Abstract
Prolactin-induced protein (PIP) is a small secreted glycoprotein carrying several N-linked carbohydrate chains. The expression of PIP is generally restricted to cells with apocrine properties. It was found in apocrine glands of the axilla, vulva, eyelid, ear canal, and seminal vesicle. Being a secretory protein, PIP is present in seminal plasma, saliva, lacrimal fluid, tears, sweat gland secretion. Little is known about the biological role of PIP. It binds to numerous proteins, however, in most cases the biological role of such interactions is poorly understood. A notable exception is its binding to CD4 receptors present on the surface of T lymphocytes, macrophages, and spermatozoa. The available data suggest that PIP can have immunomodulatory functions and plays an important role in cell-mediate adoptive immunity. PIP binds to bacteria from several genera, which suggests that this glycoprotein may participate also in innate immunity and protection of hosts against microbial infections. Increased levels of PIP were found in several types of human cancer (prostate, sweat and salivary gland cancers). It is especially common in breast cancer, however, data on the expression of PIP in normal and cancerous breast cancer tissues are to some degree conflicting. In early studies, it was shown that PIP is absent or its expression is very low in normal breast epithelium, whereas in breast cancers PIP is frequently expressed and present in large amounts. On the other hand, later study showed that expression of PIP is lower in advanced apocrine carcinomas and invasive carcinomas than in, respectively, in situ carcinomas and adjacent normal tissue. The most recent study revealed that PIP gene expression decreased gradually along with higher stage and grade of breast cancer. In agreement with these data, it was shown that that low levels or the lack of PIP expression are associated with a worse response of breast cancer cells to chemotherapy. It was proposed that PIP plays important role in the development and progression of breast cancer. However, its role in these processes is both unclear and controversial. In this review, the role of PIP in both physiological processes and carcinogenesis is discussed.
Keywords: PIP, GCDFP-15, gp-17, prolactin-induced protein, gross cystic disease fluid protein 15, breast cancer
Introduction
At the end of the 20th century, Haagensen and Mazoujian described Gross Cystic Disease Fluid Protein 15 (GCDFP-15) as one of the proteins present in the cystic fluid from mastopathy [1,2]. A few years later, Shiu and Iwasiow identified acidic protein that was present in the culture medium from breast cancer T47D cells as two glycoforms of molecular masses 16 kDa and 14 kDa [3]. They found that the level of this protein was significantly increased following prolactin (hPRL) induction, which ultimately lead to naming it prolactin-induced protein (PIP). Subsequent study confirmed that it is the same protein as GCDFP-15 [4,5]. Shortly after these reports, independent research showed that PIP/GCDFP-15 under the name glycoprotein-17 (gp-17) and seminal actin-binding protein (SABP) was present in the glandular epithelium of seminal vesicles and as extra parotid glycoprotein (EP-GP) was found in submandibular and sublingual glands [6-8]. Presently, the commonly accepted name for all these proteins in human and mouse genomic nomenclature is PIP.
Expression of PIP under physiological and pathological conditions
The expression of PIP is generally restricted to cells with apocrine properties [9,10]. Using immunohistochemistry, it was found in apocrine glands of the axilla, vulva, eyelid, ear canal, and seminal vesicle [11]. However, PIP was also detected in serous cells of salivary glands, submucosal glands of the bronchi, and accessory lacrimal glands [9,11]. These tissues do not represent typical apocrine epithelia, but phylogenetically have common properties with apocrine glands. Being a secretory protein, PIP is present in seminal plasma, saliva, lacrimal fluid, tears, sweat gland secretion, amniotic fluid, and the blood of pregnant women [2,12,13].
PIP is generally expressed by normal breast tissues, but is highly present in metaplastic/hyperplastic apocrine epithelium of breast cyst and breast cyst fluid [1,2,9,14,15]. Recently, it was shown that in keratoconus disease, the expression of PIP is highly diminished in keratoconus cells and tears, therefore it was proposed that PIP can be a potential biomarker for this disease [16]. The fact that significantly decreased expression of PIP was found in tape-stripped stratum corneum and sweat samples from atopic dermatitis patients, suggests the possibility that PIP may also be a marker for atopic dermatitis associated with decreased eccrine sweating [17].
PIP in breast cancer
In early studies, it was shown that PIP is absent in normal breast epithelium or its expression is very low and/or difficult to detect, whereas in breast cancers PIP is frequently expressed and present in large amounts [18-21]. Several early studies revealed that PIP is especially present in breast carcinomas with apocrine features [11,22-24]. Its expression was also confirmed on mRNA and the protein level in axillary node metastases [18-20,24-27]. However, others have shown that PIP mRNA was more frequently present in uninvolved breast tissue then in breast carcinoma, and metastatic breast carcinoma [28]. Also later studies showed that expression of PIP decreases in advanced apocrine carcinomas and was significantly lower in infiltrating carcinomas, especially node-positive tumors, than in situ carcinomas [29]. Similarly, significantly lower levels of PIP were found in invasive breast tissue then in adjacent normal tissue [30]. In agreement with these data, the most recent study revealed that PIP gene expression decreased gradually along with higher stage and grade of breast cancer [31]. The authors showed that PIP mRNA and protein expression in normal breast tissue were significantly higher than in breast cancer tissues. Significant downregulation of PIP was also observed in early stages of breast tumor progression.
With the introduction of new molecular classification based on gene expression profiling, the following molecular subtypes of breast cancer were discriminated: basal-like, HER2-enriched, luminal A, luminal B and normal-like [32-34]. According to this nomenclature, the highest amounts of PIP mRNA were found in the luminal A subtype, then in HER2-enriched and normal-like subtypes, and the lowest expression was observed in basal-like subtype [35,36]. In another study based on analysis of global gene expression, which identified molecular apocrine breast cancer subtype [37], it was found that such tumors are characterized by increased amounts of PIP [38]. This expression strongly correlated with the presence of AR [36,39,40]. Since in many studies, PIP expression correlated with low grade breast cancers, it was proposed that high expression of this marker is a predictor of good prognosis [25,39,41,42]. From clinical point of view, is also important that cases with high PIP expression were characterized by longer disease-free survival [43-45] and overall survival [45]. In our published study, we showed that a high level of PIP expression is positively correlated with the response of breast cancer patients to standard adjuvant chemotherapy (doxorubicin + cyclophosphamide) [46]. The data obtained indicates that low levels or the lack of PIP expression are associated with a worse response of breast cancer cells to chemotherapy. Expression DNA microarray studies show that the expression of the PIP gene is significantly lower in cases of invasive ductal carcinoma (IDC), which are poorly responsive to standard adjuvant chemotherapy [46] (Figure 1). Additionally, we showed that the levels of PIP protein and mRNA decreases along with tumor malignancy grade (G), and that PIP expression is the lowest in triple negative (ER-, PR-, HER2-) cases with poor prognosis.
Figure 1.
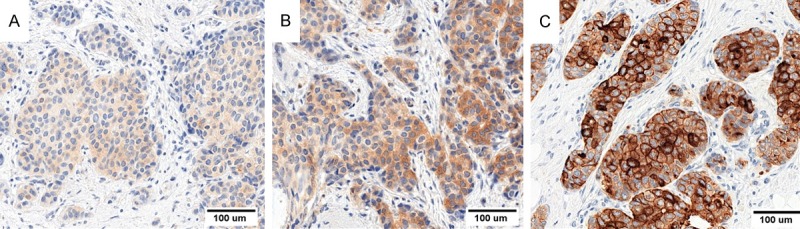
Images of immunohistochemical reactions showing expression pattern of PIP in IDC cells. PIP protein was localized in cytoplasm of tumor cells. (A) Low PIP expression, (B) Medium and (C) High intensity of PIP expression. Magnification ×200.
As PIP was not found in gastrointestinal cancers, bronchopulmonary structures, and genitourinary cancers, this specific marker of breast cancer and its metastases can be used to distinguish distant breast metastases from other primary and secondary tumors [21,41,47-53]. Furthermore, PIP may be a potential marker for breast micrometastases to auxilliary lymph nodes [25]. However, it should be remembered that PIP was also found in cancers of prostate, sweat and salivary glands [20,54]. Highly increased levels of PIP, in comparison to normal subjects, were observed in the peripheral plasma from patients with primary and metastatic breast cancer [1,19,55].
PIP protein
Human PIP is synthesized as 146 amino acid pre-protein [4]. Following cleavage of 28 amino acid signal peptide, PIP becomes an 118 amino acid secretory polypeptide of the theoretical molecular mass-13,506 kDa. SDS-PAGE experiments showed PIP apparent molecular mass to be 14-20 kDa, depending on the origin of the protein [1,12,56,57]. The difference between theoretically and experimentally determined molecular masses is a result of N-glycosylation, and differences in apparent molecular masses of PIPs isolated from various sources are most probably caused by variations in the structure of N-glycans (see below). Microheterogeneity linked to N-glycosylation is also reflected by differences in isoelectric points ranging from pH 4.8 to pH 5.4 [13,57]. At its N-terminus, PIP has a cyclic glutamine derivative-pyroglutamine [12].
