Abstract
Extracellular vesicles (EVs), named as exosomes, were recently found to play important roles in cell-cell communication by transducing various biochemical and genetic information. Exosomes, secreted from either tumor cells or stromal cells including immune cells, can eventually remodel tumor environment to promote tumor progression such as metastasis and multidrug resistance (MDR). Therefore, the detection or targeting of biochemical and genetic cargos like proteins, lipids, metabolites and various types of RNAs or DNAs are believed to be valuable for the diagnosis and treatment of human cancer. In this review, we will summarize recent progresses in the research of exosomes especially its biological and clinical relevance to MDR. By doing so, we hope it could be valuable for the prevention, detection and intervention of MDR which is one of the major challenges for the clinical management of human cancers.
Keywords: Exosomes, multidrug resistance, chemotherapy, immune suppression, signal transduction
Introduction
Cancer is a group of diseases that are characterized by uncontrolled cell growth, morphological and cellular transformation, angiogenesis, deregulation of apoptosis and metastasis [1]. With its incidence rates still rising, cancer is the second leading cause of death after cardiovascular diseases worldwide. Chemotherapy is one of most important treatments for various cancer entities. However, cancer cells often have intrinsic resistance or develop acquired multidrug resistance to chemotherapeutic drugs, thus limiting its clinical efficacy. The development of chemotherapeutic drug resistance during the course of treatment for primary and metastatic tumors is a common phenomenon. Molecular mechanism for the development of chemotherapeutics resistance in cancer treatment is a point of common interest across the globe. Many cellular and genetic factors associated with chemotherapeutics drug resistance have been disclosed. However, the exact molecular mechanism underlying the phenomenon of multidrug resistance of tumors remains to be validated.
Exosomes are small nano-molecules secreted by extracellular vesicle bodies (EVBs) which carries various biochemical or genetic information. It plays a vital role in the maintenance of stable physiological and morphological functions. The dynamic studies elucidate the contribution of exosomes to the process of tumor chemo-resistance by facilitating the drug efflux. The drug and its metabolites can be associated with the production and movement of encapsulated exosomes in the cell microenvironment [2]. Recent studies suggested that the multidrug resistance (MDR) proteins MRP, LRP, and several tumor-derived exosomes miRNAs are involved in chemotherapy-associated resistance [3]. Production of exosomes and other components may be influenced by molecular signaling, depending on the origin of the cells and types of cells. Consequently, exosomes have specific roles to play in developing MDR and transferring genetic signals to control metabolism, tumorigenesis, intercellular signaling, and the immune system [4]. Circular DNA, miRNAs and lncRNAs act as either tumor suppressor genes or oncogenes which participate in cancer progression and resistance to therapy [5].
Biogenesis of exosome
Exosomes are small lipid bilayer extracellular vesicles (EVs) secreted by the luminal membranes of the multivesicular bodies (MVBs) and released from mammalian cells by exocytosis [6,7]. Exosomes were first discovered by Trams and his colleagues in sheep reticulocytes early in 1980 [8] and later found in other mammalian including human cells [9]. Exosomes are one of the most heterogeneous groups of MVBs distinguished by their specific size of 30-100 nm and show a cup or dish-like morphology under transmission electron microscope (TEM) in numerous cells like stem cells, immune cells, neurons, cancer cells and some other body fluids like saliva, blood plasma/serum, semen, breast milk, and urine [10]. Before 1990’s, exosomes were considered as garbage bags between membranes and in cytoplasm [11], and later they were found to have a significant role in physiological as well as pathological processes [12]. They intercede cell-to-cell communication by transferring DNA, RNA, proteins and lipids among the cells [13,14]. As a biological messenger in cancer cells, exosomes can transfer both intercellular and intracellular signals. O’Brien et al. found that exosomes level in the serum of breast cancer patients is normally higher when compared to normal samples [15]. However, functions of exosomes are versatile and largely depend upon the origin of cells. For example, exosomes, originating from cancer cells, serve as vehicles for immune system regulation and other pro-cancer properties like tumor growth and propagation [16].
Malignant cells discharge specific sets of EVs that are not secreted by normal eukaryotic cells [17,18]. Further studies may lead to help the detection of specific pathways associated with biosynthesis of distinctive cancer cell-derived EVs. Some tumor cells are resistant to chemotherapy due to MDR-associated proteins MDR1 and ATP-binding cassette (ABC) transporter efflux system. It was reported by Jones that these proteins were carried by exosomes [19]. For example, docetaxel resistant prostate cancer released more exosomes than sensitive cells [20] and so did cisplatin resistant ovarian cancer [21]. Exosomes of colon cancer showed negative effects on proton irradiation and positive influence on the proliferation and metastasis of tumor cells [22]. Emerging evidence supported that exosomes from stromal cells as well as cancer cells might be potentially affected by therapeutic response via transfer of micro-RNAs (miRNAs) and proteins [23,24].
Exosomes in multidrug resistance (MDR)
The key role of exosomes in cancer cells to act as mediators of cell-cell communication with the micro-vesicles has received considerable attention. It can transfer a variety of DNA, RNA and proteins in both paracrine and autocrine manners [13,14]. For example, mesenchymal stem cells (MSC) derived exosomes significantly induce chemotherapy resistance of gastric cancer and also modulate the immune system in a suppressive mechanism [25]. Reported findings proposed that MSC exosomes have deep effects on the development of MDR-associated proteins like LRP, MRP and kinase in gastric cancer cells against 5-fluorouracil and cisplatin [20]. Recent studies have shown that chemotherapy enhances the secretion of exosomes in multiple tumor cells, which contain the chemo-resistance related mi-RNAs and mRNAs of the cells that alter their sensitivity to chemotherapeutic drugs like doxorubicin [26]. MDR genes like BCRP, MDR1 and MRP1 induce chemo-resistance and phenotypic change in breast and prostate cancer cells. They are also associated with the enhanced secretion of exosomes [20,27]. Similarly, Lv et al. found exosomes function as mediators of MDR to transfer drug resistance from drug resistant cells to sensitive cells in MCF-7 breast cancer cell lines [28]. Exosomes might function to facilitate drug efflux like cisplatin through various miRNAs and proteins [29]. The lysosomes, where cisplatin accumulates, release significant exosomes with the help of transporter proteins [21]. Ciravolo and his colleagues found that exosomes are constitutively secreted by HER2-overexpressing breast cancer cell lines both in vitro and in vivo. HER2-positive exosomes bind with trastuzumab and inhibit its anti -proliferative effect on tumor cells. In addition, exosomes released by SKBR3 and BT474, HER2-overexpressing cell lines were significantly regulated by EGF and heregulin growth factors, HER2 receptor-activating ligands present in the surrounding tumor microenvironment [30]. Zhang et al. have found emerging functions of circulating exosome-mediated miRNAs in bortezomib resistance of multiple myeloma, which provided new understanding of the intercellular crosstalk mechanism in myeloma in vivo study [31]. Lopes et al. demonstrated that drug sensitive cells have less extracellular micro-vesicles like exosomes and macrovesicles than MDR cancer cells, and also proposed the potential of exosomal proteins to be used as biomarker in cancer diagnosis [32].
Docetaxel is the first-line chemotherapy in castration-resistant prostate cancer (CRPC). However, it can induce resistance through either P-glycoprotein (P-gp) dependent drug efflux or exosome. Moreover, it has been investigated that exosomes could be used as diagnostic biomarker because they possess specific mRNAs and proteins of the cells from which they are released [33]. Kharaziha et al. reported that exosomes derived from DU145 Tax-Res cells may be a valuable diagnostic biomarker of CRPC [34]. Exosomal miRNA and proteins that mediate MDR in cancer cells are shown in the Tables 1, 2. The delivery of drug-resistant mRNA and P-gp via exosomes is an effective mechanism of exosome-mediated drug resistance transfer [28]. Previous studies have been reported that drug-resistant cancer cells are the main source of exosomes that transfer genetic signal to mediate intracellular communication, either in breast cancer or in prostate cancer [15,20].
Table 1.
Function of exosome mediated microRNA (miR) in cancer
| Type of cancer | Exosome miR | Functions | References |
|---|---|---|---|
| Breast cancer | miR-100, miR-222, miR-125a-3p | Pathogenesis, diagnosis, chemo-resistance | [35,125,126] |
| Leukemia | miR-146, miR-138, miR-203, miR-30a, 135-b, 196-b, 181-c | Autophagy, chemo-resistance | [3,127,128] |
| Prostate and endometrial carcinoma | miR-204, miR-551b, 96, 183, 182, 153, 625, miR-141, 193b, 200c, 193a-3p, 205, 708, 365, 34a | Tumor development and progression, diagnosis | [56,129] |
| Ovarian cancer | miR-21, miR-24 miR-141, miR-200a, miR-200b, miR 200c, miR-203, miR -205, and miR-214 | Diagnostic marker | [54,130] |
| Pancreatic cancer | miR-203 | IL-12, TNF-α suppression | [98] |
| Lung cancer, leukemia, head and neck cancer | miR-17-3p, 21, 106a, 146, 155, 191, 192, 203, 205, 210, 12, 214, 451 | Diagnostic marker, chemo-resistance | [131-134] |
| Esophageal cancer | miR-21, miR221 | Tumor progression, chemo-resistance | [135,136] |
| Lung cancer | miR-21 | Chemo-resistance | [137] |
| Glioblastoma multiform | miR-9 | Temozolomide resistance | [46] |
| Cervical cancer | miR-25 | Cisplatin resistance | [134] |
| Gastric cancer | miR-20, miR-19b-3p, miR-106a-5p | Cisplatin resistance | [138] |
| Liver cancer | miR-9-3p | Diagnosis biomarker | [65] |
| Ovarian cancer | mir-21, miR-100 | Cell signaling | [139,140] |
| Lung cancer | miR940, miR-23a | Tumorigenesis, intercellular communication | [141,142] |
Table 2.
