Abstract
The ubiquitin-specific protease 5 (USP5), a deubiquitinating enzyme, has been identified as a tumor promoter in several types of human cancer. However, the role of USP5 in non-small lung cancer (NSCLC) has not yet been elucidated. In this study, we found that USP5 was upregulated in NSCLC tissues compared with normal tissues. High expression of USP5 was correlated with large primary tumor size, poor differentiation and advanced TNM stage, and led to a significantly shorter overall survival (OS). USP5 overexpression enhanced, whereas USP5 silencing impaired the cell proliferation and colony formation of NSCLC cells in vitro. Moreover, knockdown of USP5 in H1299 cells inhibited tumor growth in vivo. Mechanistically, we found that USP5 deubiquitinated β-catenin, prevented ubiquitination mediated β-catenin degradation and promoted β-catenin nuclear accumulation, leading to the activation of Wnt/β-catenin signal pathway in NSCLC cells. Taken together, these findings suggest that USP5 functions as an oncogene in NSCLC and its oncogenic activity involves in part through Wnt/β-catenin signal pathway.
Keywords: USP5, NSCLC, tumorigenesis, β-catenin, deubiquitinaition
Introduction
Lung cancer is the leading cause of cancer-related death worldwide and the incidence of non-small cell lung cancer (NSCLC), the primary histologic type, is noticeably increasing [1,2]. While we have witnessed great improvement in diagnosis and synthetic therapy, the average survival time of NSCLC patients with advanced stage remains pessimistic [3]. Thus, intensive investigation of the molecular mechanism that is responsible for the tumorigenesis and development of NSCLC is of great significance and could help to identify novel tumor biomarkers for early detection and intervention.
There is considerable evidence suggesting that the canonical Wnt/β-catenin signaling pathway plays critical roles in the tumorigenesis and metastasis of lung cancer [4]. Several components of the Wnt/β-catenin pathway and β-catenin target genes including cyclin D1, c-Myc, survivin, and MMP7 are overexpressed in NSCLC [5]. Cytoplasmic β-catenin, maintained at low level through ubiquitin-mediated degradation, is a core component of this pathway. In the absence of activated Wnt signals, cytoplasmic β-catenin is rapidly phosphorylated by the APC/Axin/GSK-3 complex. Phosphorylated β-catenin then is recognized by specific E3 ligases, ubiquitinated, and degraded by the proteasome system. In addition to ubiquitination, deubiquitination also plays an important role in the regulation of β-catenin. Deubiquitinating enzymes (DUBs) deconjugate ubiquitin from target proteins and negatively regulate ubiquitination. Recently, several DUBs such as USP14 [6], USP9X [7], USP4 [8] and USP7 [9] have been implicated in regulation of β-catenin by deubiquitination.
The ubiquitin-specific protease 5 (USP5) is a member of DUBs family [10-12]. Recently, accumulating evidence has shown that USP5 function as an oncoprotein in diverse human cancers. For instance, USP5 is overexpressed in pancreatic cancer tissues and promotes pancreatic cancer cell proliferation [13]. In multiple myeloma, USP5 regulates c-Maf stability and myeloma cell survival [14]. USP5 promotes tumorigenesis and drug resistance through inhibiting p14ARF-p53 signaling pathway in hepatocellular carcinoma [15]. However, the precise role of USP5 in NSCLC and the underlying mechanism have not been explored.
In the current study, we investigated the clinical significance of USP5 in NSCLC and found its usefulness as a prognostic marker for the first time. Furthermore, we found that USP5 promoted tumor growth by deubiquitylation of β-catenin, inducing of its nuclear translocation. Our findings provided a novel insight into the tumorigenesis of NSCLC.
Materials and methods
Tissue specimens
The fresh NSCLC tissues and their corresponding normal lung tissues were collected from 56 patients between July 12 2017 and January 24 2018 at the First Affiliated Hospital of Jiaxing University. All patients provided written informed consent before enrollment and the Ethic Committee of the First Affiliated Hospital of Jiaxing University approved the study protocol. A tissue microarray (TMA), containing 136 NSCLC tissues and their corresponding normal lung tissues, was purchased from Shanghai Xinchao Biotechnology (Shanghai, China). Follow-up information was obtained by reviewing patient medical records.
To explore of USP5 transcripts in NSCLC microarray studies, the Kaplan-Meier Plotter (www.kmplot.com) was used to generate a Kaplan-Meier curve for overall survival (OS). Hazard Ratio (with 95% confidence interval) and logrank P value were calculated and presented on the graph.
Immunohistochemical staining (IHC)
Tissue chips were incubated with the anti-USP5 primary antibody (1:100; Abcam, USA) at 4°C overnight. The slides were then incubated with HRP-conjugated secondary antibody (1:500; Santa Cruz, USA) and visualized by a Dako EnVision Detection Kit (Dako, USA). The expression status of each tissue sample was assessed according to the previous report [16].
Plasmid construction
The plasmids for expressing USP5 and its active-site mutant (USP5-C335A) were generated by inserting the cDNA into a pCMV-Flag vector. USP5 shRNA and scrambled shRNA were purchased from Genepharma, China. The catalytic residue mutant (USP5-C335A) were generated using PCR mutagenesis by a site-directed mutagenesis kit (QuikChange kit; Stratagene, Agilent, Stockport, UK), The Myc-β-catenin expression plasmid was generated by inserting the cDNA into a pcDNA3.1 plasmid.
Cell culture and transfection
The normal human bronchial epithelial cell line BEAS-2B and NSCLC cell lines (H460, A549, H1299, H1944, HCC827 and H1650) were purchased from the American Type Culture Collection (ATCC) and cultured under conditions recommended by the ATCC.
