Abstract
This article aims to explore whether Wharton’s jelly (WJ) derived mesenchymal stem cells (MSCs) (WJ-MSCs) decellularized extracellular matrix (dECM) can rejuvenate MSCs during in vitro expansion. Passage 10 synovium-derived mesenchymal stem cells (SDSCs) and WJ-MSCs were expanded on plastic flasks (PL) or dECMs derived from SDSCs and WJ-MSCs. Flow cytometry was applied to evaluate surface phenotypes and proliferation capacity. Early (7 days) and late (21 days) chondrogenic potentials were assessed using histology, immunohistochemistry, and real-time polymerase chain reaction (PCR). Western blot analysis was applied to evaluate the potential involvement of MAPK and Wnts signals during the proliferation and chondrogenic processes. Cells were further evaluated for their osteogenic potential using alkaline phosphatase staining and RT-PCR and adipogenic potential using oil red O staining and RT-PCR. Compared to PL expanded cells, dECMs yielded expanded cells with better proliferation capacity as well as decreased percentage of HLA-DR positive SDSCs. Meanwhile, a decrease in CD105 median fluorescence intensity of WJ-MSCs groups were observed compared to the corresponding SDSCs groups. Moreover, both SDSCs and WJ-MSCs acquired better chondrogenic potential after dECM treatment, as evidenced by increased pellet sizes and increased expression of chondrogenic marker genes. WJ-MSCs dECM was inferior to SDSCs dECM in enhancing early stage chondrogenic differentiation, which was compensated during late stage chondrogenesis, despite causing an increased type X collagen accumulation. p-JNK and p-38 were implicated in the expansion and late chondrogenic differentiation stages, respectively. However, dECM preconditioning did not enhance either osteogenic or adipogenic potential of SDSCs and WJ-MSCs. WJ-MSCs dECM is superior to SDSCs dECM on enhancing proliferation, lowering immunogenicity and promoting late stage chondrogenesis.
Keywords: Decellularized extracellular matrix, synovium-derived mesenchymal stem cells, Wharton’s jelly-derived mesenchymal stem cells, monolayer expansion, chondrogenesis
Introduction
Trauma and degenerative diseases including osteoarthritis often result in cartilage defects. Pluripotent mesenchymal stem cells (MSCs)-based tissue engineering is an appealing method for cartilage restoration [1-3]. Synovium-derived stem cells (SDSCs) are well-recognized tissue-specific stem cells for chondrogenesis [4,5]. However, like other adult MSCs, SDSCs tend to inevitably enter senescence with decreased proliferation and chondrogenic potential after in vitro expansion [6]. Umbilical cord (UC) connects placenta and fetus during pregnancy, and is usually discarded after delivery [7]. Wharton’s jelly (WJ) is the embryonic mucous connective tissue between amniotic epithelium and umbilical vessels, and MSCs derived from WJ (WJ-MSCs) are highly purified and with low immunogenicity [8]. More interestingly, WJ-MSCs are regarded as “true” stem cells, as they have better proliferation capacity than adult MSCs even after long-term in vitro culturing [8-10]. However, the chondrogenic potential of WJ-MSCs remains controversial. WJ-MSCs express chondrocyte marker genes including Sox9 and COL2A1, and several studies have demonstrated the successful chondrogenic induction of WJ-MSCs in 3D cultures [1,11,12]. However, according to Nekanti et al., WJ-MSCs are inclined to undergo endodermal and neuronal differentiation due to the expression of some ectodermal lineage or neuronal developmental transcripts, including NESTIN, GFAP, and SEMA3A [8].
Extracellular matrix (ECM) is a critical component of cell in vivo “niches” and it functions as a pool of growth factors that guide cell remodeling [13,14]. Previously, SDSCs decellularized ECM (dECM) was reported to improve the chondrogenic potential of both SDSCs and adipose derived stem cells (ADSCs) compared with those cultured on plastic flasks (PL) [15,16]. It raises the possibility that dECM deposited by SDSCs may guide the seeded MSCs towards chondrogenic differentiation. Moreover, fetal SDSCs-deposited dECM is superior to adult SDSCs in terms of enhancing the proliferation and chondrogenic capacity of MSCs [17]. Considering the fact that WJ-MSCs share some characteristics with fetal MSCs, WJ-MSCs may generate dECM that will have better rejuvenation effect on the proliferation and chondrogenesis of the expanded MSCs.
