Abstract
Adrenergic receptors (ARs), especially β-ARs, are constitutively expressed in most mammalian cells and are associated with various malignancies including lung cancer. Epidemiologic studies have reported that activation of β-AR signalling promotes the development and progression of lung cancer and that pharmacological interference by β-AR blockers could partially reverse lung cancer progression. In this review, we mainly focus on the role of β-ARs in lung cancer and then reveal the possible application of AR blockers in anti-tumour therapy for lung cancer.
Keywords: β-adrenergic receptors, ADRB2, β-adrenergic receptor
Introduction
Cancer incidence and mortality are rapidly increasing, and lung cancer is the leading cause of cancer incidence and mortality in both sexes worldwide and in China [1-3]. Despite the great advances in the diagnosis and treatment of lung cancer, the overall 5-year survival rate of lung cancer patients is less than 20% [2,4].
The human genome encodes nine different adrenoceptor genes, which are classed into three families termed α1-, α2-, and β-adrenoceptors, each of which contains three family members. The adrenergic receptors (or adrenoceptors, ARs) are a class of G-protein-coupled receptorsthat are expressed by most cell types in humans and that function as the primary targets of the catecholamines norepinephrine (NE) and epinephrine (E), which are released from the sympathetic nervous system or are activated by stress, smoking or agonists. ARs play a central role in human disease and are key targets for widely used drugs. The main ARs have been classed according to their functions, which are described in detail in this review [5-10].
Recently, emerging data have demonstrated that the activation of adrenoceptors, and especially β-ARs, by catecholamines, chronic stress [11], smoking or AR agonists leads to pro-tumourigenic effects. Some of these effects are involved in carcinogenesis, proliferation, immune regulation [12-15], invasion [16], neoangiogenesis [17], clinical prognosis and treatment resistance [18] in various malignancies such as melanoma [7], breast cancer [19,20], prostate cancer [21,22], and pancreatic cancer [23].
Similarly to other common cancers, epidemiologic studies have reported that activation of β-AR signalling promotes the development and progression of lung cancer and that pharmacological interference by β-AR blockers could partially reverse lung cancer progression [24,25]. In regards to the role of ARs in the tumour biology of lung cancer, the evidence predominantly implicates the β-ARs (encoded by the ADRB1, ADRB2, and ADRB3 genes [25]), which are expressed to different degrees in both normal bronchial epithelial cells and lung cancer cells [24]. Many cells in the tumour microenvironment express ARs as a result of chronic stress, smoking or ARs agonists, which can activate various cancer-associated signalling pathways via both direct and indirect mechanisms [14]. In our review, the pro-cancer role of β-AR signalling in lung cancer is discussed in the following text and is summarized briefly in Figure 1 and Table 1.
Figure 1.
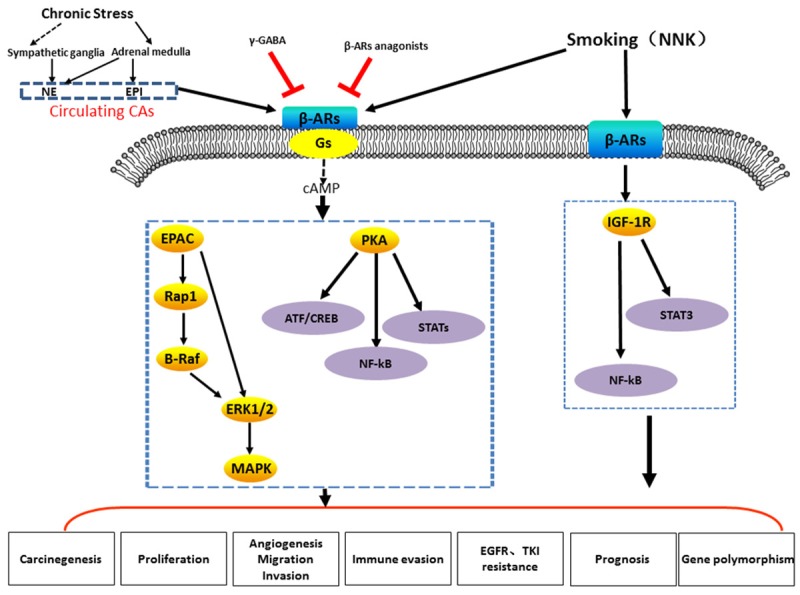
Cartoon illustrating the proposed mechanisms of the β-ARs signaling induced by chronic stress or smoking (NNK) or β-ARs agonists and suppressed by γ-GABA or β-ARs anagonists.
Table 1.