Cell proliferation and colony formation assay
Cell proliferation assays were performed by CCK-8 assay. Cells (2 × 103/well) were seeded into 96-well plates. Then, 10 µl CCK-8 solution were added and incubated for an additional 4 hours. Then, the absorbance at 450 nm was measured using a Microplate Absorbance Reader (Bio-Rad, USA).
As to colony formation assay, tumor cells (1 × 103/well) were plated into 6-well plates and incubated for 14 days. Cell colonies were fixed with 4% formaldehyde for 30 min and later stained with 0.1% crystal violet dye for 5 min.
RNA extraction and qRT-PCR
Total RNA was extracted from NSCLC tissues or cells using TRIzol reagent (Invitrogen, USA), and cDNA was then synthesized with PrimeScript RT Reagent Kit (TaKaRa, Japan) according to the manufacturer’s protocol. Quantitative RT-PCR (qRT-PCR) was conducted with SYBR Green (TaKaRa, Japan). The relative mRNA expression was calculated after normalization to GAPDH. Primers were designed and purchased from Sangon Biotech (Shanghai, China).
Immunoprecipitaion and immunoblotting analysis
Cells were lysed and the extracts were incubated with 2 μg corresponding antibodies with gentle rotation overnight at 4°C. After blended with Protein A/G agarose beads for 4 h, the immunocomplexes was resuspended and boiled with 2 × sample loading. The protocol of immunoblotting was adapted from our previous report [17]. The primary antibodies used were: USP5 (1:1000; CST, USA), cyclin D1 (1:1000; CST, USA), c-Myc (1:1000, CST, USA), β-catenin (1:1000; CST, USA), ubiquitin (1:1000, Abcam, USA) and GAPDH (1:1000; CST, USA).
Ubiquitination and protein stability assay
For ubiquitination assay, cell lysates were immunoprecipitated with β-catenin antibodies, and then subjected to immunoblotting analysis with ubiquitin antibodies. To detect β-catenin protein stability, transfected cells were treated with 80 μg/ml cycloheximide (Sigma, USA) and harvested at the indicated time points. The levels of β-catenin were detected by immunoblotting.
GST pull-down and in vitro ubiquitination assay
The GST-USP5 were expressed in E. coli BL21 and captured by glutathione-Sepharose 4B (GE Healthcare Biosciences) according to the manufacturer’s instructions. To perform direct protein-binding assay, His-β-catenin was expressed in E. coli BL21, purified by Ni-NTA agarose (Qiagen, Hilden, Germany), and incubated with purified GST or GST-USP5 baits in ice-cold lysis buffer. The protein complexes were captured by glutathione-Sepharose 4B and analyzed by western blot. As to in vitro ubiquitination assay, GST-tagged β-catenin, USP5, and USP5 (C335A) protein were expressed in E. coli BL21 and affinity-purified with glutathione-Sepharose 4B (GE Healthcare Biosciences), and then the GST tag was removed by cleavage with PreScission protease (GE Healthcare Biosciences). β-catenin protein was incubated with or without USP5 protein for 0.5 h in a 20 μL ubiquitination mixture supplemented with 50 mmol/L Tris-HCl (pH 8.3), 5 mmol/L MgCl2, 2 mmol/L DTT, 10 mmol/L phosphocreatinine, 0.2 units/mL phosphocreatinine kinase, 5 mmol/L adenosine-5’-triphosphate, 2 μL GST-ubiquitin, 50 μg/mL ubiquitin aldehyde, and MG132 (10 μM). After incubation, the reactants were subjected to western blot with anti-β-catenin antibody.
Xenograft transplantation
The protocol for the animal experiments was approved by the Animal Experimental Ethics Committee of the First Affiliated Hospital of Jiaxing University. Thirty BALB/c nude mice (4 weeks old, male) were maintained under specific pathogen-free conditions and randomly divided into six groups (five mice per group). Approximately 1 × 106 cells from stable transfected lines H1299/shUSP5 and H1299/shNC were suspended into 100 μl PBS and injected subcutaneously into the hind limbs of nude mice. After 35 days, the mice were sacrificed, and the tumor grafts were excised, weighted and examined by immunohistochemistry.
Statistical analysis
All experiments were repeated in triplicate, and all data were presented as the mean ± stand deviation (SD). All statistical analyses were performed using the SPSS 25.0 software. The significances of differences between two groups were analyzed by Student’s t test. The chi-square test was used to evaluate the relationship between the expression level of USP5 and clinicopathogical features. Kaplan-Meier cures and logrank test were used to analyze the OS of NSCLC patients. P < 0.05 was considered statistically significant.
Results
USP5 is overexpressed in NSCLC tissues and associated with poor prognosis
Initially, we examined the expression of USP5 in 56 pairs of NSCLC tissues and paired normal lung tissues. As shown in Figure 1A, USP5 was significantly increased in tumor tissues in comparison to normal tissues (P < 0.001). Interestingly, the web-based Kaplan-Meier Plotter analysis tool revealed that NSCLC patients with high USP5 transcript levels had worse OS (Figure 1B). Then, we measured USP5 expression level in 6 human NSCLC cell lines (A549, H1299, H460, H1944, H1650 and HCC827) and cultured human lung epithelial cells (BEAS-2B). The results showed that USP5 was generally elevated in NSCLC cell lines relative to that in BEAS-2B cells (Figure 1C and 1D).
Figure 1.