Mitogen-activated protein kinase (MAPK) signaling cascade, which includes extracellular regulated protein kinases (ERK), c-Jun N-terminal kinase (JNK) and p38, regulate several physiological processes including cell migration, expansion and differentiation [17,18]. Wnts are highly conservative proteins that participate in embryonic development and homeostatic mechanisms in adult tissues [19]. Wingless/Int (Wnt) have been shown to participate in chondrogenic differentiation in both embryos and adult progenitor cells [20,21]. To date, whether MAPK and Wnt pathways are involved in the expansion and chondrogenic differentiation of dECM-expanded SDSCs and WJ-MSCs is not conclusive.
Collectively, this study aims to determine whether SDSCs dECM can guide expanded MSCs for better chondrogenic differentiation, whether WJ-MSCs dECM can provide the expanded MSCs with better proliferation ability, and explore the potential involvement of MAPKs during these processes.
Materials and methods
Cell acquisition
This study was conducted according to the guidelines approved by the Ethics Committee of Zhongshan Hospital, Fudan University, and the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Synovium were obtained from four donors [two men (41 and 52 years old) and two women (42 and 46 years old), averaged 45 years old] with primary knee osteoarthritis whom underwent total knee arthroplasty. Informed consent was obtained from all participants included in the study. The synovium was sequentially digested with 0.1% collagenase II (Sigma-Aldrich, St. Louis, MO, USA) and trypsin-EDTA (0.25% trypsin, 0.4 mM EDTA, Gibco, Carlsbad, CA, USA) for 2 h and 0.5 h at 37°C, respectively. Digested cells were filtered through a 70-μm nylon filter (Becton Dickinson, Franklin Lakes, NJ, USA) and centrifuged at 400 g for 5 min to obtain a cell pellet. The pellet was then resuspended and seeded in culture flasks of alpha minimum essential medium (αMEM, Gibco) containing 10% fetal bovine serum (FBS, Gibco), 100 U/mL penicillin, 100 U/mL streptpmycin (Invitrogen, Carlsbad, CA). These SDSCs were defined as passage 0 and when the attached cells reached 90% confluency, they were passaged at a dilution rate of 1:4 for subculture. Passage 1 human WJ-MSCs were obtained from ScienCellTM Research Laboratories (Carlsbad, CA, Catalog#7530) and cultured in Dulbecco’s modified eagle medium: nutrient mixture F-12 (DMEM/F12, Gibco) containing 10% FBS (Gibco), 100 U/mL penicillin, 100 U/mL streptpmycin (Invitrogen). Both of the cells were cultured at 37°C in a humidified 5% CO2 and 21% O2 incubator. The medium was changed every three days.
dECMs preparation and in vitro expansion of SDSCs and WJ-MSCs
Plastic flasks were pre-coated with 0.2% gelatin (Sigma, St. Louis, MO) for 1 h at 37°C before being seeded with passage 5 SDSCs and WJ-MSCs at 6000 cells/cm2. When the cells reached 90% confluency, 250 μM of L-ascorbic acid phosphate (Sigma-Aldrich) was added for 7 days. dECMs were prepared with 0.5% Triton X-100 (Sigma-Aldrich) containing 20 mM ammonium hydroxide for 5 min at 37°C. After cell removal, dECMs were stored at 4°C in phosphate buffered solution (PBS) containing 100 U/mL penicillin, 100 μg/mL streptomycin and 0.25 μg/mL fungizone until use.
Passage 10 SDSCs and WJ-MSCs were seeded on plastic flasks (PL), SDSCs and WJ-MSCs dECMs at 2000 cells/cm2, referred to as SDonPL, SDonSD, SDonWJ, WJonPL, WJonSD and WJonWJ groups (Table 1; Figure 1A). The medium was changed every three days.
Table 1.
Grouping
| Cells | SD | SD | SD | WJ | WJ | WJ |
|---|---|---|---|---|---|---|
| Expanded substrate | PL | SD-deposited dECM | WJ-deposited dECM | PL | SD-deposited dECM | WJ-deposited dECM |
| Abbreviation | SDonPL | SDonSD | SDonWJ | WJonPL | WJonSD | WJonWJ |
SD: synovium-derived mesenchymal stem cells, WJ: Wharton’s Jelly-derived mesenchymal stem cells, PL: plastic flask.
Figure 1.
