Abstract
Exosomes are small membrane vesicles of endocytic origin secreted by most cell types. They play important roles in intercellular communications and many physiological processes. DCs-derived exosomes can prime naïve T cells and activate NK cells to shrink the tumor. Tumor-derived exosomes carry a variety of tumor antigens that trigger the robust tumor antigen-specific immune response. Tumor-derived exosomes also contain metastasis or invasive-related molecules, which maybe potential targets for cancer immunotherapy. Effector T cells-derived exosomes possess cytotoxic activity of their original cells, thus cause tumor cells lysis. In this review, we summarized the recent advances on the biogenesis and composition of exosomes, the functions of anti-tumor immune response, and the promising applications on cancer immunotherapy of exosomes from different origins. Exosomes schlep efficient targets homing to tumor sites and tend to be a promising new tool of immunotherapy to fight cancer in a cell-free system.
Keywords: Exosomes, cancer immunotherapy, DCs, CTLs, CAR-T cells
Introduction
Exosomes were fist described in the 1980s by Johnstone et al. They noted that vesicles shed from cultured monolayer cells retained enzymatic activity reminiscent of the parent cells [1]. Such small vesicles were formed by inward budding inside intracellular endosomes, leading to the formation of multivesicular bodies (MVBs), which could fuse with the plasma membrane (PM) and release exosomes out of the cell [2,3]. Exosomes were lipid bilayer vesicles with 30-150 nm in diameter [4] and 1.13 g/ml-1.19 g/ml in buoyant density [5]. The proteins, TSG101, ALIX, CD63, and HSP70, are exosomal markers and commonly used to identify the presence of vesicles as true exosomes [6,7].
Except the common marker proteins, exosomes also contain the specific molecules that reflect their cellular origins. Exosomes derived from professional antigen-presenting cells (APCs), such as B cells and dendritic cells (DCs), enriched in MHC/peptide complex and costimulatory molecules, therefore play an important role in anti-tumor response in immune stimulation and regulation [8-10]. Tumor-derived exosomes (Texs) carried antigens are important as a source of specific stimulus for the immune response against cancer [11]. In addition, the targeting specificity of cytotoxic T lymphocytes (CTLs) and chimeric antigen receptor engineered T (CAR-T) cells had preserved in CTLs and CAR-T cell-derived exosomes respectively [12].
Functions of exosomes in the biological process depend on the interaction between exosomes and the target cells [13]. Exosomes could directly fuse with the PM after binding to the target cells. By labeling exosomes with the lipophilic dye R18, scientists obtained direct evidence for fusion of exosomes with target cell membranes, in which self-quenching is relieved upon dilution as a consequence of fusion, resulting in an increase in the fluorescence of target cells [14]. In another way, several studies have demonstrated that exosomes also can internalized through special endocytic pathways, which depend on the actin cytoskeleton, phosphatidylinositol 3-kinase activity, and dynamin-2 function [15-17]. Therefore, exosomes can deliver their contents to target cells, and prime its biological functions.
Despite therapeutic advances in recent decades, lacks of specificity and effectiveness remain the main drawbacks of clinical cancer treatment. Exosomes have a potential effect in anti-tumor immune response and may take a place in cancer therapeutic intervention. However, more information about exosomes should be uncovered before clinical application [18], which is so attractive that an increasing number of studies about it are underway. To gain further information on this aspect to conduct and promote application of exosomes, we summarize the biogenesis, composition, functions, and clinical trials in cancer immunotherapy of exosomes.
Biogenesis and composition
Several molecules and complexes are involved in the formation of MVBs, which could fuse with the PM and release exosomes [19], including the Endosomal Sorting Complex Required for Transport (ESCRT) machinery [20], lipids (such as ceramide) [21] and the tetraspanins [22,23] (Figure 1). Components of ESCRT machinery are necessary for MVB [20]. ESCRT machinery consists of a set of cytosolic protein complexes, known as ESCRT-0, ESCRT-I, ESCRT-II, and ESCRT-III, and several associated proteins, which conserved from yeast to mammals [24,25]. ESCRT-0 complex, consisting of the hepatocyte growth factor-regulated tyrosine kinase substrate (HRS), recognizes ubiquitylated cargo during the initial step of endosomal sorting. ESCRT-I and -II are involved in inducing membrane deformation into buds with sequestered cargo. ESCRT-III induces vesicle scission [26,27], and the accessory proteins, especially the VPS4 ATPase, involves dissociation and recycling of the ESCRT machinery.
Figure 1.
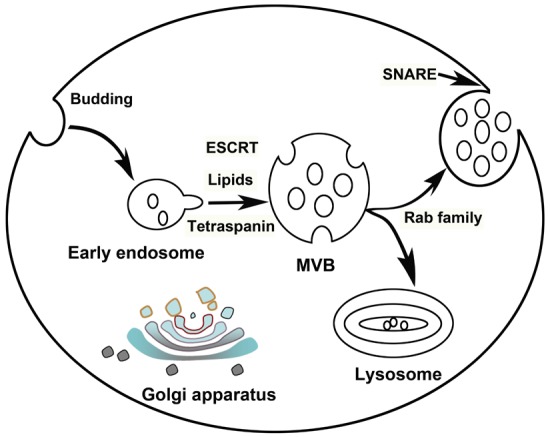
Schematic representation of the biogenesis and release of exosomes by eukaryotic cells. Exosomes firstly formed as multivesicular body (MVB) by budding into early endosomes. Several mechanisms including ESCRT, lipids and tetraspanin-dependent, are involved in MVB formation. If MVBs did not target for lysosomal degradation, they may mobilize and fuse with plasma membrane (PM) to release exosomes, depending on Rab family and soluble NSF-attachment protein receptor (SNARE) complexes respectively.
