primates hear? A meta-analysis of all known nonhuman primate behavioral audiograms. Int. J. Primatol. 2009;30:55–91. [Google Scholar]
- Moller A.R., Jannetta P.J. Auditory evoked potentials recorded from the cochlear nucleus and its vicinity in man. J. Neurosurg. 1983;59(6):1013–1018. doi: 10.3171/jns.1983.59.6.1013. [DOI] [PubMed] [Google Scholar]
- Norton T.T., Amedo A.O., Siegwart J.T., Jr. Darkness causes myopia in visually experienced tree shrews. Invest. Ophthalmol. Vis. Sci. 2006;47(11):4700–4707. doi: 10.1167/iovs.05-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeck G.W., Church M.W. Effects of tone burst frequency and intensity on the auditory brainstem response (ABR) from albino and pigmented rats. Hear. Res. 1992;59(2):129–137. doi: 10.1016/0378-5955(92)90110-9. [DOI] [PubMed] [Google Scholar]
- Parham K., Sun X.M., Kim D. 2001. Handbook of Mouse Auditory Research: from Behavior to Molecular Biology. [Google Scholar]
- Parkkonen L., Fujiki N., Makela J.P. Sources of auditory brainstem responses revisited: contribution by magnetoencephalography. Hum. Brain Mapp. 2009;30(6):1772–1782. doi: 10.1002/hbm.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E.A., Wruble S.D., Ponzoli V.I. Auditory responses in tree shrewsa nd primates. Aud. Res. 1968;8:345–355. [Google Scholar]
- Pleis J.R., Lethbridge-Cejku M. Summary health statistics for U.S. Adults: national health interview survey. 2006. Vital Health Stat. 2007;10(235):1–153. [PubMed] [Google Scholar]
- Ramsier M.A., Cunningham A.J. Social drive and the evolution of primate hearing. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012;367(1597):1860–1868. doi: 10.1098/rstb.2011.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsier M.A., Cunningham A.J. Primate communication in the pure ultrasound. Biol. Lett. 2012;8(4):508–511. doi: 10.1098/rsbl.2011.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsier M.A., Dominy N.J. A comparison of auditory brainstem responses and behavioral estimates of hearing sensitivity in Lemur catta and Nycticebus coucang. Am. J. Primatol. 2010;72(3):217–233. doi: 10.1002/ajp.20780. [DOI] [PubMed] [Google Scholar]
- Ramsier M.A., Dominy N.J. Receiver bias and the acoustic ecology of aye-ayes (Daubentonia madagascariensis) Commun. Integr. Biol. 2012;5(6):637–640. doi: 10.4161/cib.21509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmuth C., Mulsow J., Finneran J.J. Measurement and response characteristics of auditory brainstem responses in pinnipeds. Aquat. Mamm. 2007;33(1):132–150. [Google Scholar]
- S G.-S., RD F., AN P., RR F. Springer; New York: 2010. The Aging Auditory System. [Google Scholar]
- Schopf C., Zimmermann E., Tunsmeyer J. Hearing and age-related changes in the gray mouse lemur. J. Assoc. Res. Otolaryngol. 2014;15(6):993–1005. doi: 10.1007/s10162-014-0478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillein J., Heid S., Lang E. Development of brainstem-evoked responses in congenital auditory deprivation. Neural Plast. 2012;2012 doi: 10.1155/2012/182767. 182767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Yang C., Su J.J. Factors influencing long-term hepatitis B virus infection of the tree shrew (Tupaia belangeri chinensis) as an in vivo model of chronic hepatitis B. Zhonghua Gan Zang Bing Za Zhi. 2012;20(9):654–658. doi: 10.3760/cma.j.issn.1007-3418.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Xu L., Chen S.Y., Nie W.H. Evaluating the phylogenetic position of Chinese tree shrew (Tupaia belangeri chinensis) based on complete mitochondrial genome: implication for using tree shrew as an alternative experimental animal to primates in biomedical research. J. Genet. Genom. 2012;39(3):131–137. doi: 10.1016/j.jgg.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Zhao X., Tang Z.Y., Klumpp B. Primary hepatocytes of Tupaia belangeri as a potential model for hepatitis C virus infection. J. Clin. Invest. 2002;109(2):221–232. doi: 10.1172/JCI13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M., He G., Li H. The effects of the auditory brainstem response before and after fluoro-gold injection in medial geniculate body. Neuroendocrinol. Lett. 2015;36(8):779–786. [PubMed] [Google Scholar]
- Zimmermann E. Behavioral measures of auditory thresholds in developing tree shrews (Tupaia belangeri) J. Acoust. Soc. Am. 1993;94(6):3071–3075. doi: 10.1121/1.407268. [DOI] [PubMed] [Google Scholar]