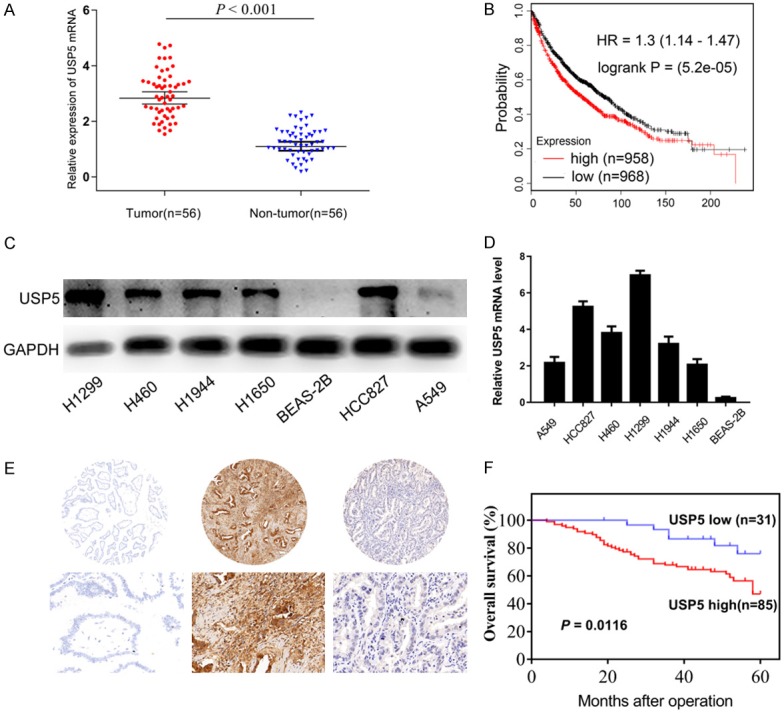
USP5 expression in NSCLC tissues and cell lines. (A) By qRT-PCR analysis, the expression of USP5 mRNA was up-regulated in 56 clinical NSCLC tissues as compared to the corresponding normal lung tissues (P < 0.001). (B) Analysis using the online Kaplan-Meier Plotter (www.kmplot.com) showed that high USP5 mRNA levels are significantly associated with shorter overall survival (P < 0.001). USP5 expression in NSCLC cell lines was examined by Western blotting (C) and qRT-PCR (D). (E) Representative images of the immunohistochemical staining for USP5 in NSCLC tissues and normal lung tissues. (F) NSCLC patients with high expression of USP5 protein showed worse overall survival compared to that with low expression of USP5 protein. (Data were presented with mean ± SD of three independent experiments, *P < 0.05, **P < 0.01).
To gain more insights into USP5 expression within lung cancer, we performed immunohistochemistry (IHC) staining of NSCLC tissue microarrays. High expression of USP5 protein was detected in 85/116 NSCLC samples and 42/116 normal tissues (P < 0.001) (Figure 1E). Correlation regression analysis revealed that high expression of USP5 protein was significantly associated with large primary tumor size (P = 0.005), poor differentiation (P = 0.038) and advanced TNM stage (P = 0.028) (Table 1). NSCLC patients with high USP5 had a worse OS compared with patients showing low expression (Figure 1F). Thus USP5 was likely to be involved in the tumorigenesis of NSCLC.
Table 1.
Association between USP5 protein levels and clinicopathologic characteristics in NSCLC patients
| Characteristics | Cases | USP5 expression | P-value | |
|---|---|---|---|---|
|
| ||||
| High | Low | |||
| Tumor | 116 | 85 | 31 | < 0.001 |
| No-tumor | 116 | 42 | 74 | |
| Age (years) | ||||
| ≤ 60 | 39 | 27 | 12 | 0.484 |
| > 60 | 77 | 58 | 19 | |
| Gender | ||||
| Male | 64 | 50 | 14 | 0.198 |
| Female | 52 | 35 | 17 | |
| Differentiation | ||||
| Well and moderate | 76 | 51 | 25 | 0.038 |
| Poor | 40 | 34 | 6 | |
| Smoking status | ||||
| No | 42 | 29 | 13 | 0.438 |
| Yes | 74 | 56 | 18 | |
| N stage | ||||
| N0 | 68 | 52 | 16 | 0.355 |
| N1~3 | 48 | 33 | 15 | |
| Tumor size (cm) | ||||
| ≤ 3 | 73 | 47 | 26 | 0.005 |
| > 3 | 43 | 38 | 5 | |
| TNM stage | ||||
| I+II | 79 | 53 | 26 | 0.028 |
| III | 37 | 32 | 5 | |
The bold represents that a P value < 0.05 and is significant.
USP5 promotes NSCLC cells growth in vitro
We confirmed that the level of USP5 was increased in A549 cells via transfection with USP5 overexpression plasmids, while reduced in H1299 cells via transfection with USP5 shRNA plasmids (Figure 2A and 2B). The CCK-8 assay showed that overexpression of USP5 in A549 cells dramatically increased the proliferation (Figure 2C), whereas knockdown of USP5 in H1299 cells significantly impaired the proliferation (Figure 2D). Plate clone formation assay revealed that A549/USP5 cells formed more colonies than that of A549/Vector cells (Figure 2E). Opposite results by USP5 loss-of-function assay showed that H1299/sh-USP5 cells formed fewer colonies than that of H1299/sh-NC control cells (Figure 3F). These results suggested that USP5 promoted NSCLC cell growth in vitro.
Figure 2.