In 2008, Hassan and co-workers determined the crystal structure of PIP in complex with ZAG protein (zinc-alpha-2-glycoprotein) [58] (Figure 2A). They showed that PIP is composed of seven parallel β-sheets and seven β-turns. Characteristic for PIP is the lack of α-helix-type structures. As a result of the highly hydrophobic character of amino acid residues, β-sheet motifs are organized as pairs in the form of hollow, sandwich-type structures. The spatial structure of PIP resembles the 7th fibronectin type III domain, although this domain has only 13% identity with PIP sequence. PIP tertiary structure analysis showed the presence of two disulphide bridges formed between cysteine residues at the positions 37 and 63 and 61 and 95 [12,58]. PIP may form dimers or aggregates of 4-12 monomers [57,59].
Figure 2.
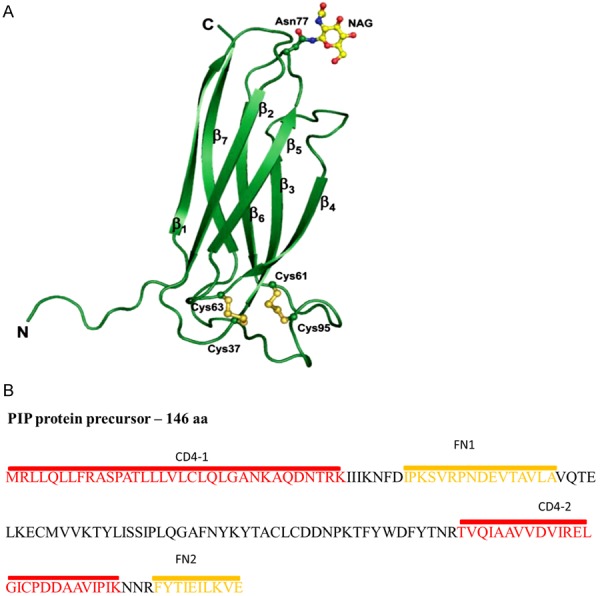
A. The three-dimensional structure of PIP. PIP is composed of seven β-sheets. The potential N-glycosylation site is at position Asn77. The tertiary structure of this protein contains two disulphide bridges formed between Cys37 and Cys63 and Cys61 and Cys95 [105,106]. B. The amino acid sequence of PIP and localization of fibronectin (FN)- and CD4-binding domains [60-62].
In PIPs isolated from human saliva and breast cystic fluid (PIP/GCDFP-15) the carbohydrate content was found to be, respectively, 13.2%, and 8.5% [56,57]. In the case of the latter, the single N-glycan linked to Asn77 represents a complex-type structure with an unusually high content of fucose [56]. However, PIP/SABP from seminal plasma was characterized by a much higher carbohydrate content (22%). The single N-glycosidic carbohydrate chain probably represents complex-type structure [12]. A more recent study showed that N-glycans from PIP/SABP are monosialylated diantennary structures with various fucosylation patterns, and are different from N-glycans linked to PIP/GCDFP-15, identified as triantennary structures [60]. According to those authors, differences in carbohydrate structures suggest that higher degree of sialylation in pathological form of PIP may better protect this glycoprotein from proteolytic degradation and affects the uptake and processing of sialylated glycoproteins by immune cells.
In addition to the actin-binding motif, which was found by in silico analysis [6], PIP showed the presence of CD4- and fibronectin-binding domains, which were found experimentally (Figure 2B). In PIP from human seminal plasma (gp17/SABP), CD4-binding domain was identified as two fragments encompassing the 1-35 N-terminal amino acids and 78-105 amino acids, and fibronectin-binding domain as two peptides encompassing 109-118 C-terminal amino acids and 42-57 amino acids [60-62].
PIP gene and regulation of its expression
The PIP gene is localized on the long arm of chromosome 7 at locus q34 and includes 7000 base pairs (bp) [5,63]. It consists of four exons and three introns [64] (Figure 3). In the promoter region a classical TATA box and a CAAT box were identified. The methylation revealed that methylation is probably necessary, but not sufficient for expression of the PIP gene [64]. Interestingly, genetic alterations within chromosome 7 are commonly found in human breast cancer [65,66]. In line with this, Ciullo et al. found that the breakage of chromosome 7 in the FRA7I area caused reversed palindromic PIP gene duplication in T47D cells, which probably is the reason for increased, constitutive PIP expression in these cells [67]. Also it was shown that the intragenic region of the PIP gene is involved in the formation of small polydispersed circular DNA, and such DNA molecules may serve to enhance genetic instability [68].
Figure 3.
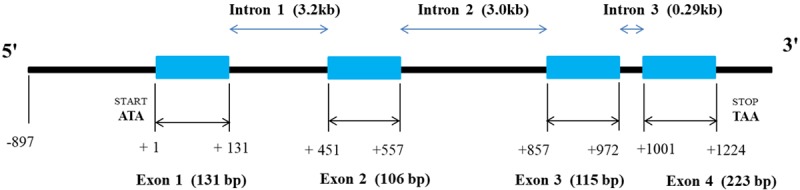
Schematic representation of the PIP gene [64].
The nucleotide sequence of PIP mRNA was determined for the first time by sequencing cDNA isolated from expression library which was constructed from mRNA from breast cancer T47D cells [4]. It was found that PIP mRNA represented a single transcript of about 591 nucleotides long (NCBI refference seq. NM_002652.2 16.04.2018).
There are few reports on the evolution of the PIP gene [69]. A comparison of nucleotide sequences in primates [chimpanzees (Pan troglodytes), gorillas (Gorilla gorilla), orangutans (Pongo pygmaeus), gibbons (Hylobates agilis)] and humans suggests that the PIP gene could have evolved as a result of positive selection caused by interaction between PIP protein and pathogens [62]. It was also found that in hominoids amino acid changes accumulated in the second fibronectin-binding domain (FN2), in contrast to the conserved first CD4-binding domain (CD4-1) (see section 4). Particularly evolutionary conserved among mammals are regions encoding four cysteines that form two pairs of disulphide bridges [70]. A phylogenetic tree indicates similarity between the PIP gene and genes coding such proteins as A2M (alfa-2-makroglobulin), PZP (pregnancy-zone-protein), A2ML1 (alpha-2-macroglobulin like 1) and OVOS2 (ovostatin 2) [62].
The expression of the PIP gene on the levels of mRNA and protein is increased by androgens, progesterone, glucorticosteroids together with prolactin or growth hormone [3,9,19,71-74], and cytokines such as IL-1α, IL-4 and IL-13 [75,76] and decreased by 17β-estradiol and IL-6 [74,77,78].
There is evidence that PIP expression is primarily regulated at the transcriptional level. Originally, it was shown that PIP gene expression in ZR-75 cells was highly increased by the simultaneous action of 5α-dihydrotestosterone and prolactin, which bind, respectively to the androgen (AR) and prolactin (PRLR) receptors [79]. Prolactin binding induces phosphorylation of STAT5A or/and STAT5B, which after dimerization are translocated from submembranous localization into the nucleus. There they bind to the STAT5-responsive element located on the PIP gene promoter and cooperate with activated AR bound to androgen responsive elements of PIP promoter to increase the transcription of the PIP gene (Figure 4A1). A similar regulatory mechanism, which involves cooperation of AR and Runx2 transcription factor was described by Baniwal et al. in human breast cancer T47D cells and prostate cancer C4-2B cells [80]. According to them, following activation by 5α-dihydrotestosterone, AR and Runx2 bind together to enhancer element of PIP promoter and by physical interaction act in a synergistic manner to highly increase the expression of the PIP gene. Runx2 and AR are recruited to four regions in the promoter sequence of PIP gene (Figure 4A2). Regions I, II, III and IV (R-I-IV) are located, respectively, -0.9 kb, -2.4 kb, -9.4 kb, and -11 kb from the transcription initiation site. Regions I and II include consensus sequences for Runx2, whereas regions III and IV contain consensus sequences for Runx2 and AR (Figure 4B). In turn, PIP positively regulates androgen signaling, facilitating translocation of AR to the nucleus and stimulation of androgen-dependent genes.
Figure 4.