ESCRT-independent mechanisms also contribute to MVBs formations. Trajkovic et al. reported in 2008 that ceramide was involved in MVBs formation [21]. Then several labs started to confirm the function of exosomes secretion in vivo through neutral sphingomyelinase inhibition [28,29]. In addition, four transmembrane domains of the tetraspanin family involve in cargoes clustering for MVBs formation. Van Niel et al. firstly found that CD63 was responsible for sorting melanosomal proteins into MVBs cargo in human melanoma cells, independent of ceramide and ESCRT [30]. TSPAN8 expression [31] and CD81 [32] were also suggested could alter both the containing of exosomes.
After biogenesis, MVBs may mobilize and fuse with the cell membrane to release their intraluminal vesicles as exosomes, if they did not target to lysosomal degradation [33,34]. RAB family proteins, such as Rab11, Rab35, and Rab27 serve a function in MVB docking to the PM [35-37], which is required for eventual fusion of the two membranes, to allow the secretion of the exosomes. After docking of the two different intracellular compartments, soluble NSF-attachment protein receptor (SNARE) complexes play an important role in fusion of the lipid bilayers [38]. The SNARE proteins SNAP-23, VAMP-7 and VAMP-8 are involved in Ca2+-regulated fusion of secretory lysosomes with the PM in different cell types [39-41].
In accordance with the biogenesis and secretion process of exosomes, the composition of exosomes includes both PM and cytosolic components, besides cytoskeleton proteins, but very few from other intracellular organelles (nucleus, mitochondria, Golgi) [6]. Studies from different cell type revealed that exosomes contain about 4,563 proteins, 194 lipids, 1639 mRNA and 764 microRNA, demonstrated their complexity [42,43]. Both ubiquitous and cell-specific proteins maybe target selectively to exosomes [44]. The former includes the components involved in exosomes biogenesis and, perhaps, in some unknown common exosomes functions cytoskeletal components, such as tubulin, actin and actin-binding proteins, as well as ANNEXINS and RAB protein family who participate in intracellular membrane fusions and transport [44]. Exosomes also contain proteins that involve in specific cell functions. Proteomics analysis of exosomes derived from DCs (Dexs) showed that it possesses MHC-I and -II molecules, which could potentially stimulate CD8+ and CD4+ T cells. Costimulatory molecules are also contained in Dexs, forming a unique molecular composition for strong immune-stimulatory functionality [6,8,45,46]. As characterized by matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF), MHC-I was found to be present together with the heat shock proteins HSC70 or HSP90 in exosomes derived from human mesothelioma cells, revealing that Texs may be involved in tumor immunity process [47].
In addition to proteins, exosomes also contain mRNAs and microRNAs (miRNAs), which can be taken up by neighboring or distant cells, subsequently repress target mRNAs of acceptor DCs and modulate recipient cells [48,49]. Transfer of exosomes represents a novel mechanism of DC-to-DC communication and post-transcriptional regulation between DCs [14]. Exosomal miRNAs also play an important role in disease progression, and can stimulate angiogenesis and facilitate metastasis in cancers [50,51]. Thus, exosomal miRNAs show potential for use as noninvasive biomarkers to indicate disease states [52,53].
DCs-derived exosomes
As the professional APC of the immune system, DCs play a critical role in initiating antigen-specific immune response and tolerance [54]. DCs are responsible to capture, process, and present antigens to naïve T cells, which are then activated, building an essential bridge between innate and adaptive immune responses [55,56]. In cancer immunity, DCs are involved in the first step of immune response that aims to eliminate tumor cells through triggering tumor-specific cytotoxic lymphocyte [57]. Therefore, DCs induced from peripheral blood mononuclear cells (PBMCs) of cancer patients are cultured in vitro and pulsed by tumor lysate or tumor antigen peptide for cancer immunotherapy [58-61]. Provenge, the first DCs vaccine product approved by FDA, which was applying for immunotherapy for castration-resistant prostate cancer, represented a 4.1-month improvement in median survival (25.8 months in the sipuleucel-T group vs. 21.7 months in the placebo group) [62]. However, DCs vaccine is living cells. The cost is very high for storage and stability over longer periods. In addition, DCs are susceptible to immunosuppressive molecules and immune regulation in the tumor microenvironment [63].
Dexs carry many immune function-associated molecules of DCs, peptide/MHC complexes that trigger antigen specific T cell response [9,10], and co-stimulatory molecules including CD80, CD83, CD86, that contribute to the T cell priming and activation [8]. Particularly, exosomes derived from tumor antigen peptide-stimulated DCs are able to prime tumor-specific CTLs in vivo, result in the tumor growth delayed, even eliminate tumor in some case of mouse models [8].
