Abstract
Organ transplantation is often the only effective treatment for patients with end-stage diseases, such as heart, liver, kidney and small bowel failure and is carried out frequently worldwide. Still the post-transplantation complications remain health- and life-threatening outcome that needed to be resolved. With the rapid development of molecular technologies in recent years, more and more researchers realize that the gut microbiota may play a critical role in human diseases. The intestinal microbiome has been proved to provide a lot of functions to the host, such as digesting food, modulating metabolism, promoting angiogenesis and regulating the immune system. Several studies have investigated the alteration of intestinal microbiota in post-transplantation patients and observed significant changes in the intestinal microbiome compared to the pre-transplant condition. Due to the abovementioned features that the gut microbiota may be used in the prognosis of clinical outcome of organ transplantation. In addition, the FMT (fecal microbiota transplantation), probiotics and prebiotics as the newest therapy methods, effectiveness of which has been verified in some diseases, such as Clostridium difficile infection, inflammatory bowel disease and other chronic disorders, might be used as the prognosis tool in organ transplantation as well. The purpose of this present review is to elucidate the relationship between gut microbiota and organ transplantation as well as the potential use of new therapies like fecal microbiota transplantation, probiotic and prebiotic administration after the transplantation, and provide some ideas for future researches in field of organ transplantation.
Keywords: Organ transplantation, gut microbiota, complication, prognosis, fecal microbiota transplantation, probiotic, prebiotic
Introduction
Numerous studies have investigated and examined the intestinal microbiomes with the latest molecular technologies and demonstrated that there are more than 1000 types of microbiota in the human gut [1], and nearly 20% of the small molecules in human blood are products of the microbiota [2]. Moreover, different researches provided a new insight into the composition and diversity of gut microbiota. The majority of bacterial taxa belong to the Firmicutes and Bacteroidetes phyla and bacteria such as Actinobacteria, Proteobacteria, Verrucomicrobia and Fusobacteria are represented as well [3]. The composition of the intestinal microbiota differs from person to person, which may implicated in health and disease [4-6]. Notably, the intestinal microbiome is involved in many processes in a host, such as digestion of food, modulation of metabolism, promotion of angiogenesis and regulation of the immune system [5,7-10]. Additionally, the imbalances of intestinal microbiota are associated with numerous disorders, for instance, inflammatory bowel disease [11,12], obesity [13,14], diabetes [13,15], colorectal cancer [16,17], cardiovascular disease [18-20], nervous system disease [21-23], and so on. The gut microbiota interacts not only with the gut epithelium but also with distant organs and body systems.
Organ transplantation is often the only possible treatment for patients with end-stage diseases [24,25]. However, infections, rejection, graft-versus-host disease (GVHD), relapse and other complications after transplantation are still the main problem needed to be resolved [26,27]. The application of immunosuppressant drugs after the transplantation, such as Tacrolimus, Mycophenolate Mofetil, is the main strategy in preventing the graft rejection. It is increased the survival chances, but at the same time bears the higher risk of post-transplant complications. Therefore, it is important to find new potentially feasible therapy methods that can attenuate the severity of post-transplant complications.
Up to now, several studies have investigated the alteration of intestinal microbiota in patients after transplantation and demonstrated a significant change in the intestinal microbiome compared to pre-transplantation condition [28-33]. They observed a decrease in the baseline predominant organism and a loss of diversity alongside with the emergence of a new dominant bacterial population, which coupled with an increased risk of post-transplant infection. Additionally, gut microbiota is proved to have an impact on distal immune response and to modulate disease in distant tissues by releasing metabolites such as short-chain fatty acids (SCFAs), tryptophan, phenylalanine and tyrosine, which have an essential role in intestinal inflammation and host resistance [34-36].
Therefore, the aim of this review is to elucidate the relationship between gut microbiota and organ transplantation from previous published outstanding publications and provide some ideas for future researches in the field of organ transplantation.
Current sights: intestinal microbiota and organ transplantation
Liver transplantation
Liver transplantation (LT) is a life-saving option for those patients who progress to end-stage liver disease including liver cirrhosis results from acute or chronic liver injury, liver carcinoma, primary sclerosing cholangitis, alcoholic liver disease, and so on [37,38]. However, the complications after liver transplantation, such as acute cell rejection (ACR), infection, graft-versus-host disease (GVHD) and chronic bile duct hyperplasia, remain the main problems of the short-term and long-term post-operation outcome.
The main focus of the modern studies lies on finding the ways to prevent or diagnose the complications early or attenuate their severity. With the development of microbial detection technologies, the evaluation of the relationship between gut microbiota and allogeneic transplantation has been investigated more intensively. Some studies have reported the change of gut microbial compositions after liver transplantation. Kato and the colleagues [39] performed a prospective study to longitudinally analyze the alteration of microbiota diversity of 38 patients during pre- and post- of liver transplantation. They found that the mean diversity index of microbiota was significantly decreased during the 21 days after liver transplantation but gradually increased during the whole observation period of 2 months post-LT, and the change of gut microbiota diversity was associated with acute cell rejection and infection. At the family level Bacteroides, Enterobacteriaceae, Streptococcaceae and Bifidobacteriaceae were increased in patients with acute cell rejection (ACR), whereas Enterococcaceae, Lactobacillaceae, Clostridiaceae, Ruminococcaceae and Peptostreptococcaceae were increased in none-ACR patients (Figure 1). Moreover, the level of Staphylococcus aureus, Enterococcus faecium, Escherichia coli, E. faecium, E. coli, Pseudomonas aeruginosa, and E. gallinarum were increased in patients with bloodstream infection. These results are conform with those from Sun [40], who analyzed the differences in the intestinal microflora of patients between pre-LT and post-LT showed a significant decrease in the abundance of Actinobacillus, Escherichia and Shigella, and a significant increase in the abundance of Micromonosporaceae, Desulfobacterales, the Sarcina genus of Eubacteriaceae and Akkermansia after liver transplantation. Additionally, Ren and his colleagues [41] established an orthotopic liver transplantation (OLT) models in rats to identify intestinal microbial profile as a biomarker for ACR and explore its potential application. In this excellent study, they found that the diversity of microbiota and species richness of key bacteria family, such as Firmicutes was decreased during ACR, whereas the level of phylum Bacteroidetes was significantly increased. Interestingly, Wu and his co-workers found that there was a significant decrease of butyrate-producing bacteria (e.g, Faecalibacterium prausnitzii) and an increase of opportunistic pathogens (e.g, Enterococcus spp.) [42], which was similar to traits in patients with diabetes, and it is may the origin of new-onset diabetes after liver transplantation [43,44]. Those results indicated that the diversity of gut microbiota was associated with the prognosis of liver transplantation.
Figure 1.
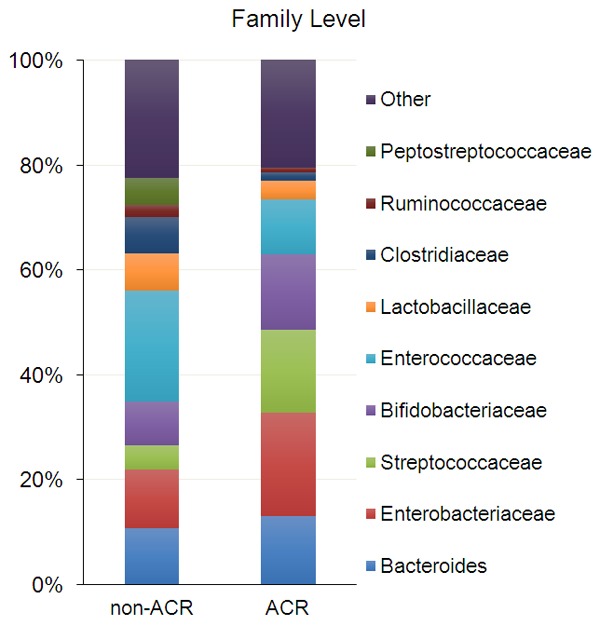
Different intestinal microbial compositions in patients with and without ACR after liver transplantation at the family level. ACR: acute cellular rejection, n = 38.