Exosomes associated proteins composition and functions
| Proteins | Name | Functions | References |
|---|---|---|---|
| Tetraspanin | CD9, CD37, CD63, CD53, CD81,CD82, CD83 | Biomarker, multidrug resistance | [143] |
| Heat-shock proteins | HSP70, Hsp84, Hsp90 αBC, HSP20, HSP27 | Multidrug resistance, immune suppression | [144-146,67] |
| Signal transduction | Gi2a, Gi3a, Gsa, FRL, Erk2, Fyn, Sh2, phosphatase, RhoA, C, GDI, syntenin, CBL, Catenin, LCK | Signal transformation | [71] |
| MVB formation | Alix, TSG 101, Gag | Cell trafficking, biomarker | [67] |
| Antigen presentation | MHC I, MHC II, CD86 | Immune system regulation | [72] |
| Adhesion molecules/targeting proteins | CD146, CD166, ICAM-1, ALCAM, MAC-1, Integrin α chain, Integrin α 4 ß1, LFA-3, CD53, CD326, CD11a, 11b, 11c, MFG-E8/lactadherin, CD171, CLDN3 | Intercellular communication | [73,146] |
| Cytoskeletal proteins | Talin, CAP1, ezrin, tubulin, cofilin, actin, moesin, rodixin, advillin, vimentin | Tumorigenesis, immune system regulation | [147] |
| Membrane transport and fusion | AP-1, Arp2/3, SNAP, syntaxin, dynamin, Rab5, 7, Rap1B, RabGDI, annexins (I, II, IV, V, VI), epidermal growth factor receptor (EGFR) | Biogenesis, cell transportation | [148] |
| Enzymes | ATP citrate lyase, ATPase, glucose 6-phosphate isomerase, peroxiredoxin 1, Asp, amino-transferase, aldehyde reductase, fatty acid synthase, pyruvate kinase, glucose 6-phosphate isomerase, ATP citrate, lyase, ATPase, fatty acid synthase (FASN) Thioredoxine peroxidase and alix | Metabolism, cell signaling | [149,71] |
| Anti-apoptosis proteins | Cell progression and angiogenesis, multidrug resistance | [69] | |
| Domain proteins | Proliferation cell nuclear antigen (PCNA) | Proliferation, angiogenesis | [73] |
MSCs have been shown to interact with many factors in mediating drug resistance and enhancing the proliferation of tumor cells [36-38]. Specifically, exosomes from mesenchymal cells transfer signals to the tumor to promote metastasis as well as chemo-resistance. Exosomes and pattern recognition receptors (PRRs) orchestrate heterotypic cellular communications to assist cancer progression, and involve in crosslink connection between cancer and the tumor microenvironment [39]. Balaj et al. have shown that non-coding RNAs that may persuade antiviral responses to influence therapy resistance were found in exosomes [40]. The whole mechanism of exosomes associated multidrug resistance in cancer cells are summarized in the (Figure 1).
Figure 1.
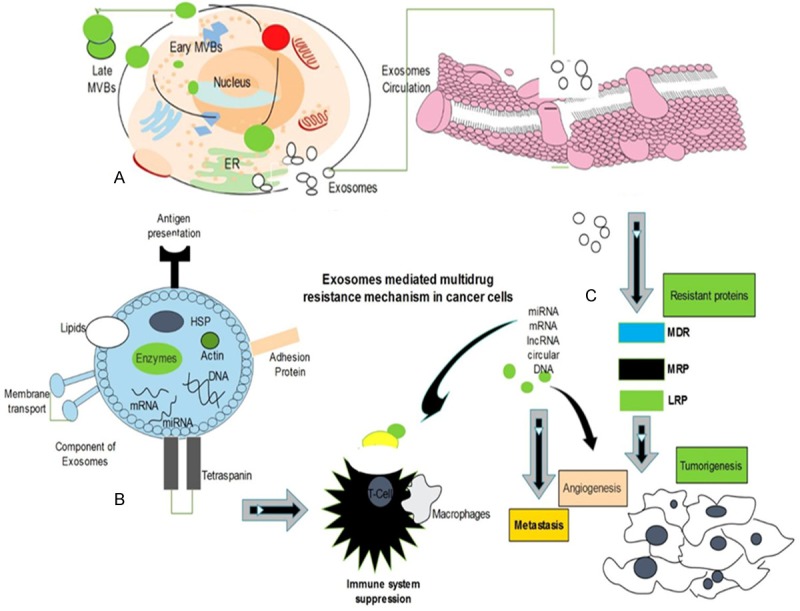
Exosomes biogenesis, morphology and composition, multidrug resistance mechanism. A. MVBs fuse by inward budding in cell and secrete exosomes through plasma membrane. B. Exosomes contain distinct components including nucleic acids, proteins, lipids and enzymes to influence on cell-to-cell communication. C. Exosomes interact with cell membrane and transfer signals to the cells via genetic materials which bind with MDR, MRP, LRP proteins and immune cell receptor and develop resistance to chemotherapy, promote angiogenesis and metastasis.
In addition, exosomal RNA transcripts can stimulate RIG-I activation, which is consistent with the known properties of RIG-I stimulatory viral RNA [41]. MDR cells produced more micro-vesicles and fewer exosomes than drug-sensitive cells because MDR cells contain P-gp, which is associated with the biogenesis of extracellular vesicles [42]. Richards et al. suggested that cancer-associated fibroblasts (CAFs) exosomes enhance chemo-resistance inducing factor Snai1, promote angiogenesis and resistance to gemcitabine in pancreatic ductal adenocarcinomas (PDACs), therefore, using exosome inhibitor could overcome chemo-resistance of PDACs [31,43].
Interestingly, a recent study adopted another method which validates that exosomes may participate in multi-drug resistance. It was found that exosomes released from cancer cells might resist the effect of antibody and chemotherapies by expressing tumor-derived cell surface antigens that confiscate the compound away from the target cell [44]. In addition, exosomes have been identified to reduce antibody dependent cell cytotoxicity (ADCC) by binding to cancer reactive antibodies. It is demonstrated that exosomes interfere with T lymphocytes to inhibit ADCC [45]. In glioblastoma multiforme (GBM) cells, miR-9 promoted resistance against temozolomide (TMZ), increased MDR1 gene expression, activated Sonic Hedgehog (SHH) pathway and suppressed PTCH1 level [46]. The development of de novo resistance acquired by radiation, chemotherapy, and other targeted therapies is still a stumbling issue in the cancer treatment and an emerging field of research [47]. The development of resistance is multi-faceted, as tumor cells may switch to retrieve secondary pathways to survive when the primary hallmark is blocked [48]. Further studies are needed to expand our understanding of molecular mechanism of multidrug resistance and role of exosomes in cancer cells.
Cargos of exosome nucleic acids
Comprehensive researches have been carried out on EVs over the period. The non-spherical membrane-bounded exosomes contain several families of proteins, lipids and nucleic acids originated from the parent cells when observed under high-resolution electron microscope. According to the database, more than 1639 mRNAs, 764 microRNAs, 4653 proteins and 194 lipids could be included in exosomes from various eukaryotic cells [49,50]. For past few years emerging evidence suggests that exosomes are involved in tumor progression and development via intercellular nucleic acid communication [51]. Cancer cells derived exosomes have different kinds of genetic materials including mRNA, microRNAs, circular DNA, small nucleolar RNAs (snoRNAs), transfer RNAs (tRNAs), long noncoding RNAs (lncRNAs), ribosomal RNAs (rRNAs) and small nuclear RNAs (snRNAs), all of these are functionally active [52]. RNA in Exosomes was first detected by Valadi from mice and human derived mast cells. Exosomal RNAs transfer signal from cell to cell; therefore they are termed as “exosomal shuttle RNA” [13]. Adriamycin and docetaxel-resistant breast cancer cells release exosomes that transfer genetic cargo and specific miRNAs between tumor cells and promote the proliferation, angiogenesis, MDR and metastasis [35]. Exosomes may also transfer circular DNA and proteins from one cell to another target cells [53]. Tumor-derived exosomes have distinct mRNA profiles across all type of cancers including ovarian [54], breast [55], prostate [56] and lung cancer [57]. Exosomes contain DNA fragments and mutated mRNA transcripts which may be involved in enhancing the growth and angiogenesis of primary and metastatic cancers [58,59]. According to Xiao et al., miR-21 and 133b are detected in exosomes and highly expressed in DDP-resistant A549 lung cancer cells compared to those in wild-type cells [60]. Leukemia cell-derived exosomes mediate miRNAs transfer to endothelial cells and play substantial roles in enhancing interactions among specific cell populations [61]. A recent research has investigated that mRNAs and miRNAs are present in EVs and they are also transferrable to recipient cells where they can be translated into functional proteins [13].