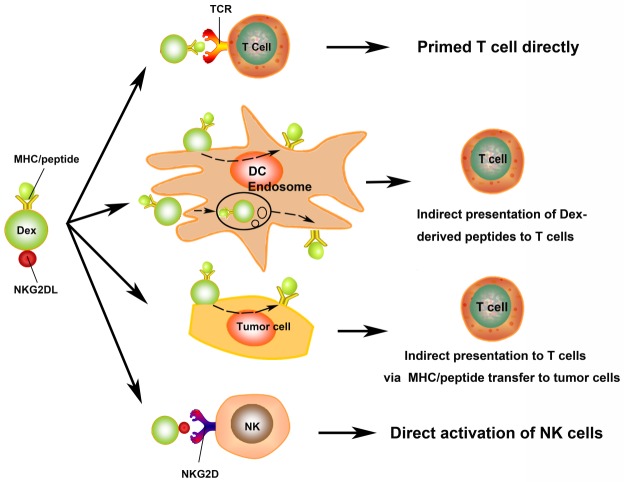
Scientists proposed several mechanisms to explain Dexs stimulate T cells via their MHC and costimulation molecules loading (Figure 2). Dexs activate T cells directly in vitro [9], however, direct Dex-to-T cell stimulation appears to be inefficient in priming naïve T cells and rarely occurs in vivo [64,65]. Rather than direct Dex-to-T cell stimulation, it is much more efficient than transfer antigenic peptide/MHC complexes in Dexs to bystander APCs. Dexs are internalized into the endosomes and processed by APCs, followed the antigen presentation on the surface of APCs indirectly [34,66-69]. Another way that Dexs mediated indirect antigen presentation is Dexs fuse with APCs surface after binding to target APCs, thereby transferring their peptide/MHC complexes to APCs surface immediately [67,70]. Dexs incorporated by tumor cells could turn tumor cells into immunogenic targets for a possibly more effective response. In a recent study, treatment of human breast adenocarcinoma cells with Dexs was able to restimulate previously primed T cells, showing significantly higher percentages of interferon-γ (IFN-γ)-secreting induction than exposure to non-Dex-treated adenocarcinoma cells [8]. These studies suggested that Dexs maintain the essential immunostimulatory faculties of DCs, may become a promising tool for cancer immunotherapy.
Figure 2.
The role of exosomes derived from DC (Dexs) in anti-tumor immunity. Dexs may stimulate T cells via direct and indirect routes. The presence of MHC/peptide complex on the surface of Dexs stimulates T cells directly. Dexs activate T cells indirectly by bystander DCs via two mechanisms, one is Dexs fuse with PM of DCs and transfer MHC/peptide complexes to DCs surface immediately, the other one, Dexs are internalised into the endosomes and processed by APCs, following the antigen presentation on the surface of APCs indirectly. Dexs may also present MHC/peptide complexes to host T cells by MHC/peptide transfer to tumor cells. Dexs were shown to possess NKG2D ligand (NKG2DL), which can interact with NKG2D and activate NK cells.
In addition, Dexs have the bilayer membrane to keep the bioactivity stable in -80°C for at least 6 months with a phenotype and function preserved [71]. Used as a cell-free cancer immunotherapy tool, Dexs maybe more resistant to the immunosuppressive microenvironment in the tumor, which can down-regulate costimulatory molecules on DCs, therefore block T cell response [72]. Compared to DCs, Dexs surface possess natural killer (NK) cell lectin-like receptor subfamily K, member 1 ligands (NKG2D-L), which can engage with NKG2D expressed on NK cells, resulting in NK cells activation (Figure 2) [73]. Dexs express BCL2-associated athanogene 6 (BAG6, also known as BAT3) on their surface, which has been shown to enhance NK cell cytokine release [74]. Additionally, tumor necrosis factor (TNF) in Dexs induces NK cell-mediated IFN-γ production [75].
Because of their high potential and benefits for immunotherapy, Dexs have developed as clinical cell-free cancer vaccines. Two phase I clinical trials [76,77] and one phase II trial [78] of Dexs in advanced cancer patients have been completed. The results suggested that Dexs are safe, and a mild T cell response and a potential increase in NK cell lysis ability observed. So combined Dexs with NK cells for cancer immunotherapy might be considerable.
Tumor-derived exosomes
Texs bear MHC-I molecules, HSP70, and antigens, that are considered to be a source of specific stimulus for the immune response against cancer. Texs trigger anti-tumor responses more efficiently than irradiated tumor cells, apoptotic bodies, or lysate of cancer cells [11]. Stress-inducible exosomal HSP70 functions as an endogenous danger signal, promotes NK cell activation and cancer cell lysis via granzyme B [79]. Texs can efficiently deliver a variety of tumor antigens to DCs (Figure 3) and, thus, have employed as antigen carriers for cancer immunotherapy [80]. Rao et al. demonstrated that hepatocellular carcinoma (HCC) cell-derived exosomes, carried an array of HCC antigens, can elicit a stronger DC-mediated immune response than cell lysates in vitro and in vivo. In addition, HCC tumor microenvironment was improved by HCC cell-derived exosomes, demonstrated by increased numbers of T lymphocytes, elevated levels of IFN-γ, and decreased levels of interleukin-10 and tumor growth factor-β in the tumor (Figure 3) [81,82].
Figure 3.
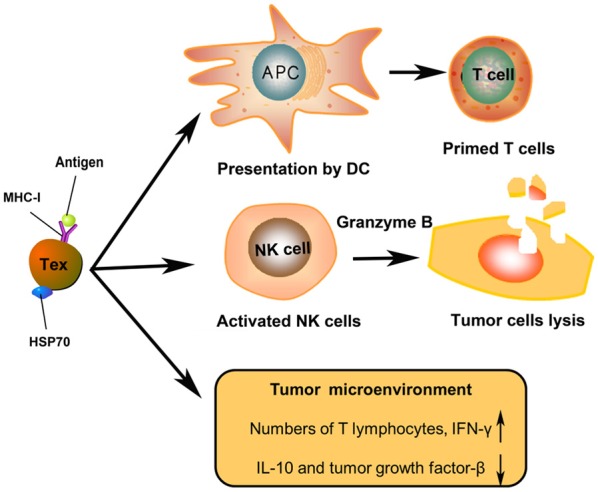
The role of tumor cells secreted exosomes (Texs) in anti-tumor immunity. Texs expressed tumor MHC/antigen complexes internalised by or fused with antigen-presenting cells (APCs) to prime T cells. Stress-inducible exosomal HSP70 functions as an endogenous danger signal, promotes NK cell activation and cancer cell lysis via granzyme B. Texs improve the HCC tumor microenvironment in vivo after infusion.