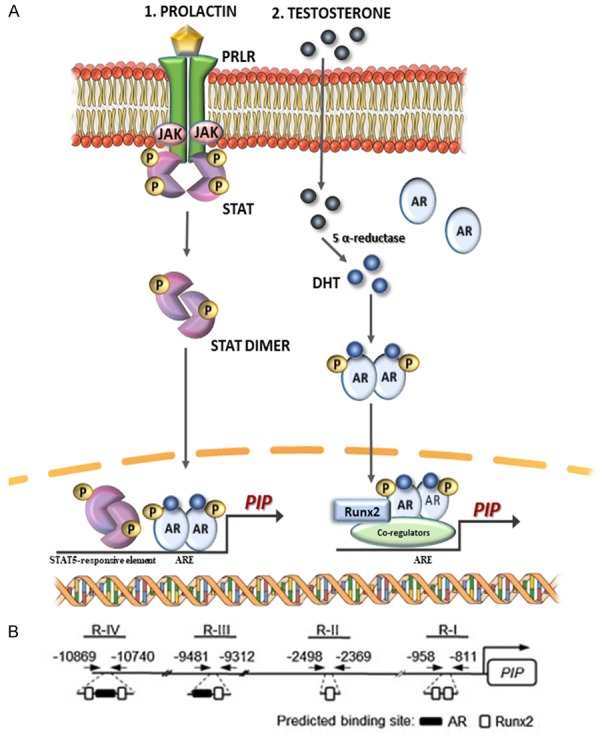
A. Regulation of PIP expression. 1. Synergistic action of prolactin and 5α-dihydrotestosterone leads to an increased PIP expression by activation of STAT5 and AR transcription factors that bind to the promotor region of the PIP gene. 2. Synergistic action of AR activated by 5α-dihydrotestosterone and Runx2 that bind to the promotor region of the PIP gene leads to increased PIP expression [80,81]. AR-androgen receptor, ARE-androgen response element, DHT-dihydrotestosterone, ErbB2-Her2, human epidermal growth factor receptor 2, Jak-Janus-activated kinases, PIP-prolactin-induced protein, PRLR-prolactin receptor, Runx2-Runt-related transcription factor 2, STAT5-signal transducer. B. At the promoter site of PIP gene, regions I, II, III and IV are located -0.9 kb, -2.4 kb, -9.4 kb, and -11 kb from the transcription initiation site [80].
In ER-negative breast cancer, the expression of PIP is auto-regulated by the positive feedback loop between PIP and ERK, Akt/PkB signaling (Figure 5) [81]. In human molecular apocrine breast cells, the secreted PIP, by its proteolytic activity degrades fibronectin to peptides (see section 5), which activates β1-integrins to interact with integrin-linked kinase 1 (ILK1) and ErbB2 (Her2-neu) molecules. ILK1 binding activates Akt/PkB and ERK signaling pathways, while ErbB2 binding activates MAPK/ERK signaling. Both of these both signaling pathways, by phosphorylation of the RSK and MSK families of kinases, by phosphorylation activate the CREB1 transcription factor, which binds to the PIP gene promoter and increases its transcription. In ER-negative breast cancer cells, PIP expression is also regulated by positive AR, ERK positive feedback loop, since CREB1 also activates transcription of the AR gene. The resulting AR protein increases expression of ErbB2, which in turn activates the ERK, CREB1 axis.
Figure 5.
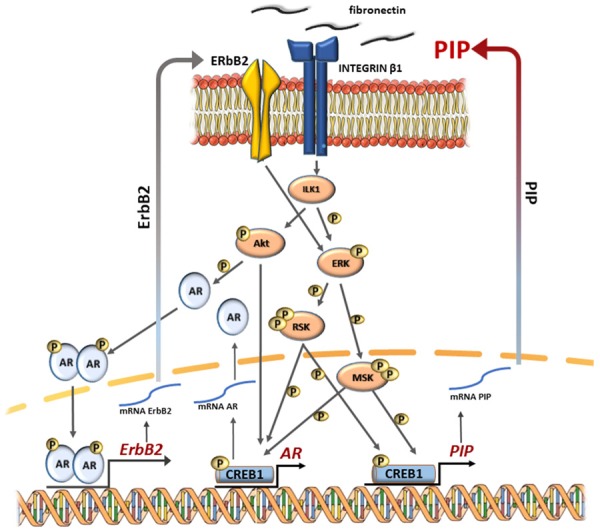
Regulation of PIP expression. In ER-negative breast cancer, the expression of PIP is auto-regulated by the positive feedback loop between PIP and ERK, Akt/PkB signaling [81,107,108]. AR-androgen receptor, CREB1-cAMP responsive element binding protein 1, DHT-dihydrotestosterone, ErbB2-Her2, human epidermal growth factor receptor 2, ERK1/2-extracellular signal-regulated kinases 1 and 2, ILK1-integrin-linked kinase 1, Jak-Janus-activated kinases, MSK-mitogen- and stress-activated protein kinase, PIP-prolactin-induced protein, PKB/AKT-protein kinase B/serine/threonine kinase, PRLR-prolactin receptor, RSK-ribosomal S6 kinase, Runx2-runt-related transcription factor 2, STAT5-signal transducer.
In summary, all the available data shows that positive regulation of PIP expression in breast cancer cells on the level of transcription is dependent on the cooperation of AR with different transcription factors, such as STAT5, Runx2, and CREB1.
Biological functions of PIP
PIP has an aspartyl protease activity [82]. This function results from the presence of aspartate residue at position 22 (Asp22), which shows homology to aspartate residue 32 in other known aspartyl proteases, such as cathepsin D, pepsin or renin. As fibronectin is one of the substrates of PIP, it is believed that PIP participates in extracellular matrix degradation, and therefore is engaged in breast cancer progression. In addition to fibronectin, PIP binds numerous proteins including: actin, fibrinogen, β-tubulin, serum albumin and hydroxyapatite (a major component of tooth enamel), zinc α2-glycoprotein and Fc fragment of IgGs [8,12,36,57,83-85]. However, in most cases the biological role of such interactions is poorly understood.
PIP/EP-GP from human saliva binds to bacteria from the genera Gemella, Streptococcus and Staphylococcus, and causes their aggregation [8,86,87]. Similar properties were shown by murine salivary PIP, which bound to bacteria from the genus Streptococcus [88]. Based on these results, it was suggested that PIP is a part of the oral defense mechanism against bacterial pathogens. The idea that PIP participates in innate immunity and protection of host against microbial infections is supported by the fact that it is present in significant amounts in mucosal-type tissues, bronchial submucosal glands, apocrine glands of the skin, saliva, and lacrimal fluid, all of which represent ports of pathogen entry.
Several studies revealed that PIP isolated from different sources such as human seminal fluid (gp17/SABP) and breast cyst fluid (GCDF-15) binds to CD4 receptors present on the surface of T lymphocytes, macrophages, and spermatozoa [7,59,60,89]. However, using surface plasmon resonance (SPR), it was found that PIP/GCDF-15 derived from pathological sources (breast cyst fluid) binds to CD4 with lower affinity than physiological form of PIP/gp17/SABP from human seminal plasma [60]. These data suggest that depending on the tissue and physiological v. pathological conditions, PIP may interact differently with other molecules and therefore can have distinct functions. CD4 plays a key role in immune response, acting as TCR co-receptor essential for recognizing peptides presented by major histocompatibility complex II (MHC II) on the antigen presenting cells [90]. This molecule is also involved in T cell activation by interaction with the protein-tyrosine kinase p56lck [91]. CD4 is a receptor for human immunodeficiency virus (HIV-1) gp120 envelope glycoprotein and it has been suggested that such interaction can increase HIV-induced T cell apoptosis [92]. The binding of PIP to CD4 inhibited its interaction with gp120 and syncytium formation by cell expressing gp120 and CD4, which strongly suggests that PIP may be involved in pathogenesis of HIV-1 infection [92]. In fact, further study revealed that PIP strongly inhibits T cell apoptosis induced by sequential gp120/CD4 and TCR/CD3 activation [93]. It was also shown that the binding of gp17 significantly increased the expression of anti-apoptotic Bcl-2 protein. These data suggest that PIP can have immunomodulatory function in some virus infections and generally modulate adaptive immune response.
PIP gene knockout mice showed enlarged lymph nodes around the parotid glands, lymphocytic aggregations within the prostate lobes, and an enlargement of thymic medulla, however, their development was normal and animals were fertile [94]. knock-out mice revealed the presence of many differentially expressed genes related to cell death and survival, inflammation, immune response and cancer in comparison to parental mice. This further supports the involvement of PIP in immunological processes. The loss of PIP expression in mice leads to a significant decrease in the CD4 positive T cell population in spleen, and has a negative impact on their differentiation to CD4 positive Th1-cells, resulting in a highly decreased production of IFNγ [95]. As the result of low numbers of CD4 positive Th1-cells, such mice are highly susceptible to Leishmania major infection. Taking into account that differentiation of Th1 cells and the outcome of infection depend on the production of IL-12 by antigen presenting cells (APC), the authors found that bone marrow derived macrophages from PIP-knock-out produce significantly lower amounts of IL-6, IL-12p40 and TNF than wild-type animals after in vitro stimulation with LPS and polyI: C. These findings suggest that PIP indirectly affect maturation of naive CD4 positive T cells to Th1-cells by decreasing production of cytokines by antigen presenting cells. The increased sensitivity of PIP-knock-out mice to L. major infection is also associated with highly decreased production of NO by bone marrow-derived macrophages after LPS and IFNγ stimulation. It was found recently that decreased production of pro-inflammatory cytokines by macrophages from PIP-negative mice is associated with decreased phosphorylation of mitogen-activated protein kinase (MAPK) and signal transducer of activation of transcription (STAT) proteins. In such mice, the expression of suppressors of cytokine signaling (SOCS) 1 and 3 proteins was higher than wild type mice [96]. All these data further support the idea that PIP plays an important role in cell-mediated immunity. Independently, it was also shown that the level of PIP expression increases following stimulation with IL-4 and IL-13, which play a key role in the regulation of immune cells activity [75].