Outstandingly, miRNAs have been shown to be shuttled between cells by EVs, leading to the repression of mRNA expression in recipient cells [62], although current evidence suggests that miRNAs can also be transported and delivered through other mechanisms [63]. Through exchange of genetic information, EVs are thought to show pleiotropic biological functions and significances in a variety of fundamental physiological and pathological processes [64]. Wang et al. have identified novel exosomal miR-19b-3p and miR-106a-5p in gastric cancer cells and suggest that these miRNAs can be used as biomarkers for diagnosis of gastric cancer [65]. As more miRNAs in exosomes released by human tumor cells and cancer cell lines are characterized [66], it is shown that all these genetic cargos are associated to MDR mechanism in tumor cells. miRNAs as genetic cargo in exosomes and their functions in cancer cells are shown in the Table 1.
Cargos of exosomal proteins and lipids
Exosome proteins are quite different from intercellular proteins secreted by apoptotic cells. They contain specific sets of protein families arising from endocytic pathways [67]. Numerous reports have already shown that exosomes carry membrane transport and fission proteins like Rab, GTPases, Tsg101 and annexin. Furthermore, they included tetraspanin proteins that are associated with lipid microdomains, such as CD9, CD63, CD81, CD82, CD8 [68] and heat shock proteins (HSPs) [51]. Tetraspanin proteins (CD9 and CD63) and HSPs are specifically the most prominent conserved proteins in exosomes that highlight potential biomarkers [69,70]. Mathivanan and his co-workers have reported 11,000 proteins associated with exosomes according to ExoCarta [50]. Some of these proteins are metabolic enzymes like pyruvate dehydrogenases, peroxidases, enolases and lipid kinases [71], while others include major histocompatibility complex (MHC) and signal transduction proteins [72]. Several exosomal proteins overexpressed in plasma of ovarian cancer patients compared to patients with initial stages of tumors and healthy individual controls, including proliferation cell nuclear antigen (PCNA), epithelial cell surface antigen (EpCAM), epidermal growth factor receptor (EGFR), tubulin beta-3 chain (TUBB3), ERBB2, and L1CAM (CD171), apolipoprotein E (APOE), claudin 3 (CLDN3) and fatty acid synthase (FASN). Cellular apoptosis susceptibility (CSE1L/CAS) gene is highly expressed in several tumors that are associated with cell signal transformation and angiogenesis [73,74]. Exosomal proteins have fundamental physiological as well as pathological functions in cell communications, immune suppression, tumor cell prolongation and multidrug resistance to cancer therapy. Exosome cargos with high incidence in cancer cells have been commonly used as biomarkers for the detection and confirmation of exosomes [75]. The composition and functions of exosome-associated proteins are listed in the Table 2.
Moreover, the composition of exosomes from the parental cells can be different due to the distinctive categories of the cargo elements. They are made up of lipid bilayer membranes surrounding part of cytosol in the cells. The structural lipids not only give specific shape to exosomes, but also have significant roles in signal transduction. Lipid is also a major component of exosomes, such as cholesterol, diglycerides, sphingolipids, phospholipids, glycerol phospholipids, phosphatidylserine (PS), phosphatidylethanolamine (PE), phosphatidylinositol (PI) and poly glycerol-phospholipids (i.e. bisphosphate) [49]. A previous research has found evidence that the incidence of phosphoinositides in the inner leaflet of a cell membrane is involved in multiple intracellular signaling pathways, which could be used as biomarker of lipid diseases [76].
Exosomes with bioactive components of lipids such as leukotrienes and prostaglandins can insulate from the parent cells, but they also have some active enzymes of lipid metabolism which are able to produce these bioactive compounds [77,78]. Therefore, exosomes may function as lipid transporters, allowing the passage of the bioactive lipid molecules they carry to recipient cells. This progression of exosome trafficking, predominantly in the context of the tumor microenvironment, may lead to the enhancement of certain tumor progressive/immunosuppressive lipids, such as prostaglandins [79]. However, it may also guide to a supplementary of beneficial exosomal lipid contents, such as docosahexaenoic acid, an omega-3 polyunsaturated fatty acid with many healthy and anticancer benefits, which can affect cell-to-cell signaling, increase sensitivity to therapeutic interventions and reduce tumor cell growth [6,80].
Immune modulation by exosome and MDR
Previous studies elucidating the biological functions of exosomes have revealed important roles of exosomes in modulating immune responses owning to their biochemical and genetical cargos. The immune modulatory function of exosomes was first found in dendritic cells (DCs) through dynamic studies of major histocompatibility complex (MHC) class II. MHC II released from DCs-derived exosomes was capable of stimulating antigen-specific responses both in vitro and in vivo via different pathways, such as cytokine modulation and chemokine transport [81]. Exosomes have several integral, peripheral cytosolic associated proteins induced as antitumor immune responses in vivo and also identify low exosomes production in mature DCs [82], while high amount of exosomes only released by immature DCs. DCs-derived exosomes as key regulators of immune system have several functions. They transport MHCs between dendritic cells, promote the initiation of adaptive immunity, enhance the tumorigenesis and promote resistance to chemotherapy [83]. Mast cell-derived exosomes indirectly activate B and T cells through cell differentiation and specific immune responses [44,84]. Kim et al. reported IL-10 treated DCs derived exosomes reduced inflammation in autoimmune diseases like arthritis [85]. Salivary glands epithelial cells (SGECs) derived exosomes may be associated with autoimmune suppression since they have auto-antigens Ro/SSA, La/SSB, and Sm [86]. Tumor cells commonly cause autoimmune suppression, angiogenesis and progression of tumorigenesis. Tumor cells mediated exosomes have a specific mechanism that controls the activation of immune systems and enhances the tumor cell growth. Synovial fibroblast derived exosomal proteins and TNF-α may involve in the development of apoptosis resistance and suppress T-cells activation induced cell death [87]. OVA protein has stimulatory effects on CD8+ T-cell progression [88]. It induces and controls humoral immunity of specific antigen response to DCs in vivo [89], natural and autoimmunity against bacterial infection in vivo [90]. Exosomes are mobile elements with cargos of proteins and miRNA that suppress the immune system and promote tumor metastasis, cell proliferation, and therapeutic resistance. Cancer-derived exosomes may inhibit natural killer cells proliferation and natural killer group 2 and member D (NKG2D), CD8 (+), and gamma delta (+) T cells [92].
Interestingly, exosomes mediate regulation to immune tolerance or immune activation and suppress hypersensitivity type-IV antigen-specific response. CD80 and CD86, which are co-stimulatory molecules via exosomes through direct interactions with lymphocytes, control natural killer cell activation [93,94]. HCC cells exosomes contain rich amount of HSP60, 70 and 90, which act as a negative signal to inhibit NK cells mediated antitumor responses and activate MDR proteins to enhance resistance to therapy [95]. Recent studies showed that macrophages induce tumor invasion after stimulation by exosomes. Macrophages reduce levels of IL-16, TIMP1, IFNg, and induce a high level of CCL2, IL-8, MIP2 and IL-1Ra, all of which are associated with tumor cell progression [96]. Exosomes based vaccines have been used to induce antitumor immunity [97]. Zhou et al. reported interleukin IL-12 and tumor necrosis factor-α (TNF-α) expression are inhibited by exosomes and cytokines are down-regulated through pancreatic cancer derived exosomes containing miR-203 [98]. Tumor-derived exosomes have cargo elements that are capable of inducing immune suppression through various pathways. In vivo immune system suppression modulated by exosomes drives tumor progression and metastasis development [99]. The exosomes derived from dendritic cells and other immune cells have been implicated in the regulation of immune response [100], while immunological activities of exosomes in tumors are dynamic and complex [101].
Previous studies found that exosomal miRNAs are not only diagnostic biomarkers but also associated with cell signaling pathways between microvesicles. miR-24 enriched in breast cancer-mediated exosomes are involved in T-cell inhibition and tumor angiogenesis. Tumor-mediated exosomal miR-24 regulates tumor angiogenesis by inducing T-cell suppression through FGF11 cell signaling and also acts as a prognostic biomarker in nasopharyngeal malignant cells [102]. Whiteside reported in 2016 that tumor-derived exosomes have potential ability of immune suppression since they carry immunosuppressive factors like DNA, mRNA, and micro RNAs. Exosomes associated signaling pathways contribute to the down-regulation of anticancer immunity and increase MDR resistance to tumor therapy [103].
To better understand mechanisms of cancer development, most of the experimental evidences investigate the immunosuppressive role of tumor-derived exosomes in cancer progression and therapy resistance. For example, overexpression of immunoglobulin B-cell receptor in mice serum and 5T33MM cells by exosomes and identified the relationship between B-cells malignancy and MDR in multiple myeloma [104]. Immune suppression and prolonged angiogenesis induced by melanoma mediated exosomes are regulated by vascular endothelial mediated TNF-α [105]. As for the whole cancer microenvironment, the interaction between the cellular immune system and exosomes can promote tumorigenesis and pathological states by induction of CD4+CD25- T-cells to CD4+CD25+Foxp3+ and T-reg through phosphorylation of Stat3 and Smad2/3 [106,107]. Exosomal tetraspanin family has the ability to induce immune suppression, metastasis and resistance to chemotherapy by systemic induction of angiogenesis signals in cancer and non-cancer cells [108]. In summary, exosomes have the ability to stop anti-tumor immune response and promote tumor development and progression (Figure 2).