The same as Dexs, Texs may also be the potential candidate used as a cell-free tumor vaccine. However, the isolation of autologous Texs may require culture and expansion of patients’ cancer cells in vitro, which may be inconvenient in clinical applications. Ascites-derived exosomes (Aexs) of advanced colorectal carcinoma (CRC) patients are considered be released from CRC cells [83]. Aexs contain MHC-I and -II molecules, co-stimulatory molecules, ICAMs, and the immunogenic carcinoembryonic antigen (CEA) of CRC, which maybe recognized by APCs. In a phase I clinical trial, Aexs were used to treat 40 advanced CRC patients with Aexs alone or Aexs plus GM-CSF [83]. Safety of Aexs on patients has confirmed, but no significant therapeutic effect observed when Aexs used alone. However, Aexs plus GM-CSF can activate CD8+ CTL thus elicit CEA-specific antitumor immune response. It is suggested that Aexs in combination with GM-CSF maybe a potential method for cancer immunotherapy in the future [83]. Nevertheless, the immunogenic potential and anti-tumor efficiency of Aexs still need to investigate in clinical trials.
Studies unraveled that Texs possess immunosuppressive properties and directly modify tumor cells’ intrinsic motility and invasiveness capacity. Texs has been shown to suppress T cell and NK cell response, stimulate myeloid-derived suppressor cells (MDSCs), resulting in facilitating tumor growth [84,85]. Texs could induce T cell apoptosis that facilitate evasion of immune surveillance [86]. In particular, Texs directly enhance tumor cells’ metastasis and invasiveness through some mechanisms [18]. Integrins distributed on the Texs surface, which bind to extracellular matrix (ECM) components, such as fibronectin, providing a substrate favoring cell adhesion and enhancing cell mobility [87]. Texs are involved in the biogenesis and activity of an invasive structure called invadopodia of tumor cells via the MVB-dependent delivery of metalloproteinases and other exosomes cargoes [88]. Cellular physiology of both surrounding and distant normal cells can also change by Texs to facilitate metastasis and growth of cancer cells [89,90]. Therefore, it is vital to distinguish the immune-activated and immunosuppressive Texs, in order to remove the latter ones when technology is available, ensuring the curative effect of Tex vaccine. Alternatively, the immune-inhibitory effect of Texs successfully suppressed by combining Texs with appropriate, immune-stimulatory adjuvants, thus an anti-tumor response might be developed [91]. On the other hand, Texs included different molecules from donors’ tumors that are involved in enhancing metastasis and invasiveness of tumor or inhibiting anti-tumor immune response. These molecules in Texs are useful markers to block as potential targets for cancer immunotherapy, but a great deal of additional research will be required to develop these therapies for clinical use.
CTLs and CAR-T cells derived exosomes
Adoptive cell therapy (ACT) relieves tumors with infused CTLs. Mature DCs that pulsed by tumor lysates or tumor antigen peptides activate naïve T cells isolated from PBMC, trigger CTLs induction. Most cytotoxic T cells express T-cell receptors (TCRs) that can recognize specific antigens. TCRs bind to the MHC/antigen complex, and T cell destroys the target cell through releasing the cytotoxins perforin, granzymes, and granulysin (Figure 4) [92]. As early as in 1989, Peters et al. suggested that it’s essential for human T cell-derived exosomes to participate in the process of CTLs and target cell interaction [12,93,94]. As specifically expressed on CTLs surface, the presence of CD3, CD8, and TCR on CTLs-derived exosomes suggested that exosomes could deliver cytotoxicity to targeted cells. Exosomes can interact with target cell by TCR binding to antigen/MHC-I complex, resulting in target cell death [12]. The cytotoxicity to target cells achieved by the cytotoxic compounds in the exosomes, including perforin, granzymes, and lysosomal enzymes [93]. In 2002, it was unraveled that the secretion of exosomes by CTLs accelerated by TCR activation and the TCR/CD3ζ complex existed on the surface membrane of exosomes derived from human CTLs [95].
Figure 4.
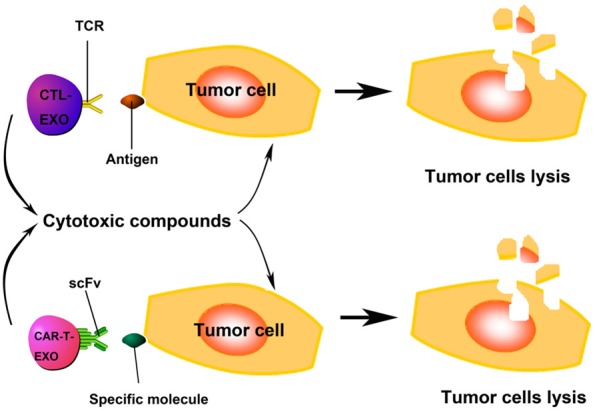
The role of exosomes derived from CTLs (CTL-EXO) and CAR-T cells (CAR-T-EXO) in anti-tumor immunity. The presence of TCR/CD3/CD8 on CTL-EXO enable CTL-EXO target antigen expressing tumor cells specificly, and cytotoxic compounds contained in CTL-EXO kill the target cells. CAR-T-EXO may possess antibody-derived single-chain variable fragment (scFv), which determines the targeting specificity of CAR-T cells, can recognize specific molecules on target cells (such as CD19 on B-cell neoplasms) and kill them by cytotoxic compounds release.