Besides, other factors like malnutrition, ischemia-reperfusion injury and immunosuppression therapy after liver transplantation may lead to the dysbiosis, disrupted intestinal barrier, alterations in innate immunity response as well as to bacterial translocation. It is associated with early infections, graft failure and decreased survival in patients [45]. Under these pathological conditions, due to the decrease of beneficial bacteria and the increase of pathogenic species, the altered gut microbiota responds to the host with higher endotoxin levels and increased bacterial translocation. Previous studies suggest that the intestinal microbiota regulate liver tumorigenesis or inflammatory reactions through altering the activity of pro-inflammatory microorganism-associated molecular patterns, bacterial metabolites, NKT cells-mediated bile acid metabolism and PGE2-mediated suppression of antitumor immunity [46-49]. However, few studies have elucidated the mechanism of how intestinal microbiota influence the prognosis of liver transplantation, and the further studies are needed to investigate it.
Taken together, these studies indicate that gut microbiota plays a crucial role in the prognosis of clinical outcomes of liver transplantation, and might be a potential marker that predicts acute/chronic rejection in early phase and become an assistant therapeutic target to improve rejection after liver transplantation in future.
Kidney transplantation
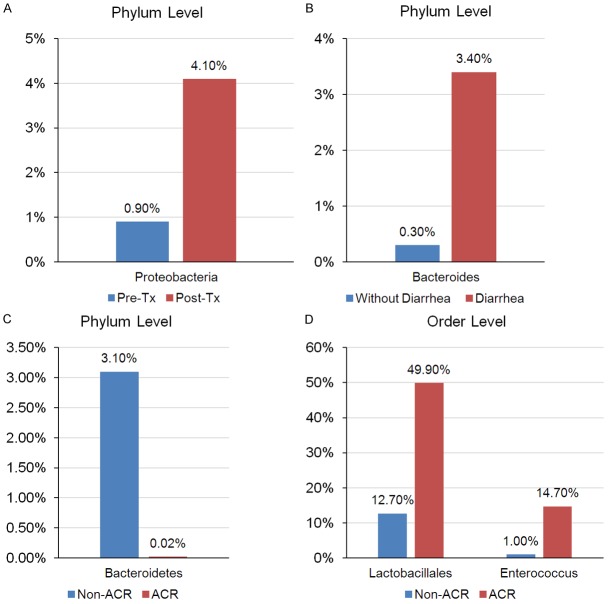
Kidney transplantation has been widely used as an effective therapy for the persons with chronic kidney diseases [50]. However, immunological and non-immunological factors, including genetic variation, epigenetic, pharmacogenetics, infection, injuries, hormones, environment [51,52] and gut microbiota have been reported to be associated with increased risk of graft failure in renal transplants, especially the intestinal microbiota, has drawn significant attention in the immunity of transplant, gut microbiota may influence the dosing of immunosuppressant (e.g. Everolimus, Tacrolimus and Mycophenolate Mofetil) medications [53]. Some of them indicate that the gut microbiome may play an important role in the prognosis of kidney transplantation outcome [54-56]. Lee and co-workers [29] performed an excellent study to clarify the alteration of gut microbiota in pre- and post-kidney transplantation patients. They used the polymerase chain reaction amplification of the 16S rRNA V4-V5 variable region to analyze the bacterial composition of fecal specimens from 26 kidney transplant recipients during the first three months of transplantation. The results demonstrated that the abundance of Proteobacteria at phylum level was significantly increased in the post-transplantation specimens compared to pre-transplantation specimens (Figure 2A). Using the linear discriminant analysis effect size (LEfSe) method, they showed that Bacteroides as well as Ruminococcus, Coprococcus and Dorea were significantly lower in the patients with post-transplantation diarrhea compared to those without diarrhea (Figure 2B). Further they demonstrated significant differences between the groups with and without acute rejection (AR) with Bacteroidetes being conspicuous lower at the phylum level in the AR group compared to no-AR group (Figure 2C), whereas the Lactobacillales, Enterococcus, Anaerofilum, Clostidium tertium were higher in the AR group at the order level (Figure 2D). They also found that the high abundance of Enterococcus was associated with the urinary tract infection (UTI). Additionally, Janice and his teammates [57] used pyrosequencing analysis of 16S rRNA genes to investigate the intestinal microbiota in pediatric patients with end-stage renal disease. They found that, at the family level, Bifidobacteria showed a significant decrease in transplant patients compared to healthy control group.
Figure 2.
Different intestinal microbial compositions in patients with kidney transplantation. A. The alteration level of Proteobacteria after kidney transplantation, n = 5; B. The alteration level of Bacteroides in patients with or without post-transplant diarrhea, without diarrhea, n = 9, diarrhea, n = 6; C and D. The alteration phylum level of Bacteroidetes and order level of Lactobacillales and Enterococcus in patients with or without acute rejection, acute rejection, n = 3, without rejection, n = 23. Pre-Tx: pre-transplantation, Post-Tx: post-transplantation, ACR: acute cellular rejection.
Besides, gut microbiota can also influence the Tacrolimus dosing in kidney transplantation. John and his co-workers [58] performed a pilot study by used the deep sequencing of PCR amplified 16S rRNA V4-V5 region, initial tacrolimus dosing was similar in the dose escalation group (4.2 ± 1.1 mg/day) and in the stable group (3.8 ± 0.8 mg/day). After the end of first transplantation month, the dosing of tacrolimus became higher in the dose escalation group (9.6 ± 2.4 mg/day), while became decreased in the dose stable group (3.3 ± 1.5 mg/day). They characterized the intestinal microbiota composition and identified that fecal Faecalibacterium prausnitzii abundance in the first week of transplantation was 11.8% in the dose escalation group and 0.8% in the dose stable group. Thus, intestinal microbiota can influence the dosing of tacrolimus in the kidney transplantation.
In summary, intestinal microbiota might play a critical role in the prognosis of renal transplantation outcomes; further researches are certainly needed to investigate it.
Small bowel transplantation
Small bowel transplantation (SBT) is an effective life-preserving treatment for patients with irreversible intestinal failure, such as intestinal failure and complications from parenteral nutrition [59,60]. The long-term survival rates of small bowel transplantation are not satisfied because of severe rejection after transplantation [61,62]. The intestinal is a unique organ containing a high dense microbial population that promotes the development and maturation of the immune system of a host and once the microbiota dysbiosis may results irreversible bowel disease, allergies and rheumatoid arthritis [63,64]. Therefore, the microbiota may impact on the prognosis of small bowel transplantation. Many recent studies explored the relationship between gut microbiota and small bowel transplantation. Oh and his co-workers [65] enrolled 19 small bowel transplantation recipients to characterize the ileal microbiota in rejecting and non-rejecting recipients. Their findings demonstrated that the phylum Proteobacteria (Figure 3A), particularly the family Enterobacteriaceae (Figure 3B), was strongly expanded in patients with active rejection, whereas the commensal Firmicutes, especially Lactobacillales was dramatically decreased relative to non-rejecting recipients (Figure 3C). At the genus level, abundance of the genera Escherichia and Klebsiella was significant expansion during the acute rejection (Figure 3D). This finding suggests that acute rejection is closely associated with the changes in the ileal microbial populations in recipients in SBT. Li and his colleagues characterized the composition of the gut microbiota during the chronic rejection by established a rat model with small bowel transplantation [66]. Their research demonstrated that the genera Bacteroides and Clostridium were remarkable expansion during the chronic rejection in the rat model, while deeply decreased of Lactobacillales. Above all, those results may provide novel insight into the roles of the intestinal microbiota in the pathophysiology of the transplanted intestine.
Figure 3.