Figure 2.
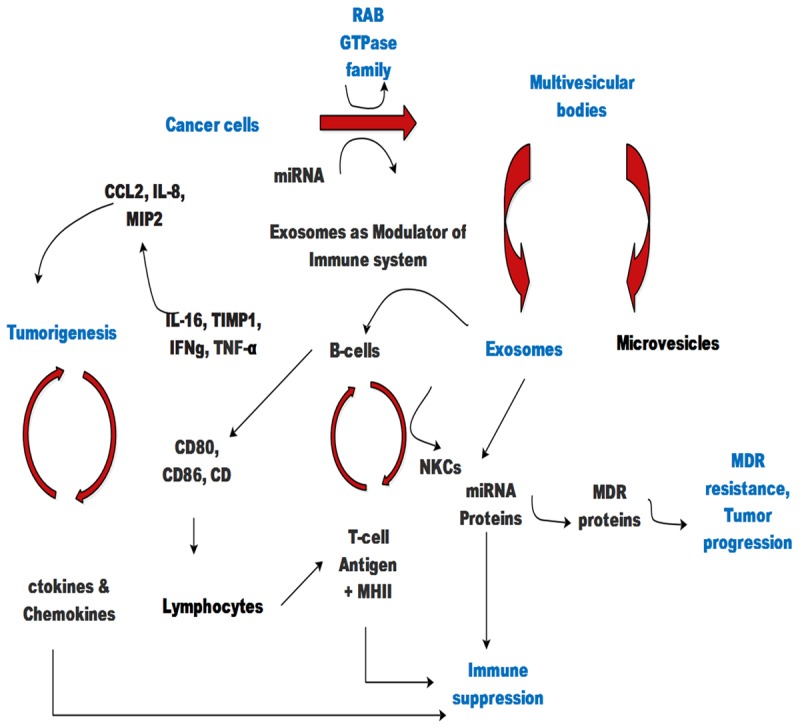
Schematic representation of exosomes mediated cellular effects. MVBs secrete exosomes and transfer signals constituted by proteins and miRNAs and subsequently modulate immune system and promote tumor progression as well as multidrug resistance.
Signal transduction by exosome
One of the most outstanding features of cancer-related exosomes is co-development of phenotypic and genotypic changes in tumor microenvironment through signal transduction between cancer cells and tumor associated stroma. For instance, the well-known mechanism of exosomes-mediated intercellular communication is via signaling molecules such as proteins and mi-RNAs to interact with the surface receptors on target cells, thereby activating intercellular pathways [109]. For example, there are several exosomal mRNAs and mi-RNAs reported to be shuttled between cells that can lead to direct stimulation of target cells through bioactive lipids as potential mediators of signaling pathway [13]. Inclusive studies in the past 30 years have revealed a few signaling pathways activated by transcriptional regulators during cell development and progression such as Notch, Hedgehog (HH) family of secreted proteins, Wingless/WNT, epidermal growth factor (EGF), and fibroblast growth factor (FGF) [110]. Pikkarainen et al. have identified that WNT signaling and Mac-2BP expressions are upregulated in HEK293 cells by induction of exosomes [111]. Further, they suggested that the four-domains of Mac-2BP play a vital role in WNT binding with the C-terminal domain [112]. In vitro experiments reported that WNT signaling pathway was upregulated after treatment by exosomes in human mesenchymal stem cell (MSC) and breast cancer MCF7 cell line [113]. Bretz et al. have shown ex-vivo exosomes induce the cell signaling effects in THP-1 cells by producing IL-1β, TNF-α, and IL-6 to suppress the immune system and enhance resistance to cancer therapy [113].
Signal transduction is essential to existence and maintenance of homeostasis in all multicellular organisms through membrane adhesion molecules, gap junctions and nanotubes, or via soluble signal transduction such as cytokines, growth factors, and hormones secreted by both cancer and non-cancer cells [114]. Tumor-derived exosomes (TEX) carry cargos of several stimulatory and inhibitory biomolecules, serving as a signal network in cancer micro-environment in vivo and in vitro. The TEX induced Ca2+ influx in cancer cells and subsequent signaling are important for T-reg to function. Thus, modulation of T-reg suppressor by TEX is through cell signaling-dependent mechanisms and does not need TEX internalization by recipient cells [115]. A recent research has investigated that chemotherapy-induced exosomes in myeloma cancer cells lead to the stimulation of ERK signaling. Moreover, an increase in flaking of the syndecan-1 proteoglycan and anti-myeloma therapy triggers a release of exosomes containing a huge amount of heparanase that remodels extracellular matrix and modifies tumor- host cell interaction, probably contributing to chemo-resistance and ultimate disease recurrence [116]. In addition, the key mediators in intercellular communications are small circular molecules like EVs secreted by host cells. They mediate the exchange of genetic materials and proteins associated with tumor angiogenesis. Exosomes derived from tumor-associated macrophages (TAMs) mediate cisplatin resistance in gastric cancer via signal molecule miR-21. Furthermore, miR-21 suppresses cell apoptosis and enhances upregulation of PI3K/AKT signaling pathway by down-regulation of PTEN in gastric cancer [117]. Transforming growth factor-beta (TGFβ) signaling mediated by exosomes in squamous cell carcinoma (SCC) is also reported. Exosomes transferring components of the TGFβ signaling pathway between tumor cells and stromal fibroblasts provide possible mechanism for MDR [118].
Translational values of exosome
In the uprising research of cancer, diagnosis and treatment of MDR is a big challenge. Analysis of exosomes may present potential biomarkers to monitor the progression or emergence of MDR during cancer treatment. Exosomal RNAs stimulate the receptor RIG1 to activate STAT1 signaling pathway in breast cancer cells (BrCa). In parallel, stromal cells also activate NOTCH3 in BrCa cells as STAT1 facilitates transcriptional responses to NOTCH3 and increases chemotherapy-resistant tumorigenesis [23]. Ristorcelli et al. have reported the first evidence that exosomes are involved in apoptosis pathway, where it can up-regulate the expression of Bax but down-regulate the expression of Bcl-2 in human pancreatic cancer cells. Furthermore, exosomes can induce the increased activity of phosphatase, tensin homologue (PTEN) and glycogen synthase kinase (GSK)-3β and decrease the expression of keto acid dehydrogenase [119]. Recent studies anticipated that exosomal miR-222 directs target MDR gene expression and could regulate resistance to breast cancer therapy in MCF-7 cultured cells [35], and GLT1 (astrological glutamate transporter) expression is also regulated by neuronal exosomes miRNAs [120]. Melo et al. have reported that cancer mediated exosomes contain RISC-Loading complex miRNAs along with AGO2, TRBP, and Dicer proteins, which are capable of rapidly silencing of mRNAs and reprograming the target cell transcriptome. These findings provide significant opportunities for the development of exosomes-based diagnostic biomarkers and cancer therapy [121]. However, an additional mechanism has recently emerged, based on the exosomes released by breast cancer cells. They revealed that psoralen can affect the exosomes and it persuades the reduction of MDR resistance proteins transmission via exosomes probably through p53 and PPAR signaling pathways [122]. This study might provide a novel approach to deal with resistance to chemotherapy in breast cancer. Further advances in our understanding with regard to translational values of exosomes and other EVs on target cells highlight the significance of establishing essential knowledge in this field.
MSC-exosomes play significant roles in post-transcriptional and translational level of genes in various carcinoma. Ji et al. demonstrated that exosomes triggered the activation of Raf/MEK/ERK kinase cascade and calcium/calmodulin-dependent protein kinases (CaMKs) in gastric cancer cells, while blocking these kinases could be achieved by inhibiting the promoting role of MSC-exosomes in chemo-resistance gastric cancer cells [25]. Exosomal proteins AnxA2 contributes to metastatic cell transformation and its overexpression and phosphorylation are related to cancer progression and tumorigenesis. However, regulation of AnxA2 function is an essential feature of cellular stability, and the prospective regulations of exosomal AnxA2 by phosphorylation or other PTMs are the most interesting studies in future [123]. Further evidence found that ubiquitinated proteins in exosomes are shed by myeloid-derived suppressor cells (MDSC). These five proteins associated with endosomal trafficking, i.e., keratin, histone, heat shock proteins, ezrin and pyruvate kinase isozyme, were detected as ubiquitinated proteins as a post-translational modification of histones in cancer cells [124].
Conclusions and perspectives
The predictive value of exosomes in cancer has expanded rapidly over the past few decades. This has led to the understanding of the fundamental role of exosomes in tumor growth and multidrug resistance. Exosomes are small but skilled mediators of immune responses, intercellular communications and tumorigenesis. The precise understanding of physiological functions of exosomes may allow us to develop novel diagnostics, therapeutics, and targeted drug delivery approaches to overcome multiple drug resistance. However, many opening questions still exist, such as the heterogeneity of cancer exosomes, exosome cargos effects on donor and recipient cells, the biogenesis of exosomes and molecular mechanisms of de novo and adopted resistance to therapy. Future studies will focus on several unknown functions of exosomes in cancer biology and need to further explore the co-contribution of nucleic acids, proteins, and lipids to the genotype as well as the phenotype of the cancer. We can further elucidate novel and useful therapeutic approaches for MDR, based on the inhibition of exosomes-facilitated intercellular communication in cancer cells.