The role and mechanisms of adrenergic receptors in lung cancer
| Agonists | Receptor | Signalling | Patients/cells | References |
|
| ||||
| The expression of β-ARs in lung cancer | ||||
|
| ||||
| / | ADRB2 | / | PAC | [26] |
| / | β2-AR | / | NSCLC tissues | [27] |
| / | β-ARs | NSCLC tissues | [24] | |
| β1-AR, β2-AR | NCI-H322 and NCI-H441 | [28] | ||
| β2-AR | NSCLC tissues | [29] | ||
| NNK | β2-AR not β1-AR | / | Lung cancer cells in hamsters | [30] |
|
| ||||
| β-ARs signaling promotes lung tumorigenesis | ||||
|
| ||||
| Norepinephrine | β2-AR | PKA-VDCC-IGF-1R | Airway epithelial cells | [31] |
| Stress | Catecholamines | FVB mice initiated for LC | ||
| NNK | β-ARs | IGF-1R via IGF2 transcription | BEAS-2B cells (vitro) | [34] |
| NNK | β-ARs | A/J mice AC models | ||
| NNK | β-ARs | Arachidonic acid | NCI-H322 and NCI-H441 cells | [35] |
| NNK | β-ARs | cAMP | PAC in hamsters | [36] |
| Soproterenol | β-ARs | cAMP | PAC derived from Clara cell | [37] |
|
| ||||
| β-ARs signaling increases proliferation of lung cancer | ||||
|
| ||||
| / | β2-AR | Ki-67 expression | NSCLC, AC and non-AC patients | [29] |
| NNK, estrogen | β1-AR alpha7nAChR | cAMP | HPL1D cells | [24] |
| NNK | β-ARs | Bad and PKCiota | A549 cells | [39] |
| NNK | β-ARs | Bad | A549 cells | [38] |
| Epinephrine propranolol tolazoline | β-ARs | cAMP | Human peripheral adenocarcinomas that present Clara cells features | [41] |
| ISO | β2-AR | ERK1/2/CREB, | A549 cells | [42] |
| Epinephrine | β-ARs | cAMP | CSCs from NSCLC | [43] |
| Isoproterenol antagonist | β2-AR | GIRK1 | NCI-H69 | [25] |
| β2-AR | SCLC cells | |||
|
| ||||
| β-ARs signaling facilities angiogenesis and invasion of lung cancer | ||||
|
| ||||
| β2-AR | CD34 | NSCLC, AC and non-AC patients | [36] | |
| ISO | β2-AR | ERK1/2/CREB,MMP2,9,VEGF | A549 cells | [42] |
| Chronic stress | β-ARs | VEGF | CSCs from NSCLC | [43] |
| ISO | / | HDAC6/PKA/Epac/ERK- | H1299 cells | [48] |
|
| ||||
| β-ARs signaling promote immune evasion of lung cancer | ||||
|
| ||||
| Stress | β-ARs | NK cells | Mice of lung cancer models | [58] |
|
| ||||
| The role of β-ARs in the clinical prognosis of lung cancer | ||||
|
| ||||
| / | β2-AR | Worse outcomes | Stage I AC patients | [29] |
| β-ARs-blockers | β-ARs | Decreased DMFS, DFS, and OS | NSCLC received definitive radiotherapy | [60] |
| β-ARs-blockers | β-ARs | Decreased mortality | Stage III NSCLC | [61] |
| Metoprolol | β-ARs | Favour OS | Metastatic NSCLC patients | [62] |
| β-ARs-blockers | β-ARs | Reduced OS | Patients with resection of NSCLC | [63] |
| β-ARs-blockers | β-ARs | No association with OS | Lung cancer | [64] |
| α1-AR-blockers | α1-AR | Significant OS benefit | Stage 4 SCLC | [66] |
|
| ||||
| The role of β-ARs in the treatment resistance of lung cancer | ||||
|
| ||||
| Stress | β2-AR | LKB1/CREB/IL-6 | EGFR TKI resistance in NSCLC | [24] |
|
| ||||
| ADRB2 single-nucleotide polymorphisms (SNPs) in lung cancer | ||||
|
| ||||
| NNK | ADRB2 | Induced ADRB2 SNPs | AC from NNK-injected olden hamsters | [69] |
| ADRB2 | SNPs (no association) | AC risk | [70] | |
In this paper, we will review the current evidence of the role of ARs, especially β2-ARs in lung cancer, which have not yet been published in a review. We will also provide new insight into the use of AR blockers in anti-tumour therapy for lung cancer.
The expression of β-ARs in lung cancer
Several studies have demonstrated β-AR expression in lung cancer, but the expression of α-ARs has not yet been reported. A study by X. Wu et al. combined two PAC data sets (GSE2514 and GSE7670) to find novel target genes by bioinformatics analysis. They identified 1030 differentially expressed genes (DEGs) in their meta-analysis and revealed that ADRB2 was expressed to a high degree in PAC [26]. In the study by Z.Q. Tian, 1063 DEGs were identified between normal control (NC) tissues and non-small lung cancer (NSCLC) tissues by integration of 15 microarray datasets. It was revealed that ADRB2 was one of the significant hub proteins. They then used qRT-PCR to demonstrate that ADRB2 expression was downregulated 3.4-fold in five NSCLC tissues compared with NC tissues [27]. However, further experiments are still needed to confirm these conflicting results.
All three β-AR subtypes are expressed in lung tumour specimens and lung cancer-derived cell lines, but β2-AR is expressed at a relatively higher level and is more widely investigated compared with β1-AR and β3-AR. Data from a study by Monique B et al. showed that β-AR mRNA was expressed in 159 NSCLC clinical samples and 116 lung cancer cell lines by qRT-PCR and, specifically, that β2-AR was highly expressed [24]. Schuller HM et al. also showed the mRNA expression of β1-AR and β2-AR in NCI-H322 and NCI-H441 cells by RT-PCR [28]. Additionally, using immunohistochemistry (IHC), T. Yazawa et al. also found that β2-AR was positively expressed in 27% of 328 tumour sections from patients with surgically resected NSCLC, in 29% of PAC patients, and in 24% of non-PAC patients [29]. Based on research by Schuller HM et al., β2-AR protein or the phosphorylation at Ser-355/Ser-356 of β2-AR in tumour cells was profoundly increased compared with corresponding normal airway epithelia and alveolar epithelia of NNK-treated or untreated control hamsters. However, β1-AR was not observed to be over-expressed in tumour cells [30], which suggests that β-ARs, especially β2-Ars, play important roles in lung cancer. Next, we will summarize the current knowledge of the details of β2-ARs in lung cancer.
β-ARs promotes lung tumourigenesis
It is widely accepted that activation of β-AR signalling pathways induced by chronic stress, NNK or β-AR agonists promotes lung carcinogenesis via various mechanisms and that β-AR blockers can reverse this effect.
First, emerging data have demonstrated the relationship between ARs induced by chronic stress and lung cancer. Jang HJ et al. showed that chronic stress in FVB mice initiated lung tumourigenesis via the production of catecholamines, especially NE, and then markedly promoted urethane- or KrasG12D/+- -induced lung tumourigenesis and development. Mechanistically, the NE-increased β2-AR activation facilitates VDCC-mediated Ca2+ influx, which then induces IGF-1R activation via the release of IGF2 by airway epithelial cells; this in turn leads to lung epithelial cell transformation and lung tumour formation. Importantly, clinical drugs that block L-type VDCC decrease the effects of chronic stress or norepinephrine on the β2-AR-PKA-VDCC-IGF-1R signalling cascade, along with lung epithelial cell transformation and lung tumourigenesis [31]. This finding is consistent with the results of another study by Wu X et Al., who demonstrated that C57BL/6 mice exposed to repeated social defeat stress for 10 days followed by subcutaneous inoculation with Lewis lung carcinoma cells for seven days exhibited significantly increased weight and volume of the primary tumour as well as the number of lung metastatic nodules [32].