PIP protein, present in high amounts in human seminal plasma, is an IgG-reacting protein, which binds to the Fc fragment of antisperm antibodies (ASAs) that recognize seminal proteins and can cause infertility in men [84]. But the total levels of PIP as well as levels of different PIP isoforms in seminal plasma did not correlated with fertility status. This, however does not exclude its immunoregulatory functions.
The role of PIP in breast cancer progression
The biological functions of PIP were primarily studied using established in vitro breast cancer cell lines. Early studies performed on tumor cells of different origin revealed that only breast cancer cells respond to exogenous PIP by increased proliferation [97]. The important role of PIP in proliferation of breast cancer cells was further confirmed by knockdown of the PIP gene in T47D and MDA-MB-453 cells [80,81]. The inhibition of PIP expression highly decreased the proliferative potential of such cells. Interestingly, with T47D cells, proliferation stimulated by serum growth factors or dihydrotestosterone was fully dependent on the presence of PIP [80]. When more breast cancer cell lines with PIP knockdown were studied, it was found that generally proliferation of cell lines representing luminal A, luminal B and apocrine subtypes of breast cancer is dependent on the presence of PIP [36].
Studies on the molecular mechanisms connecting PIP expression with increased proliferative potential of breast cancer cells revealed that PIP knockdown was associated with decreased phosphorylation of focal adhesion kinase (FAK), ephrin B3 (EphB3), tyrosine kinase of the Src family (FYN), and hemopoietic cell kinase (HCK) [35]. The absence of PIP also made the activation of serine/threonine kinases AKT, ERK1/2 and JNK1 by serum factors impossible. Such changes in intracellular signaling resulted in the loss of cytoskeletal stress fiber formation, which was associated with decreased adhesion of cells to fibronectin and diminished protein secretion. Using the same experimental approach-breast cancer cell lines with suppressed expression of the PIP gene [36], found that the absence of PIP protein causes cell cycle arrest in the G1 or mitotic phase and cytokinesis defect. Furthermore, a correlation between expression of PIP and cell cycle-related genes, especially those involved in mitotic transition was observed. Among analyzed breast cancer cell lines, T47D and MDA-MB-453 cells with silenced expression of the PIP gene underwent G1 arrest manifested by a 10% to 20% increase in the G0-G1 cell population, which correlated with a reduction in expression levels of cyclin D1 and cyclin E1 genes, and decreased phosphorylation of ERK. A second group of cell lines, comprised of MFM-223, SK-BR3, HCC-1954, HCC-202 and BT-474 cells after PIP gene knockdown, was characterized by a significant increase in the G2/M phase and the percentage of aneuploidy, which was associated with decreased expression of cyclin D1 and cyclin B1 genes, a reduction in total and phospho-Cdc2 protein levels, and a significant decreased in FOXM1, TTK, BUB1, and CDC20 genes expression. In summary, these observations suggest that in breast cancer cells PIP is involved in the progression of cell cycle, especially during the mitotic phase primarily as a regulator of the transcription of key cell cycle genes. Proteomic studies performed on the same cells revealed that PIP interacts with β-tubulin and facilitates tubulin polymerization, which can further explain its role in cell proliferation, since microtubules are involved in mitotic transition, spindle assembly and cytokinesis. PIP also interacts with Arp2/3 protein, which participates in actin polymerization and facilitates talin binding to integrins, which can explain why PIP is required for inside-out activation and signaling effects of integrin-β1. In contrast to the results of Cassoni et al. (1995), the studies on breast cancer cells with silenced PIP suggest that stimulated effects of PIP on their proliferative potential are mediated by intracellular PIP interacting with cytosol proteins, not an exogenous form of PIP. However, this suggestion should be treated with caution because electron microscopy showed that PIP is present in secretory vesicles [98], and therefore its presence in cytoplasm is rather unlikely.
In contrast to the studies described above, others research has shown that increased expression of PIP in ZR-75-1 cells caused by androgens was associated with their growth arrest [74,99]. In line with these results, recent microarray analysis revealed a significant increase in the expression of genes associated with an anti-proliferative and pro-apoptotic effects in PIP-expressing breast cancer cells in comparison to breast cancer cells with no expression of PIP [100].
The silencing of PIP expression in breast cancer cells inhibited their invasive properties through modulating the integrin signaling pathway [81]. It was proposed that PIP as asapartyl protease increases the invasion of cancer cells by generating fibronectin peptides, which bind to integrin β1. Also, microarray analysis revealed a significant increase in the expression of genes associated with migratory properties in PIP-expressing breast cancer cells in comparison to breast cancer cells with no expression of PIP [100]. There is also some evidence that PIP is involved in cell-cell and cell-matrix (fibronectin) adhesion of breast cancer cells [101].
In breast cancer, an efficient Th1 response is associated with smaller tumor size [102] and better prognosis [103]. Therefore, it was proposed recently that PIP may play an advantageous role during the progression of breast cancer, especially during early stages, because it positively affects differentiation of T lymphocytes to CD4 positive Th1-cells [104]. However, there is lack of direct experimental data supporting such a hypothesis.
Conclusions
PIP is produced and excreted by normal cells and tissues characterized by apocrine-type secretion. However, it is also found in large amounts in some pathological conditions such as cystic fluid from mastopathy and some cancer cells, especially breast cancer cells. PIP plays a role in cell proliferation, migration and adhesion, and is generally described as a protein with immunomodulatory properties. However, its functions in both cancerous and normal cells have not yet been fully elucidated and in many aspects are controversial. Therefore, further studies regarding this glycoprotein, which is undoubtedly important for cell functioning are still necessary.
Acknowledgements
“The project was financed from the funds of the National Science Center granted on the basis of decision No. DEC-2016/23/B/N25/02647”. Figure 2A Reprinted by permission from Springer Nature (The Licensor): [Journal Publisher (Springer)] [JOURNAL NAME: Cellular and Molecular Life Sciences] [REFERENCE CITATION: (Prolactin inducible protein in cancer, fertility and immunoregulation: structure, function and its clinical implications, Md. I. Hassan, A. Waheed, S. Yadav et al.), [COPYRIGHT] (2008), 2009 Feb; 66 (3): 447-59, (doi: 10.1007/s00018-008-8463-x. [Cell Mol Life Sci.]). Figure 4B Reprinted by permission from: John Wiley and Sons (The Licensor): [Journal Publisher (John Wiley and Sons)] [JOURNAL NAME: Journal of Cellular Physiology] [REFERENCE CITATION: (Runx2 controls a feed-forward loop between androgen and prolactin-induced protein (PIP) in stimulating T47D cell proliferation, Sanjeev K. Baniwal, Gillian H. Little, Nyam-Osor Chimge, et al.), [COPYRIGHT] (2012), 2012 May; 227 (5): 2276-82, (doi: 10.1002/jcp.22966 [J Cell Physiol.]).
Disclosure of conflict of interest
None.