Acknowledgements
This work was supported by Natural Science Foundation of China (81572715; 81772944), Zhejiang Natural Science Foundation (LZ18H160001) and Department of Healthcare in Zhejiang. The authors acknowledge the input of Dr. Qurat-ul-ain Arif, Oxford University, U.K. for proofreading this review.
Disclosure of conflict of interest
None.
References
- 1.Boveri T. Concerning the origin of malignant tumours by Theodor Boveri. Translated and annotated by Henry Harris. J Cell Sci. 2008;121(Suppl 1):1–84. doi: 10.1242/jcs.025742. [DOI] [PubMed] [Google Scholar]
- 2.Chen KG, Valencia JC, Lai B, Zhang G, Paterson JK, Rouzaud F, Berens W, Wincovitch SM, Garfield SH, Leapman RD, Hearing VJ, Gottesman MM. Melanosomal sequestration of cytotoxic drugs contributes to the intractability of malignant melanomas. Proc Natl Acad Sci U S A. 2006;103:9903–9907. doi: 10.1073/pnas.0600213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu Y, Yang L, Zhao M, Zhu S, Kang R, Vernon P, Tang D, Cao L. Targeting microRNA-30amediated autophagy enhances imatinib activity against human chronic myeloid leukemia cells. Leukemia. 2012;26:1752–1760. doi: 10.1038/leu.2012.65. [DOI] [PubMed] [Google Scholar]
- 4.Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics. 2009;6:267–283. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- 5.Yang Q, Diamond MP, Al-Hendy A. The emerging role of extracellular vesicle-derived miRNAs: implication in cancer progression and stem cell related diseases. J Clin Epigenet. 2016;2 [PMC free article] [PubMed] [Google Scholar]
- 6.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 7.Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak M. Membranederived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487–1495. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- 8.Trams EG, Lauter CJ, Salem JN, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;645:63–70. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- 9.Denzer K, Kleijmeer MJ, Heijnen H, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113:3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- 10.Hesari A, Moghadam SA, Siasi A, Rahmani M, Behboodi N, Rastgar-Moghadam A, Ferns GA, Ghasemi F, Avan A. Tumor-derived exosomes: potential biomarker or therapeutic target in breast cancer? J Cell Biochem. 2018;119:4236–4240. doi: 10.1002/jcb.26364. [DOI] [PubMed] [Google Scholar]
- 11.Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: from biogenesis and secretion to biological function. Immunol Lett. 2006;107:102–108. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief C, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 14.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Brien K, Rani S, Corcoran C, Wallace R, Hughes L, Friel AM, McDonnell S, Crown J, Radomski MW, O’Driscoll L. Exosomes from triple-negative breast cancer cells can transfer phenotypic traits representing their cells of origin to secondary cells. Eur J Cancer. 2013;49:1845–1859. doi: 10.1016/j.ejca.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Lespagnol A, Duflaut D, Beekman C, Blanc L, Fiucci G, Marine JC, Vidal M, Amson R, Telerman A. Exosome secretion, including the DNA damage-induced p53-dependent secretory pathway, is severely compromised in TSAP6/Steap3-null mice. Cell Death Differ. 2008;15:1723–1733. doi: 10.1038/cdd.2008.104. [DOI] [PubMed] [Google Scholar]
- 17.Palma J, Yaddanapudi SC, Pigati L, Havens MA, Jeong S, Weiner GA, Weimer KM, Stern B, Hastings ML, Duelli DM. MicroRNAs are exported from malignant cells in customized particles. Nucleic Acids Res. 2012;40:9125–9138. doi: 10.1093/nar/gks656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Vizio D, Morello M, Dudley AC, Schow PW, Adam RM, Morley S, Mulholland D, Rotinen M, Hager MH, Insabato L, Moses MA, Demichelis F, Lisanti MP, Wu H, Klagsbrun M, Bhowmick NA, Rubin MA, D’Souza-Schorey C, Freeman MR. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am J Pathol. 2012;181:1573–1584. doi: 10.1016/j.ajpath.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones PM, George AM. The ABC transporter structure and mechanism: perspectives on recent research. Cell Mol Life Sci. 2004;61:682–699. doi: 10.1007/s00018-003-3336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corcoran C, Rani S, O’Brien K, O’Neill A, Prencipe M, Sheikh R, Webb G, McDermott R, Watson W, Crown J, O’Driscoll L. Docetaxel-resistance in prostate cancer: evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS One. 2012;7:e50999. doi: 10.1371/journal.pone.0050999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Safaei R, Larson BJ, Cheng TC, Gibson MA, Otani S, Naerdemann W, Howell SB. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol Cancer Ther. 2005;4:1595–1604. doi: 10.1158/1535-7163.MCT-05-0102. [DOI] [PubMed] [Google Scholar]
- 22.Khan S, Jutzy JM, Aspe JR, McGregor DW, Neidigh JW, Wall NR. Survivin is released from cancer cells via exosomes. Apoptosis. 2011;16:1–12. doi: 10.1007/s10495-010-0534-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y, Yoon T, Azzam DJ, Twyman-Saint Victor C, Wiemann BZ, Ishwaran H, Ter Brugge PJ, Jonkers J, Slingerland J, Minn AJ. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell. 2014;159:499–513. doi: 10.1016/j.cell.2014.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Challagundla KB, Wise PM, Neviani P, Chava H, Murtadha M, Xu T, Kennedy R, Ivan C, Zhang X, Vannini I, Fanini F, Amadori D, Calin GA, Hadjidaniel M, Shimada H, Jong A, Seeger RC, Asgharzadeh S, Goldkorn A, Fabbri M. Exosome-mediated transfer of microRNAs within the tumor microenvironment and neuroblastoma resistance to chemotherapy. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji R, Zhang B, Zhang X, Xue J, Yuan X, Yan Y, Wang M, Zhu W, Qian H, Xu W. Exosomes derived from human mesenchymal stem cells confer drug resistance in gastric cancer. Cell Cycle. 2015;14:2473–2483. doi: 10.1080/15384101.2015.1005530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shedden K, Xie XT, Chandaroy P, Chang YT, Rosania GR. Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer Res. 2003;63:4331–4337. [PubMed] [Google Scholar]
- 27.Li WJ, Zhong SL, Wu YJ, Xu WD, Xu JJ, Tang JH, Zhao JH. Systematic expression analysis of genes related to multidrug-resistance in isogenic docetaxel- and adriamycin-resistant breast cancer cell lines. Mol Biol Rep. 2013;40:6143–6150. doi: 10.1007/s11033-013-2725-x. [DOI] [PubMed] [Google Scholar]
- 28.Lv MM, Zhu XY, Chen WX, Zhong SL, Hu Q, Ma TF, Zhang J, Chen L, Tang JH, Zhao JH. Exosomes mediate drug resistance transfer in MCF-7 breast cancer cells and a probable mechanism is delivery of P-glycoprotein. Tumour Biol. 2014;35:10773–10779. doi: 10.1007/s13277-014-2377-z. [DOI] [PubMed] [Google Scholar]
- 29.Zhang G, Sun L, Lu X, Chen Z, Duerksen-Hughes PJ, Hu H, Zhu X, Yang J. Cisplatin treatment leads to changes in nuclear protein and microRNA expression. Mutat Res. 2012;746:66–77. doi: 10.1016/j.mrgentox.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Ciravolo V, Huber V, Ghedini GC, Venturelli E, Bianchi F, Campiglio M, Morelli D, Villa A, Della Mina P, Menard S, Filipazzi P, Rivoltini L, Tagliabue E, Pupa SM. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J Cell Physiol. 2012;227:658–667. doi: 10.1002/jcp.22773. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Pan L, Xiang B, Zhu H, Wu Y, Chen M, Guan P, Zou X, Valencia CA, Dong B, Li J, Xie L, Ma H, Wang F, Dong T, Shuai X, Niu T, Liu T. Potential role of exosome-associated microRNA panels and in vivo environment to predict drug resistance for patients with multiple myeloma. Oncotarget. 2016;7:30876–30891. doi: 10.18632/oncotarget.9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopes-Rodrigues V, Di Luca A, Sousa D, Seca H, Meleady P, Henry M, Lima RT, O’Connor R, Vasconcelos MH. Data supporting the shedding of larger extracellular vesicles by multidrug resistant tumour cells. Data Brief. 2016;6:1023–1027. doi: 10.1016/j.dib.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kato T, Mizutani K, Kameyama K, Kawakami K, Fujita Y, Nakane K, Kanimoto Y, Ehara H, Ito H, Seishima M, Deguchi T, Ito M. Serum exosomal P-glycoprotein is a potential marker to diagnose docetaxel resistance and select a taxoid for patients with prostate cancer. Urol Oncol. 2015;33:385, e315–320. doi: 10.1016/j.urolonc.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 34.Kharaziha P, Chioureas D, Rutishauser D, Baltatzis G, Lennartsson L, Fonseca P, Azimi A, Hultenby K, Zubarev R, Ullen A, Yachnin J, Nilsson S, Panaretakis T. Molecular profiling of prostate cancer derived exosomes may reveal a predictive signature for response to docetaxel. Oncotarget. 