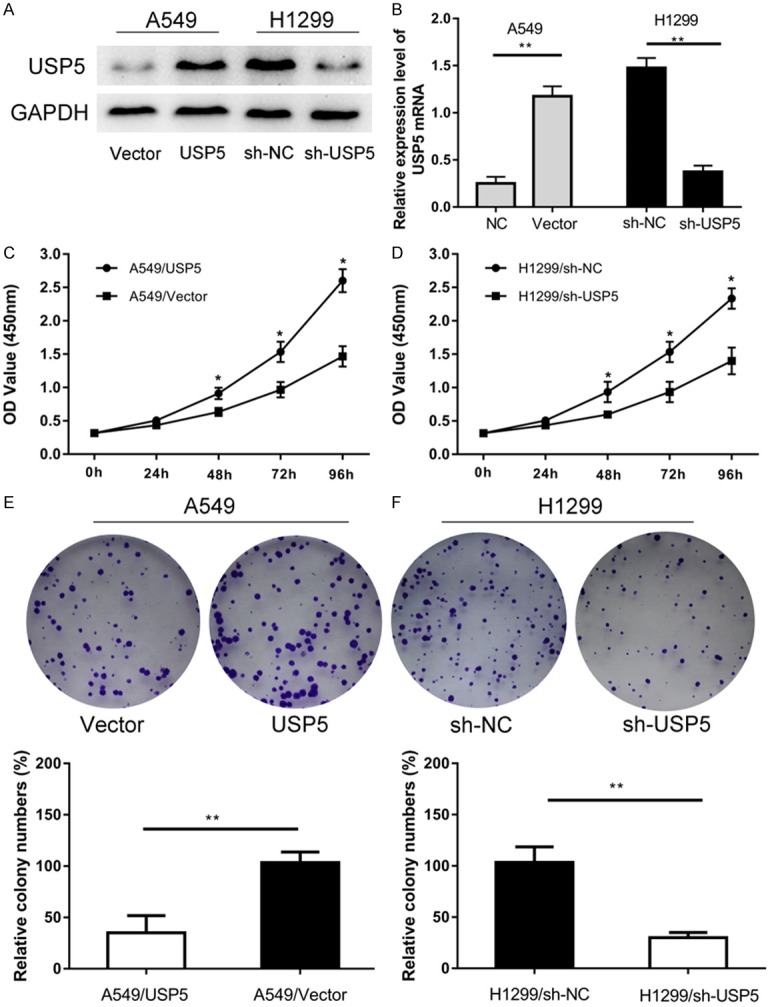
USP5 promotes NSCLC cells growth in vitro. A, B. USP5 protein levels in H1299 and A549 cells transfected with USP5 shRNA or USP5 expression plasmid were determined by western blot and qRT-PCR analysis. C, D. The effect of USP5 knockdown and overexpression on the proliferation of H1299 and A549 cells was assessed by CCK8 assay respectively. E, F. Knockdown of USP5 impaired the formation of colonies, while overexpression of USP5 promoted the formation of colonies. (Data were presented with mean ± SD of three independent experiments, *P < 0.05, **P < 0.01).
Figure 3.
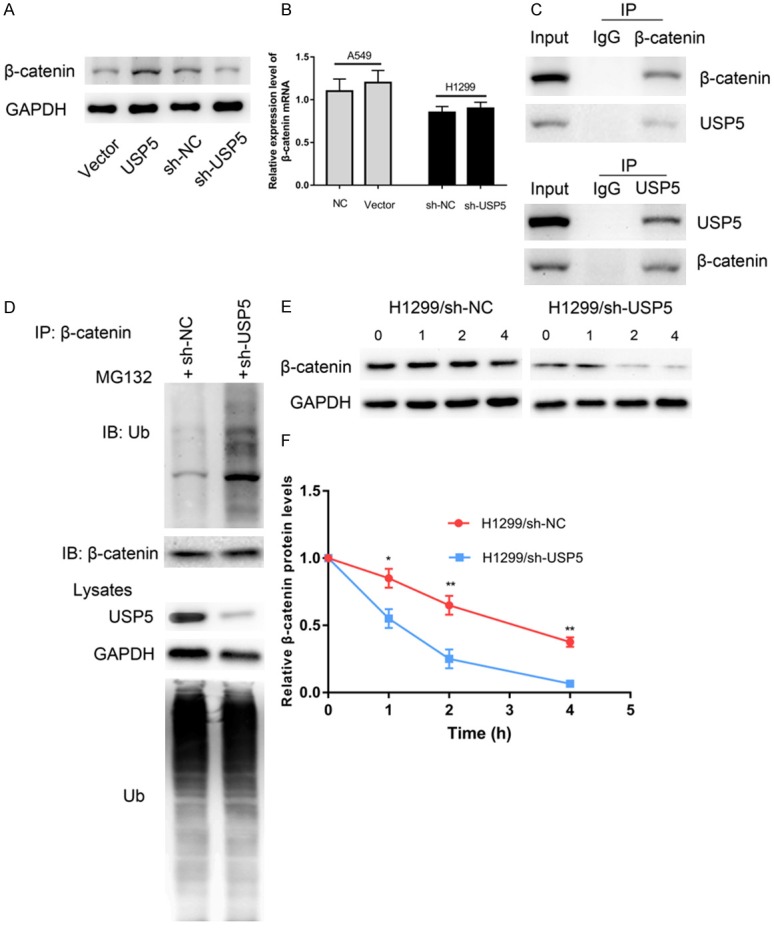
USP5 regulated the expression of β-catenin by controlling its protein stability. (A) Overexpression of USP5 in A549 cells elevated the protein level of β-catenin, and knockdown of USP5 in H1299 cells decrease the protein level of β-catenin. (B) However, USP5 did not affect the expression of β-catenin mRNA. After treated with MG132 (10 μM), the endogenous association of USP5 and β-catenin was detected by immunoprecipitation with indicated antibodies (C). Knockdown of USP5 promoted the ubiquitination and proteasomal degradation of β-catenin (D). Knockdown of USP5 in A549 cells significantly accelerated the degradation of β-catenin (E, F).