References
- 1.Haagensen DE, Mazoujian G, Dilley WG, Pedersen CE, Kister SJ, Wells SA. Breast gross cystic disease fluid analysis. I. Isolation and radioimmunoassay for a major component protein. J Natl Cancer Inst. 1979;62:239–247. [PubMed] [Google Scholar]
- 2.Haagensen DE, Gall SA, Brazy JE, Giannola J, Wells SA. Analysis of amniotic fluid, maternal plasma, and cord blood for a human breast gross cystic disease fluid protein. Am J Obstet Gynecol. 1980;138:25–32. doi: 10.1016/0002-9378(80)90007-1. [DOI] [PubMed] [Google Scholar]
- 3.Shiu RP, Iwasiow BM. Prolactin-inducible proteins in human breast cancer cells. J Biol Chem. 1985;260:11307–11313. [PubMed] [Google Scholar]
- 4.Murphy LC, Tsuyuki D, Myal Y, Shiu RP. Isolation and sequencing of a cDNA clone for a prolactin-inducible protein (PIP). Regulation of PIP gene expression in the human breast cancer cell line, T-47D. J Biol Chem. 1987;262:15236–15241. [PubMed] [Google Scholar]
- 5.Myal Y, Gregory C, Wang H, Hamerton JL, Shiu RP. The gene for prolactin-inducible protein (PIP), uniquely expressed in exocrine organs, maps to chromosome 7. Somat Cell Mol Genet. 1989;15:265–270. doi: 10.1007/BF01534877. [DOI] [PubMed] [Google Scholar]
- 6.Akiyama K, Kimura H. Isolation of a new actin-binding protein from human seminal plasma. Biochim Biophys Acta. 1990;1040:206–210. doi: 10.1016/0167-4838(90)90077-s. [DOI] [PubMed] [Google Scholar]
- 7.Autiero M, Abrescia P, Guardiola J. Interaction of seminal plasma proteins with cell surface antigens: presence of a CD4-binding glycoprotein in human seminal plasma. Exp Cell Res. 1991;197:268–271. doi: 10.1016/0014-4827(91)90432-t. [DOI] [PubMed] [Google Scholar]
- 8.Schenkels LC, Schaller J, Walgreen-Weterings E, Schadee-Eestermans IL, Veerman EC, Nieuw Amerongen AV. Identity of human extra parotid glycoprotein (EP-GP) with secretory actin binding protein (SABP) and its biological properties. Biol Chem Hoppe Seyler. 1994;375:609–615. doi: 10.1515/bchm3.1994.375.9.609. [DOI] [PubMed] [Google Scholar]
- 9.Haagensen DE, Dilley WG, Mazoujian G, Wells SA. Review of GCDFP-15. An apocrine marker protein. Ann N Y Acad Sci. 1990;586:161–173. doi: 10.1111/j.1749-6632.1990.tb17804.x. [DOI] [PubMed] [Google Scholar]
- 10.Schenkels LC, Veerman EC, Nieuw Amerongen AV. EP-GP and the lipocalin VEGh, two different human salivary 20-kDa proteins. J Dent Res. 1995;74:1543–1550. doi: 10.1177/00220345950740090701. [DOI] [PubMed] [Google Scholar]
- 11.Mazoujian G, Pinkus GS, Davis S, Haagensen DE. Immunohistochemistry of a gross cystic disease fluid protein (GCDFP-15) of the breast. A marker of apocrine epithelium and breast carcinomas with apocrine features. Am J Pathol. 1983;110:105–112. [PMC free article] [PubMed] [Google Scholar]
- 12.Schaller J, Akiyama K, Kimura H, Hess D, Affolter M, Rickli EE. Primary structure of a new actin-binding protein from human seminal plasma. Eur J Biochem. 1991;196:743–750. doi: 10.1111/j.1432-1033.1991.tb15873.x. [DOI] [PubMed] [Google Scholar]
- 13.Schenkels LC, Rathman WM, Veerman EC, Nieuw Amerongen AV. Detection of proteins related to a salivary glycoprotein (EP-GP). Concentrations in human secretions (saliva, sweat, tears, nasal mucus, cerumen, seminal plasma) Biol Chem Hoppe Seyler. 1991;372:325–329. doi: 10.1515/bchm3.1991.372.1.325. [DOI] [PubMed] [Google Scholar]
- 14.Collette J, Hendrick JC, Jaspar JM, Franchimont P. Presence of alpha-lactalbumin, epidermal growth factor, epithelial membrane antigen, and gross cystic disease fluid protein (15,000 daltons) in breast cyst fluid. Cancer Res. 1986;46:3728–3733. [PubMed] [Google Scholar]
- 15.Sciarra A, Lopez G, Corti C, Runza L, Ercoli G, Bonometti A, Despini L, Blundo C, Gambini D, Fusco N. Columnar cell lesion and apocrine hyperplasia of the breast: is there a common origin? The role of prolactin-induced protein. Appl Immunohistochem Mol Morphol. 2017 doi: 10.1097/PAI.0000000000000604. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Priyadarsini S, Hjortdal J, Sarker-Nag A, Sejersen H, Asara JM, Karamichos D. Gross cystic disease fluid protein-15/prolactin-inducible protein as a biomarker for keratoconus disease. PLoS One. 2014;9:e113310. doi: 10.1371/journal.pone.0113310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamiya K, Sakabe J, Yamaguchi H, Suzuki T, Yatagai T, Aoshima M, Ito T, Tokura Y. Gross cystic disease fluid protein 15 in stratum corneum is a potential marker of decreased eccrine sweating for atopic dermatitis. PLoS One. 2015;10:e0125082. doi: 10.1371/journal.pone.0125082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Doussal V, Zangerle PF, Collette J, Spyratos F, Hacene K, Briere M, Franchimont P, Gest J. Immunohistochemistry of a component protein of the breast cystic disease fluid with mol. wt 15,000. Eur J Cancer Clin Oncol. 1985;21:715–725. doi: 10.1016/0277-5379(85)90269-x. [DOI] [PubMed] [Google Scholar]
- 19.Murphy LC, Lee-Wing M, Goldenberg GJ, Shiu RP. Expression of the gene encoding a prolactin-inducible protein by human breast cancers in vivo: correlation with steroid receptor status. Cancer Res. 1987;47:4160–4164. [PubMed] [Google Scholar]
- 20.Wick MR, Lillemoe TJ, Copland GT, Swanson PE, Manivel JC, Kiang DT. Gross cystic disease fluid protein-15 as a marker for breast cancer: immunohistochemical analysis of 690 human neoplasms and comparison with alpha-lactalbumin. Hum Pathol. 1989;20:281–287. doi: 10.1016/0046-8177(89)90137-8. [DOI] [PubMed] [Google Scholar]
- 21.Fiel MI, Cernaianu G, Burstein DE, Batheja N. Value of GCDFP-15 (BRST-2) as a specific immunocytochemical marker for breast carcinoma in cytologic specimens. Acta Cytol. 1996;40:637–641. doi: 10.1159/000333931. [DOI] [PubMed] [Google Scholar]
- 22.Eusebi V, Millis RR, Cattani MG, Bussolati G, Azzopardi JG. Apocrine carcinoma of the breast. A morphologic and immunocytochemical study. Am J Pathol. 1986;123:532–541. [PMC free article] [PubMed] [Google Scholar]
- 23.Miller WR, Shivas AA, Franchimont P, Haagensen DE. Breast gross cystic disease protein 15 in human breast cancer in culture. Eur J Cancer Clin Oncol. 1988;24:223–228. doi: 10.1016/0277-5379(88)90257-x. [DOI] [PubMed] [Google Scholar]
- 24.Mazoujian G, Bodian C, Haagensen DE, Haagensen CD. Expression of GCDFP-15 in breast carcinomas. Relationship to pathologic and clinical factors. Cancer. 1989;63:2156–2161. doi: 10.1002/1097-0142(19890601)63:11<2156::aid-cncr2820631115>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 25.Clark JW, Snell L, Shiu RP, Orr FW, Maitre N, Vary CP, Cole DJ, Watson PH. The potential role for prolactin-inducible protein (PIP) as a marker of human breast cancer micrometastasis. Br J Cancer. 1999;81:1002–1008. doi: 10.1038/sj.bjc.6690799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lockett MA, Baron PL, O’Brien PH, Elliott BM, Robison JG, Maitre N, Metcalf JS, Cole DJ. Detection of occult breast cancer micrometastases in axillary lymph nodes using a multimarker reverse transcriptase-polymerase chain reaction panel. J Am Coll Surg. 1998;187:9–16. doi: 10.1016/s1072-7515(98)00130-6. [DOI] [PubMed] [Google Scholar]
- 27.Bhargava R, Beriwal S, Dabbs DJ. Mammaglobin vs GCDFP-15: an immunohistologic validation survey for sensitivity and specificity. Am J Clin Pathol. 2007;127:103–113. doi: 10.1309/TDP92PQLDE2HLEET. [DOI] [PubMed] [Google Scholar]
- 28.Hähnel E, Harvey J, Robbins P, Sterrett G, Hähnel R. Hormone-regulated genes (pS2, PIP, FAS) in breast cancer and nontumoral mammary tissue. Pathobiology. 1994;62:82–89. doi: 10.1159/000163882. [DOI] [PubMed] [Google Scholar]
- 29.Honma N, Takubo K, Akiyama F, Sawabe M, Arai T, Younes M, Kasumi F, Sakamoto G. Expression of GCDFP-15 and AR decreases in larger or node-positive apocrine carcinomas of the breast. Histopathology. 2005;47:195–201. doi: 10.1111/j.1365-2559.2005.02181.x. [DOI] [PubMed] [Google Scholar]