2015;6:21740–21754. doi: 10.18632/oncotarget.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen WX, Liu XM, Lv MM, Chen L, Zhao JH, Zhong SL, Ji MH, Hu Q, Luo Z, Wu JZ, Tang JH. Exosomes from drug-resistant breast cancer cells transmit chemoresistance by a horizontal transfer of microRNAs. PLoS One. 2014;9:e95240. doi: 10.1371/journal.pone.0095240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Houthuijzen JM, Daenen LG, Roodhart JM, Voest EE. The role of mesenchymal stem cells in anti-cancer drug resistance and tumour progression. Br J Cancer. 2012;106:1901–1906. doi: 10.1038/bjc.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez CG, Penfornis P, Oskowitz AZ, Boonjindasup AG, Cai DZ, Dhule SS, Rowan BG, Kelekar A, Krause DS, Pochampally RR. Activation of autophagy in mesenchymal stem cells provides tumor stromal support. Carcinogenesis. 2011;32:964–972. doi: 10.1093/carcin/bgr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Boeck A, Pauwels P, Hensen K, Rummens JL, Westbroek W, Hendrix A, Maynard D, Denys H, Lambein K, Braems G, Gespach C, Bracke M, De Wever O. Bone marrow-derived mesenchymal stem cells promote colorectal cancer progression through paracrine neuregulin 1/HER3 signalling. Gut. 2013;62:550–560. doi: 10.1136/gutjnl-2011-301393. [DOI] [PubMed] [Google Scholar]
- 39.Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M, Wrana JL. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151:1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 40.Balaj L, Lessard R, Dai L, Cho YJ, Pomeroy SL, Breakefield XO, Skog J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun. 2011;2:180. doi: 10.1038/ncomms1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ireton RC, Gale M Jr. RIG-I like receptors in antiviral immunity and therapeutic applications. Viruses. 2011;3:906–919. doi: 10.3390/v3060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopes-Rodrigues V, Di Luca A, Sousa D, Seca H, Meleady P, Henry M, Lima RT, O’Connor R, Vasconcelos MH. Multidrug resistant tumour cells shed more microvesicle-like EVs and less exosomes than their drug-sensitive counterpart cells. Biochim Biophys Acta. 2016;1860:618–627. doi: 10.1016/j.bbagen.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 43.Richards KE, Zeleniak AE, Fishel ML, Wu J, Littlepage LE, Hill R. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene. 2017;36:1770–1778. doi: 10.1038/onc.2016.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aung T, Chapuy B, Vogel D, Wenzel D, Oppermann M, Lahmann M, Weinhage T, Menck K, Hupfeld T, Koch R, Trumper L, Wulf GG. Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. Proc Natl Acad Sci U S A. 2011;108:15336–15341. doi: 10.1073/pnas.1102855108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Battke C, Ruiss R, Welsch U, Wimberger P, Lang S, Jochum S, Zeidler R. Tumour exosomes inhibit binding of tumour-reactive antibodies to tumour cells and reduce ADCC. Cancer Immunol Immunother. 2011;60:639–648. doi: 10.1007/s00262-011-0979-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Munoz JL, Rodriguez-Cruz V, Walker ND, Greco SJ, Rameshwar P. Temozolomide resistance and tumor recurrence: halting the Hedgehog. Cancer Cell Microenviron. 2015;2 doi: 10.14800/ccm.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer. 2009;9:665–674. doi: 10.1038/nrc2714. [DOI] [PubMed] [Google Scholar]
- 48.Shain KH, Landowski TH, Dalton WS. The tumor microenvironment as a determinant of cancer cell survival: a possible mechanism for de novo drug resistance. Curr Opin Oncol. 2000;12:557–563. doi: 10.1097/00001622-200011000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Hannafon BN, Ding WQ. Intercellular communication by exosome-derived microRNAs in cancer. Int J Mol Sci. 2013;14:14240–14269. doi: 10.3390/ijms140714240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40:D1241–1244. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bang C, Thum T. Exosomes: new players in cell-cell communication. Int J Biochem Cell Biol. 2012;44:2060–2064. doi: 10.1016/j.biocel.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 52.Gajos-Michniewicz A, Duechler M, Czyz M. MiRNA in melanoma-derived exosomes. Cancer Lett. 2014;347:29–37. doi: 10.1016/j.canlet.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 53.Loyer X, Vion AC, Tedgui A, Boulanger CM. Microvesicles as cell-cell messengers in cardiovascular diseases. Circ Res. 2014;114:345–353. doi: 10.1161/CIRCRESAHA.113.300858. [DOI] [PubMed] [Google Scholar]
- 54.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 55.Collignon J, Struman I, Tabruyn S, Josse C, Boukerroucha M, Jerusalem G, Bours V. [Molecular and genetic aspects of triple negative breast cancer and therapeutic implications] . Rev Med Liege. 2011;66:393–396. [PubMed] [Google Scholar]
- 56.Hessvik NP, Phuyal S, Brech A, Sandvig K, Llorente A. Profiling of microRNAs in exosomes released from PC-3 prostate cancer cells. Biochim Biophys Acta. 2012;1819:1154–1163. doi: 10.1016/j.bbagrm.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 57.Rosell R, Wei J, Taron M. Circulating MicroRNA signatures of tumor-derived exosomes for early diagnosis of non-small-cell lung cancer. Clin Lung Cancer. 2009;10:8–9. doi: 10.3816/CLC.2009.n.001. [DOI] [PubMed] [Google Scholar]
- 58.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dvorakova M, Karafiat V, Pajer P, Kluzakova E, Jarkovska K, Pekova S, Krutilkova L, Dvorak M. DNA released by leukemic cells contributes to the disruption of the bone marrow microenvironment. Oncogene. 2013;32:5201–5209. doi: 10.1038/onc.2012.553. [DOI] [PubMed] [Google Scholar]
- 60.Xiao X, Yu S, Li S, Wu J, Ma R, Cao H, Zhu Y, Feng J. Exosomes: decreased sensitivity of lung cancer A549 cells to cisplatin. PLoS One. 2014;9:e89534. doi: 10.1371/journal.pone.0089534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Umezu T, Ohyashiki K, Kuroda M, Ohyashiki JH. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene. 2013;32:2747–2755. doi: 10.1038/onc.2012.295. [DOI] [PubMed] [Google Scholar]
- 62.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, de Gruijl TD, Wurdinger T, Middeldorp JM. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.EL Andaloussi S, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 65.Wang N, Wang L, Yang Y, Gong L, Xiao B, Liu X. A serum exosomal microRNA panel as a potential biomarker test for gastric cancer. Biochem Biophys Res Commun. 2017;493:1322–1328. doi: 10.1016/j.bbrc.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 66.Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, Rivoltini L. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 2008;15:80–88. doi: 10.1038/sj.cdd.4402237. [DOI] [PubMed] [Google Scholar]
- 67.Bobrie A, Colombo M, Raposo G, Thery C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 68.Harshman SW, Canella A, Ciarlariello PD, Rocci A, Agarwal K, Smith EM, Talabere T, Efebera YA, Hofmeister CC, Benson DM Jr, Paulaitis ME, Freitas MA, Pichiorri F. Characterization of multiple myeloma vesicles by label-free relative quantitation. Proteomics. 2013;13:3013–3029. doi: 10.1002/pmic.201300142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Simpson RJ, Kalra H, Mathivanan S. ExoCarta as a resource for exosomal research. J Extracell Vesicles. 2012:1. doi: 10.3402/jev.v1i0.18374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Logozzi M, De Milito A, Lugini L, Borghi M, Calabro L, Spada M, Perdicchio M, Marino ML, Federici C, Iessi E, Brambilla D, Venturi G, Lozupone F, Santinami M, Huber V, Maio M, Rivoltini L, Fais S. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS One. 2009;4:e5219. doi: 10.1371/journal.pone.0005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hegmans JP, Bard MP, Hemmes A, Luider TM, Kleijmeer MJ, Prins JB, Zitvogel L, Burgers SA, Hoogsteden HC, Lambrecht BN. Proteomic analysis of exosomes secreted by human mesothelioma cells. Am J Pathol. 2004;164:1807–1815. doi: 10.1016/S0002-9440(10)63739-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beach A, Zhang HG, Ratajczak MZ, Kakar SS. Exosomes: an overview of biogenesis, composition and role in ovarian cancer. J Ovarian Res. 2014;7:14. doi: 10.1186/1757-2215-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liang B, Peng P, Chen S, Li L, Zhang M, Cao D, Yang J, Li H, Gui T, Li X, Shen K. Characterization and proteomic analysis of ovarian cancer-derived exosomes. J Proteomics. 2013;80:171–182. doi: 10.1016/j.jprot.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 74.Jiang MC. CAS (CSE1L) signaling pathway in tumor progression and its potential as a biomarker and target for targeted therapy. Tumour Biol. 2016;37:13077–13090. doi: 10.1007/s13277-016-5301-x. [DOI] [PubMed] [Google Scholar]