USP5 enhances the stability of β-catenin via deubiqutination
The canonical Wnt/β-catenin signaling pathway is essential for tumorigenesis of lung cancer. The key component of this pathway, β-catenin, is regulated by the ubiquitin-proteasome system. Previous studies reported that DUBs USP14, USP9X, USP4 and USP7 could control the stability of β-catenin by deubiqutination. Thus, we hypothesized that USP5 might regulate the stability of β-catenin via deubiquitination in NSCLC. To this end, we first evaluated whether USP5 could regulate β-catenin expression. We observed that overexpression of USP5 elevated the protein level of β-catenin, while knockdown of USP5 decreased the protein level of β-catenin (Figure 3A). However, the results of qRT-PCR showed that neither USP5 overexpression nor knockdown affected β-catenin mRNA levels (Figure 3B). Additionally, reciprocal immunoprecipitation analyses showed that USP5 could bind to β-catenin (Figure 3C). To examine the effect of USP5 silencing on the ubiquitination of β-catenin, H1299-shRNA and H1299-shNC cells were treated with M132 for 4 h. The results showed that knockdown of USP5 noticeably decreased the ubiquitination of β-catenin (Figure 3D).
To investigate the effect of USP5 on β-catenin protein stability, H1299-shRNA cells and H1299-shNC cells were treatment with 80 µg/ml cycloheximide to block protein synthesis, and the cells were harvested at the indicated time points. The protein level of β-catenin decreased overtime in cychoheximide-treated cells, and the cellular degradation of β-catenin was dramatically faster in H1299-shRNA cells (Figure 3E and 3F). These findings suggested that USP5 deubiquitinated β-catenin and prevented ubiquitination mediated β-catenin degradation in NSCLC cells.
We then evaluated whether USP5 overexpression promoted β-catenin deubiquitination. As shown in Figure 4A, co-transfection of wild type USP5 and β-catenin significantly promoted β-catenin deubiquitination. A lot of studies had proved that C335 is the key catalytic residue of USP5 [18-23]. In our study, the ubiquitination of β-catenin were virtually unaffected by C335A USP5 (Figure 4A), suggesting that USP5 enzyme activity is critical for its function.
Figure 4.
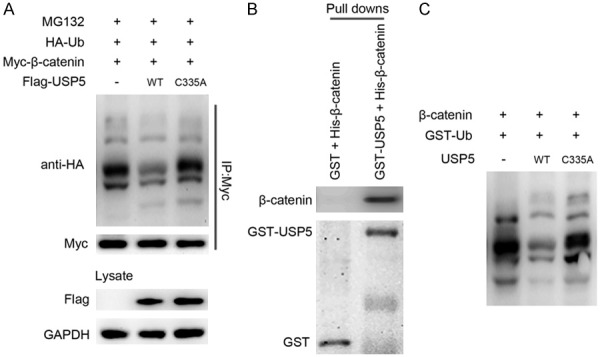
USP5 can interact with β-catenin and deubiquitinate ubiquitinated-β-catenin. A. Overexpression of wild type (WT) USP5 inhibited the ubiquitination and proteasomal degradation of β-catenin. B. Bacterially expressed GST fusion USP5 protein bound to glutathione-Sepharose beads and incubated with recombinant His-β-catenin. Bound His-β-catenin was detected by western blot. C. USP5 directly deubiquitinates β-catenin in vitro. Recombinant β-catenin was incubated with or without recombinant USP5 and GST-ubiquitin in the in vitro ubiquitination mixture as described in materials and methods. After the ubiquitination reaction, the samples were analyzed by western blot with anti-β-catenin antibody.
Based on the in vivo results, we then conducted in vitro experiments using purified USP5 and β-catenin proteins to demonstrate that the two proteins are able to bind to each other directly and that USP5 can deubiquitinate ubiquitinated-β-catenin. In the GST pull-down assay, GST-USP5, but not GST, interacted with β-catenin (Figure 4B). In the in vitro ubiquitination assay, USP5 mediated deubiquitination of β-catenin was observed only with all of the reaction components and depended on the C335 of USP5 (Figure 4C). Taken together, USP5 promotes deubiquitination of β-catenin in vivo, as well as in vitro, and that β-catenin is a direct substrate for USP5.
Furthermore, we observed that USP5 increased the level of total β-catenin, nuclear β-catenin, c-Myc and cyclin D1 (Figure 5). These data suggested that USP5 enhanced the nuclear transduction of β-catenin, subsequently activated the Wnt-target-gene cyclin D1 and c-Myc.
Figure 5.
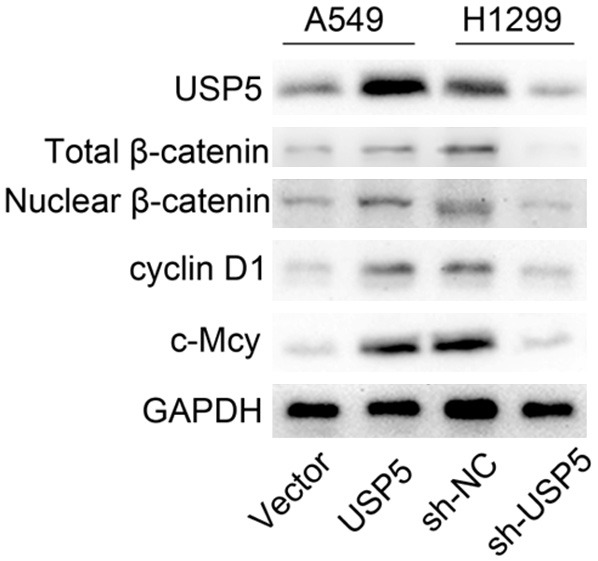
USP5 induces the nuclear translocation of β-catenin. Overexpression of USP5 in A549 cells elevated the level of total β-catenin, nuclear β-catenin, cyclin D1 and c-Myc, and knockdown of USP5 in H1299 cells decreased the levels of total β-catenin, nuclear β-catenin, cyclin D1 and c-Myc.