Second, current preclinical and clinical data that indicate that smoking facilitates NSCLC progression via direct and indirect mechanisms that involve nicotinic receptor-regulated β-AR signalling are summarized in a previous study [33]. Moreover, targeted pharmacological interference with β-AR blockers or by γ-GABA can reduce the role of β-AR signalling. For instance, research by Min HY et al. revealed that NNK-inducedIGF-1R activation through β-ARs was vital for lung carcinogenesis. Notably, the IGF2 level increase through β-ARs, NF-κB, and stat3 was dependent on NNK-mediated IGF-1R activation. Finally, treatment with β-AR antagonists inhibited the transformation of lung epithelial cell sand lung tumour formation in mice [34], which implies that blocking β-AR-induced IGF-1R activation might be an effective method for lung cancer prevention in smokers. Data from a study by Schuller HM et al. also indicated that NNK facilitated the growth of NCI-H322 and NCI-H441 cells via the binding of an agonist to β-ARs, which resulted in the release of arachidonic acid (AA). RT-PCR revealed that NNK induced the expression of β1-AR and β2-AR in two cell lines, accelerated DNA synthesis and induced the release of AA. β-AR antagonists completely blocked the release of AA and the increase in DNA synthesis [35]. Experiments performed by Schuller HM et al. indicated a notable increase in NNK-mediated AC multiplicity in hamsters that were chronically exposed to the β-AR agonist epinephrine or theophylline, which led to intracellular accumulation of the beta-adrenergic second messenger, cAMP. Moreover, propranolol before NNK injection significantly decreased the development of ACs [36].
Moreover, Adissu HA et al. found that human lung adenocarcinomas ofa Clara cell lineage were highly sensitive to the tumour-promoting effects of β-AR agonists such as isoproterenol, which could promote cAMP expression. However, lung adenocarcinomas that develop from alveolar type II cells are resistant to β-AR agonists such as isoproterenol and respond to stimulation with cAMP with a reduction in cell growth [37]. This finding suggests the important role of β-AR signalling in lung carcinogenesis and indicates that the application of AR blockers could be an effective means to prevent lung carcinogenesis.
β-ARs increase the proliferation of lung cancer cells
Currently, several studies have revealed that NNK or β-AR agonists facilitate NSCLC proliferation via direct and indirect mechanisms that involve β-AR signalling and that pharmacological interference by AR blockers or γ-GABA can suppress the abovementioned effects. It has also been shown that β2-AR is positively expressed in tumour sections from patients with surgically resected NSCLC (in both AC and non-AC) by β2-AR IHC. Additionally, β2-AR expression was found to be significantly correlated with Ki-67 expression in NSCLC and AC, but this correlation was not observed in patients without AC [29], which implies that the role of β2-AR in proliferation in different histopathological types of NSCLC requires further investigation.
Al-Wadei HA et al. previously reported that a single dose of NNK stimulated the proliferation of immortalized human small airway epithelial cells (HPL1D) via the actions of cAMP on the β1-AR and that oestrogen enhanced this response. Then, they reported that γ-GABA could prevent the combined signalling of NNK and oestrogen in HPL1D cells. Additionally, chronic NNK and oestrogen also decrease γ-GABA via the desensitization of its regulatory alpha4beta2nAChR [24]. In another study, Jin Z et al. revealed that treatment of human lung cancer cells with a PKC inhibitor (staurosporine) or a Src-specific inhibitor (PP2) could block NNK-induced Bad phosphorylation to facilitate apoptotic cell death. Propranolol decreases both NNK-induced Bad phosphorylation and PKCiota activation as well as cell survival, while it induces apoptosis of A549 cells, which suggests that NNK-mediates these effects at least partly via the upstream β-AR pathway [38,39].
Several studies have shown that β-AR agonists facilitate NSCLC proliferation and that AR blockers or γ-GABA can suppress the above effects. Epinephrine increases the proliferation of and DNA synthesis in NSCLC cells, and this effect could be blocked by propranolol [40]. According to data from Park PG et al., a β-AR-induced mitogenic pathway, which activates the cAMP down-stream pathway in cell lines, arose from human peripheral adenocarcinomas that presented features of Clara cells. β-AR agonists such as epinephrine strongly promote cell proliferation, while propranolol, but not antagonists of α-ARs (e.g., tolazoline) or antagonists of cAMP, can inhibit this effect. However, in their recent study, the SCLC cell line NCI-H69 exhibited no cell proliferation effect as a result of stimulators of this pathway [41], which implies differences in different pathological types. However, the mechanism remains unclear. Hu P et al. also reported that β2-adrenergic receptor activation by isoproterenol (ISO) promoted A549 proliferation via the ERK1/2/CREB pathway [42]. Using spheroid formation assays, Banerjee J et al. also demonstrated that epinephrine enhanced the number of cancer stem cells derived from three NSCLC cell lines (NCI-H322, NCI-H441, NCI-H1299), while intracellular cAMP levels and the levels of stem cell markers such as sonic hedgehog (SHH) and aldehyde dehydrogenase-1 (ALDH-1) were also enhanced. These effects were reversed by γ-GABA or cAMP inhibition [43]. Plummer HK et al. observed GIRK1 mRNA and protein expression in SCLC cell lines such as NCI-H69 but did not observe expression in any NSCLC cell lines. They also observed that SCLC cells treated with a β2-AR antagonist daily for seven days exhibited slight decreases in the GIRK1 mRNA level and decreased proliferation. NCI-H69 cells stimulated with the β2-adrenergic agonist isoproterenol exhibited increased growth rates. The GIRK inhibitor U50488H also inhibited NCI-H69 cell proliferation, but this inhibition was reversed by isoproterenol [25].