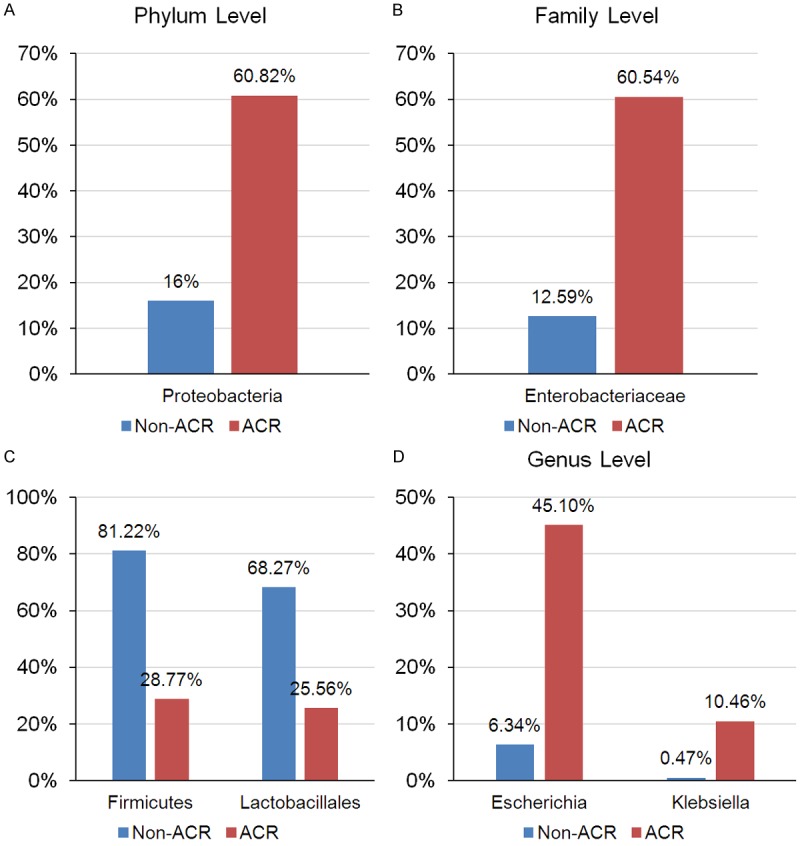
Different intestinal microbial compositions in patients with small bowel transplantation. The alteration phylum level of Proteobacteria, family level of Enterobacteriaceae, level of Firmicutes and Lactobacillales and genus level of Escherichia and Klebsiella in patients with or without acute rejection. ACR, acute cellular rejection.
Recently, Wood and his teammates [30] made a point that the intestinal microbiota profile might function as a diagnostic biomarker of small bowel transplant rejection. In this research, they observed a significant reduction in Firmicutes from 81% to 29% during the rejection process that was due to a reduction in the order Lactobacillales as well as to decrease of Streptococcaceae, Enterococcaceae and Lactobacillaceae at the family level. At the same time, the significant rise in Proteobacteria from 16% to 61% was associated with the expansion of the order Enterobacteriales, family Enterobacteriaceae. These results reveal the crucial role that the gut microbiota might play in the prognosis of small bowel transplantation.
Heart transplantation
Heart transplantation is a gold standard treatment for patients with end-stage heart diseases, such as dilated cardiomyopathy, advanced heart failure induced by valve diseases, end-stage coronary artery diseases and complexity congenital heart diseases [67,68]. However, graft rejection, cardiac allograft vasculopathy, graft dysfunction, chronic kidney disease, infection and malignancy are the most significant complications that almost all patients face after the transplantation. However, there was no studies connected to the heart transplantation and gut microbiota. Fortunately, previous studies demonstrated that gut microbiota acted as critical role in the cardiovascular disease [69-74]. Trimethylamine-N-oxide (TMAO) as a metabolite of gut microbiota has raised up a huge attention because of the potential role for increase cardiovascular risk [69,75-78]. Li and his colleagues [79] performed two independent cohort studies to investigate the relationship between TMAO level and incident cardiovascular risk among sequential patients presenting with acute coronary syndromes (ACS). They found that the elevated plasma TMAO level was associated with risk of major adverse cardiac events, additionally, it is also can be a significant predictor for the long term mortality. Similar conclusion was concluded by Marius [80], they found that TMAO may directly enhance atherosclerosis by interfering with cholesterol transportation and foam cell formation, as well as inducing platelet reactivity, promoting thrombosis and acute coronary events. Gut microbiota metabolize dietary phosphatidylcholine and generated trimethylamine, then delivered to the liver and converted to TMAO by Flavin-containing monooxygenases, finally, the activity TMAO influenced the prognosis of cardiovascular events [76].
Additionally, Marques and his teammates [81] established a DOCA-salt hypertensive mice model to investigate the potential mechanisms that dietary intake of fruit and vegetables is associated with low risk of heart failure. They found that high consumption of fiber modified the gut microbiota populations and increased the abundance of acetate-producing bacteria and surprising decreased the systolic and diastolic blood pressure, cardiac fibrosis, left ventricular hypertrophy and the down-regulated the mitogen-activated protein kinases (MAPK) signaling in the heart.
Taken together, intestinal microbiota is associated with the function of heart, the metabolites may influence the outcome of cardiovascular events. However, it should be noted that there is no study that explores the relationship between heart transplantation and gut microbiota, similar to the investigations on the other organ transplantation (e.g. liver, kidney, small bowel) and intestinal microbiota. We would issue a hypothesis that gut microbiota due to its crucial role in the regulation the immunity might be play a key role in the prognosis of heart transplantation as well. To elucidate this mechanism, further or new studies are necessary.
Potential views and future challenges
Difference in gender, ethnicity, and age
As we all know, the composition of the intestinal microbiota differs from person to person [4], and might be dependent on gender and ethnicity as well.
To date, some researchers investigated the difference between gut microbiota of males and females [82-88]. Yurkovetskiy and his teammates [83] established a mice model to analysis the relationship of gender bias and intestinal microbiota. They set up two groups to verify their hypothesis, the specific pathogen-free nonobese diabetic (NOD) mice and germ-free (GF) mice. They found that the female NOD mice have 1.3-4.4 times higher incidence of type 1 diabetes compared with male NOD mice while no gender bias was found in GF mice (female-to-male ratio 1.1-1.2). Then, they used 16S rRNA analysis to detect the microbiota from each groups and found that gut microbiota was different in males and females NOD mice, however, no significant difference was found in males and females GF mice. Additionally, they found a trend reversed of gut microbiota that no significant difference was found in males and females when male NOD mice castration, and confirmed that androgens influenced by gut microbiota. Those results demonstrated that gender bias is influenced by gut microbiota. Similar conclusion was conclude by Bolnick and his colleagues [84], they found that individual diet has sex-dependent effects on human gut microbiota. Additionally, Mueller and his co-workers [85] performed a cross-sectional study to investigate the difference in fecal microbiota in different European populations related to gender and country. They found that males have higher levels in the Bacteroides-Prevotella group than females independent from location, but no gender-related differences were observed in the Bacteroides vulgatus and Bacteroides putredinis group. In other previous study of fungal components of gut microbiota, Strati and his colleagues [86] revealed that the human gut microbiota differs in function of individuals’ life stage in a gender-related fashion. Female subjects showed a higher number of fungal isolates and fungal species compared to male subjects. The aforementioned results demonstrated that gender difference have an influence on the gut microbiota.
Ageing may be a potential risk factor that influence the human gut microbiota. Recently, a surprising study conducted by Arya and his co-workers [89] demonstrated that ageing is associated with a reduction in the beneficial commensal microbes. Reduction of beneficial muciniphola, prausnitzii, lactobacilli and bifidobacteria and an increase of staphylococci, clostridia, streptococci and enerobacteria were found in ageing rodents gut microbiota. Those results were same with O’Toole [90] and Claesson [91] conclusions, a significantly reduction of Lactobacillus and Faecalibacterium and the increase of Eubacteriaceae family and Oscillibacter and Alistipes genera was found in the gut microbiota of frailty elderly people. Those results conclude that ageing is probably associated with the gut microbiota.