The function of USP5 is mediated by β-catenin in NSCLC cells
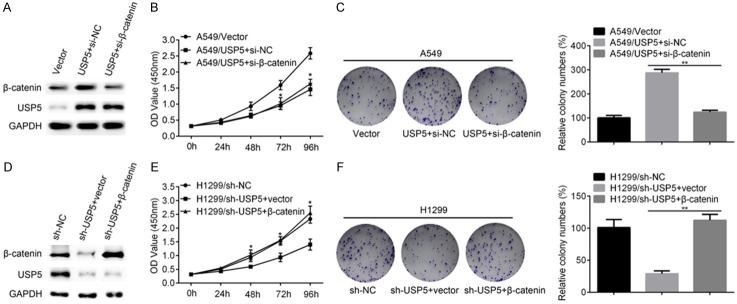
Given the fact that USP5 promoted the in vitro proliferation of NSCLC cells, and that USP5 can deubiquitinate ubiquitinated-β-catenin, it is assumed that β-catenin mediated the function of USP5. Indeed, co-transfection of USP5 and si-β-catenin in A549 cells significantly decreased the level of β-catenin compared with the control group (Figure 6A). Meanwhile, co-transfection of USP5 and si-β-catenin also decreased the proliferation (Figure 6B) and colony formation (Figure 6C) of A549 cells compared with the control group. These results were also confirmed in H1299 cells. Re-expressing β-catenin in H1299/shUSP5 cells promoted the protein level of β-catenin (Figure 6D) as well as the cell proliferation (Figure 6E) and colony formation (Figure 6F) ability. These data indicated that β-catenin indeed mediates the function of USP5 in NSCLC cells.
Figure 6.
The function of USP5 is mediated by β-catenin. A. USP5 expressing plasmid was co-transfected with si-NC or si-β-catenin into A549 cells. Then, the protein levels of β-catenin and USP5 were evaluated by western blot. B. The proliferation ability of A549/vector, A549/USP5+si-NC and A549/USP5+β-catenin cells were evaluated by CCK8 assay. C. The colony formation ability of A549/vector, A549/USP5+si-NC and A549/USP5+β-catenin cells were evaluated. D. β-catenin expressing plasmid or the empty vector was transfected into H1299/sh-USP5 cells and the protein levels of β-catenin and USP5 were detected by western blot. E. The proliferation ability of H1299/sh-NC, H1299/sh-USP5+vector and H1299/sh-USP5+β-catenin cells were evaluated by CCK8 assay. F. The colony formation ability of H1299/sh-NC, H1299/sh-USP5+vector and H1299/sh-USP5+β-catenin cells were evaluated. (Data were presented with mean ± SD of three independent experiments, *P < 0.05, **P < 0.01).
USP5 enhances NSCLC cells growth in vitro
In order to confirm the effect of USP5 on tumorigenesis in vivo, H1299/sh-USP5 cells and H1299/sh-NC cells were subcutaneously injected into mice. As presented in Figure 7A, we observed that tumors derived from H1299 shRNA cells grew significantly slower than those derived from control cells. The average tumor weight derived from H1299/sh-USP5 cells was remarkably lighter than the control group (Figure 7B). In addition, the expression of USP5, β-catenin, Ki-67, cyclin D1 and c-Myc in tumors was examined by IHC staining (Figure 7C). The tumors derived from H1299/sh-USP5 cells showed significantly lower expression of USP5, β-catenin, Ki-67, cyclin D1 and c-Myc compared to that of tumors derived from H1299/sh-NC cells (Figure 7D). Thus, USP5 had capability to promote tumor growth in vivo.
Figure 7.
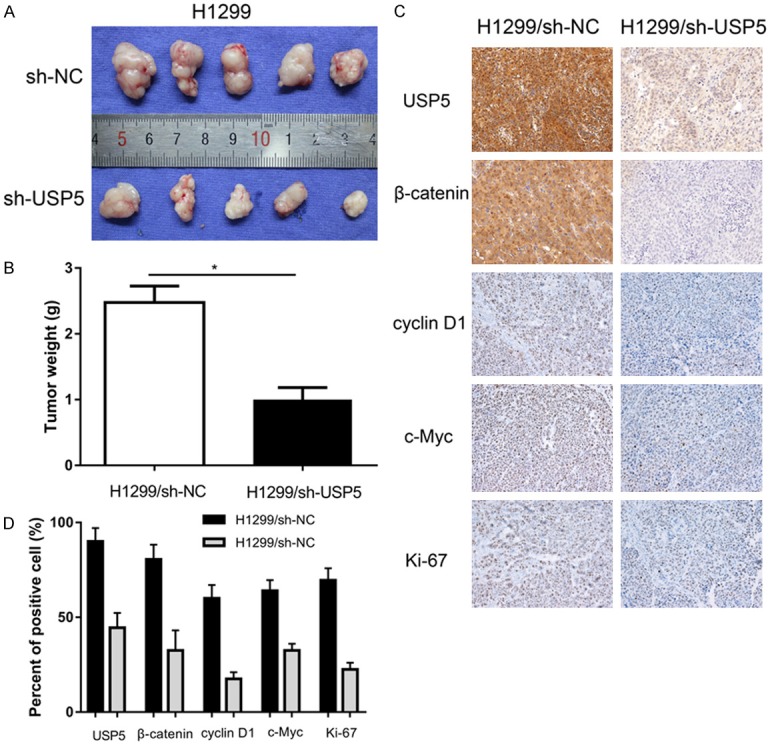
Knockdown of USP5 impaired the tumorigenesis of NSCLC cells in vivo. H1299-shUSP5 and H1299-shNC cells were injected subcutaneously into the hind limbs of nude mice. A. After 5 weeks, the tumor nodules were removed and photographed. B. Average weight of tumors in nude mice. C, D. Immunohistochemical analysis of USP5, b-catenin, c-Myc, cyclin D1 and Ki-67 in tumor nodules and comparison of expression was shown below. (Data were presented with mean ± SD of three independent experiments, *P < 0.05, **P < 0.01).