β-ARs facilitate angiogenesis and promote invasiveness in lung cancer
ARs are expressed by most cell types such as tumour cells and immune cells in the tumour microenvironment. They play an important role in the process of cancer metastasis by inducing degradation of basement membranes, cancer cell invasiveness, migration, extravasation and colonization [44].
β-AR activation in recent in vitro experiments and in vivo animal models has shown that β-ARs induced by chronic stress and the stress-related neurotransmitters significantly promote the metastatic potential of malignancies [45] including lung cancer. According to β2-AR IHC experiments, β2-AR expression is significantly correlated with lymphatic permeation, vascular invasion (CD34) in tumour sections from patients with surgically resected NSCLC, AC and non-AC [36], which implies that the role of β2-AR in angiogenesis in different histopathologic types of NSCLC requires further investigation. Hu P et al. have reported that β2-AR activation by isoproterenol (ISO) in A549 cells increased the expression of MMP family proteins such as MMP-2, MMP-9 and VEGF in, which could be blocked by knockdown of CREB [42]. These findings are similar to those of other studies in pancreatic cancer [23] and gastric cancer [46]. Banerjee J et al. demonstrated that chronic stress induced by epinephrine enhanced VEGF expression and the number of cancer stem cells derived from three NSCLC cell lines (NCI-H322, NCI-H441, NCI-H1299) [43], which is similar to the finding that tumours in stressed animals showed significantly increased VEGFR-2 and L1CAM expression, increased VEGF protein secretion, and increased pERK, MMP-2 and MMP-9 protein expression [32]. These results are also in agreement with the latest evidence from a study on prostate cancer [47]. Lim JA et al. reported that isoproterenol led to HDAC6 mRNA and protein expression via a PKA/Epac/ERK-dependent pathway, the induction of the deacetylation of α-tubulin and stimulation of H1299 cell migration. Moreover, ISO enhances H1299 cell migration via the induction of HDAC6 expression [48]. The pro-angiogenic and invasive role of β2-AR signalling in other tumour types has also been reported [17,44,45,47,49,50].
β-ARs promote immune regulation in lung cancer
In general, both stressors and the depression of AR signalling are associated with decreased cytotoxic T-cell and natural-killer-cell activities, which affects processes such as the immune surveillance of tumours [51-54]. Although the role and mechanisms of ARs in immune regulation in other malignancies such as B-cell lymphoma [15] and pancreatic carcinoma [55] have been widely investigated [14,51,56,57], reports on the immune modulation of ARs in the lungs are rare. It has been reported that stress stimulation could induce a tumour-resistant phenotype via the infiltration of NK cells into tumours in mouse models of lung cancer when they are housed in an enriched environment. Exposure to an enriched environment could enhance the cytotoxic activity of NK cells against tumours. Moreover, β-AR blockade or chemical sympathectomy inhibited the effects of the enriched environment on the cytotoxic activity of NK cells and subsequently decreased the antitumour effect of the enriched environment [58]. However, the determination of the effect and mechanisms of ARs on immune regulation in lung cancer still requires extensive investigation.
The role of β-ARs in the clinical prognosis and treatment resistance of lung cancer
The data from previous studies on the association between β-AR-blocker use and overall survival in patients with lung cancer are currently controversial, which is similar to what is seen in other types of cancers [59].
Thus far, several reports have demonstrated beneficial progress in β-AR blocker use in patients with lung cancer. T. Yazawa et al. also found that positive β2-AR protein expression was a negative predictor of worse outcomes in AC patients, particularly in patients with stage I tumours. A multivariate analysis showed that β2-AR protein expression was an independent factor for predicting poor progression-free survival in patients with stage I AC [29]. A univariate analysis in another study on NSCLC patients who received definitive radiotherapy (RT) demonstrated that β-AR blockers were associated with improved distant metastasis-free survival (DMFS), disease-free survival (DFS), and overall survival (OS) but not locoregional progression-free survival (LRPFS) compared with patients who were not treated with β-AR blockers. Additionally, in a multivariate analysis, the uptake of β-AR blockers was found to be related to a notably better DMFS, DFS, and OS with adjustments for age, stage, histologic type, and treatment with chemotherapy and radiation. No relationship was observed between β-AR blockers and LRPFS [60]. Moreover, those authors also found a 22% decrease in mortality, but this result was derived only from hospital-based data of 673 patients with stage III NSCLC [61]. According to a univariate analysis in a retrospective study of 35 patients with metastatic NSCLC who were treated with the β-AR blocker metoprolol, the treated patients exhibited a favourable overall survival compared with controls (72 patients with metastatic NSCLC who were not treated with metoprolol). However, the benefit of β-AR blockers on OS was not observed in the multivariate analysis, which suggests that use of β-AR blockers during chemotherapy may be associated with an increased OS for patients with metastatic NSCLC [62]. Univariate analyses showed that selective and nonselective β-AR blockers were related to reduced recurrence-free survival and OS in patients undergoing resection of NSCLC. However, the results were not always consistent with those discussed above even after adjustments for possible variables in the multivariate analysis, which illustrates that nonselective or selective β-AR blockers may not increase recurrence-free survival or OS [63].
In contrast, several studies have not illustrated any association between β-AR blockers and the survival of patients with lung cancer. A large retrospective cohort study consisting of 436 patients with lung cancer showed no correlation between the use of β-AR blockers and OS. Compared with cancer patients who received other antihypertensive medications, patients who received β-AR blockers experienced a slightly poorer survival; this poorer OS was seen in patients with pancreatic and prostate cancer but was not observed in patients with lung, breast or colorectal cancer, which suggests that not all the β-AR blockers used will result in a favourable survival in patients with malignancies [64]. In the largest study thus far, the relationship between β-AR blockers and OS was analysed by a Cox proportional hazards regression model. However, pre-diagnostic β-AR blockers were not related to OS of patients with lung cancer in the adjusted model. In contrast, some notable associations with β-AR blockers were observed when stratified by subtype, stage, site, dose or duration of use, but these associations did not follow a consistent direction [65]. Importantly, Lohinai Z et al. confirmed that aspirin, statins, SSRIs, ADRA1, and TCA were administered to a total of 876 patients with stage 4 SCLC (138, 72, 20, 28, and 5 cases, respectively, were given aspirin, statins, SSRIs, ADRA1 and TCA), and it was found that administration of α1-adrenergic receptor antagonists (doxazosin and prazosin) did not result in a statistically significant OS benefit [66].