Moreover, ethnic also have an important influence to gut microbiota. Yazici and his co-workers [92] found that race-dependent association of sulfidogenic bacteria with colorectal cancer. They used 16S rDNA sequence to confirm that sulfidogenic bacteria was associated with colorectal cancer, and examine the microbiota of African Americans and non-Hispanic whites. To their surprise, they found that African Americans harboured a great abundance of sulfidogenic bacteria compared with non-Hispanic whites regardless of disease status.
Recently, new and outstanding studies [93,94] defined three enterotypes of gut microbiota in human, which were independent of age, gender, cultural background and geography. An enterotype was described as “densely populated area in a multidimensional space of community composition”. Of them, Bacteroides are the dominant bacterial in enterotype 1, Prevotella are the driving factor of enterotype 2, and Ruminococcus are dominant in enterotype 3. These enterotypes have close relationship with different acute or chronic diseases in different people. Thus, different therapies should be applied based on the different enterotypes.
Taken together, those results demonstrated that the composition of gut microbiota might be influenced by gender, ethnicity and age. Therefore, future research should take gender, age and race into consideration when investigating the association of gut microbiota and organ transplantation.
Probiotics and prebiotics
Probiotics were defined as “live micro-organisms, which when administered in adequate amounts confer a health benefit on the host” by FAO/WHO (2001) [95]. The purpose of probiotics use in treatment of gut microbiota-related disease is to restore the intestinal homeostasis by a beneficial microbe. Lactobacilli and Bifidobacteria are the strains which most frequently used as probiotics [96]. Previous studies suggested that their potential functions and effects include the inhibition of microbial adherence and translocation, the establishment of a restrictive luminal environment (such as modification of the luminal pH), the production of peptides with antibacterial properties (such as bacteriocins), or the induction of the host’s immune response (such as expression of human defensins) [97-100]. In clinical practice, probiotics have been used for the treatments in many diseases, such as clostridium difficile infection [101,102], hematopoietic cell transplantation [103], inflammatory bowel disease [104], small bowel transplantation [105], kidney transplantation [106] and liver transplantation [107]. Xie and his colleagues [108] found that the quantity of Lactobacillus and Bifidobacterium after the treatment with probiotic substance (probiotic group) was significantly higher than antibiotic groups of Brown-Norway rats after allograft liver transplantation. Liver injury was significantly reduced in the probiotic group compared with the allograft group. Moreover, this study revealed that probiotics mediated their beneficial effects through increase of Treg cells and TGF-β and reduction of CD4/CD8 in rats with acute rejection after liver transplantation.
The concept of prebiotics was defined for the first time in 1995 [109] and revised in 2007 [110] by Gibson and Roberfroid as following “A prebiotic is a selectively fermented ingredient that allows specific changes, both in the composition or activity in the gastrointestinal microflora that confer benefits upon host well-being and health” [110,111]. To be classified as a prebiotic, a food ingredient needs to fulfill the following criteria: resistance to gastric acid secretion and to hydrolysis by digestive enzymes; absorption in the upper gastrointestinal tract and fermentation by the intestinal gut microbiota; stimulation of the growth or activity of beneficial microbes. According to current studies, only insulin and trans-galacto-oligosaccharides completely match or meet such a definition [112]. The potential mechanisms of the action of prebiotics include an increase in the production of short-chain fatty acids (SCFA) or a decrease of intestinal pH [113]. Recently, Huaman and his co-workers [114] performed a randomized, parallel, double-blind study to investigate the influence of prebiotics in patients with functional gut disorder. They set up two groups, prebiotic group (prebiotic supplement plus Mediterranean-type diet) and FODMAP group (glucose plus a diet low in fermentable oligo-, di-, mono-saccharides and polyols) for 4 weeks, and then patients were followed 2 weeks. After 6 weeks, they found that both groups had statistically significant reductions in all symptom scores, although the decrease in symptoms persisted for 2 weeks after patients discontinued prebiotic supplementation, symptoms reappeared immediately after patients discontinued the low-FODMAP diet. They conclude that prebiotic administration might be a potential treatment strategy for patients with functional gut symptoms.
Taken together, probiotics and prebiotics seem to be effective in positively altering the diversity of gut microbiota, especially in probiotics administration. The administration of antibiotics and immunosuppressive drugs after organ transplantation causes intestinal microbiota disorder and bacterial translocation in the patients. Therefore, further researches are necessary to clarify the possible clinical application of probiotics and prebiotics in the therapy of post-transplantation outcomes.
Fecal microbiota transplantation (FMT)
Fecal microbiota transplantation (FMT), defined as the transfer of a microbial community from a healthy donor to a patient, has emerged as a promising treatment option for a range of chronic disorders [115-119], especially of the Clostridium difficile infection [116,120]. Kassam with colleagues [120] performed a meta-analysis and systematic review to investigate the efficacy and safety profile of fecal microbiota transplantation in Clostridium difficile infection. They included 11 studies with 273 patients in their review and came to the conclusion that FMT were useful for the treatment of Clostridium difficile infection with no reported adverse events associated with this therapy. Besides, van Nood and his teammates [116] conducted a random trail to study the effect of duodenal infusion of donor feces in patients with recurrent Clostridium difficile infection. Surprisingly, they found that infusion with donor feces was significantly more effective for the treatment of recurrent Clostridium difficile infection than the use of vancomycin. The success of treatment of recurrent Clostridium difficile infection with donor feces made fecal microbiota transplantation emerge as a promising effective treatment option for a range of chronic disorders, such as ulcerative colitis [121,122], inflammatory bowel disease [123], Crohn’s disease [124], obesity [125], irritable bowel syndrome [126,127] and other indications. Those results demonstrated that FMT has an essential role in treating some chronic diseases; however, little is known about the potential treatments of post-transplantation complications. Recently, Kakihana and his colleagues [117] performed a pilot study to evaluate the safety of fecal microbiota transplantation in stem cell transplantation. The study revealed that no severe adverse events attributed to FMT, and suggested FMT as a potential novel therapeutic option for a graft-versus-host disease. Still there is no definite evidence of the effectiveness of FMT in treating organ transplantations, such as liver, kidney or heart transplantation. Therefore, further researches are necessary to be conducted to clarify it.
Besides, it should be kept in mind that FMT has also some adverse events, although rare. Most of them are related to the procedure used for the administration of the FMT, such as the endoscopy complications (perforation and bleeding) and side effects from sedation (aspiration) [128]. Additionally, transmission of donor enteric pathogens via FMT is also an important point of concern, but appears to be rare.