- 75.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 76.Record M, Carayon K, Poirot M, Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta. 2014;1841:108–120. doi: 10.1016/j.bbalip.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 77.Hsieh AL, Walton ZE, Altman BJ, Stine ZE, Dang CV. MYC and metabolism on the path to cancer. Semin Cell Dev Biol. 2015;43:11–21. doi: 10.1016/j.semcdb.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Van Dyke T, Jacks T. Cancer modeling in the modern era: progress and challenges. Cell. 2002;108:135–144. doi: 10.1016/s0092-8674(02)00621-9. [DOI] [PubMed] [Google Scholar]
- 79.Hu G, Drescher KM, Chen XM. Exosomal miRNAs: biological properties and therapeutic potential. Front Genet. 2012;3:56. doi: 10.3389/fgene.2012.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 81.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 82.Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 83.Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 84.Skokos D, Botros HG, Demeure C, Morin J, Peronet R, Birkenmeier G, Boudaly S, Mecheri S. Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J Immunol. 2003;170:3037–3045. doi: 10.4049/jimmunol.170.6.3037. [DOI] [PubMed] [Google Scholar]
- 85.Kim SH, Lechman ER, Bianco N, Menon R, Keravala A, Nash J, Mi Z, Watkins SC, Gambotto A, Robbins PD. Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. J Immunol. 2005;174:6440–6448. doi: 10.4049/jimmunol.174.10.6440. [DOI] [PubMed] [Google Scholar]
- 86.Kapsogeorgou EK, Abu-Helu RF, Moutsopoulos HM, Manoussakis MN. Salivary gland epithelial cell exosomes: a source of autoantigenic ribonucleoproteins. Arthritis Rheum. 2005;52:1517–1521. doi: 10.1002/art.21005. [DOI] [PubMed] [Google Scholar]
- 87.Zhang HG, Liu C, Su K, Yu S, Zhang L, Zhang S, Wang J, Cao X, Grizzle W, Kimberly RP. A membrane form of TNF-alpha presented by exosomes delays T cell activation-induced cell death. J Immunol. 2006;176:7385–7393. doi: 10.4049/jimmunol.176.12.7385. [DOI] [PubMed] [Google Scholar]
- 88.Hao S, Bai O, Yuan J, Qureshi M, Xiang J. Dendritic cell-derived exosomes stimulate stronger CD8+ CTL responses and antitumor immunity than tumor cell-derived exosomes. Cell Mol Immunol. 2006;3:205–211. [PubMed] [Google Scholar]
- 89.Colino J, Snapper CM. Exosomes from bone marrow dendritic cells pulsed with diphtheria toxoid preferentially induce type 1 antigen-specific IgG responses in naive recipients in the absence of free antigen. J Immunol. 2006;177:3757–3762. doi: 10.4049/jimmunol.177.6.3757. [DOI] [PubMed] [Google Scholar]
- 90.Colino J, Snapper CM. Dendritic cell-derived exosomes express a streptococcus pneumoniae capsular polysaccharide type 14 cross-reactive antigen that induces protective immunoglobulin responses against pneumococcal infection in mice. Infect Immun. 2007;75:220–230. doi: 10.1128/IAI.01217-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kedrin D, van Rheenen J, Hernandez L, Condeelis J, Segall JE. Cell motility and cytoskeletal regulation in invasion and metastasis. J Mammary Gland Biol Neoplasia. 2007;12:143–152. doi: 10.1007/s10911-007-9046-4. [DOI] [PubMed] [Google Scholar]
- 92.Clayton A, Mitchell JP, Court J, Linnane S, Mason MD, Tabi Z. Human tumor-derived exosomes down-modulate NKG2D expression. J Immunol. 2008;180:7249–7258. doi: 10.4049/jimmunol.180.11.7249. [DOI] [PubMed] [Google Scholar]
- 93.Ruffner MA, Kim SH, Bianco NR, Francisco LM, Sharpe AH, Robbins PD. B7-1/2, but not PD-L1/2 molecules, are required on IL-10-treated tolerogenic DC and DC-derived exosomes for in vivo function. Eur J Immunol. 2009;39:3084–3090. doi: 10.1002/eji.200939407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Viaud S, Terme M, Flament C, Taieb J, Andre F, Novault S, Escudier B, Robert C, Caillat-Zucman S, Tursz T, Zitvogel L, Chaput N. Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15 Ralpha. PLoS One. 2009;4:e4942. doi: 10.1371/journal.pone.0004942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lv LH, Wan YL, Lin Y, Zhang W, Yang M, Li GL, Lin HM, Shang CZ, Chen YJ, Min J. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J Biol Chem. 2012;287:15874–15885. doi: 10.1074/jbc.M112.340588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Menck K, Klemm F, Gross JC, Pukrop T, Wenzel D, Binder C. Induction and transport of Wnt 5a during macrophage-induced malignant invasion is mediated by two types of extracellular vesicles. Oncotarget. 2013;4:2057–2066. doi: 10.18632/oncotarget.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yao Y, Chen L, Wei W, Deng X, Ma L, Hao S. Tumor cell-derived exosome-targeted dendritic cells stimulate stronger CD8+ CTL responses and antitumor immunities. Biochem Biophys Res Commun. 2013;436:60–65. doi: 10.1016/j.bbrc.2013.05.058. [DOI] [PubMed] [Google Scholar]
- 98.Zhou M, Chen J, Zhou L, Chen W, Ding G, Cao L. Pancreatic cancer derived exosomes regulate the expression of TLR4 in dendritic cells via miR-203. Cell Immunol. 2014;292:65–69. doi: 10.1016/j.cellimm.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 99.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014:3. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McCoy-Simandle K, Hanna SJ, Cox D. Exosomes and nanotubes: control of immune cell communication. Int J Biochem Cell Biol. 2016;71:44–54. doi: 10.1016/j.biocel.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ventimiglia LN, Alonso MA. Biogenesis and function of T cell-derived exosomes. Front Cell Dev Biol. 2016;4:84. doi: 10.3389/fcell.2016.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ye SB, Zhang H, Cai TT, Liu YN, Ni JJ, He J, Peng JY, Chen QY, Mo HY, Jun C, Zhang XS, Zeng YX, Li J. Exosomal miR-24-3p impedes T-cell function by targeting FGF11 and serves as a potential prognostic biomarker for nasopharyngeal carcinoma. J Pathol. 2016;240:329–340. doi: 10.1002/path.4781. [DOI] [PubMed] [Google Scholar]
- 103.Whiteside TL. Exosomes and tumor-mediated immune suppression. J Clin Invest. 2016;126:1216–1223. doi: 10.1172/JCI81136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Iaccino E, Mimmi S, Dattilo V, Marino F, Candeloro P, Di Loria A, Marimpietri D, Pisano A, Albano F, Vecchio E, Ceglia S, Golino G, Lupia A, Fiume G, Quinto I, Scala G. Monitoring multiple myeloma by idiotype-specific peptide binders of tumor-derived exosomes. Mol Cancer. 2017;16:159. doi: 10.1186/s12943-017-0730-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hood JL. Melanoma exosomes enable tumor tolerance in lymph nodes. Med Hypotheses. 2016;90:11–13. doi: 10.1016/j.mehy.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Szajnik M, Czystowska M, Szczepanski MJ, Mandapathil M, Whiteside TL. Tumor-derived microvesicles induce, expand and upregulate biological activities of human regulatory T cells (Treg) PLoS One. 2010;5:e11469. doi: 10.1371/journal.pone.0011469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bu N, Wu H, Zhang G, Zhan S, Zhang R, Sun H, Du Y, Yao L, Wang H. Exosomes from dendritic cells loaded with chaperone-rich cell lysates elicit a potent t cell immune response against intracranial glioma in mice. J Mol Neurosci. 2015;56:631–643. doi: 10.1007/s12031-015-0506-9. [DOI] [PubMed] [Google Scholar]
- 108.Gesierich S, Berezovskiy I, Ryschich E, Zoller M. Systemic induction of the angiogenesis switch by the tetraspanin D6.1A/CO-029. Cancer Res. 2006;66:7083–7094. doi: 10.1158/0008-5472.CAN-06-0391. [DOI] [PubMed] [Google Scholar]
- 109.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 110.Perrimon N, Pitsouli C, Shilo BZ. Signaling mechanisms controlling cell fate and embryonic patterning. Cold Spring Harb Perspect Biol. 2012;4:a005975. doi: 10.1101/cshperspect.a005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pikkarainen T, Nurmi T, Sasaki T, Bergmann U, Vainio S. Role of the extracellular matrixlocated Mac-2 binding protein as an interactor of the Wnt proteins. Biochem Biophys Res Commun. 2017;491:953–957. doi: 10.1016/j.bbrc.2017.07.141. [DOI] [PubMed] [Google Scholar]
- 112.Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14:1036–1045. doi: 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- 113.Lin R, Wang S, Zhao RC. Exosomes from human adipose-derived mesenchymal stem cells promote migration through Wnt signaling pathway in a breast cancer cell model. Mol Cell Biochem. 2013;383:13–20. doi: 10.1007/s11010-013-1746-z. [DOI] [PubMed] [Google Scholar]
