Abstract
Application of surgical endoscope, used alone or in combination with the surgical microscope, for the operative management of ear and temporal bone conditions may allow improved access and clearance of disease. Preservation of normal structures may also be improved.
As the use of this tool is increasing, the need for better understanding of the anatomy of the ear is becoming evident. This is particularly so for endoscopic surgery aiming at removal of lesions involving the infra-cochlear corridor and/or petrous apex.
Human temporal bone-derived labyrinth casts (molds), originally made for endolymphatic duct and sac analysis which genuinely represent the membranous labyrinth and its adjacent soft tissues, were morphometrically analyzed in terms of the anatomic relations between structures in and around the infra-cochlear corridor. The distance between the petrous carotid artery (PCA) and the basal turn of the cochlea, the distance between PCA and infra-cochlear vein (ICV)/cochlear aqueduct (CA), and the distance between the lower surface of basal cochlear turn and the point where the carotid artery and jugular vein (JV) meet close to the jugular foramen, were measured to be around 1.3 mm, 6 mm and 8 mm respectively, thus constituting an approximate 6 × 8 mm2 infra-cochlear corridor. This analysis and further study with larger samples might be helpful for operation via this corridor led to the petrous apex where cholesterol granuloma, cholesteatoma and other lesions are not uncommon.
Keywords: Infra-cochlear corridor, Petrous apex, Anatomical study, Ear endoscopy, Surgery
1. Introduction
New techniques and new approaches are developed to improve the completeness of disease resection in the temporal bone and the preservation of function of important structures in or near the surgical field. The increasing use of surgical endoscopes in the management of ear and temporal bone diseases is related to its promise of improved intra-operative visualization leading to improved disease management.
The endoscopic approach to the petrous apex via the external auditory canal has been explored and further developed recently (Wick et al., 2017; Sugimoto et al., 2017; Iannella et al., 2016). A combined microscopic/endoscopic method for ablation of petrous apex lesions makes use of the advantages of these two distinct tools. As with all surgery, the most important issue pertinent to endoscopic surgery of the temporal bone is a thorough knowledge of anatomy but this needs to be understood from a new perspective quite different to that seen with traditional microscopic approaches.
The infra-cochlear corridor provides a trans-canal infra-cochlear approach to the petrous apex, an efficient route for drainage of the cyst or ablation of cholesteatoma in the apex. Infra-cochlear corridor is often described as bordered by the carotid artery anteriorly, the jugular bulb posteriorly and the cochlear basal turn superiorly (Wick et al., 2017; Mosnier et al., 2002; Leung et al., 2010; Giddings et al., 1991). When we analyzed the relations between these structures, the infra-cochlear vein and the cochlear aqueduct are also included considering this complex to be part of the cochlea and significant for hearing preservation surgery.
2. Materials and methods
We have evaluated the anatomical relationships between structures possibly associated with the infra-cochlear corridor. The structures studied included the basal turn of the cochlea, infra-cochlear vein (ICV)/cochlear aqueduct (CA), petrous carotid artery (PCA) and jugular bulb (JB) and vein (JV). 17 silicone and plastic temporal bone (labyrinth) molds were studied in detail and the distances between these structures analyzed.
The distance between the wall of the petrous carotid artery (PCA) and the initial segment of the cochlear aqueduct (CA)/infra-cochlear vein (ICV), the one between lowest point of the basal cochlear turn and the point where the PCA and internal jugular vein (JV) meet close to the jugular foramen, and the shortest distance between the PCA and the cochlear basal turn were measured in 17 labyrinth molds (casts), 10 made of silicone and 7 of plastic. The means of the measured values and ranges regarding the distances were obtained. Soft rulers were used for the measurement of the distances, under direct visualization using a dissecting microscope, between the structures of interest in the casts. The microscope was equipped with photography and an automatic scale bar setting function, the latter being helpful for confirming the directly measured parameters using the rulers. Soft rulers with different lengths were able to be inserted to the deep part of the casts for accuracy of measurement.