FMT is the first way to alter the intestinal microbiome, and both patients and donors have become more aware of this highly effective option in treatment of chronic diseases. Though FMT appears to be safe, yet few short-term adverse effects and complications attributed to the procedure were reported. Based on that, more supporting data is certainly needed for better understanding of the mechanisms of FMT efficacy and safety for its application in organ transplantation.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (NSFC81570427). We would like to thank all the participants for their contribution to this study. We also like to thank Ms. Svetlana Gasimova (Department of Traumatology, BG Trauma center, Eberhard Karls University of Tübingen) for editing the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Dore J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, Bork P, Ehrlich SD, Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rook G, Bäckhed F, Levin BR, McFall-Ngai MJ, McLean AR. Evolution, human-microbe interactions, and life history plasticity. Lancet. 2017;390:521–530. doi: 10.1016/S0140-6736(17)30566-4. [DOI] [PubMed] [Google Scholar]
- 3.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynch SV, Phimister EG, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375:2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 6.Hall A, Tolonen A, Xavier R. Human genetic variation and the gut microbiome in disease. Nat Rev Genet. 2017;18:690–699. doi: 10.1038/nrg.2017.63. [DOI] [PubMed] [Google Scholar]
- 7.Manfredo Vieira S, Hiltensperger M, Kumar V, Zegarra-Ruiz D, Dehner C, Khan N, Costa F, Tiniakou E, Greiling T, Ruff W, Barbieri A, Kriegel C, Mehta S, Knight J, Jain D, Goodman A, Kriegel M. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science. 2018;359:1156–1161. doi: 10.1126/science.aar7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thaiss C, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature. 2016;535:65–74. doi: 10.1038/nature18847. [DOI] [PubMed] [Google Scholar]
- 9.Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65–80. doi: 10.1053/j.gastro.2008.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, Reyes JA, Shah SA, LeLeiko N, Snapper SB, Bousvaros A, Korzenik J, Sands BE, Xavier RJ, Huttenhower C. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13:R79. doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ray K. Understanding gut microbiota in newonset Crohn’s disease. Nat Rev Gastroenterol Hepatol. 2014;11:268. doi: 10.1038/nrgastro.2014.45. [DOI] [PubMed] [Google Scholar]
- 12.Bye W, Ishaq N, Bolin TD, Duncombe VM, Riordan SM. Overgrowth of the indigenous gut microbiome and irritable bowel syndrome. World J Gastroenterol. 2014;20:2449–2455. doi: 10.3748/wjg.v20.i10.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komaroff A. The microbiome and risk for obesity and diabetes. JAMA. 2017;317:355–356. doi: 10.1001/jama.2016.20099. [DOI] [PubMed] [Google Scholar]
- 14.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 15.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K, Wang J. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 16.Tilg H, Adolph TE, Gerner RR, Moschen AR. The intestinal microbiota in colorectal cancer. Cancer Cell. 2018;33:954–964. doi: 10.1016/j.ccell.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Ahn J, Sinha R, Pei Z, Dominianni C, Wu J, Shi J, Goedert JJ, Hayes RB, Yang L. Human gut microbiome and risk for colorectal cancer. J Natl Cancer Inst. 2013;105:1907–1911. doi: 10.1093/jnci/djt300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang W, Wang Z, Levison B, Koeth R, Britt E, Fu X, Wu Y, Hazen S. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komaroff AL. The microbiome and risk for atherosclerosis. JAMA. 2018;319:2381. doi: 10.1001/jama.2018.5240. [DOI] [PubMed] [Google Scholar]
- 20.Brown JM, Hazen SL. Microbial modulation of cardiovascular disease. Nat Rev Microbiol. 2018;16:171–181. doi: 10.1038/nrmicro.2017.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olson C, Vuong H, Yano J, Liang Q, Nusbaum D, Hsiao E. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell. 2018;173:1728–1741. e1713. doi: 10.1016/j.cell.2018.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola-Rautio J, Pohja M, Kinnunen E, Murros K, Auvinen P. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2015;30:350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- 23.Cignarella F, Cantoni C, Ghezzi L, Salter A, Dorsett Y, Chen L, Phillips D, Weinstock G, Fontana L, Cross A, Zhou Y, Piccio L. Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota. Cell Metab. 2018;27:1222–1235. e1226. doi: 10.1016/j.cmet.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stehlik J, Kobashigawa J, Hunt SA, Reichenspurner H, Kirklin JK. Honoring 50 years of clinical heart transplantation in circulation: indepth state-of-the-art review. Circulation. 2018;137:71–87. doi: 10.1161/CIRCULATIONAHA.117.029753. [DOI] [PubMed] [Google Scholar]
- 25.Zarrinpar A, Busuttil RW. Liver transplantation: past, present and future. Nat Rev Gastroenterol Hepatol. 2013;10:434–440. doi: 10.1038/nrgastro.2013.88. [DOI] [PubMed] [Google Scholar]
- 26.Chong AS, Alegre ML. The impact of infection and tissue damage in solid-organ transplantation. Nat Rev Immunol. 2012;12:459–471. doi: 10.1038/nri3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mori DN, Kreisel D, Fullerton JN, Gilroy DW, Goldstein DR. Inflammatory triggers of acute rejection of organ allografts. Immunol Rev. 2014;258:132–144. doi: 10.1111/imr.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bajaj JS, Fagan A, Sikaroodi M, White MB, Sterling RK, Gilles H, Heuman D, Stravitz RT, Matherly SC, Siddiqui MS, Puri P, Sanyal AJ, Luketic V, John B, Fuchs M, Ahluwalia V, Gillevet PM. Liver transplant modulates gut microbial dysbiosis and cognitive function in cirrhosis. Liver Transpl. 2017;23:907–914. doi: 10.1002/lt.24754. [DOI] [PubMed] [Google Scholar]
- 29.Lee JR, Muthukumar T, Dadhania D, Toussaint NC, Ling L, Pamer E, Suthanthiran M. Gut microbial community structure and complications after kidney transplantation: a pilot study. Transplantation. 2014;98:697–705. doi: 10.1097/TP.0000000000000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wood NJ. Transplantation: potential of intestinal microbiota profile as a diagnostic biomarker of small bowel transplant rejection. Nat Rev Gastroenterol Hepatol. 2012;9:61. doi: 10.1038/nrgastro.2011.261. [DOI] [PubMed] [Google Scholar]
- 31.Peled JU, Jenq RR, Holler E, van den Brink MR. Role of gut flora after bone marrow transplantation. Nat Microbiol. 2016;1:16036. doi: 10.1038/nmicrobiol.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staffas A, Burgos da Silva M, van den Brink M. The intestinal microbiota in allogeneic hematopoietic cell transplant and graft-versushost disease. Blood. 2017;129:927–933. doi: 10.1182/blood-2016-09-691394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chalmers J. Microbial dysbiosis after lung transplantation. Am J Respir Crit Care Med. 2016;194:1184–1186. doi: 10.1164/rccm.201606-1178ED. [DOI] [PubMed] [Google Scholar]
- 34.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489:231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K. Induction of colonic regulatory T cells by indigenous clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, Le A, Cowan TM, Nolan GP, Fischbach MA, Sonnenburg JL. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. 2017;551:648–652. doi: 10.1038/nature24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, Le Chatelier E, Yao J, Wu L, Zhou J, Ni S, Liu L, Pons N, Batto J, Kennedy S, Leonard P, Yuan C, Ding W, Chen Y, Hu X, Zheng B, Qian G, Xu W, Ehrlich S, Zheng S, Li L. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513:59–64. doi: 10.1038/nature13568. [DOI] [PubMed] [Google Scholar]