- 30.Parris TZ, Kovács A, Aziz L, Hajizadeh S, Nemes S, Semaan M, Forssell-Aronsson E, Karlsson P, Helou K. Additive effect of the AZGP1, PIP, S100A8 and UBE2C molecular biomarkers improves outcome prediction in breast carcinoma. Int J Cancer. 2014;134:1617–1629. doi: 10.1002/ijc.28497. [DOI] [PubMed] [Google Scholar]
- 31.Gangadharan A, Nyirenda T, Patel K, Jaimes-Delgadillo N, Coletta D, Tanaka T, Walland AC, Jameel Z, Vedantam S, Tang S, Mannion C, Lee GY, Goy A, Pecora A, Suh KS. Prolactin induced protein (PIP) is a potential biomarker for early stage and malignant breast cancer. Breast. 2018;39:101–109. doi: 10.1016/j.breast.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 32.Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lønning PE, Børresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 34.Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lønning PE, Børresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baniwal SK, Chimge NO, Jordan VC, Tripathy D, Frenkel B. Prolactin-induced protein (PIP) regulates proliferation of luminal A type breast cancer cells in an estrogen-independent manner. PLoS One. 2014;8:e62361. doi: 10.1371/journal.pone.0062361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naderi A, Vanneste M. Prolactin-induced protein is required for cell cycle progression in breast cancer. Neoplasia. 2014;16:329–342. e1–14. doi: 10.1016/j.neo.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farmer P, Bonnefoi H, Becette V, Tubiana-Hulin M, Fumoleau P, Larsimont D, Macgrogan G, Bergh J, Cameron D, Goldstein D, Duss S, Nicoulaz AL, Brisken C, Fiche M, Delorenzi M, Iggo R. Identification of molecular apocrine breast tumours by microarray analysis. Oncogene. 2005;24:4660–4671. doi: 10.1038/sj.onc.1208561. [DOI] [PubMed] [Google Scholar]
- 38.Lewis GH, Subhawong AP, Nassar H, Vang R, Illei PB, Park BH, Argani P. Relationship between molecular subtype of invasive breast carcinoma and expression of gross cystic disease fluid protein 15 and mammaglobin. Am J Clin Pathol. 2011;135:587–591. doi: 10.1309/AJCPMFR6OA8ICHNH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darb-Esfahani S, von Minckwitz G, Denkert C, Ataseven B, Högel B, Mehta K, Kaltenecker G, Rüdiger T, Pfitzner B, Kittel K, Fiedler B, Baumann K, Moll R, Dietel M, Eidtmann H, Thomssen C, Loibl S. Gross cystic disease fluid protein 15 (GCDFP-15) expression in breast cancer subtypes. BMC Cancer. 2014;14:546. doi: 10.1186/1471-2407-14-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lehmann-Che J, Hamy AS, Porcher R, Barritault M, Bouhidel F, Habuellelah H, Leman-Detours S, de Roquancourt A, Cahen-Doidy L, Bourstyn E, de Cremoux P, de Bazelaire C, Albiter M, Giacchetti S, Cuvier C, Janin A, Espié M, de Thé H, Bertheau P. Molecular apocrine breast cancers are aggressive estrogen receptor negative tumors overexpressing either HER2 or GCDFP15. Breast Cancer Res. 2013;15:R37. doi: 10.1186/bcr3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Satoh F, Umemura S, Osamura RY. Immunohistochemical analysis of GCDFP-15 and GCDFP-24 in mammary and non-mammary tissue. Breast Cancer. 2000;7:49–55. doi: 10.1007/BF02967188. [DOI] [PubMed] [Google Scholar]
- 42.Luo MH, Huang YH, Ni YB, Tsang JY, Chan SK, Shao MM, Tse GM. Expression of mammaglobin and gross cystic disease fluid protein-15 in breast carcinomas. Hum Pathol. 2013;44:1241–1250. doi: 10.1016/j.humpath.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 43.Pagani A, Sapino A, Eusebi V, Bergnolo P, Bussolati G. PIP/GCDFP-15 gene expression and apocrine differentiation in carcinomas of the breast. Virchows Arch. 1994;425:459–465. doi: 10.1007/BF00197548. [DOI] [PubMed] [Google Scholar]
- 44.Fritzsche FR, Thomas A, Winzer KJ, Beyer B, Dankof A, Bellach J, Dahl E, Dietel M, Kristiansen G. Co-expression and prognostic value of gross cystic disease fluid protein 15 and mammaglobin in primary breast cancer. Histol Histopathol. 2007;22:1221–1230. doi: 10.14670/HH-22.1221. [DOI] [PubMed] [Google Scholar]
- 45.Hähnel R, Hähnel E. Expression of the PIP/GCDFP-15 gene and survival in breast cancer. Virchows Arch. 1996;429:365–369. doi: 10.1007/BF00198441. [DOI] [PubMed] [Google Scholar]
- 46.Jablonska K, Grzegrzolka J, Podhorska-Okolow M, Stasiolek M, Pula B, Olbromski M, Gomulkiewicz A, Piotrowska A, Rys J, Ambicka A, Ong SH, Zabel M, Dziegiel P. Prolactin-induced protein as a potential therapy response marker of adjuvant chemotherapy in breast cancer patients. Am J Cancer Res. 2016;6:878–893. [PMC free article] [PubMed] [Google Scholar]
- 47.Monteagudo C, Merino MJ, LaPorte N, Neumann RD. Value of gross cystic disease fluid protein-15 in distinguishing metastatic breast carcinomas among poorly differentiated neoplasms involving the ovary. Hum Pathol. 1991;22:368–372. doi: 10.1016/0046-8177(91)90084-3. [DOI] [PubMed] [Google Scholar]
- 48.de Almeida PC, Pestana CB. Immunohistochemical markers in the identification of metastatic breast cancer. Breast Cancer Res Treat. 1992;21:201–210. doi: 10.1007/BF01975003. [DOI] [PubMed] [Google Scholar]
- 49.Akasofu M, Kawahara E, Kurumaya H, Nakanishi I. Immunohistochemical detection of breast specific antigens and cytokeratins in metastatic breast carcinoma in the liver. Acta Pathol Jpn. 1993;43:736–744. doi: 10.1111/j.1440-1827.1993.tb02560.x. [DOI] [PubMed] [Google Scholar]
- 50.Perry A, Parisi JE, Kurtin PJ. Metastatic adenocarcinoma to the brain: an immunohistochemical approach. Hum Pathol. 1997;28:938–943. doi: 10.1016/s0046-8177(97)90009-5. [DOI] [PubMed] [Google Scholar]
- 51.Yim H, Jin YM, Shim C, Park HB. Gastric metastasis of mammary signet ring cell carcinoma--a differential diagnosis with primary gastric signet ring cell carcinoma. J Korean Med Sci. 1997;12:256–261. doi: 10.3346/jkms.1997.12.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wick MR, Ockner DM, Mills SE, Ritter JH, Swanson PE. Homologous carcinomas of the breasts, skin, and salivary glands. A histologic and immunohistochemical comparison of ductal mammary carcinoma, ductal sweat gland carcinoma, and salivary duct carcinoma. Am J Clin Pathol. 1998;109:75–84. doi: 10.1093/ajcp/109.1.75. [DOI] [PubMed] [Google Scholar]
- 53.Ormsby AH, Snow JL, Su WP, Goellner JR. Diagnostic immunohistochemistry of cutaneous metastatic breast carcinoma: a statistical analysis of the utility of gross cystic disease fluid protein-15 and estrogen receptor protein. J Am Acad Dermatol. 1995;32:711–716. doi: 10.1016/0190-9622(95)91447-1. [DOI] [PubMed] [Google Scholar]
- 54.Tian W, Osawa M, Horiuchi H, Tomita Y. Expression of the prolactin-inducible protein (PIP/GCDFP15) gene in benign epithelium and adenocarcinoma of the prostate. Cancer Sci. 2004;95:491–495. doi: 10.1111/j.1349-7006.2004.tb03238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haagensen DE, Kister SJ, Panick J, Giannola J, Hansen HJ, Wells SA. Comparative evaluation of carcinoembryonic antigen and gross cystic disease fluid protein as plasma markers for human breast carcinoma. Cancer. 1978;42:1646–1652. doi: 10.1002/1097-0142(197809)42:3+<1646::aid-cncr2820420844>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 56.Toth CA, Haagensen DE, Davis S, Zamcheck N, Thomas P. Hepatic clearance and metabolism in the rat of a human breast cancer associated glycoprotein (GCDFP-15) Breast Cancer Res Treat. 1988;12:235–243. doi: 10.1007/BF01805944. [DOI] [PubMed] [Google Scholar]
- 57.Rathman WM, Van Zeyl MJ, Van den Keybus PA, Bank RA, Veerman EC, Nieuw Amerongen AV. Isolation and characterization of three non-mucinous human salivary proteins with affinity for hydroxyapatite. J Biol Buccale. 1989;17:199–208. [PubMed] [Google Scholar]
- 58.Hassan MI, Bilgrami S, Kumar V, Singh N, Yadav S, Kaur P, Singh TP. Crystal structure of the novel complex formed between zinc alpha2-glycoprotein (ZAG) and prolactin-inducible protein (PIP) from human seminal plasma. J Mol Biol. 2008;384:663–672. doi: 10.1016/j.jmb.2008.09.072. [DOI] [PubMed] [Google Scholar]
- 59.Autiero M, Cammarota G, Friedlein A, Zulauf M, Chiappetta G, Dragone V, Guardiola J. A 17-kDa CD4-binding glycoprotein present in human seminal plasma and in breast tumor cells. Eur J Immunol. 1995;25:1461–1464. doi: 10.1002/eji.1830250550. [DOI] [PubMed] [Google Scholar]