3. Results
The distance between the lowest point of the basal cochlear turn and the point where the PCA and jugular vein approach near the jugular foramen was defined as series 1 (Fig. 1). The measured value of the distance between the PCA and CA/ICV was defined as series 2 (Fig. 2). We chose to measure PCA-ICV/CA distance out of a consideration that intact cochlear aqueduct and infra-cochlear vein might be significant for normal hearing. The cochlear aqueduct forms a short curve as it exits the scala tympani anterior to the round window membrane (at a distance around 0.3 mm). This curved segment, also the thinnest part, passes parallel to the IAM, and becomes straighter and thicker as it turns down and ends at the jugular foramen. We set this point for measuring the distance between ICV/CA and the medial surface of PCA at its genu (Fig. 2). The distance between petrous carotid artery (PCA) and basal turn of the cochlea was defined as series 3 (Fig. 2). The mean values of the three series are given in Table 1. Fig. 3 demonstrates in chart form the measurement of these distances in each of the 17 temporal bone casts.
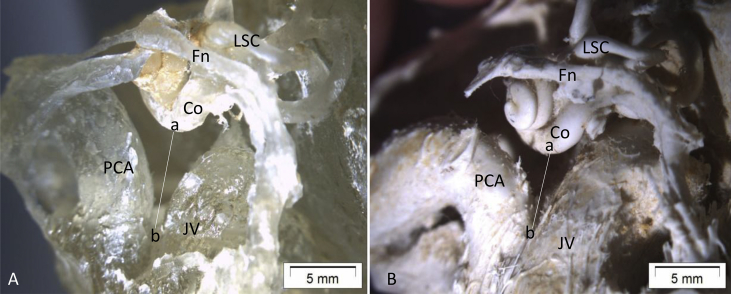
Fig. 1.
In A, the distance between the basal cochlear turn and the point where the PCA and jugular vein (JV) approach (a–b). This distance in A looks apparently bigger than the one in B, although both have a shorter a-b distance than average because of the high position of the jugular bulbs that render the cochlear aqueduct/infra-cochlear vein (CA/ICV) invisible. Fn, facial nerve; Co, cochlear basal turn; IAM, internal acoustic meatus; PCV, petrous carotid artery; JV, jugular vein.
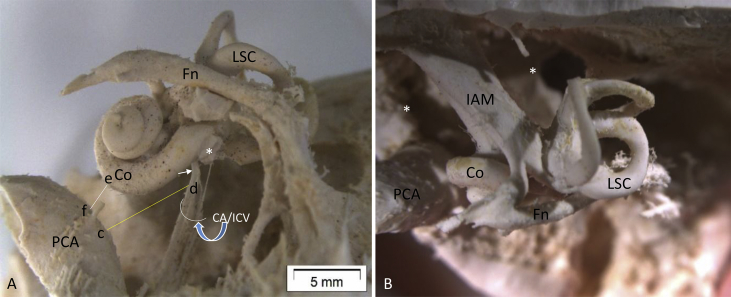
Fig. 2.
The lateral and superior view of silicon temporal bone casts. In A, the distance between the PCA and the CA/ICV (c–d) and the one between the PCA and basal turn of the cochlea (e–f) are included in our analysis. Note the CA/ICV forms a short curve (short white arrow in A), as it exits the scala tympani anterior to the round window (* in A) membrane (at a distance around 0.3 mm). This curved segment, also the thinnest part, passes parallel to the IAM, and becomes straighter and thicker as it turns down and ends at the jugular foramen. In B, one can see the upper side of the PCA, cochlear turns and the petrous apex that is divided by the content in the internal acoustic meatus (IAM) into anterior and posterior parts (*). Fn, facial nerve; Co, cochlear basal turn; IAM, internal acoustic meatus; PCV, petrous coratid artery; CA/ICV, cochlear aqueduct/infra-cochlear vein.
Table 1.
Values of the three distances measured (mm).
| distance a-b (series 1) | distance c-d (series 2) | distance e-f (series 3) | |
|---|---|---|---|
| Mean | 7.976 mm | 6.05 3 mm | 1.31 mm |
| Range | 4.5–11.5 mm | 5–8 mm | 0.3–1.6 mm |
Three distances in 17 temporal bone casts (Silicone and plastic). The series 1 represents the distance between lowest margin of the basal cochlear turn and the bifurcation point of PCA and jugular vein; series 2 represents the shortest distance between the medial aspect of PCA and the infra-cochlear vein/cochlear aqueduct; series 3 represents the shortest distance between the medial aspect of the PCA and the anterior cochlear margin.