- 38.Mieli-Vergani G, Vergani D, Czaja A, Manns M, Krawitt E, Vierling J, Lohse A, Montano-Loza A. Autoimmune hepatitis. Nat Rev Dis Primers. 2018;4:18017. doi: 10.1038/nrdp.2018.17. [DOI] [PubMed] [Google Scholar]
- 39.Kato K, Nagao M, Miyamoto K, Oka K, Takahashi M, Yamamoto M, Matsumura Y, Kaido T, Uemoto S, Ichiyama S. Longitudinal analysis of the intestinal microbiota in liver transplantation. Transplant Direct. 2017;3:e144. doi: 10.1097/TXD.0000000000000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun LY, Yang YS, Qu W, Zhu ZJ, Wei L, Ye ZS, Zhang JR, Sun XY, Zeng ZG. Gut microbiota of liver transplantation recipients. Sci Rep. 2017;7:3762. doi: 10.1038/s41598-017-03476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren Z, Jiang J, Lu H, Chen X, He Y, Zhang H, Xie H, Wang W, Zheng S, Zhou L. Intestinal microbial variation may predict early acute rejection after liver transplantation in rats. Transplantation. 2014;98:844–852. doi: 10.1097/TP.0000000000000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu Z, Ling Z, Lu H, Zuo J, Sheng J, Zheng S, Li L. Changes of gut bacteria and immune parameters in liver transplant recipients. HBPD INT. 2012;11:40–50. doi: 10.1016/s1499-3872(11)60124-0. [DOI] [PubMed] [Google Scholar]
- 43.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto J, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich S, Nielsen R, Pedersen O, Kristiansen K, Wang J. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X, Shen D, Fang Z, Jie Z, Qiu X, Zhang C, Chen Y, Ji L. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS One. 2013;8:e71108. doi: 10.1371/journal.pone.0071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chassaing B, Etienne-Mesmin L, Gewirtz AT. Microbiota-liver axis in hepatic disease. Hepatology. 2014;59:328–339. doi: 10.1002/hep.26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, Agdashian D, Terabe M, Berzofsky J, Fako V, Ritz T, Longerich T, Theriot C, McCulloch J, Roy S, Yuan W, Thovarai V, Sen S, Ruchirawat M, Korangy F, Wang X, Trinchieri G, Greten T. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018:360. doi: 10.1126/science.aan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu LX, Yan HX, Liu Q, Yang W, Wu HP, Dong W, Tang L, Lin Y, He YQ, Zou SS, Wang C, Zhang HL, Cao GW, Wu MC, Wang HY. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology. 2010;52:1322–1333. doi: 10.1002/hep.23845. [DOI] [PubMed] [Google Scholar]
- 48.Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A, Bataller R, Lefkowitch JH, Bower M, Friedman R, Sartor RB, Rabadan R, Schwabe RF. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–516. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loo TM, Kamachi F, Watanabe Y, Yoshimoto S, Kanda H, Arai Y, Nakajima-Takagi Y, Iwama A, Koga T, Sugimoto Y, Ozawa T, Nakamura M, Kumagai M, Watashi K, Taketo MM, Aoki T, Narumiya S, Oshima M, Arita M, Hara E, Ohtani N. Gut microbiota promotes obesity-associated liver cancer through PGE2-mediated suppression of antitumor immunity. Cancer Discov. 2017;7:522–538. doi: 10.1158/2159-8290.CD-16-0932. [DOI] [PubMed] [Google Scholar]
- 50.Robinson BM, Akizawa T, Jager KJ, Kerr PG, Saran R, Pisoni RL. Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: differences in access to renal replacement therapy, modality use, and haemodialysis practices. Lancet. 2016;388:294–306. doi: 10.1016/S0140-6736(16)30448-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zununi Vahed S, Ardalan M, Samadi N, Omidi Y. Pharmacogenetics and drug-induced nephrotoxicity in renal transplant recipients. Bioimpacts. 2015;5:45–54. doi: 10.15171/bi.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zununi Vahed S, Samadi N, Mostafidi E, Ardalan M, Omidi Y. Genetics and epigenetics of chronic allograft dysfunction in kidney transplants. Iran J Kidney Dis. 2016;10:1–9. [PubMed] [Google Scholar]
- 53.Zaza G, Dalla Gassa A, Felis G, Granata S, Torriani S, Lupo A. Impact of maintenance immunosuppressive therapy on the fecal microbiome of renal transplant recipients: comparison between an everolimus- and a standard tacrolimus-based regimen. PLoS One. 2017;12:e0178228. doi: 10.1371/journal.pone.0178228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ardalan M, Vahed SZ. Gut microbiota and renal transplant outcome. Biomed Pharmacother. 2017;90:229–236. doi: 10.1016/j.biopha.2017.02.114. [DOI] [PubMed] [Google Scholar]
- 55.Ahmad S, Bromberg JS. Current status of the microbiome in renal transplantation. Curr Opin Nephrol Hypertens. 2016;25:570–576. doi: 10.1097/MNH.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fricke WF, Maddox C, Song Y, Bromberg JS. Human microbiota characterization in the course of renal transplantation. Am J Transplant. 2014;14:416–427. doi: 10.1111/ajt.12588. [DOI] [PubMed] [Google Scholar]
- 57.Crespo-Salgado J, Vehaskari V, Stewart T, Ferris M, Zhang Q, Wang G, Blanchard E, Taylor C, Kallash M, Greenbaum L, Aviles D. Intestinal microbiota in pediatric patients with end stage renal disease: a midwest pediatric nephrology consortium study. Microbiome. 2016;4:50. doi: 10.1186/s40168-016-0195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee J, Muthukumar T, Dadhania D, Taur Y, Jenq R, Toussaint N, Ling L, Pamer E, Suthanthiran M. Gut microbiota and tacrolimus dosing in kidney transplantation. PLoS One. 2015;10:e0122399. doi: 10.1371/journal.pone.0122399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Middleton SJ, Jamieson NV. The current status of small bowel transplantation in the UK and internationally. Gut. 2005;54:1650–1657. doi: 10.1136/gut.2004.062612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quigley EM. Small intestinal transplantation: reflections on an evolving approach to intestinal failure. Gastroenterology. 1996;110:2009–2012. doi: 10.1053/gast.1996.v110.agast962009. [DOI] [PubMed] [Google Scholar]
- 61.Abu-Elmagd K, Kosmach-Park B, Costa G, Zenati M, Martin L, Koritsky D, Emerling M, Murase N, Bond G, Soltys K, Sogawa H, Lunz J, Al Samman M, Shaefer N, Sindhi R, Mazariegos G. Long-term survival, nutritional autonomy, and quality of life after intestinal and multivisceral transplantation. Ann Surg. 2012;256:494–508. doi: 10.1097/SLA.0b013e318265f310. [DOI] [PubMed] [Google Scholar]
- 62.Hilst CS, Ijtsma AJ, Bottema JT, Hoek BV, Dubbeld J, Metselaar HJ, Kazemier G, Berg AP, Porte RJ, Slooff MJ. The price of donation after cardiac death in liver transplantation: a prospective cost-effectiveness study. Transpl Int. 2013;26:411–418. doi: 10.1111/tri.12059. [DOI] [PubMed] [Google Scholar]
- 63.Cotter P. Small intestine and microbiota. Curr Opin Gastroenterol. 2011;27:99–105. doi: 10.1097/MOG.0b013e328341dc67. [DOI] [PubMed] [Google Scholar]
- 64.Wu H, Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. 2012;3:4–14. doi: 10.4161/gmic.19320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oh PL, Martinez I, Sun Y, Walter J, Peterson DA, Mercer DF. Characterization of the ileal microbiota in rejecting and nonrejecting recipients of small bowel transplants. Am J Transplant. 2012;12:753–762. doi: 10.1111/j.1600-6143.2011.03860.x. [DOI] [PubMed] [Google Scholar]
- 66.Li Q, Zhang Q, Wang C, Tang C, Zhang Y, Li N, Li J. Fish oil enhances recovery of intestinal microbiota and epithelial integrity in chronic rejection of intestinal transplant. PLoS One. 2011;6:e20460. doi: 10.1371/journal.pone.0020460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mancini D, Lietz K. Selection of cardiac transplantation candidates in 2010. Circulation. 2010;122:173–183. doi: 10.1161/CIRCULATIONAHA.109.858076. [DOI] [PubMed] [Google Scholar]
- 68.Hullin R. Heart transplantation: current practice and outlook to the future. Swiss Med Wkly. 2014;144:w13977. doi: 10.4414/smw.2014.13977. [DOI] [PubMed] [Google Scholar]