- 60.Caputo E, Camarca A, Moharram R, Tornatore P, Thatcher B, Guardiola J, Martin BM. Structural study of GCDFP-15/gp17 in disease versus physiological conditions using a proteomic approach. Biochemistry. 2003;42:6169–6178. doi: 10.1021/bi034038a. [DOI] [PubMed] [Google Scholar]
- 61.Basmaciogullari S, Autiero M, Culerrier R, Mani JC, Gaubin M, Mishal Z, Guardiola J, Granier C, Piatier-Tonneau D. Mapping the CD4 binding domain of gp17, a glycoprotein secreted from seminal vesicles and breast carcinomas. Biochemistry. 2000;39:5332–5340. doi: 10.1021/bi992398l. [DOI] [PubMed] [Google Scholar]
- 62.Kitano T, Tian W, Umetsu K, Yuasa I, Yamazaki K, Saitou N, Osawa M. Origin and evolution of gene for prolactin-induced protein. Gene. 2006;383:64–70. doi: 10.1016/j.gene.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 63.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, Stange-Thomann Y, Stojanovic N, Subramanian A, Wyman D, Rogers J, Sulston J, Ainscough R, Beck S, Bentley D, Burton J, Clee C, Carter N, Coulson A, Deadman R, Deloukas P, Dunham A, Dunham I, Durbin R, French L, Grafham D, Gregory S, Hubbard T, Humphray S, Hunt A, Jones M, Lloyd C, McMurray A, Matthews L, Mercer S, Milne S, Mullikin JC, Mungall A, Plumb R, Ross M, Shownkeen R, Sims S, Waterston RH, Wilson RK, Hillier LW, McPherson JD, Marra MA, Mardis ER, Fulton LA, Chinwalla AT, Pepin KH, Gish WR, Chissoe SL, Wendl MC, Delehaunty KD, Miner TL, Delehaunty A, Kramer JB, Cook LL, Fulton RS, Johnson DL, Minx PJ, Clifton SW, Hawkins T, Branscomb E, Predki P, Richardson P, Wenning S, Slezak T, Doggett N, Cheng JF, Olsen A, Lucas S, Elkin C, Uberbacher E, Frazier M, Gibbs RA, Muzny DM, Scherer SE, Bouck JB, Sodergren EJ, Worley KC, Rives CM, Gorrell JH, Metzker ML, Naylor SL, Kucherlapati RS, Nelson DL, Weinstock GM, Sakaki Y, Fujiyama A, Hattori M, Yada T, Toyoda A, Itoh T, Kawagoe C, Watanabe H, Totoki Y, Taylor T, Weissenbach J, Heilig R, Saurin W, Artiguenave F, Brottier P, Bruls T, Pelletier E, Robert C, Wincker P, Smith DR, Doucette-Stamm L, Rubenfield M, Weinstock K, Lee HM, Dubois J, Rosenthal A, Platzer M, Nyakatura G, Taudien S, Rump A, Yang H, Yu J, Wang J, Huang G, Gu J, Hood L, Rowen L, Madan A, Qin S, Davis RW, Federspiel NA, Abola AP, Proctor MJ, Myers RM, Schmutz J, Dickson M, Grimwood J, Cox DR, Olson MV, Kaul R, Shimizu N, Kawasaki K, Minoshima S, Evans GA, Athanasiou M, Schultz R, Roe BA, Chen F, Pan H, Ramser J, Lehrach H, Reinhardt R, McCombie WR, de la Bastide M, Dedhia N, Blöcker H, Hornischer K, Nordsiek G, Agarwala R, Aravind L, Bailey JA, Bateman A, Batzoglou S, Birney E, Bork P, Brown DG, Burge CB, Cerutti L, Chen HC, Church D, Clamp M, Copley RR, Doerks T, Eddy SR, Eichler EE, Furey TS, Galagan J, Gilbert JG, Harmon C, Hayashizaki Y, Haussler D, Hermjakob H, Hokamp K, Jang W, Johnson LS, Jones TA, Kasif S, Kaspryzk A, Kennedy S, Kent WJ, Kitts P, Koonin EV, Korf I, Kulp D, Lancet D, Lowe TM, McLysaght A, Mikkelsen T, Moran JV, Mulder N, Pollara VJ, Ponting CP, Schuler G, Schultz J, Slater G, Smit AF, Stupka E, Szustakowki J, Thierry-Mieg D, Thierry-Mieg J, Wagner L, Wallis J, Wheeler R, Williams A, Wolf YI, Wolfe KH, Yang SP, Yeh RF, Collins F, Guyer MS, Peterson J, Felsenfeld A, Wetterstrand KA, Patrinos A, Morgan MJ, de Jong P, Catanese JJ, Osoegawa K, Shizuya H, Choi S, Chen YJ International Human Genome Sequencing Consortium. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 64.Myal Y, Robinson DB, Iwasiow B, Tsuyuki D, Wong P, Shiu RP. The prolactin-inducible protein (PIP/GCDFP-15) gene: cloning, structure and regulation. Mol Cell Endocrinol. 1991;80:165–175. doi: 10.1016/0303-7207(91)90153-j. [DOI] [PubMed] [Google Scholar]
- 65.Bièche I, Khodja A, Driouch K, Lidereau R. Genetic alteration mapping on chromosome 7 in primary breast cancer. Clin Cancer Res. 1997;3:1009–1016. [PubMed] [Google Scholar]
- 66.Bièche I, Champème MH, Matifas F, Hacène K, Callahan R, Lidereau R. Loss of heterozygosity on chromosome 7q and aggressive primary breast cancer. Lancet. 1992;339:139–143. doi: 10.1016/0140-6736(92)90208-k. [DOI] [PubMed] [Google Scholar]
- 67.Ciullo M, Debily MA, Rozier L, Autiero M, Billault A, Mayau V, El Marhomy S, Guardiola J, Bernheim A, Coullin P, Piatier-Tonneau D, Debatisse M. Initiation of the breakage-fusionbridge mechanism through common fragile site activation in human breast cancer cells: the model of PIP gene duplication from a break at FRA7I. Hum Mol Genet. 2002;11:2887–2894. doi: 10.1093/hmg/11.23.2887. [DOI] [PubMed] [Google Scholar]
- 68.Autiero M, Camarca A, Ciullo M, Debily MA, El Marhomy S, Pasquinelli R, Capasso I, D’Aiuto G, Anzisi AM, Piatier-Tonneau D, Guardiola J. Intragenic amplification and formation of extrachromosomal small circular DNA molecules from the PIP gene on chromosome 7 in primary breast carcinomas. Int J Cancer. 2002;99:370–377. doi: 10.1002/ijc.10368. [DOI] [PubMed] [Google Scholar]
- 69.Osawa M, Horiuchi H, Tian W, Kaneko M. Divergent evolution of the prolactin-inducible protein gene and related genes in the mouse genome. Gene. 2004;325:179–186. doi: 10.1016/j.gene.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 70.Filip LC, Mundy NI. Rapid evolution by positive Darwinian selection in the extracellular domain of the abundant lymphocyte protein CD45 in primates. Mol Biol Evol. 2004;21:1504–1511. doi: 10.1093/molbev/msh111. [DOI] [PubMed] [Google Scholar]
- 71.Loos S, Schulz KD, Hackenberg R. Regulation of GCDFP-15 expression in human mammary cancer cells. Int J Mol Med. 1999;4:135–140. doi: 10.3892/ijmm.4.2.135. [DOI] [PubMed] [Google Scholar]
- 72.Chalbos D, Haagensen D, Parish T, Rochefort H. Identification and androgen regulation of two proteins released by T47D human breast cancer cells. Cancer Res. 1987;47:2787–2792. [PubMed] [Google Scholar]
- 73.Haagensen DE, Stewart P, Dilley WG, Wells SA. Secretion of breast gross cystic disease fluid proteins by T47D breast cancer cells in culture--modulation by steroid hormones. Breast Cancer Res Treat. 1992;23:77–86. doi: 10.1007/BF01831479. [DOI] [PubMed] [Google Scholar]
- 74.Labrie F, Simard J, Poulin R, Hatton AC, Labrie C, Dauvois S, Zhao HF, Petitclerc L, Couët J, Dumont M. Potent antagonism between estrogens and androgens on GCDFP-15 expression and cell growth in the ZR-75-1 human breast cancer cells. Ann N Y Acad Sci. 1990;586:174–187. doi: 10.1111/j.1749-6632.1990.tb17805.x. [DOI] [PubMed] [Google Scholar]
- 75.Blais Y, Gingras S, Haagensen DE, Labrie F, Simard J. Interleukin-4 and interleukin-13 inhibit estrogen-induced breast cancer cell proliferation and stimulate GCDFP-15 expression in human breast cancer cells. Mol Cell Endocrinol. 1996;121:11–18. doi: 10.1016/0303-7207(96)03843-9. [DOI] [PubMed] [Google Scholar]
- 76.Myal Y, Iwasiow B, Cosby H, Yarmill A, Blanchard A, Tsuyuki D, Fresnoza A, Duckworth ML, Shiu RP. Analysis of tissue- and hormone-specific regulation of the human prolactin-inducible protein/gross cystic disease fluid protein-15 gene in transgenic mice. J Mol Endocrinol. 1998;21:217–223. doi: 10.1677/jme.0.0210217. [DOI] [PubMed] [Google Scholar]
- 77.Simard J, Hatton AC, Labrie C, Dauvois S, Zhao HF, Haagensen DE, Labrie F. Inhibitory effect of estrogens on GCDFP-15 mRNA levels and secretion in ZR-75-1 human breast cancer cells. Mol Endocrinol. 1989;3:694–702. doi: 10.1210/mend-3-4-694. [DOI] [PubMed] [Google Scholar]
- 78.Dauvois S, Simard J, Dumont M, Haagensen DE, Labrie F. Opposite effects of estrogen and the progestin R5020 on cell proliferation and GCDFP-15 expression in ZR-75-1 human breast cancer cells. Mol Cell Endocrinol. 1990;73:171–178. doi: 10.1016/0303-7207(90)90130-z. [DOI] [PubMed] [Google Scholar]
- 79.Carsol JL, Gingras S, Simard J. Synergistic action of prolactin (PRL) and androgen on PRL-inducible protein gene expression in human breast cancer cells: a unique model for functional cooperation between signal transducer and activator of transcription-5 and androgen receptor. Mol Endocrinol. 2002;16:1696–1710. doi: 10.1210/mend.16.7.0875. [DOI] [PubMed] [Google Scholar]