In several pancreatic tumour models housed at 22 degrees Celsius, a beta-adrenergic receptor antagonist could facilitate the sensitivity of tumours to cytotoxic therapies [18]. In terms of the relationship between ARs and treatment resistance in lung cancer, a study by Monique B et al. revealed that stress hormones (E and NE) promoted EGFR TKI resistance in vitro and in vivo. Their data on confocal microscopy revealed NE-induced interactions between β-AR blockers and mutant EGFR but not wild-type EGFR. They further found that chronic stress hormones facilitated EGFR TKI resistance through β2-AR activation via an LKB1/CREB/IL-6-dependent mechanism. They also found that the inhibition of β-AR blockers by PPL or IL-6 may have increased the response to erlotinib and may have been related to a better outcome in EGFR TKI-treated NSCLC patients. Finally, they observed that β-Arb locker use was associated with lower IL-6 concentrations and an improved benefit from EGFR inhibitors. These findings imply that combinations of β-AR blockers with EGFR TKIs merit further investigation as a strategy to abrogate resistance in NSCLC patients. This combination could delay the emergence of resistance to the EGFRTKI afatinib [24]. The majority of EGFR TKIs have demonstrated a survival benefit in patients with a variety of cancers, including lung cancer, who were randomized to psychologically effective interventions. This is highlighted by a recent randomized clinical trial of palliative care for NSCLC patients [67].
ADRB2 single-nucleotide polymorphisms (SNPs) in lung cancer
AR variants and their receptor functions have been reported to play important roles in physiology and disease [68]. Two studies have revealed the association between ADRB2 and SNPs in lung cancer. One study reported that NNK induced ADRB2 single-nucleotide polymorphisms (SNPs) in AC tumours in NNK-injected golden hamsters. In their animal model, the authors found that both groups of hamsters contained SNPs but remarkably more ADRB2 SNPs in NNK-injected hamsters compared with controls. The majority of these SNPs were novel, nonsynonymous mutations found in regions near the ADRB2 gene that are known to be involved in ligand binding, G-protein coupling, and desensitization/downregulation [69]. In another study by Wang H et al., ADRB2 polymorphisms were not considered to play a major independent role in lung AC risk. To determine the relationship between the genetic variants of ADRB2 and how they modify risk of AC, they compared three common SNPs of ADRB2 between 313 patients with AC and 321 controls. They revealed no association between ADRB2 alterations that modify risk and any one of the three SNPs or their combined haplotypes. However, in the subgroup of young subjects ≤50 years of age, a notable association was seen for G-1023A, A46G (Gly16Arg), and the haplotype A(-1023)A(46) [70].
Conclusions and perspectives
This review aims to provide a comprehensive overview of AR signalling induced by chronic stress, NNK or AR agonists, which has been described in various malignancies including lung cancer in association with tumourigenesis, proliferation, angiogenesis, migration and invasion, immune invasion, survival and treatment resistance. Blockade of ARs or γ-GABA could partially reverse the effects of ARs.
Although the prognostic significance of β-ARs in lung cancer is not widely recognized and the use of AR blockade is not widely used in patients with lung cancer, β-AR blockers will be a promising treatment based on their pro-tumour effects on the progression and development of lung cancer. Finally, further work is required to clarify the role and mechanisms of ARs on several aspects such as immune regulation.
Acknowledgements
This paper was supported by the National Natural Science Foundation of China (no. 81572942, no. 81770096), Hubei province technical innovation special major project (2017ACA094), the Natural Science Foundation of Hubei Province (no. 2015CFB178), the Health and Planning Commission Fund of Hubei Province (WJ2017M096), the Science and Technology Support Program of Hubei Province (YSF2015001294), the Special Fund for Industrial Transformation and Upgrading, the Independent Innovation Research Fund for Huazhong University of Science and Technology (no. 2016YXMS231), and the National major new drug discovery technology major special projects (2018ZX09301001001).