- 114.Rak J. Extracellular vesicles - biomarkers and effectors of the cellular interactome in cancer. Front Pharmacol. 2013;4:21. doi: 10.3389/fphar.2013.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Muller L, Simms P, Hong CS, Nishimura MI, Jackson EK, Watkins SC, Whiteside TL. Human tumor-derived exosomes (TEX) regulate Treg functions via cell surface signaling rather than uptake mechanisms. Oncoimmunology. 2017;6:e1261243. doi: 10.1080/2162402X.2016.1261243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bandari SK, Purushothaman A, Ramani VC, Brinkley GJ, Chandrashekar DS, Varambally S, Mobley JA, Zhang Y, Brown EE, Vlodavsky I, Sanderson RD. Chemotherapy induces secretion of exosomes loaded with heparanase that degrades extracellular matrix and impacts tumor and host cell behavior. Matrix Biol. 2018;65:104–118. doi: 10.1016/j.matbio.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zheng P, Chen L, Yuan X, Luo Q, Liu Y, Xie G, Ma Y, Shen L. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J Exp Clin Cancer Res. 2017;36:53. doi: 10.1186/s13046-017-0528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Languino LR, Singh A, Prisco M, Inman GJ, Luginbuhl A, Curry JM, South AP. Exosomemediated transfer from the tumor microenvironment increases TGFbeta signaling in squamous cell carcinoma. Am J Transl Res. 2016;8:2432–2437. [PMC free article] [PubMed] [Google Scholar]
- 119.Ristorcelli E, Beraud E, Verrando P, Villard C, Lafitte D, Sbarra V, Lombardo D, Verine A. Human tumor nanoparticles induce apoptosis of pancreatic cancer cells. FASEB J. 2008;22:3358–3369. doi: 10.1096/fj.07-102855. [DOI] [PubMed] [Google Scholar]
- 120.Morel L, Regan M, Higashimori H, Ng SK, Esau C, Vidensky S, Rothstein J, Yang Y. Neuronal exosomal miRNA-dependent translational regulation of astroglial glutamate transporter GLT1. J Biol Chem. 2013;288:7105–7116. doi: 10.1074/jbc.M112.410944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Melo SA, Sugimoto H, O’Connell JT, Kato N, Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, Lucci A, Ivan C, Calin GA, Kalluri R. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26:707–721. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang X, Xu C, Hua Y, Sun L, Cheng K, Jia Z, Han Y, Dong J, Cui Y, Yang Z. Exosomes play an important role in the process of psoralen reverse multidrug resistance of breast cancer. J Exp Clin Cancer Res. 2016;35:186. doi: 10.1186/s13046-016-0468-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Grindheim AK, Saraste J, Vedeler A. Protein phosphorylation and its role in the regulation of Annexin A2 function. Biochim Biophys Acta. 2017;1861:2515–2529. doi: 10.1016/j.bbagen.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 124.Burke MC, Oei MS, Edwards NJ, Ostrand-Rosenberg S, Fenselau C. Ubiquitinated proteins in exosomes secreted by myeloid-derived suppressor cells. J Proteome Res. 2014;13:5965–5972. doi: 10.1021/pr500854x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu X, Lv YG, Yan CY, Yi J, Ling R. Enforced expression of hsa-miR-125a-3p in breast cancer cells potentiates docetaxel sensitivity via modulation of BRCA1 signaling. Biochem Biophys Res Commun. 2016;479:893–900. doi: 10.1016/j.bbrc.2016.09.087. [DOI] [PubMed] [Google Scholar]
- 126.Peng F, Xiong L, Tang H, Peng C, Chen J. Regulation of epithelial-mesenchymal transition through microRNAs: clinical and biological significance of microRNAs in breast cancer. Tumour Biol. 2016;37:14463–14477. doi: 10.1007/s13277-016-5334-1. [DOI] [PubMed] [Google Scholar]
- 127.Ho TT, He X, Mo YY, Beck WT. Transient resistance to DNA damaging agents is associated with expression of microRNAs-135b and -196b in human leukemia cell lines. Int J Biochem Mol Biol. 2016;7:27–47. [PMC free article] [PubMed] [Google Scholar]
- 128.Zhao L, Li Y, Song X, Zhou H, Li N, Miao Y, Jia L. Upregulation of miR-181c inhibits chemoresistance by targeting ST8SIA4 in chronic myelocytic leukemia. Oncotarget. 2016;7:60074–60086. doi: 10.18632/oncotarget.11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li T, Pan H, Li R. The dual regulatory role of miR-204 in cancer. Tumour Biol. 2016;37:11667–11677. doi: 10.1007/s13277-016-5144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Taylor DD, Gercel-Taylor C. Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. Br J Cancer. 2005;92:305–311. doi: 10.1038/sj.bjc.6602316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rabinowits G, Gercel-Taylor C, Day JM, Taylor DD, Kloecker GH. Exosomal microRNA: a diagnostic marker for lung cancer. Clin Lung Cancer. 2009;10:42–46. doi: 10.3816/CLC.2009.n.006. [DOI] [PubMed] [Google Scholar]
- 132.Tutar Y. miRNA and cancer; computational and experimental approaches. Curr Pharm Biotechnol. 2014;15:429. doi: 10.2174/138920101505140828161335. [DOI] [PubMed] [Google Scholar]
- 133.Cheng D, Xu Y, Sun C, He Z. MicroRNA-451 sensitizes lung cancer cells to cisplatin through regulation of Mcl-1. Mol Cell Biochem. 2016;423:85–91. doi: 10.1007/s11010-016-2827-6. [DOI] [PubMed] [Google Scholar]
- 134.Song J, Li Y. miR-25-3p reverses epithelialmesenchymal transition via targeting Sema4C in cisplatin-resistance cervical cancer cells. Cancer Sci. 2017;108:23–31. doi: 10.1111/cas.13104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tanaka Y, Kamohara H, Kinoshita K, Kurashige J, Ishimoto T, Iwatsuki M, Watanabe M, Baba H. Clinical impact of serum exosomal microRNA-21 as a clinical biomarker in human esophageal squamous cell carcinoma. Cancer. 2013;119:1159–1167. doi: 10.1002/cncr.27895. [DOI] [PubMed] [Google Scholar]
- 136.Wang Y, Zhao Y, Herbst A, Kalinski T, Qin J, Wang X, Jiang Z, Benedix F, Franke S, Wartman T, Camaj P, Halangk W, Kolligs FT, Jauch KW, Nelson PJ, Bruns CJ. miR-221 mediates chemoresistance of esophageal adenocarcinoma by direct targeting of DKK2 expression. Ann Surg. 2016;264:804–814. doi: 10.1097/SLA.0000000000001928. [DOI] [PubMed] [Google Scholar]
- 137.Gao W, Lu X, Liu L, Xu J, Feng D, Shu Y. MiRNA-21: a biomarker predictive for platinum-based adjuvant chemotherapy response in patients with non-small cell lung cancer. Cancer Biol Ther. 2012;13:330–340. doi: 10.4161/cbt.19073. [DOI] [PubMed] [Google Scholar]
- 138.Zhu M, Zhou X, Du Y, Huang Z, Zhu J, Xu J, Cheng G, Shu Y, Liu P, Zhu W, Wang T. miR-20a induces cisplatin resistance of a human gastric cancer cell line via targeting CYLD. Mol Med Rep. 2016;14:1742–1750. doi: 10.3892/mmr.2016.5413. [DOI] [PubMed] [Google Scholar]
- 139.Tang J, Li Y, Liu K, Zhu Q, Yang WH, Xiong LK, Guo DL. Exosomal miR-9-3p suppresses HBGF-5 expression and is a functional biomarker in hepatocellular carcinoma. Minerva Med. 2018;109:15–23. doi: 10.23736/S0026-4806.17.05167-9. [DOI] [PubMed] [Google Scholar]
- 140.Pakravan K, Babashah S, Sadeghizadeh M, Mowla SJ, Mossahebi-Mohammadi M, Ataei F, Dana N, Javan M. MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1alpha/VEGF signaling axis in breast cancer cells. Cell Oncol (Dordr) 2017;40:457–470. doi: 10.1007/s13402-017-0335-7. [DOI] [PubMed] [Google Scholar]
- 141.Chen X, Ying X, Wang X, Wu X, Zhu Q, Wang X. Exosomes derived from hypoxic epithelial ovarian cancer deliver microRNA-940 to induce macrophage M2 polarization. Oncol Rep. 2017;38:522–528. doi: 10.3892/or.2017.5697. [DOI] [PubMed] [Google Scholar]
- 142.Hsu YL, Hung JY, Chang WA, Lin YS, Pan YC, Tsai PH, Wu CY, Kuo PL. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene. 2017;36:4929–4942. doi: 10.1038/onc.2017.105. [DOI] [PubMed] [Google Scholar]
- 143.Garner JM, Herr MJ, Hodges KB, Jennings LK. The utility of tetraspanin CD9 as a biomarker for metastatic clear cell renal cell carcinoma. Biochem Biophys Res Commun. 2016;471:21–25. doi: 10.1016/j.bbrc.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 144.Jagadish N, Agarwal S, Gupta N, Fatima R, Devi S, Kumar V, Suri V, Kumar R, Suri V, Sadasukhi TC, Gupta A, Ansari AS, Lohiya NK, Suri A. Heat shock protein 70-2 (HSP70-2) overexpression in breast cancer. J Exp Clin Cancer Res. 2016;35:150. doi: 10.1186/s13046-016-0425-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Reddy VS, Madala SK, Trinath J, Reddy GB. Extracellular small heat shock proteins: exosomal biogenesis and function. Cell Stress Chaperones. 2018;23:441–454. doi: 10.1007/s12192-017-0856-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 147.Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, Bernad A, Sanchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Futter CE, White IJ. Annexins and endocytosis. Traffic. 2007;8:951–958. doi: 10.1111/j.1600-0854.2007.00590.x. [DOI] [PubMed] [Google Scholar]
- 149.Schorey JS, Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic. 2008;9:871–881. doi: 10.1111/j.1600-0854.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