- 69.Trøseid M, Ueland T, Hov J, Svardal A, Gregersen I, Dahl C, Aakhus S, Gude E, Bjørndal B, Halvorsen B, Karlsen T, Aukrust P, Gullestad L, Berge R, Yndestad A. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med. 2015;277:717–726. doi: 10.1111/joim.12328. [DOI] [PubMed] [Google Scholar]
- 70.Tang WH, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res. 2017;120:1183–1196. doi: 10.1161/CIRCRESAHA.117.309715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Otto CM, Rahimi K. Heartbeat: the gut microbiota and heart failure. Heart. 2016;102:811–812. doi: 10.1136/heartjnl-2016-309848. [DOI] [PubMed] [Google Scholar]
- 72.Tang WH, Hazen SL. The contributory role of gut microbiota in cardiovascular disease. J Clin Invest. 2014;124:4204–4211. doi: 10.1172/JCI72331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schiattarella G, Sannino A, Toscano E, Giugliano G, Gargiulo G, Franzone A, Trimarco B, Esposito G, Perrino C. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur Heart J. 2017;38:2948–2956. doi: 10.1093/eurheartj/ehx342. [DOI] [PubMed] [Google Scholar]
- 74.Kelly T, Bazzano L, Ajami N, He H, Zhao J, Petrosino J, Correa A, He J. Gut microbiome associates with lifetime cardiovascular disease risk profile among bogalusa heart study participants. Circ Res. 2016;119:956–964. doi: 10.1161/CIRCRESAHA.116.309219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Senthong V, Li X, Hudec T, Coughlin J, Wu Y, Levison B, Wang Z, Hazen S, Tang W. Plasma trimethylamine n-oxide, a gut microbe-generated phosphatidylcholine metabolite, is associated with atherosclerotic burden. J Am Coll Cardiol. 2016;67:2620–2628. doi: 10.1016/j.jacc.2016.03.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Howitt M, Garrett W. A complex microworld in the gut: gut microbiota and cardiovascular disease connectivity. Nat Med. 2012;18:1188–1189. doi: 10.1038/nm.2895. [DOI] [PubMed] [Google Scholar]
- 77.Tang W, Wang Z, Fan Y, Levison B, Hazen J, Donahue L, Wu Y, Hazen S. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64:1908–1914. doi: 10.1016/j.jacc.2014.02.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Loscalzo J. Gut microbiota, the genome, and diet in atherogenesis. N Engl J Med. 2013;368:1647–1649. doi: 10.1056/NEJMe1302154. [DOI] [PubMed] [Google Scholar]
- 79.Li X, Obeid S, Klingenberg R, Gencer B, Mach F, Räber L, Windecker S, Rodondi N, Nanchen D, Muller O, Miranda M, Matter C, Wu Y, Li L, Wang Z, Alamri H, Gogonea V, Chung Y, Tang W, Hazen S, Lüscher T. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J. 2017;38:814–824. doi: 10.1093/eurheartj/ehw582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Trøseid M. Gut microbiota and acute coronary syndromes: ready for use in the emergency room? Eur Heart J. 2017;38:825–827. doi: 10.1093/eurheartj/ehx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marques F, Nelson E, Chu P, Horlock D, Fiedler A, Ziemann M, Tan J, Kuruppu S, Rajapakse N, El-Osta A, Mackay C, Kaye D. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135:964–977. doi: 10.1161/CIRCULATIONAHA.116.024545. [DOI] [PubMed] [Google Scholar]
- 82.Ding T, Schloss P. Dynamics and associations of microbial community types across the human body. Nature. 2014;509:357–360. doi: 10.1038/nature13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yurkovetskiy L, Burrows M, Khan A, Graham L, Volchkov P, Becker L, Antonopoulos D, Umesaki Y, Chervonsky A. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39:400–412. doi: 10.1016/j.immuni.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bolnick D, Snowberg L, Hirsch P, Lauber C, Org E, Parks B, Lusis A, Knight R, Caporaso J, Svanbäck R. Individual diet has sex-dependent effects on vertebrate gut microbiota. Nat Commun. 2014;5:4500. doi: 10.1038/ncomms5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mueller S, Saunier K, Hanisch C, Norin E, Alm L, Midtvedt T, Cresci A, Silvi S, Orpianesi C, Verdenelli MC, Clavel T, Koebnick C, Zunft HJ, Dore J, Blaut M. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a crosssectional study. Appl Environ Microbiol. 2006;72:1027–1033. doi: 10.1128/AEM.72.2.1027-1033.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Strati F, Di Paola M, Stefanini I, Albanese D, Rizzetto L, Lionetti P, Calabro A, Jousson O, Donati C, Cavalieri D, De Filippo C. Age and gender affect the composition of fungal population of the human gastrointestinal tract. Front Microbiol. 2016;7:1227. doi: 10.3389/fmicb.2016.01227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bridgewater LC, Zhang C, Wu Y, Hu W, Zhang Q, Wang J, Li S, Zhao L. Gender-based differences in host behavior and gut microbiota composition in response to high fat diet and stress in a mouse model. Sci Rep. 2017;7:10776. doi: 10.1038/s41598-017-11069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li X, Yan Q, Ringo E, Wu X, He Y, Yang D. The influence of weight and gender on intestinal bacterial community of wild largemouth bronze gudgeon (Coreius guichenoti, 1874) BMC Microbiol. 2016;16:191. doi: 10.1186/s12866-016-0809-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Biragyn A, Ferrucci L. Gut dysbiosis: a potential link between increased cancer risk in ageing and inflammaging. Lancet Oncol. 2018;19:e295–e304. doi: 10.1016/S1470-2045(18)30095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.O’Toole P, Jeffery I. Gut microbiota and aging. Science. 2015;350:1214–1215. doi: 10.1126/science.aac8469. [DOI] [PubMed] [Google Scholar]
- 91.Claesson M, Jeffery I, Conde S, Power S, O’Connor E, Cusack S, Harris H, Coakley M, Lakshminarayanan B, O’Sullivan O, Fitzgerald G, Deane J, O’Connor M, Harnedy N, O’Connor K, O’Mahony D, van Sinderen D, Wallace M, Brennan L, Stanton C, Marchesi J, Fitzgerald A, Shanahan F, Hill C, Ross R, O’Toole P. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 92.Yazici C, Wolf P, Kim H, Cross T, Vermillion K, Carroll T, Augustus G, Mutlu E, Tussing-Humphreys L, Braunschweig C, Xicola R, Jung B, Llor X, Ellis N, Gaskins H. Race-dependent association of sulfidogenic bacteria with colorectal cancer. Gut. 2017;66:1983–1994. doi: 10.1136/gutjnl-2016-313321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende D, Fernandes G, Tap J, Bruls T, Batto J, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichanh C, Nielsen H, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, Torrents D, Ugarte E, Zoetendal E, Wang J, Guarner F, Pedersen O, de Vos W, Brunak S, Doré J, Antolín M, Artiguenave F, Blottiere H, Almeida M, Brechot C, Cara C, Chervaux C, Cultrone A, Delorme C, Denariaz G, Dervyn R, Foerstner K, Friss C, van de Guchte M, Guedon E, Haimet F, Huber W, van Hylckama-Vlieg J, Jamet A, Juste C, Kaci G, Knol J, Lakhdari O, Layec S, Le Roux K, Maguin E, Mérieux A, Melo Minardi R, M’rini C, Muller J, Oozeer R, Parkhill J, Renault P, Rescigno M, Sanchez N, Sunagawa S, Torrejon A, Turner K, Vandemeulebrouck G, Varela E, Winogradsky Y, Zeller G, Weissenbach J, Ehrlich S, Bork P. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Costea PI, Hildebrand F, Manimozhiyan A, Backhed F, Blaser MJ, Bushman FD, de Vos WM, Ehrlich SD, Fraser CM, Hattori M, Huttenhower C, Jeffery IB, Knights D, Lewis JD, Ley RE, Ochman H, O’Toole PW, Quince C, Relman DA, Shanahan F, Sunagawa S, Wang J, Weinstock GM, Wu GD, Zeller G, Zhao L, Raes J, Knight R, Bork P. Enterotypes in the landscape of gut microbial community composition. Nat Microbiol. 2018;3:8–16. doi: 10.1038/s41564-017-0072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.(WHO-FAO)Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria [Google Scholar]
- 96.Dinleyici EC, Eren M, Ozen M, Yargic ZA, Vandenplas Y. Effectiveness and safety of saccharomyces boulardii for acute infectious diarrhea. Expert Opin Biol Ther. 2012;12:395–410. doi: 10.1517/14712598.2012.664129. [DOI] [PubMed] [Google Scholar]