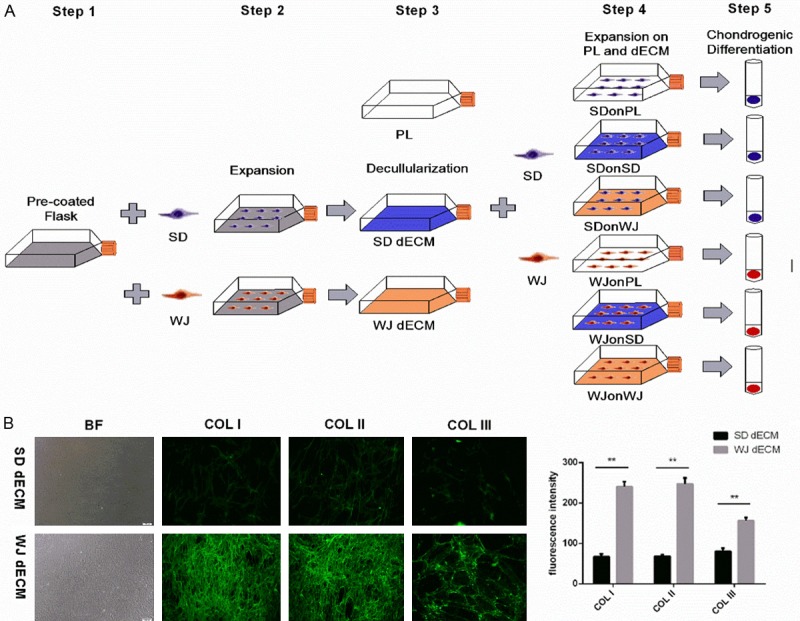
Decellularized cell matrix (dECM) fabrication and cell expansion and physiochemical properties of dECMs. A. Flow diagram of dECM fabrication and cell expansion. Step 1. surface preparation with 0.2% gelatin. Step 2. synovium-derived stem cell (SDSCs) and Wharton’s jelly-derived stem cell (WJ-MSCs) culture. Step 3. dECM fabrication using SDSCs and WJ-MSCs. Step 4. cell seed and harvest after dECM expansion. Step 5. chondrogenic differentiation. SD: SDSCs, WJ: WJ-MSCs. B. Representative dECMs appearance and immunofluorescence staining for COL I, COL II and COL III in dECMs deposited by SDSCs and WJ-MSCs. Magnification: 100×. ImageJ software was used to quantify the immunofluorescence intensity. The experiments were conducted in triplicate. **P < 0.05.
Immunofluorescence staining of dECMs
After decellularization, dECMs were fixed in 4% paraformaldehyde (Yeasen, China), blocked in 1% bovine serum albumin (BSA) and incubated with primary antibodies against type I collagen, (ab34710, 1:100, Abcam, Cambridge, UK), type II collagen (ab34712, 1:100, Abcam), and type III collagen (ab7778, 1:100, Abcam) overnight at 4°C. After being washed with PBS, dECMs were incubated with a secondary antibody (FITC goat anti-rabbit IgG, Yeasen) for 1 h at room temperature (RT). Fluorescence images were acquired with an Olympus 1X51 microscope (Olympus Corporation, Tokyo, Japan). The experiments were conducted in triplicate and ImageJ software was used to quantify the intensity of immunostaining.
Fluorescein diacetate (FDA) staining and population doubling time (PDT)
Six groups of SDSCs and WJ-MSCs were cultured at 37°C in a 5% humidified CO2 incubator for 7 days with cell viability evaluated at day 1, 3 and 7 using FDA staining (Yeasen). Briefly, after being washed three times with PBS, the cells were incubated with 5 μg/mL FDA solution under dark conditions for 5 min. Afterwards, fluorescence images were captured with an Olympus 1X51 microscope (Olympus). The experiments were performed in triplicate.
For population doubling (PD), all six groups of cells were serially passaged and the initial and final number of cells were counted using the Countess® II cell counter (Invitrogen). The cell PD and PDT were calculated using the following formula [22]:
PD = [log (Nf/NO)]/log2, PDT = duration of culture/PD.
Where Nf is the final number of cells, and NO is the initial number of cells seeded. The experiments were conducted in triplicate.