Adoptive transfer of CAR-T cells suggests a promising new method in cancer immunotherapy. The targeting specificity of CAR-T cells are determined by an antibody-derived single-chain variable fragment (scFv) in the CAR structure. However, a major limitation of CAR-T cells application in clinic is poor therapeutic effects in solid tumors rather than in lymphoid malignancies. CAR-T cell-derived exosomes might possess antibody-derived scFv, being the promising alternative of cell therapy. The cell-to-cell contact between CAR-T cells or CTLs and tumor cells is necessary for the anticancer effect of CAR-T cells and CTLs. Unlike lymphoid malignancies, CAR-T cells and CTLs must penetrate stroma rich matrixes of the solid tumor to interact with tumor cells. However, tumor microenvironment may limit the activity of CAR-T cells and CTLs [96]. While cell-free exosomes are small nanometer-sized particles and were able to be located at specific antigen-targeted tumor in situ and to attack the tumor cells, since exosomes have the ability to cross biological barriers such as the blood-brain barrier (BBB) [97-99] and blood-tumor barrier (BTB) which was confirmed by the large quantity of Texs in body fluids [100]. The enhanced angiogenesis effect and leaky vasculature of the tumor are also attractive for intravenously injected exosomes to migrate to the tumor [100].
Conclusion and prospects
Exosomes are easily available through ultracentrifugation process generally. Ultracentrifuges are widespread and straightforward to use, suggesting that the protocol of exosomes isolation and infusion can implement in clinical laboratories as a routine immunotherapy procedure for the cancer patients. But we now know that which isolated production is a mixture of exosomes and other extracellular vesicles (EVs). Here is impossible to distinguish them on the basis of a single property, such as size, structure, buoyant density, or presence of a given protein, so purification of exosomes is technically unavailable so far [7]. Novel isolation methods are required to enrichment of the specific subtypes [18]. Therefore, better knowledge of specific markers of EVs subtypes is required. Extensive quantitative proteomic analysis have shown that several classically used exosomes markers, like MHC, flotillin, and HSP70 proteins, are similarly present in all EVs [101]. Nevertheless, a few new specific markers for different subtypes of exosomes are proposed, which are tetraspanins CD9/CD63/CD81, TSG101, and syntenin-1 for endosome-derived exosomes, thus providing guidelines to define subtypes of exosomes precisely for future functional studies.
Two areas for improvement of cancer treatment are targeting and effectiveness. Exosomes schlep efficient targets homing to tumor sites. There is abundant source of exosomes in the culture supernatant of DCs and tumor cells. CTLs and CAR-T cells possess mighty expansion ability, ensuring the outlay of exosomes to be satisfied. With the remarkable effects in anti-tumor immune response mentioned above, exosomes could substitute or boost other strategies of cell-based immunotherapy or be used as a maintenance vaccine in the future. Exosomes derived from different sources hold diverse applications in cancer therapy. In addition to the detailed discussed above, there are exosomes derived from other sources, such as mesenchymal stem cells-derived exosomes, HEK293T cells-derived exosomes, macrophage-derived exosomes, cerebral endothelial cell-derived exosomes, reticulocyte-derived exosomes and so on. Thus, it is now vital to understand exosomes derived from different sources themselves with the aim of choosing the appropriate exosomes for cancer therapy.
However, these issues should not interfere with the diffusion of such clinically important therapy for cancer. The potential development of exosomes for efficient therapeutic strategies in cancer depends on further progression.
Acknowledgements
This work was supported by grants from the Basic Research Program of Shenzhen (JCYJ20130326110139687, JSGG20160226161357949); the China Postdoctoral Science Foundation (2014M552234).
Disclosure of conflict of interest
None.
References
- 1.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–9420. [PubMed] [Google Scholar]
- 2.Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101:942–948. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, Manel N, Moita LF, Thery C, Raposo G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126:5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 5.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 6.Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 7.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Bi. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 8.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 9.Admyre C, Johansson SM, Paulie S, Gabrielsson S. Direct exosome stimulation of peripheral human T cells detected by ELISPOT. Eur J Immunol. 2006;36:1772–1781. doi: 10.1002/eji.200535615. [DOI] [PubMed] [Google Scholar]
- 10.Utsugi-Kobukai S, Fujimaki H, Hotta C, Nakazawa M, Minami M. MHC class I-mediated exogenous antigen presentation by exosomes secreted from immature and mature bone marrow derived dendritic cells. Immunol Lett. 2003;89:125–131. doi: 10.1016/s0165-2478(03)00128-7. [DOI] [PubMed] [Google Scholar]
- 11.Wolfers J, Lozier A, Raposo G, Regnault A, Théry C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 12.Peters PJ, Geuze HJ, Van der Donk HA, Slot JW, Griffith JM, Stam NJ, Clevers HC, Borst J. Molecules relevant for T cell-target cell interaction are present in cytolytic granules of human T lymphocytes. Eur J Immunol. 1989;19:1469–1475. doi: 10.1002/eji.1830190819. [DOI] [PubMed] [Google Scholar]