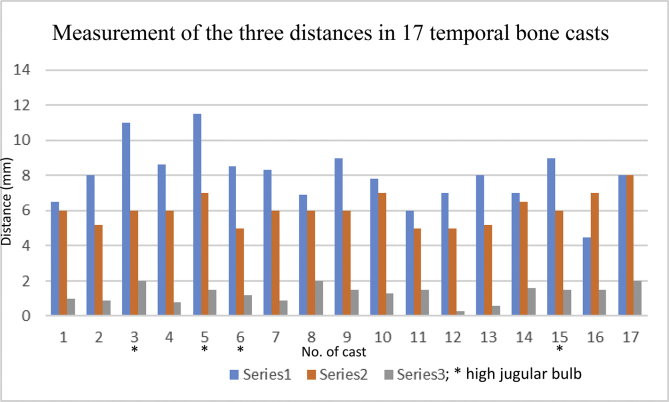
Fig. 3.
A chart shows measurement of the three distances in 17 temporal bone casts (silicone and plastic). The series 1 represents the distance between lowest margin of the basal cochlear turn and the bifurcation point of PCA and the jugular vein; series 2 represents the shortest distance between the medial aspect of PCA and the infra-cochlear vein/cochlear aqueduct; series 3 represents the shortest distance between the medial aspect of the PCA and the anterior cochlear margin. The mean value of series 1 is 7.976 mm, range from 4.5 to 11.5 mm; the mean value of series 2 is 6.053 mm, range 5–8 mm; the mean value of series 3 is 1.31 mm, range 0.3–1.6 mm. * high jugular bulb.
4. Discussion
The application of surgical endoscopes, used alone or combined with the surgical microscope, in the management of ear and temporal bone diseases is increasing (Wick et al., 2017; Sugimoto et al., 2017; Iannella et al., 2016; Marchioni et al., 2015; Presutti et al., 2014; Scopel et al., 2012; Mattox, 2004). The advantages of using the endoscope in treatment of diseases in the petrous apex are being actively assessed and improved anatomical knowledge is evident (Wick et al., 2017; Mosnier et al., 2002). In the present study, temporal bone casts (molds), originally made for endolymphatic duct/sac analysis and genuinely representing the membranous labyrinth and its adjacent soft tissue, allow morphometric analysis of the anatomical components in the temporal bone. Using the casts we analyzed the relationships between the petrous carotid artery, cochlea, infra-cochlear vein/cochlear aqueduct and jugular bulb/jugular vein (JB/JV). These components are connected to the infra-cochlear corridor that is being used nowadays for endoscopic approaches to petrous apex disease.
The molds provide the contours of soft tissue which has been replaced with polyester or silicone (they were also called labyrinth molds, 13). The soft tissue seen in the casts had been separated from the enveloping hard bone that was macerated and removed during preparation. The shrinkage index of the materials used for replacing the soft tissue is at a negligible level.
The distance between the petrous carotid artery (PCA) and the basal turn of the cochlea measured 1.3 mm. The distance between PCA and infra-cochlear vein (ICV)/cochlear aqueduct (CA) and distance between the inferior surface of cochlear basal turn and the bifurcation point between carotid artery and jugular vein near the jugular foramen measured 6 mm and 8 mm respectively, thus constituting roughly a 6 × 8 mm2 infra-cochlear corridor leading to the petrous apex where cholesterol granuloma, cholesteatoma and other lesions are not uncommon.