Disclosure of conflict of interest
None.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 4.Hong QY, Wu GM, Qian GS, Hu CP, Zhou JY, Chen LA, Li WM, Li SY, Wang K, Wang Q, Zhang XJ, Li J, Gong X, Bai CX Lung Cancer Group of Chinese Thoracic Society; Chinese Alliance Against Lung Cancer. Prevention and management of lung cancer in China. Cancer. 2015;121(Suppl 17):3080–3088. doi: 10.1002/cncr.29584. [DOI] [PubMed] [Google Scholar]
- 5.Tank AW, Lee Wong D. Peripheral and central effects of circulating catecholamines. Compr Physiol. 2015;5:1–15. doi: 10.1002/cphy.c140007. [DOI] [PubMed] [Google Scholar]
- 6.Lohse MJ. The ins and outs of adrenergic signaling. J Mol Med (Berl) 2015;93:955–962. doi: 10.1007/s00109-015-1323-x. [DOI] [PubMed] [Google Scholar]
- 7.Cole SW, Sood AK. Molecular pathways: betaadrenergic signaling in cancer. Clin Cancer Res. 2012;18:1201–1206. doi: 10.1158/1078-0432.CCR-11-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hein L. Structure and evolution of adrenergic receptors. Pharm Unserer Zeit. 2011;40:470–473. doi: 10.1002/pauz.201100441. [DOI] [PubMed] [Google Scholar]
- 9.Mizobe T. Current aspects of research on adrenergic receptor. Masui. 2008;57:22–38. [PubMed] [Google Scholar]
- 10.Wallukat G. The beta-adrenergic receptors. Herz. 2002;27:683–690. doi: 10.1007/s00059-002-2434-z. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald PJ. Beta blockers, norepinephrine, and cancer: an epidemiological viewpoint. Clin Epidemiol. 2012;4:151–156. doi: 10.2147/CLEP.S33695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiao G, Chen M, Bucsek M, Repasky E, Hylander B. Adrenergic signaling: a targetable checkpoint limiting development of the antitumor immune response. Front Immunol. 2018;9:164. doi: 10.3389/fimmu.2018.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Repasky EA, Eng J, Hylander BL. Stress, metabolism and cancer: integrated pathways contributing to immune suppression. Cancer J. 2015;21:97–103. doi: 10.1097/PPO.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eng JW, Kokolus KM, Reed CB, Hylander BL, Ma WW, Repasky EA. A nervous tumor microenvironment: the impact of adrenergic stress on cancer cells, immunosuppression, and immunotherapeutic response. Cancer Immunol Immunother. 2014;63:1115–1128. doi: 10.1007/s00262-014-1617-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nissen MD, Sloan EK, Mattarollo SR. Beta-adrenergic signaling impairs antitumor CD8(+) Tcell responses to b-cell lymphoma immunotherapy. Cancer Immunol Res. 2018;6:98–109. doi: 10.1158/2326-6066.CIR-17-0401. [DOI] [PubMed] [Google Scholar]
- 16.Kim TH, Gill NK, Nyberg KD, Nguyen AV, Hohlbauch SV, Geisse NA, Nowell CJ, Sloan EK, Rowat AC. Cancer cells become less deformable and more invasive with activation of betaadrenergic signaling. J Cell Sci. 2016;129:4563–4575. doi: 10.1242/jcs.194803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Entschladen F, Drell TLt, Lang K, Joseph J, Zaenker KS. Tumour-cell migration, invasion, and metastasis: navigation by neurotransmitters. Lancet Oncol. 2004;5:254–258. doi: 10.1016/S1470-2045(04)01431-7. [DOI] [PubMed] [Google Scholar]
- 18.Eng JW, Reed CB, Kokolus KM, Pitoniak R, Utley A, Bucsek MJ, Ma WW, Repasky EA, Hylander BL. Housing temperature-induced stress drives therapeutic resistance in murine tumour models through beta2-adrenergic receptor activation. Nat Commun. 2015;6:6426. doi: 10.1038/ncomms7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barron TI, Sharp L, Visvanathan K. Beta-adrenergic blocking drugs in breast cancer: a perspective review. Ther Adv Med Oncol. 2012;4:113–125. doi: 10.1177/1758834012439738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen H, Liu D, Guo L, Cheng X, Guo N, Shi M. Chronic psychological stress promotes lung metastatic colonization of circulating breast cancer cells by decorating a pre-metastatic niche through activating beta-adrenergic signaling. J Pathol. 2018;244:49–60. doi: 10.1002/path.4988. [DOI] [PubMed] [Google Scholar]
- 21.Zahalka AH, Arnal-Estape A. Adrenergic nerves activate an angio-metabolic switch in prostate. Cancer. 2017;358:321–326. doi: 10.1126/science.aah5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu J, Cao Q, Mehra R, Laxman B, Yu J, Tomlins SA, Creighton CJ, Dhanasekaran SM, Shen R, Chen G, Morris DS, Marquez VE, Shah RB, Ghosh D, Varambally S, Chinnaiyan AM. Integrative genomics analysis reveals silencing of beta-adrenergic signaling by polycomb in prostate cancer. Cancer Cell. 2007;12:419–431. doi: 10.1016/j.ccr.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 23.Partecke LI, Speerforck S, Kading A, Seubert F, Kuhn S, Lorenz E, Schwandke S, Sendler M, Kessler W, Trung DN, Oswald S, Weiss FU, Mayerle J, Henkel C, Menges P, Beyer K, Lerch MM, Heidecke CD, von Bernstorff W. Chronic stress increases experimental pancreatic cancer growth, reduces survival and can be antagonised by beta-adrenergic receptor blockade. Pancreatology. 2016;16:423–433. doi: 10.1016/j.pan.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson MB, Sun H, Diao L, Tong P, Liu D, Li L, Fan Y, Poteete A, Lim SO, Howells K, Haddad V, Gomez D, Tran H, Pena GA, Sequist LV, Yang JC, Wang J, Kim ES, Herbst R, Lee JJ, Hong WK, Wistuba I, Hung MC, Sood AK, Heymach JV. Stress hormones promote EGFR inhibitor resistance in NSCLC: implications for combinations with beta-blockers. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aao4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plummer HK 3rd, Dhar MS, Cekanova M, Schuller HM. Expression of G-protein inwardly rectifying potassium channels (GIRKs) in lung cancer cell lines. BMC Cancer. 2005;5:104. doi: 10.1186/1471-2407-5-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu X, Zang W, Cui S, Wang M. Bioinformatics analysis of two microarray gene-expression data sets to select lung adenocarcinoma marker genes. Eur Rev Med Pharmacol Sci. 2012;16:1582–1587. [PubMed] [Google Scholar]