Discussion
NSCLC remains one of the most lethal cancers worldwide. NSCLC patients have high malignancy and poor prognosis, especially those with advanced stage. Thus, there is an urgent need to identify new diagnostic and prognostic marker for this disease.
USP5 has been reported to be involved in tumorgenesis in various types of human cancers, including pancreatic cancer, liver cancer and multiple myeloma [13,15,24,25]. However, the relationship between USP5 and NSCLC remains largely unknown. In this context, we found that USP5 is significantly upregulated in NSCLC tumor tissues compared to normal tissues, and high expression of USP5 is significantly associated with adverse clinicopathological characteristics and poor prognosis. Hence, we can postulate that USP5 also plays a critical role in tumorigenesis in NSCLC. To explore the functions of USP5 in NSCLC, USP5 was overexpressed or knocked down in the gain/low-of-function analysis. Consistent with its role in other kinds of cancers, USP5 promotes cell proliferation, colony formation in vitro and tumor growth in vivo. These results demonstrate that USP5 function as a tumor promoter in NSCLC cells. Overexpression of USP5 augments its simulative role on cell proliferation, leading to unrestricted cell growth and tumorigenesis in NSCLC.
The canonical Wnt/β-catenin signaling pathway has been demonstrated to be involved in various kinds of human cancers including NSCLC. The key component of this signaling, β-catenin, is overexpressed in many cancer such as gastric cancer [26], liver cancer [27] and lung cancer [28]. Nuclear translocation of β-catenin activates the Wnt-target-genes, such as c-Myc and cyclin D1, leading to incontrollable cancer proliferation. In the context, we identified USP5 as a regulator of β-catenin protein stability by deubiquitylation assay in vitro and in vivo. Knockdown of USP5 enhanced ubiquitination mediated β-catenin degradation, while USP5 overexpression promoted β-catenin deubiquitination. Subsequently, USP5 induced accumulation of nuclear β-catenin and upreuglated the levels of Wnt-target genes cyclin D1 and c-Myc, prompting NSCLC cell growth.
Taken together, these findings suggest that USP5 can promote cell growth of NSCLC cells by stabilizing β-catenin by deubiquitination, may play an essential role in NSCLC progression. Thus, USP5 has potential prognostic value as a tumor biomarker and may be a promising therapeutic target for NSCLC.
Acknowledgements
This study was supported by the program of Zhejiang province medical and health technology (2018KY800) and Jiaxing Science and Technology Bureau (2017AY33007).
Disclosure of conflict of interest
None.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 4.Fang L, Cai J, Chen B, Wu S, Li R, Xu X, Yang Y, Guan H, Zhu X, Zhang L, Yuan J, Wu J, Li M. Aberrantly expressed miR-582-3p maintains lung cancer stem cell-like traits by activating Wnt/beta-catenin signalling. Nat Commun. 2015;6:8640. doi: 10.1038/ncomms9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart DJ. Wnt signaling pathway in non-small cell lung cancer. J Natl Cancer Inst. 2014;106:djt356. doi: 10.1093/jnci/djt356. [DOI] [PubMed] [Google Scholar]
- 6.Wu N, Liu C, Bai C, Han YP, Cho WC, Li Q. Over-expression of deubiquitinating enzyme USP14 in lung adenocarcinoma promotes proliferation through the accumulation of betacatenin. Int J Mol Sci. 2013;14:10749–10760. doi: 10.3390/ijms140610749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang B, Zhang S, Wang Z, Yang C, Ouyang W, Zhou F, Zhou Y, Xie C. Deubiquitinase USP9X deubiquitinates beta-catenin and promotes high grade glioma cell growth. Oncotarget. 2016;7:79515–79525. doi: 10.18632/oncotarget.12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yun SI, Kim HH, Yoon JH, Park WS, Hahn MJ, Kim HC, Chung CH, Kim KK. Ubiquitin specific protease 4 positively regulates the WNT/beta-catenin signaling in colorectal cancer. Mol Oncol. 2015;9:1834–1851. doi: 10.1016/j.molonc.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novellasdemunt L, Foglizzo V, Cuadrado L, Antas P, Kucharska A, Encheva V, Snijders AP, Li VSW. USP7 is a tumor-specific WNT activator for APC-Mutated colorectal cancer by mediating beta-catenin deubiquitination. Cell Rep. 2017;21:612–627. doi: 10.1016/j.celrep.2017.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avvakumov GV, Walker JR, Xue S, Allali-Hassani A, Asinas A, Nair UB, Fang X, Zuo X, Wang YX, Wilkinson KD, Dhe-Paganon S. Two ZnFUBP domains in isopeptidase T (USP5) Biochemistry. 2012;51:1188–1198. doi: 10.1021/bi200854q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dayal S, Sparks A, Jacob J, Allende-Vega N, Lane DP, Saville MK. Suppression of the deubiquitinating enzyme USP5 causes the accumulation of unanchored polyubiquitin and the activation of p53. J Biol Chem. 2009;284:5030–5041. doi: 10.1074/jbc.M805871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang CH, Huang YC, Chen PY, Cheng YJ, Kao HH, Pi H, Chien CT. USP5/Leon deubiquitinase confines postsynaptic growth by maintaining ubiquitin homeostasis through Ubiquilin. Elife. 2017:6. doi: 10.7554/eLife.26886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li XY, Wu HY, Mao XF, Jiang LX, Wang YX. USP5 promotes tumorigenesis and progression of pancreatic cancer by stabilizing FoxM1 protein. Biochem Biophys Res Commun. 2017;492:48–54. doi: 10.1016/j.bbrc.2017.08.040. [DOI] [PubMed] [Google Scholar]