- 80.Baniwal SK, Little GH, Chimge NO, Frenkel B. Runx2 controls a feed-forward loop between androgen and prolactin-induced protein (PIP) in stimulating T47D cell proliferation. J Cell Physiol. 2012;227:2276–2282. doi: 10.1002/jcp.22966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Naderi A, Meyer M. Prolactin-induced protein mediates cell invasion and regulates integrin signaling in estrogen receptor-negative breast cancer. Breast Cancer Res. 2012;14:R111. doi: 10.1186/bcr3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Caputo E, Manco G, Mandrich L, Guardiola J. A novel aspartyl proteinase from apocrine epithelia and breast tumors. J Biol Chem. 2000;275:7935–7941. doi: 10.1074/jbc.275.11.7935. [DOI] [PubMed] [Google Scholar]
- 83.Hassan MI, Kumar V, Singh TP, Yadav S. Purification and characterization of zinc alpha2-glycoprotein-prolactin inducible protein complex from human seminal plasma. J Sep Sci. 2008;31:2318–2324. doi: 10.1002/jssc.200700686. [DOI] [PubMed] [Google Scholar]
- 84.Chiu WW, Chamley LW. Human seminal plasma prolactin-inducible protein is an immunoglobulin G-binding protein. J Reprod Immunol. 2003;60:97–111. doi: 10.1016/s0165-0378(03)00084-6. [DOI] [PubMed] [Google Scholar]
- 85.Kumar S, Tomar AK, Singh S, Saraswat M, Singh TP, Yadav S. Human serum albumin as a new interacting partner of prolactin inducible protein in human seminal plasma. Int J Biol Macromol. 2012;50:317–322. doi: 10.1016/j.ijbiomac.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 86.Schenkels LC, Walgreen-Weterings E, Oomen LC, Bolscher JG, Veerman EC, Nieuw Amerongen AV. In vivo binding of the salivary glycoprotein EP-GP (identical to GCDFP-15) to oral and non-oral bacteria detection and identification of EP-GP binding species. Biol Chem. 1997;378:83–88. doi: 10.1515/bchm.1997.378.2.83. [DOI] [PubMed] [Google Scholar]
- 87.Schenkels LC, Ligtenberg AJ, Veerman EC, Van Nieuw Amerongen A. Interaction of the salivary glycoprotein EP-GP with the bacterium Streptococcus salivarius HB. J Dent Res. 1993;72:1559–1565. doi: 10.1177/00220345930720120501. [DOI] [PubMed] [Google Scholar]
- 88.Nistor A, Bowden G, Blanchard A, Myal Y. Influence of mouse prolactin-inducible protein in saliva on the aggregation of oral bacteria. Oral Microbiol Immunol. 2009;24:510–513. doi: 10.1111/j.1399-302X.2009.00543.x. [DOI] [PubMed] [Google Scholar]
- 89.Bergamo P, Balestrieri M, Cammarota G, Guardiola J, Abrescia P. CD4-mediated anchoring of the seminal antigen gp17 onto the spermatozoon surface. Hum Immunol. 1997;58:30–41. doi: 10.1016/s0198-8859(97)00213-9. [DOI] [PubMed] [Google Scholar]
- 90.König R. Interactions between MHC molecules and co-receptors of the TCR. Curr Opin Immunol. 2002;14:75–83. doi: 10.1016/s0952-7915(01)00300-4. [DOI] [PubMed] [Google Scholar]
- 91.Veillette A, Bookman MA, Horak EM, Samelson LE, Bolen JB. Signal transduction through the CD4 receptor involves the activation of the internal membrane tyrosine-protein kinase p56lck. Nature. 1989;338:257–259. doi: 10.1038/338257a0. [DOI] [PubMed] [Google Scholar]
- 92.Autiero M, Gaubin M, Mani JC, Castejon C, Martin M, el Marhomy S, Guardiola J, Piatier-Tonneau D. Surface plasmon resonance analysis of gp17, a natural CD4 ligand from human seminal plasma inhibiting human immunodeficiency virus type-1 gp120-mediated syncytium formation. Eur J Biochem. 1997;245:208–213. doi: 10.1111/j.1432-1033.1997.00208.x. [DOI] [PubMed] [Google Scholar]
- 93.Gaubin M, Autiero M, Basmaciogullari S, Métivier D, Mis hal Z, Culerrier R, Oudin A, Guardiola J, Piatier-Tonneau D. Potent inhibition of CD4/TCR-mediated T cell apoptosis by a CD4-binding glycoprotein secreted from breast tumor and seminal vesicle cells. J Immunol. 1999;162:2631–2638. [PubMed] [Google Scholar]
- 94.Blanchard A, Nistor A, Castaneda FE, Martin D, Hicks GG, Amara F, Shiu RP, Myal Y. Generation and initial characterization of the prolactin-inducible protein (PIP) null mouse: accompanying global changes in gene expression in the submandibular gland. Can J Physiol Pharmacol. 2009;87:859–872. doi: 10.1139/Y09-077. [DOI] [PubMed] [Google Scholar]
- 95.Li J, Liu D, Mou Z, Ihedioha OC, Blanchard A, Jia P, Myal Y, Uzonna JE. Deficiency of prolactin-inducible protein leads to impaired Th1 immune response and susceptibility to Leishmania major in mice. Eur J Immunol. 2015;45:1082–1091. doi: 10.1002/eji.201445078. [DOI] [PubMed] [Google Scholar]
- 96.Ihedioha O, Blanchard AA, Balhara J, Okwor I, Jia P, Uzonna J, Myal Y. The human breast cancer-associated protein, the prolactin-inducible protein (PIP), regulates intracellular signaling events and cytokine production by macrophages. Immunol Res. 2018;66:245–254. doi: 10.1007/s12026-018-8987-6. [DOI] [PubMed] [Google Scholar]
- 97.Cassoni P, Sapino A, Haagensen DE, Naldoni C, Bussolati G. Mitogenic effect of the 15-kDa gross cystic disease fluid protein (GCDFP-15) on breast-cancer cell lines and on immortal mammary cells. Int J Cancer. 1995;60:216–220. doi: 10.1002/ijc.2910600215. [DOI] [PubMed] [Google Scholar]
- 98.Mazoujian G, Warhol MJ, Haagensen DE. The ultrastructural localization of gross cystic disease fluid protein (GCDFP-15) in breast epithelium. Am J Pathol. 1984;116:305–310. [PMC free article] [PubMed] [Google Scholar]
- 99.Dumont M, Dauvois S, Simard J, Garcia T, Schachter B, Labrie F. Antagonism between estrogens and androgens on GCDFP-15 gene expression in ZR-75-1 cells and correlation between GCDFP-15 and estrogen as well as progesterone receptor expression in human breast cancer. J Steroid Biochem. 1989;34:397–402. doi: 10.1016/0022-4731(89)90115-5. [DOI] [PubMed] [Google Scholar]
- 100.Debily MA, Marhomy SE, Boulanger V, Eveno E, Mariage-Samson R, Camarca A, Auffray C, Piatier-Tonneau D, Imbeaud S. A functional and regulatory network associated with PIP expression in human breast cancer. PLoS One. 2009;4:e4696. doi: 10.1371/journal.pone.0004696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vanneste M, Naderi A. Prolactin-Induced Protein regulates cell adhesion in breast cancer. Biochem Biophys Res Commun. 2015;468:850–856. doi: 10.1016/j.bbrc.2015.11.043. [DOI] [PubMed] [Google Scholar]
- 102.Emens LA. Breast cancer immunobiology driving immunotherapy: vaccines and immune checkpoint blockade. Expert Rev Anticancer Ther. 2012;12:1597–1611. doi: 10.1586/era.12.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, Rugo HS, Hwang ES, Jirström K, West BL, Coussens LM. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ihedioha OC, Shiu RP, Uzonna JE, Myal Y. Prolactin-inducible protein: from breast cancer biomarker to immune modulator-novel insights from knockout mice. DNA Cell Biol. 2016;35:537–541. doi: 10.1089/dna.2016.3472. [DOI] [PubMed] [Google Scholar]
- 105.Hassan MI, Waheed A, Yadav S, Singh TP, Ahmad F. Prolactin inducible protein in cancer, fertility and immunoregulation: structure, function and its clinical implications. Cell Mol Life Sci. 2009;66:447–459. doi: 10.1007/s00018-008-8463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xie YL, Hassan SA, Qazi AM, Tsai-Morris CH, Dufau ML. Intramolecular disulfide bonds of the prolactin receptor short form are required for its inhibitory action on the function of the long form of the receptor. Mol Cell Biol. 2009;29:2546–2555. doi: 10.1128/MCB.01716-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Naderi A, Hughes-Davies L. A functionally significant cross-talk between androgen receptor and ErbB2 pathways in estrogen receptor negative breast cancer. Neoplasia. 2008;10:542–548. doi: 10.1593/neo.08274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chia KM, Liu J, Francis GD, Naderi A. A feedback loop between androgen receptor and ERK signaling in estrogen receptor-negative breast cancer. Neoplasia. 2011;13:154–166. doi: 10.1593/neo.101324. [DOI] [PMC free article] [PubMed] [Google Scholar]