- 97.Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC. Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis. 2009;15:300–310. doi: 10.1002/ibd.20602. [DOI] [PubMed] [Google Scholar]
- 98.Strunk T, Kollmann T, Patole S. Probiotics to prevent early-life infection. Lancet Infect Dis. 2015;15:378–379. doi: 10.1016/S1473-3099(15)70088-5. [DOI] [PubMed] [Google Scholar]
- 99.Slade E, Dale A, Mahtani K. Probiotics in very preterm infants: the PiPS trial. Lancet. 2016;388:656. doi: 10.1016/S0140-6736(16)31269-7. [DOI] [PubMed] [Google Scholar]
- 100.Tancredi D. Global health: probiotic prevents infections in newborns. Nature. 2017;548:404–405. doi: 10.1038/nature23540. [DOI] [PubMed] [Google Scholar]
- 101.Shen N, Maw A, Tmanova L, Pino A, Ancy K, Crawford C, Simon M, Evans A. Timely use of probiotics in hospitalized adults prevents clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152:1889–1900. e1889. doi: 10.1053/j.gastro.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 102.Dauby N. Risks of saccharomyces boulardiicontaining probiotics for the prevention of clostridium difficile infection in the elderly. Gastroenterology. 2017;153:1450–1451. doi: 10.1053/j.gastro.2017.04.054. [DOI] [PubMed] [Google Scholar]
- 103.Ladas EJ, Bhatia M, Chen L, Sandler E, Petrovic A, Berman DM, Hamblin F, Gates M, Hawks R, Sung L, Nieder M. The safety and feasibility of probiotics in children and adolescents undergoing hematopoietic cell transplantation. Bone Marrow Transplant. 2016;51:262–266. doi: 10.1038/bmt.2015.275. [DOI] [PubMed] [Google Scholar]
- 104.Tsilingiri K, Barbosa T, Penna G, Caprioli F, Sonzogni A, Viale G, Rescigno M. Probiotic and postbiotic activity in health and disease: comparison on a novel polarised ex-vivo organ culture model. Gut. 2012;61:1007–1015. doi: 10.1136/gutjnl-2011-300971. [DOI] [PubMed] [Google Scholar]
- 105.Zhang W, Frankel WL, Bain A, Choi D, Klurfeld DM, Rombeau JL. Glutamine reduces bacterial translocation after small bowel transplantation in cyclosporine-treated rats. J Surg Res. 1995;58:159–164. doi: 10.1006/jsre.1995.1025. [DOI] [PubMed] [Google Scholar]
- 106.Guida B, Cataldi M, Memoli A, Trio R, di Maro M, Grumetto L, Capuano I, Federico S, Pisani A, Sabbatini M. Effect of a short-course treatment with synbiotics on plasma p-cresol concentration in kidney transplant recipients. J Am Coll Nutr. 2017;36:586–591. doi: 10.1080/07315724.2017.1334602. [DOI] [PubMed] [Google Scholar]
- 107.Koretz R. Probiotics in gastroenterology: how pro is the evidence in adults? Am J Gastroenterol. 2018;113:1125–1136. doi: 10.1038/s41395-018-0138-0. [DOI] [PubMed] [Google Scholar]
- 108.Xie Y, Chen H, Zhu B, Qin N, Chen Y, Li Z, Deng M, Jiang H, Xu X, Yang J, Ruan B, Li L. Effect of intestinal microbiota alteration on hepatic damage in rats with acute rejection after liver transplantation. Microb Ecol. 2014;68:871–880. doi: 10.1007/s00248-014-0452-z. [DOI] [PubMed] [Google Scholar]
- 109.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 110.Roberfroid M. Prebiotics: the concept revisited. J Nutr. 2007;137:830S–837S. doi: 10.1093/jn/137.3.830S. [DOI] [PubMed] [Google Scholar]
- 111.Langlands SJ, Hopkins MJ, Coleman N, Cummings JH. Prebiotic carbohydrates modify the mucosa associated microflora of the human large bowel. Gut. 2004;53:1610–1616. doi: 10.1136/gut.2003.037580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 2013;5:1417–1435. doi: 10.3390/nu5041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.de Vrese M, Marteau PR. Probiotics and prebiotics: effects on diarrhea. J Nutr. 2007;137:803S–811S. doi: 10.1093/jn/137.3.803S. [DOI] [PubMed] [Google Scholar]
- 114.Huaman J, Mego M, Manichanh C, Cañellas N, Cañueto D, Segurola H, Jansana M, Malagelada C, Accarino A, Vulevic J, Tzortzis G, Gibson G, Saperas E, Guarner F, Azpiroz F. Effects of prebiotics vs a diet low in fodmaps in patients with functional gut disorder. Gastroenterology. 2018;155:1004–1007. doi: 10.1053/j.gastro.2018.06.045. [DOI] [PubMed] [Google Scholar]
- 115.Li SS, Zhu A, Benes V, Costea PI, Hercog R, Hildebrand F, Huerta-Cepas J, Nieuwdorp M, Salojarvi J, Voigt AY, Zeller G, Sunagawa S, de Vos WM, Bork P. Durable coexistence of donor and recipient strains after fecal microbiota transplantation. Science. 2016;352:586–589. doi: 10.1126/science.aad8852. [DOI] [PubMed] [Google Scholar]
- 116.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, Speelman P, Dijkgraaf MG, Keller JJ. Duodenal infusion of donor feces for recurrent clostridium difficile. N Engl J Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 117.Kakihana K, Fujioka Y, Suda W, Najima Y, Kuwata G, Sasajima S, Mimura I, Morita H, Sugiyama D, Nishikawa H, Hattori M, Hino Y, Ikegawa S, Yamamoto K, Toya T, Doki N, Koizumi K, Honda K, Ohashi K. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood. 2016;128:2083–2088. doi: 10.1182/blood-2016-05-717652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kelly CR, Kahn S, Kashyap P, Laine L, Rubin D, Atreja A, Moore T, Wu G. Update on fecal microbiota transplantation 2015: indications, methodologies, mechanisms, and outlook. Gastroenterology. 2015;149:223–237. doi: 10.1053/j.gastro.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Khoruts A. Faecal microbiota transplantation in 2013: developing human gut microbiota as a class of therapeutics. Nat Rev Gastroenterol Hepatol. 2014;11:79–80. doi: 10.1038/nrgastro.2013.231. [DOI] [PubMed] [Google Scholar]
- 120.Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for clostridium difficile infection: systematic review and metaanalysis. Am J Gastroenterol. 2013;108:500–508. doi: 10.1038/ajg.2013.59. [DOI] [PubMed] [Google Scholar]
- 121.Bennet JD, Brinkman M. Treatment of ulcerative colitis by implantation of normal colonic flora. Lancet. 1989;1:164. doi: 10.1016/s0140-6736(89)91183-5. [DOI] [PubMed] [Google Scholar]
- 122.Narula N, Kassam Z, Yuan Y, Colombel JF, Ponsioen C, Reinisch W, Moayyedi P. Systematic review and meta-analysis: fecal microbiota transplantation for treatment of active ulcerative colitis. Inflamm Bowel Dis. 2017;23:1702–1709. doi: 10.1097/MIB.0000000000001228. [DOI] [PubMed] [Google Scholar]
- 123.Colman RJ, Rubin DT. Fecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic review and metaanalysis. J Crohns Colitis. 2014;8:1569–1581. doi: 10.1016/j.crohns.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang FM, Wang HG, Wang M, Cui BT, Fan ZN, Ji GZ. Fecal microbiota transplantation for severe enterocolonic fistulizing Crohn’s disease. World J Gastroenterol. 2013;19:7213–7216. doi: 10.3748/wjg.v19.i41.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liou AP, Paziuk M, Luevano JM Jr, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5:178ra141. doi: 10.1126/scitranslmed.3005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McEvoy R. Bowel-flora alteration: a potential cure for inflammatory bowel disease and irritable bowel syndrome? Med J Aust. 1989;151:112. doi: 10.5694/j.1326-5377.1989.tb101176.x. [DOI] [PubMed] [Google Scholar]
- 127.Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, Poggioli G, Miglioli M, Campieri M. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305–309. doi: 10.1053/gast.2000.9370. [DOI] [PubMed] [Google Scholar]
- 128.Kelly CR, Ihunnah C, Fischer M, Khoruts A, Surawicz C, Afzali A, Aroniadis O, Barto A, Borody T, Giovanelli A, Gordon S, Gluck M, Hohmann EL, Kao D, Kao JY, McQuillen DP, Mellow M, Rank KM, Rao K, Ray A, Schwartz MA, Singh N, Stollman N, Suskind DL, Vindigni SM, Youngster I, Brandt L. Fecal microbiota transplant for treatment of clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109:1065–1071. doi: 10.1038/ajg.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]