We chose PCA, JV, cochlear soft tissue wall, jugular bulb and CA/ICV for analysis as they need to be preserved during surgery via the infra-cochlear corridor to prevent hearing loss or significant bleeding. The choice of CA and ICV as the medial border for the measurement is based on the fact that these structures have potential importance in perilymph regulation (CA) and drainage of blood from the inner ear to the venous sinuses (ICV) (Scopel et al., 2012; Mattox, 2004). Damage to either of the two during surgery should be avoided if possible. The CA and ICV have been studied regarding their anatomy using dissected human temporal bones as well as human labyrinth casts (molds) (Siebenmann, 1894; Rask-Andersen et al., 1977; Guo et al., 2016). These two structures are parallel and close to each other. The CA and its accessory canal (the accessory canal (AC) is a bony channel that contains the ICV) were analyzed using photo stereomicroscopy, especially documenting the distance between the proximal portion of the AC and the anterior border of the round window membrane (Guo et al., 2016); the possibility of damaging the vein during cochlear implant (CI) electrode array insertion was highlighted for hearing preservation surgery by the authors of the study and others (Guo et al., 2016; Li et al., 2007; Wright and Roland, 2013). The ICV and CA have been studied for more than 50 years (Anson et al., 1965; Ritter and Lawrence, 1965; Atturo et al., 2018).
In Sugimoto's cases using combined endoscopy/microscopy approaches through the infra-cochlear corridor for treating petrous apex cholesteatoma, it was not necessary to mobilize the PCA in any case (Leung et al., 2010). It is desired that the PCA and a functioning cochlea are not injured. It has been shown that even localized drilling into the spiral ligament of the cochlear lateral wall, causing mechanic and heating injury, could lead to a widespread damage to the syncytium of the ion-transporting mechanism including the connexin gap junction channels on which endolymph potential maintenance is dependent (Liu et al., 2017). The height of the jugular bulb varies widely within the corridor. In the cases with a high JB, this delicate structure may be possibly pressed away during operation, and preoperative evaluation of imaging is important.
In the present study, the samples were randomly taken from several hundred casts. The number of samples was rather small. More extensive study will be undertaken, including using dissected bones and modern imaging techniques to provide more detailed information, with an ultimate goal of improving the safety and efficacy of endoscopic ear surgery.
5. Conclusion
We measured the shortest distance between the petrous carotid artery (PCA) and cochlear basal turn, the distance between the PCA and infra-cochlear vein (ICV)/cochlear aqueduct (CA), the distance between PCA/JV and cochlear basal turn. With the aim of preserving the ICV/CA medially, the petrous carotid artery anteriorly, the basal cochlear turn superiorly and the jugular bulb posteriorly this study has identified that the infra-cochlear corridor has a cross sectional area of approximately 6 × 8 mm2. The height of jugular bulb seems to be the most variable factor which influences the dimension of the infra-cochlear corridor. In trans-canal endoscopic surgery dealing with lesions in the petrous apex, meticulous preoperative evaluation of the temporal bone anatomy with imaging, including the structures analyzed in the present study, cannot be overemphasized.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
References
- Anson B.J., Donaldson J.A., Warpeha R.L., Winch T.R. The vestibular and cochlear aqueducts: their variational anatomy in the adult human ear. Laryngoscope. 1965;75:1203–1223. doi: 10.1288/00005537-196508000-00001. [DOI] [PubMed] [Google Scholar]
- Atturo F., Schart-Morén N., Larsson S., Rask-Andersen H., Li H. The human cochlear aqueduct and accessory canals: a micro-CT analysis using a 3D reconstruction paradigm. Otol. Neurotol. 2018;39(6):e429–e435. doi: 10.1097/MAO.0000000000001831. [DOI] [PubMed] [Google Scholar]