- 27.Tian ZQ, Li ZH, Wen SW, Zhang YF, Li Y, Cheng JG, Wang GY. Identification of commonly dysregulated genes in non-small-cell lung cancer by integrated analysis of microarray data and qRT-PCR validation. Lung. 2015;193:583–592. doi: 10.1007/s00408-015-9726-6. [DOI] [PubMed] [Google Scholar]
- 28.Schuller HM, Plummer HK, Bochsler PN, Dudric P, Bell JL, Harris RE. Co-expression of betaadrenergic receptors and cyclooxygenase-2 in pulmonary adenocarcinoma. Int J Oncol. 2001;19:445–449. [PubMed] [Google Scholar]
- 29.Yazawa T, Kaira K, Shimizu K, Shimizu A, Mori K, Nagashima T, Ohtaki Y, Oyama T, Mogi A, Kuwano H. Prognostic significance of beta2-adrenergic receptor expression in non-small cell lung cancer. Am J Transl Res. 2016;8:5059–5070. [PMC free article] [PubMed] [Google Scholar]
- 30.Schuller HM, Cekanova M. NNK-induced hamster lung adenocarcinomas over-express beta2-adrenergic and EGFR signaling pathways. Lung Cancer. 2005;49:35–45. doi: 10.1016/j.lungcan.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Jang HJ, Boo HJ, Lee HJ, Min HY, Lee HY. Chronic stress facilitates lung tumorigenesis by promoting exocytosis of IGF2 in lung epithelial cells. Cancer Res. 2016;76:6607–6619. doi: 10.1158/0008-5472.CAN-16-0990. [DOI] [PubMed] [Google Scholar]
- 32.Wu X, Liu BJ, Ji S, Wu JF, Xu CQ, Du YJ, You XF, Li B, Le JJ, Xu HL, Duan XH, Dong JC. Social defeat stress promotes tumor growth and angiogenesis by upregulating vascular endothelial growth factor/extracellular signal-regulated kinase/matrix metalloproteinase signaling in a mouse model of lung carcinoma. Mol Med Rep. 2015;12:1405–1412. doi: 10.3892/mmr.2015.3559. [DOI] [PubMed] [Google Scholar]
- 33.Schuller HM. Effects of tobacco constituents and psychological stress on the beta-adrenergic regulation of non-small cell lung cancer and pancreatic cancer: implications for intervention. Cancer Biomark. 2013;13:133–144. doi: 10.3233/CBM-130323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Min HY, Boo HJ, Lee HJ, Jang HJ, Yun HJ, Hwang SJ, Smith JK, Lee HJ, Lee HY. Smoking-associated lung cancer prevention by blockade of the beta-adrenergic receptor-mediated insulin-like growth factor receptor activation. Oncotarget. 2016;7:70936–70947. doi: 10.18632/oncotarget.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuller HM, Tithof PK, Williams M, Plummer H 3rd. The tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone is a beta-adrenergic agonist and stimulates DNA synthesis in lung adenocarcinoma via beta-adrenergic receptor-mediated release of arachidonic acid. Cancer Res. 1999;59:4510–4515. [PubMed] [Google Scholar]
- 36.Schuller HM, Porter B, Riechert A. Beta-adrenergic modulation of NNK-induced lung carcinogenesis in hamsters. J Cancer Res Clin Oncol. 2000;126:624–630. doi: 10.1007/pl00008474. [DOI] [PubMed] [Google Scholar]
- 37.Adissu HA, Schuller HM. Antagonistic growth regulation of cell lines derived from human lung adenocarcinomas of clara cell and aveolar type II cell lineage: implications for chemoprevention. Int J Oncol. 2004;24:1467–1472. [PubMed] [Google Scholar]
- 38.Jin Z, Gao F, Flagg T, Deng X. Nicotine induces multi-site phosphorylation of bad in association with suppression of apoptosis. J Biol Chem. 2004;279:23837–23844. doi: 10.1074/jbc.M402566200. [DOI] [PubMed] [Google Scholar]
- 39.Jin Z, Xin M, Deng X. Survival function of protein kinase C{iota} as a novel nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-activated bad kinase. J Biol Chem. 2005;280:16045–16052. doi: 10.1074/jbc.M413488200. [DOI] [PubMed] [Google Scholar]
- 40.Al-Wadei HA, Al-Wadei MH, Schuller HM. Cooperative regulation of non-small cell lung carcinoma by nicotinic and beta-adrenergic receptors: a novel target for intervention. PLoS One. 2012;7:e29915. doi: 10.1371/journal.pone.0029915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park PG, Merryman J, Orloff M, Schuller HM. Beta-adrenergic mitogenic signal transduction in peripheral lung adenocarcinoma: implications for individuals with preexisting chronic lung disease. Cancer Res. 1995;55:3504–3508. [PubMed] [Google Scholar]
- 42.Hu P, He J, Liu S, Wang M, Pan B, Zhang W. Beta2-adrenergic receptor activation promotes the proliferation of A549 lung cancer cells via the ERK1/2/CREB pathway. Oncol Rep. 2016;36:1757–1763. doi: 10.3892/or.2016.4966. [DOI] [PubMed] [Google Scholar]
- 43.Banerjee J, Papu John AM, Schuller HM. Regulation of nonsmall-cell lung cancer stem cell like cells by neurotransmitters and opioid peptides. Int J Cancer. 2015;137:2815–2824. doi: 10.1002/ijc.29646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li S, Sun Y, Gao D. Role of the nervous system in cancer metastasis. Oncol Lett. 2013;5:1101–1111. doi: 10.3892/ol.2013.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moreno-Smith M, Lutgendorf SK, Sood AK. Impact of stress on cancer metastasis. Future Oncol. 2010;6:1863–1881. doi: 10.2217/fon.10.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu Y, Xu Q, Zuo Y, Liu L, Liu S, Chen L, Wang K, Lei Y, Zhao X, Li Y. Isoprenaline/beta2-AR activates Plexin-A1/VEGFR2 signals via VEGF secretion in gastric cancer cells to promote tumor angiogenesis. BMC Cancer. 2017;17:875. doi: 10.1186/s12885-017-3894-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zahalka A, Arnal-Estapé A, Maryanovich M, Nakahara F, Cruz C, Finley L, Frenette P. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science. 2017;358:321–326. doi: 10.1126/science.aah5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lim JA, Juhnn YS. Isoproterenol increases histone deacetylase 6 expression and cell migration by inhibiting ERK signaling via PKA and Epac pathways in human lung cancer cells. Exp Mol Med. 2016;48:e204. doi: 10.1038/emm.2015.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park SY, Kang JH, Jeong KJ, Lee J, Han JW, Choi WS, Kim YK, Kang J, Park CG, Lee HY. Norepinephrine induces VEGF expression and angiogenesis by a hypoxia-inducible factor-1alpha protein-dependent mechanism. Int J Cancer. 2011;128:2306–2316. doi: 10.1002/ijc.25589. [DOI] [PubMed] [Google Scholar]