- 14.Wang S, Juan J, Zhang Z, Du Y, Xu Y, Tong J, Cao B, Moran MF, Zeng Y, Mao X. Inhibition of the deubiquitinase USP5 leads to c-Maf protein degradation and myeloma cell apoptosis. Cell Death Dis. 2017;8:e3058. doi: 10.1038/cddis.2017.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Wang WM, Zou LY, Li L, Feng L, Pan MZ, Lv MY, Cao Y, Wang H, Kung HF, Pang JX, Fu WM, Zhang JF. Ubiquitin specific peptidase 5 mediates histidine-rich protein Hpn induced cell apoptosis in hepatocellular carcinoma through P14-P53 signaling. Proteomics. 2017;17 doi: 10.1002/pmic.201600350. [DOI] [PubMed] [Google Scholar]
- 16.Ma X, Huang M, Wang Z, Liu B, Zhu Z, Li C. ZHX1 inhibits gastric cancer cell growth through inducing cell-cycle arrest and apoptosis. J Cancer. 2016;7:60–68. doi: 10.7150/jca.12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma X, Zhao J, Yang F, Liu H, Qi W. Ubiquitin conjugating enzyme E2 L3 promoted tumor growth of NSCLC through accelerating p27kip1 ubiquitination and degradation. Oncotarget. 2017;8:84193–84203. doi: 10.18632/oncotarget.20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manzi L, Barrow AS, Scott D, Layfield R, Wright TG, Moses JE, Oldham NJ. Carbene footprinting accurately maps binding sites in protein-ligand and protein-protein interactions. Nat Commun. 2016;7:13288. doi: 10.1038/ncomms13288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reyes-Turcu FE, Horton JR, Mullally JE, Heroux A, Cheng X, Wilkinson KD. The ubiquitin binding domain ZnF UBP recognizes the C-terminal diglycine motif of unanchored ubiquitin. Cell. 2006;124:1197–1208. doi: 10.1016/j.cell.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 20.Reyes-Turcu FE, Shanks JR, Komander D, Wilkinson KD. Recognition of polyubiquitin isoforms by the multiple ubiquitin binding modules of isopeptidase T. J Biol Chem. 2008;283:19581–19592. doi: 10.1074/jbc.M800947200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott D, Layfield R, Oldham NJ. Ion mobility-mass spectrometry reveals conformational flexibility in the deubiquitinating enzyme USP5. Proteomics. 2015;15:2835–2841. doi: 10.1002/pmic.201400457. [DOI] [PubMed] [Google Scholar]
- 22.Scott D, Layfield R, Oldham NJ. Structural insights into interactions between ubiquitin specific protease 5 and its polyubiquitin substrates by mass spectrometry and ion mobility spectrometry. Protein Sci. 2015;24:1257–1263. doi: 10.1002/pro.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang YH, Zhou CJ, Zhou ZR, Song AX, Hu HY. Domain analysis reveals that a deubiquitinating enzyme USP13 performs non-activating catalysis for Lys63-linked polyubiquitin. PLoS One. 2011;6:e29362. doi: 10.1371/journal.pone.0029362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SY, Kwon SK, Lee SY, Baek KH. Ubiquitin-specific peptidase 5 and ovarian tumor deubiquitinase 6A are differentially expressed in p53+/+ and p53-/- HCT116 cells. Int J Oncol. 2018 doi: 10.3892/ijo.2018.4302. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Potu H, Peterson LF, Pal A, Verhaegen M, Cao J, Talpaz M, Donato NJ. Usp5 links suppression of p53 and FAS levels in melanoma to the BRAF pathway. Oncotarget. 2014;5:5559–5569. doi: 10.18632/oncotarget.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li LF, Wei ZJ, Sun H, Jiang B. Abnormal beta-catenin immunohistochemical expression as a prognostic factor in gastric cancer: a meta-analysis. World J Gastroenterol. 2014;20:12313–12321. doi: 10.3748/wjg.v20.i34.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patil MA, Lee SA, Macias E, Lam ET, Xu C, Jones KD, Ho C, Rodriguez-Puebla M, Chen X. Role of cyclin D1 as a mediator of c-Met- and beta-catenin-induced hepatocarcinogenesis. Cancer Res. 2009;69:253–261. doi: 10.1158/0008-5472.CAN-08-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang X, Gu P, Zhou C, Liang A, Ren S, Liu F, Zeng Y, Wu Y, Zhao Y, Huang B, Zhang Z, Yi X. Beta-Catenin overexpression is associated with gefitinib resistance in non-small cell lung cancer cells. Pulm Pharmacol Ther. 2014;28:41–48. doi: 10.1016/j.pupt.2013.05.005. [DOI] [PubMed] [Google Scholar]