- 13.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM, Baty CJ, Gibson GA, Erdos G, Wang Z, Milosevic J, Tkacheva OA, Divito SJ, Jordan R, Lyons-Weiler J, Watkins SC, Morelli AE. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119:756–766. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morelli AE. The immune regulatory effect of apoptotic cells and exosomes on dendritic cells: its impact on transplantation. Am J Transplant. 2006;6:254–261. doi: 10.1111/j.1600-6143.2005.01197.x. [DOI] [PubMed] [Google Scholar]
- 16.Barres C, Blanc L, Bette-Bobillo P, Andre S, Mamoun R, Gabius HJ, Vidal M. Galectin-5 is bound onto the surface of rat reticulocyte exosomes and modulates vesicle uptake by macrophages. Blood. 2010;115:696–705. doi: 10.1182/blood-2009-07-231449. [DOI] [PubMed] [Google Scholar]
- 17.Tian T, Wang Y, Wang H, Zhu Z, Xiao Z. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J Cell Biol. 2010;111:488–496. doi: 10.1002/jcb.22733. [DOI] [PubMed] [Google Scholar]
- 18.Tkach M, Thery C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164:1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 19.Kowal J, Tkach M, Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Boil. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Henne WM, Stenmark H, Emr SD. Molecular mechanisms of the membrane sculpting ESCRT pathway. CSH Perspect Biol. 2013;5:1288–1302. doi: 10.1101/cshperspect.a016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 22.van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, Marks MS, Rubinstein E, Raposo G. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21:708–721. doi: 10.1016/j.devcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273:20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- 24.Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 25.Roxrud I, Stenmark H, Malerod L. ESCRT & Co. Biol Cell. 2010;102:293–318. doi: 10.1042/BC20090161. [DOI] [PubMed] [Google Scholar]
- 26.Hurley JH, Hanson PI. Membrane budding and scission by the ESCRT machinery: it’s all in the neck. Nat Rev Mol Cell Bi. 2010;11:556–566. doi: 10.1038/nrm2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wollert T, Wunder C, Lippincott-Schwartz J, Hurley JH. Membrane scission by the ESCRT-III complex. Nature. 2009;458:172–177. doi: 10.1038/nature07836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dreux M, Garaigorta U, Boyd B, Decembre E, Chung J, Whitten-Bauer C, Wieland S, Chisari FV. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe. 2012;12:558–570. doi: 10.1016/j.chom.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, Marks MS, Rubinstein E, Raposo G. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21:708–721. doi: 10.1016/j.devcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nazarenko I, Rana S, Baumann A, McAlear J, Hellwig A, Trendelenburg M, Lochnit G, Preissner KT, Zoller M. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010;70:1668–1678. doi: 10.1158/0008-5472.CAN-09-2470. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Hernandez D, Gutierrez-Vazquez C, Jorge I, Lopez-Martin S, Ursa A, Sanchez-Madrid F, Vazquez J, Yanez-Mo M. The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J Biol Chem. 2013;288:11649–11661. doi: 10.1074/jbc.M112.445304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harding CV, Heuser JE, Stahl PD. Exosomes: looking back three decades and into the future. J Cell Biol. 2013;200:367–371. doi: 10.1083/jcb.201212113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savina A, Fader CM, Damiani MT, Colombo MI. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic. 2005;6:131–143. doi: 10.1111/j.1600-0854.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- 36.Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, Bakhti M, Grønborg M, Möbius W, Rhee J, Barr FA, Simons M. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189:223–232. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Goud B, Benaroch P, Hacohen N, Fukuda M, Desnos C, Seabra MC, Darchen F, Amigorena S, Moita LF, Thery C. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19–30. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 38.Zylbersztejn K, Galli T. Vesicular traffic in cell navigation. FEBS J. 2011;278:4497–4505. doi: 10.1111/j.1742-4658.2011.08168.x. [DOI] [PubMed] [Google Scholar]
- 39.Puri N, Roche PA. Mast cells possess distinct secretory granule subsets whose exocytosis is regulated by different SNARE isoforms. Proc Natl Acad Sci U S A. 2008;105:2580–2585. doi: 10.1073/pnas.0707854105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rao SK, Huynh C, Proux-Gillardeaux V, Galli T, Andrews NW. Identification of SNAREs involved in synaptotagmin VII-regulated lysosomal exocytosis. J Biol Chem. 2004;279:20471–20479. doi: 10.1074/jbc.M400798200. [DOI] [PubMed] [Google Scholar]
- 41.Tiwari N, Wang CC, Brochetta C, Ke G, Vita F, Qi Z, Rivera J, Soranzo MR, Zabucchi G, Hong W, Blank U. VAMP-8 segregates mast cellpreformed mediator exocytosis from cytokine trafficking pathways. Blood. 2008;111:3665–3674. doi: 10.1182/blood-2007-07-103309. [DOI] [PubMed] [Google Scholar]
- 42.Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40:D1241–1244. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mathivanan S, Simpson RJ. ExoCarta: a compendium of exosomal proteins and RNA. Proteomics. 2009;9:4997–5000. doi: 10.1002/pmic.200900351. [DOI] [PubMed] [Google Scholar]
- 44.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 45.Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tamura Y, Torigoe T, Kukita K, Saito K, Okuya K, Kutomi G, Hirata K, Sato N. Heat-shock proteins as endogenous ligands building a bridge between innate and adaptive immunity. Immunotherapy. 2012;4:841–852. doi: 10.2217/imt.12.75. [DOI] [PubMed] [Google Scholar]