- Giddings N.A., Brackmann D.E., Kwartler J.A. Transcanal infracochlear approach to the petrous apex. Otolaryngology-Head Neck Surg. (Tokyo) 1991;104(1):29–36. doi: 10.1177/019459989110400107. [DOI] [PubMed] [Google Scholar]
- Guo R., Zhang H.L., Chen W., Zhu X.Q., Liu W., Helge Rask-Andersen H. The inferior cochlear vein: surgical aspects in cochlear implantation. Eur. Arch. Oto-Rhino-Laryngol. 2016;273:355–361. doi: 10.1007/s00405-015-3549-1. [DOI] [PubMed] [Google Scholar]
- Iannella G., Savastano E., Pasquariello B., Re M., Magliulo G. Giant petrous bone cholesteatoma: combined microscopic surgery and an adjuvant endoscopic approach. J. Neurol. Surg. Rep. 2016;77(1):e46–49. doi: 10.1055/s-0035-1571205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung R., Samy R.N., Leach J.L., Murugappan S., Stredney D., Wiet G. Radiographic anatomy of the infracochlear approach to the petrous apex for computer-assisted surgery. Otol. Neurotol. 2010;31(3):1. doi: 10.1097/MAO.0b013e3181c99524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P.M., Wang H., Northrop C., Merchant S.N., Nadol J.B., Jr. Anatomy of the round window and hook region of the cochlea with implications for cochlear implantation and other endocochlear surgical procedures. Otol. Neurotol. 2007;28:641–648. doi: 10.1097/mao.0b013e3180577949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Schrott-Fischer A., Glueckert R., Benav H., Rask-Andersen H. The human “cochlear battery” - claudin-11 barrier and ion transport proteins in the lateral wall of the cochlea. Front. Mol. Neurosci. 2017;10:239. doi: 10.3389/fnmol.2017.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchioni D., Alicandri-Ciufelli M., Rubini A., Presutti L. Endoscopic transcanal corridors to the lateral skull base: initial experiences. Laryngoscope. 2015;125(Suppl. 5):S1–S13. doi: 10.1002/lary.25203. Epub 2015 Feb 20. [DOI] [PubMed] [Google Scholar]
- Mattox D.E. Endoscopy-assisted surgery of the petrous apex. Otolaryngol. Head Neck Surg. 2004;130(2):229–241. doi: 10.1016/j.otohns.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Mosnier S., Cyna-Gorse F., Grayeli A.B., Fraysse B., Martin C., Robier A., Gardini B., Chelikh L., Sterkers O. Management of cholesterol granulomas of the petrous apex based on clinical and radiologic evaluation. Otol. Neurotol. 2002;23:522–528. doi: 10.1097/00129492-200207000-00022. [DOI] [PubMed] [Google Scholar]
- Presutti L., Alicandri-Ciufelli M., Rubini A., Gioacchini F.M., Marchioni D. Combined lateral microscopic/endoscopic approaches to petrous apex lesions: pilot clinical experiences. Ann. Otol. Rhinol. Laryngol. 2014;123(8):550–559. doi: 10.1177/0003489414525342. [DOI] [PubMed] [Google Scholar]
- Rask-Andersen H., Stahle J., Wilbrand H. Human cochlear aqueduct and its accessory canals. Ann. Otol. Rhinol. Laryngol. Suppl. 1977;86(5 Pt 2 Suppl. 42):1–16. doi: 10.1177/00034894770860s501. [DOI] [PubMed] [Google Scholar]
- Ritter F.N., Lawrence M. A histological and experimental study of cochlear aqueduct patency in the adult human. Laryngoscope. 1965;75:1224–1233. doi: 10.1288/00005537-196508000-00002. [DOI] [PubMed] [Google Scholar]
- Scopel T.F., Fernandez-Miranda J.C., Pinheiro-Neto C.D., Peris-Celda M., Paluzzi A., Gardner P.A., Hirsch B.E., Snyderman C.H. Petrous apex cholesterol granulomas: endonasal versus infracochlear approach. Laryngoscope. 2012;122(4):751–761. doi: 10.1002/lary.22448. [DOI] [PubMed] [Google Scholar]
- Siebenmann F. JF Berkmann; Wiesbaden: 1894. Die Blutgefä ssa im Labyriinthe des Menschlichen Ohres. [Google Scholar]
- Sugimoto H., Hatano M., Noda M., Hasegawa H., Yoshizaki T. Endoscopic management of petrous apex cholesteatoma. Eur. Arch. Oto-Rhino-Laryngol. 2017;274(12):4127–4130. doi: 10.1007/s00405-017-4763-9. [DOI] [PubMed] [Google Scholar]
- Wick C.C., Hansen A.R., Kutz J.W., Jr., Isaacson B. Endoscopic infracochlear approach for drainage of petrous apex cholesterol granulomas: a case series. Otol. Neurotol. 2017;38(6):876–881. doi: 10.1097/MAO.0000000000001422. [DOI] [PubMed] [Google Scholar]
- Wright C.G., Roland P.S. Vascular trauma during cochlear implantation: a contributor to residual hearing loss? Otol. Neurotol. 2013;34:402–407. doi: 10.1097/MAO.0b013e318278509a. [DOI] [PubMed] [Google Scholar]