- 50.Sood AK, Lutgendorf SK. Stress influences on anoikis. Cancer Prev Res. 2011;4:481–485. doi: 10.1158/1940-6207.CAPR-10-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5:617–625. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- 52.Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16:300–317. doi: 10.1159/000216188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nagaraja AS, Sadaoui NC, Dorniak PL, Lutgendorf SK, Sood AK. Snapshot: stress and disease. Cell Metab. 2016;23:388–388.e1. doi: 10.1016/j.cmet.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 54.Dhabhar FS. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol Res. 2014;58:193–210. doi: 10.1007/s12026-014-8517-0. [DOI] [PubMed] [Google Scholar]
- 55.Wang L, Liu H, Chen X, Zhang M, Xie K, Ma Q. Immune sculpting of norepinephrine on MHC-I, B7-1, IDO and B7-H1 expression and regulation of proliferation and invasion in pancreatic carcinoma cells. PLoS One. 2012;7:e45491. doi: 10.1371/journal.pone.0045491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sarkar C, Chakroborty D, Basu S. Neurotransmitters as regulators of tumor angiogenesis and immunity: the role of catecholamines. J Neuroimmune Pharmacol. 2013;8:7–14. doi: 10.1007/s11481-012-9395-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reiche EM, Morimoto HK, Nunes SM. Stress and depression-induced immune dysfunction: implications for the development and progression of cancer. Int Rev Psychiatry. 2005;17:515–527. doi: 10.1080/02646830500382102. [DOI] [PubMed] [Google Scholar]
- 58.Song Y, Gan Y, Wang Q, Meng Z, Li G, Shen Y, Wu Y, Li P, Yao M, Gu J, Tu H. Enriched housing environment enhances nk cell antitumor immunity via sympathetic nerves-dependent regulation of nkg2d and ccr5 in mice. Cancer Res. 2017;77:1611–1622. doi: 10.1158/0008-5472.CAN-16-2143. [DOI] [PubMed] [Google Scholar]
- 59.Weberpals J, Jansen L, Carr PR, Hoffmeister M, Brenner H. Beta blockers and cancer prognosis - The role of immortal time bias: a systematic review and meta-analysis. Cancer Treat Rev. 2016;47:1–11. doi: 10.1016/j.ctrv.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 60.Wang HM, Liao ZX, Komaki R, Welsh JW, O’Reilly MS, Chang JY, Zhuang Y, Levy LB, Lu C, Gomez DR. Improved survival outcomes with the incidental use of beta-blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Ann Oncol. 2013;24:1312–1319. doi: 10.1093/annonc/mds616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang H, Liao Z, Zhuang Y, Liu Y, Levy LB, Xu T, Yusuf SW, Gomez DR. Incidental receipt of cardiac medications and survival outcomes among patients with stage III non-small-cell lung cancer after definitive radiotherapy. Clin Lung Cancer. 2015;16:128–136. doi: 10.1016/j.cllc.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 62.Aydiner A, Ciftci R, Karabulut S, Kilic L. Does beta-blocker therapy improve the survival of patients with metastatic non-small cell lung cancer? Asian Pac J Cancer Prev. 2013;14:6109–6114. doi: 10.7314/apjcp.2013.14.10.6109. [DOI] [PubMed] [Google Scholar]
- 63.Cata JP, Villarreal J, Keerty D, Thakar DR, Liu DD, Sood AK, Gottumukkala V. Perioperative beta-blocker use and survival in lung cancer patients. J Clin Anesth. 2014;26:106–117. doi: 10.1016/j.jclinane.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 64.Shah SM, Carey IM, Owen CG, Harris T, Dewilde S, Cook DG. Does beta-adrenoceptor blocker therapy improve cancer survival? Findings from a population-based retrospective cohort study. Br J Clin Pharmacol. 2011;72:157–161. doi: 10.1111/j.1365-2125.2011.03980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weberpals J, Jansen L, Haefeli WE, Hoffmeister M, Wolkewitz M, Herk-Sukel MPPV, Vissers PAJ, Brenner H. Pre- and post-diagnostic betablocker use and lung cancer survival: a population-based cohort study. Sci Rep. 2017;7:2911. doi: 10.1038/s41598-017-02913-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lohinai Z, Dome P, Szilagyi Z, Ostoros G, Moldvay J, Hegedus B, Dome B, Weiss GJ. From bench to bedside: attempt to evaluate repositioning of drugs in the treatment of metastatic small cell lung cancer (SCLC) PLoS One. 2016;11:e0144797. doi: 10.1371/journal.pone.0144797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spiegel D. Mind matters in cancer survival. Psychooncology. 2012;21:588–593. doi: 10.1002/pon.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ahles A, Engelhardt S. Polymorphic variants of adrenoceptors: pharmacology, physiology, and role in disease. Pharmacol Rev. 2014;66:598–637. doi: 10.1124/pr.113.008219. [DOI] [PubMed] [Google Scholar]
- 69.Masi T, Cekanova M, Walker K, Bernert H, Majidi M, Becker JM, Schuller HM. Nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced pulmonary adenocarcinomas in Syrian golden hamsters contain beta 2-adrenergic receptor single-nucleotide polymorphisms. Genes Chromosomes Cancer. 2005;44:212–217. doi: 10.1002/gcc.20228. [DOI] [PubMed] [Google Scholar]
- 70.Wang H, Hao B, Chen X, Zhao N, Cheng G, Jiang Y, Liu Y, Lin C, Tan W, Lu D, Wei Q, Jin L, Lin D, He F. Beta-2 adrenergic receptor gene (ADRB2) polymorphism and risk for lung adenocarcinoma: a case-control study in a Chinese population. Cancer Lett. 2006;240:297–305. doi: 10.1016/j.canlet.2005.09.018. [DOI] [PubMed] [Google Scholar]