- 47.Hegmans JP, Bard MP, Hemmes A, Luider TM, Kleijmeer MJ, Prins JB, Zitvogel L, Burgers SA, Hoogsteden HC, Lambrecht BN. Proteomic analysis of exosomes secreted by human mesothelioma cells. Am J Surg Pathol. 2004;164:1807–1815. doi: 10.1016/S0002-9440(10)63739-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, Li S, Li L, Sjostrand M, Lee JJ, Lotvall JO. Exosome and exosomal microRNA: trafficking, sorting, and function. Genom Proteom Bioinf. 2015;13:17–24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Png KJ, Halberg N, Yoshida M, Tavazoie SF. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature. 2012;481:190–194. doi: 10.1038/nature10661. [DOI] [PubMed] [Google Scholar]
- 51.Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, Yu Y, Chow A, O’Connor ST, Chin AR, Yen Y, Wang Y, Marcusson EG, Chu P, Wu J, Wu X, Li AX, Li Z, Gao H, Ren X, Boldin MP, Lin PC, Wang SE. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25:501–515. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rabinowits G, Gercel-Taylor C, Day JM, Taylor DD, Kloecker GH. Exosomal microRNA: a diagnostic marker for lung cancer. Clin Lung Cancer. 2009;10:42–46. doi: 10.3816/CLC.2009.n.006. [DOI] [PubMed] [Google Scholar]
- 54.Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 55.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 56.Steinman RM. Dendritic cells and the control of immunity: enhancing the efficiency of antigen presentation. Mt Sinai J Med. 2001;68:160–166. [PubMed] [Google Scholar]
- 57.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 58.Berzofsky JA, Terabe M, Oh S, Belyakov IM, Ahlers JD, Janik JE, Morris JC. Progress on new vaccine strategies for the immunotherapy and prevention of cancer. J Clin Invest. 2004;113:1515–1525. doi: 10.1172/JCI21926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murphy G, Tjoa B, Ragde H, Kenny G, Boynton A. Phase I clinical trial: T-cell therapy for prostate cancer using autologous dendritic cells pulsed with HLA-A0201-specific peptides from prostate-specific membrane antigen. Prostate. 1996;29:371–380. doi: 10.1002/(SICI)1097-0045(199612)29:6<371::AID-PROS5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 60.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 61.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 63.Pitt JM, Andre F, Amigorena S, Soria JC, Eggermont A, Kroemer G, Zitvogel L. Dendritic cell-derived exosomes for cancer therapy. J Clin Invest. 2016;126:1224–1232. doi: 10.1172/JCI81137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muntasell A, Berger AC, Roche PA. T cellinduced secretion of MHC class II-peptide complexes on B cell exosomes. EMBO J. 2007;26:4263–4272. doi: 10.1038/sj.emboj.7601842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Montecalvo A, Shufesky WJ, Stolz DB, Sullivan MG, Wang Z, Divito SJ, Papworth GD, Watkins SC, Robbins PD, Larregina AT, Morelli AE. Exosomes as a short-range mechanism to spread alloantigen between dendritic cells during T cell allorecognition. J Immunol. 2008;180:3081–3090. doi: 10.4049/jimmunol.180.5.3081. [DOI] [PubMed] [Google Scholar]
- 66.Vincent-Schneider H, Stumptner-Cuvelette P, Lankar D, Pain S, Raposo G, Benaroch P, Bonnerot C. Exosomes bearing HLA-DR1 molecules need dendritic cells to efficiently stimulate specific T cells. Int Immunol. 2002;14:713–722. doi: 10.1093/intimm/dxf048. [DOI] [PubMed] [Google Scholar]
- 67.Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 68.Andre F, Chaput N, Schartz NE, Flament C, Aubert N, Bernard J, Lemonnier F, Raposo G, Escudier B, Hsu DH, Tursz T, Amigorena S, Angevin E, Zitvogel L. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol. 2004;172:2126–2136. doi: 10.4049/jimmunol.172.4.2126. [DOI] [PubMed] [Google Scholar]
- 69.Wakim LM, Bevan MJ. Cross-dressed dendritic cells drive memory CD8+ T-cell activation after viral infection. Nature. 2011;471:629–632. doi: 10.1038/nature09863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakayama M. Antigen presentation by MHCDressed cells. Front Immuno. 2014;5:672. doi: 10.3389/fimmu.2014.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Andre F, Escudier B, Angevin E, Tursz T, Zitvogel L. Exosomes for cancer immunotherapy. Ann Oncol. 2004;15:iv141–144. doi: 10.1093/annonc/mdh918. [DOI] [PubMed] [Google Scholar]
- 72.Gabrilovich DI, Ciernik IF, Carbone DP. Dendritic cells in antitumor immune responses. I. Defective antigen presentation in tumor-bearing hosts. Cell Immunol. 1996;170:101–110. doi: 10.1006/cimm.1996.0139. [DOI] [PubMed] [Google Scholar]
- 73.Viaud S, Terme M, Flament C, Taieb J, André F, Novault S, Escudier B, Robert C, Caillat-Zucman S, Tursz T, Zitvogel L, Chaput N. Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha. PLoS One. 2009;4:e4942. doi: 10.1371/journal.pone.0004942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Simhadri VR, Reiners KS, Hansen HP, Topolar D, Simhadri VL, Nohroudi K, Kufer TA, Engert A, Pogge von Strandmann E. Dendritic cells release HLA-B-associated transcript-3 positive exosomes to regulate natural killer function. PLoS One. 2008;3:e3377. doi: 10.1371/journal.pone.0003377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Munich S, Sobo-Vujanovic A, Buchser WJ, Beer-Stolz D, Vujanovic NL. Dendritic cell exosomes directly kill tumor cells and activate natural killer cells via TNF superfamily ligands. Oncoimmunology. 2012;1:1074–1083. doi: 10.4161/onci.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Escudier B, Dorval T, Chaput N, André F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, Boccaccio C, Bonnerot C, Dhellin O, Movassagh M, Piperno S, Robert C, Serra V, Valente N, Le Pecq JB, Spatz A, Lantz O, Tursz T, Angevin E, Zitvogel L. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J Transl Med. 2005;3:10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A, Hsu DH, Le Pecq JB, Lyerly HK. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med. 2005;3:9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, Le Chevalier T, Livartoski A, Barlesi F, Laplanche A, Ploix S, Vimond N, Peguillet I, Théry C, Lacroix L, Zoernig I, Dhodapkar K, Dhodapkar M, Viaud S, Soria JC, Reiners KS, Pogge von Strandmann E, Vély F, Rusakiewicz S, Eggermont A, Pitt JM, Zitvogel L, Chaput N. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology. 2016;5:e1071008. doi: 10.1080/2162402X.2015.1071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Elsner L, Muppala V, Gehrmann M, Lozano J, Malzahn D, Bickeböller H, Brunner E, Zientkowska M, Herrmann T, Walter L, Alves F, Multhoff G, Dressel R. The heat shock protein HSP70 promotes mouse NK cell activity against tumors that express inducible NKG2D ligands. J Immunol. 2007;179:5523–5533. doi: 10.4049/jimmunol.179.8.5523. [DOI] [PubMed] [Google Scholar]
- 80.Pitt JM, Charrier M, Viaud S, Andre F, Besse B, Chaput N, Zitvogel L. Dendritic cell-derived exosomes as immunotherapies in the fight against cancer. J Immunol. 2014;193:1006–1011. doi: 10.4049/jimmunol.1400703. [DOI] [PubMed] [Google Scholar]
- 81.Zeelenberg IS, Ostrowski M, Krumeich S, Bobrie A, Jancic C, Boissonnas A, Delcayre A, Le Pecq JB, Combadière B, Amigorena S, Théry C. Targeting tumor antigens to secreted membrane vesicles in vivo induces efficient antitumor immune responses. Cancer Res. 2008;68:1228–1235. doi: 10.1158/0008-5472.CAN-07-3163. [DOI] [PubMed] [Google Scholar]
- 82.Rao Q, Zuo B, Lu Z, Gao X, You A, Wu C, Du Z, Yin H. Tumor-derived exosomes elicit tumor suppression in murine hepatocellular carcinoma models and human in vitro. Hepatology. 2016;64:456. doi: 10.1002/hep.28549. [DOI] [PubMed] [Google Scholar]
- 83.Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G. Phase I clinical trial of autologous ascitesderived exosomes combined with GM-CSF for colorectal cancer. Mol Ther. 2008;16:782–790. doi: 10.1038/mt.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gutierrez-Vazquez C, Villarroya-Beltri C, Mittelbrunn M, Sanchez-Madrid F. Transfer of extracellular vesicles during immune cell-cell interactions. Immunol Rev. 2013;251:125–142. doi: 10.1111/imr.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bobrie A, Colombo M, Raposo G, Thery C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 86.Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P, Squarcina P, Accornero P, Lozupone F, Lugini L, Stringaro A, Molinari A, Arancia G, Gentile M, Parmiani G, Fais S. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med. 2002;195:1303–1316. doi: 10.1084/jem.20011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sung BH, Ketova T, Hoshino D, Zijlstra A, Weaver AM. Directional cell movement through tissues is controlled by exosome secretion. Nat Commun. 2015;6:7164. doi: 10.1038/ncomms8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hoshino D, Kirkbride KC, Costello K, Clark ES, Sinha S, Grega-Larson N, Tyska MJ, Weaver AM. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep. 2013;5:1159–1168. doi: 10.1016/j.celrep.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen TM, García-Santos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Shen TL, Labori KJ, Lothe IM, Kure EH, Hernandez J, Doussot A, Ebbesen SH, Grandgenett PM, Hollingsworth MA, Jain M, Mallya K, Batra SK, Jarnagin WR, Schwartz RE, Matei I, Peinado H, Stanger BZ, Bromberg J, Lyden D. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Adams M, Navabi H, Croston D, Coleman S, Tabi Z, Clayton A, Jasani B, Mason MD. The rationale for combined chemo/immunotherapy using a Toll-like receptor 3 (TLR3) agonist and tumour-derived exosomes in advanced ovarian cancer. Vaccine. 2005;23:2374–2378. doi: 10.1016/j.vaccine.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 92.Milstein O, Hagin D, Lask A, Reich-Zeliger S, Shezen E, Ophir E, Eidelstein Y, Afik R, Antebi YE, Dustin ML, Reisner Y. CTLs respond with activation and granule secretion when serving as targets for T-cell recognition. Blood. 2011;117:1042–1052. doi: 10.1182/blood-2010-05-283770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peters PJ, Borst J, Oorschot V, Fukuda M, Krahenbuhl O, Tschopp J, Slot JW, Geuze HJ. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J Exp Med. 1991;173:1099–1109. doi: 10.1084/jem.173.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peters PJ, Geuze HJ, van der Donk HA, Borst J. A new model for lethal hit delivery by cytotoxic T lymphocytes. Immunol Today. 1990;11:28–32. doi: 10.1016/0167-5699(90)90008-w. [DOI] [PubMed] [Google Scholar]
- 95.Blanchard N, Lankar D, Faure F, Regnault A, Dumont C, Raposo G, Hivroz C. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J Immunol. 2002;168:3235–3241. doi: 10.4049/jimmunol.168.7.3235. [DOI] [PubMed] [Google Scholar]
- 96.Burgents JE, Moran TP, West ML, Davis NL, Johnston RE, Serody JS. The immunosuppressive tumor environment is the major impediment to successful therapeutic vaccination in Neu transgenic mice. J Immunother. 2010;33:482–491. doi: 10.1097/CJI.0b013e3181d756bb. [DOI] [PubMed] [Google Scholar]
- 97.Tran TH, Mattheolabakis G, Aldawsari H, Amiji M. Exosomes as nanocarriers for immunotherapy of cancer and inflammatory diseases. Clin Immunol. 2015;160:46–58. doi: 10.1016/j.clim.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 98.Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, Yin VP, Lockman P, Bai S. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm Res. 2015;32:2003–2014. doi: 10.1007/s11095-014-1593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 100.Gyorgy B, Hung ME, Breakefield XO, Leonard JN. Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu Rev Pharmacol. 2015;55:439–464. doi: 10.1146/annurev-pharmtox-010814-124630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Thery C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. P Natl Acad Sci U S A. 2016;113:E968–977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]