Cell surface phenotypes and cell proliferation analysis using flow cytometry
The following primary antibodies were used in flow cytometry for the evaluation of surface phenotypes: eFluor 506-conjugated CD45 (eBioscience), eFluor 506-conjugated HLA-DR (eBioscience), fluorescein isothyocyanate-conjugated (FITC)-conjugated CD73 (biolegend), FITC-conjugated CD90 (biolegend), allophcocyanin (APC)-CD105 (eBioscience). 0.3×106 of expanded cells from each group were incubated in 0.1% ChromPure Human IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) at 4°C and 1% NaN3 (Sigma-Aldrich) for 30 min, before incubation with primary antibodies for another 30 min under dark conditions for flow cytometry detection.
Evaluation of cell proliferation was done with the Click-iT 5-ethynyl-2’-deoxyuridine (Edu) cell proliferation assay (Invitrogen). SDSCs and WJ-MSCs (passage 10) were seeded at a density of 3000 cells/cm2 in T75 plastic flasks. When cells reached 50% confluency, Edu at a final concentration of 10 μM was added and cells were incubated at 37°C for 18 h before fixation with 4% paraformaldehyde. Afterwards, samples were incubated with Click-iT reaction cocktail for 30 min at RT for flow cytometry detection. The experiments were conducted in triplicate and fluorescence was analyzed by FACS Calibur system (BD Biosciences) and FCS Express software package (De Novo Software, Glendale, CA).
Chondrogenic differentiation
For chondrogenic differentiation, aliquots of 0.3×106 SDSCs and WJ-MSCs were centrifuged at 500 g for 5 min in a 15-ml polypropylene tube to form a pellet. After overnight incubation at 37°C in a humidified 5% CO2, the pellets were cultured in serum-free chondrogenic medium (Cyagen Biosciences, Santa Clara, CA, USA) with 10 ng/mL recombinant human transforming growth factor beta 1 (rhTGF-β1, PeproTech) to induce chondrogenesis. Pellets were collected at specific time points (day 7 and day 21) and evaluated using histology, immunostaining, biochemical analysis, and real-time polymerase chain reaction (real-time PCR). The primer sequences used are listed (Table 2).
Table 2.
Primer list
| Genes | Forward primer sequence (5’ to 3’) | Reverse primer sequence (5’ to 3’) |
|---|---|---|
| β-actin | CCCCAAGGCCAACCGCGAGAAGATG | AGGTCCCGGCCAGCCAGGTCCAG |
| SOX9 | TATGACTGGACCCTGGTG | TGTGGCTTGTTCTTGCTGG |
| COL2A1 | CAACACTGCCAAACGTCCAGAT | CTGCTTCGTCCAGATAGGCAAT |
| ACAN | GCAGAGACGCATCTAGAAATTG | GGTAATTGCAGGGAACATCATT |
| RUNX2 | ATTCCTGTAGATCCGAGCACC | GCTCACGTCGCTCATTTTGC |
| ALP | ATGGGATGGGTGTCTCCACA | CCACGAAGGGGAACTTGTC |
| PPARγ | CCTATTGACCCAGAAAGCGATT | CATTACGGAGAGATCCACGGA |
| LPL | TCATTCCCGGAGTAGCAGAGT | GGCCACAAGTTTTGGCACC |
Histology, immunohistochemistry and immunofluorescence staining
Three representative pellets from each group were fixed in 4% paraformaldehyde before being dehydrated in a gradient ethanol series and cleared with xylene. After being embedded in paraffin blocks, pellets were cut into 5-μm-thick sections. To detect the expression of sulfated glycosaminoglycan (sGAG), sections were stained with Alcian blue (Sigma-Aldrich) for 30 min at RT before being counterstained with fast red. ImageJ software was used to quantify the intensity of immunostaining.
For immunohistochemical analysis, sections were incubated with primary antibodies against type II collagen (ab34712, 1:100, Abcam) overnight at 4°C. Staining was achieved with the application of an immunohistochemistry kit (Yeasen). As a control experiment, an identical immunohistochemical procedure was performed with omission of the primary antibody. For immunofluorescence staining, antibodies against type I collagen (ab34710, 1:100, Abcam) and type X collagen (ab58632, 1:100, Abcam) were used as the primary antibody. After incubation with primary antibodies overnight at 4°C, the sections were incubated with goat anti-rabbit Alexa Fluor 488 (1:200, Molecular Probes, USA) in PBS for 1 h at RT. 4,6-Diamino-2-phenylindole (DAPI; Molecular Probes) was used as a nuclear marker. ImageJ software was used to quantify the intensity of immunostaining.
Western blot
Cells were lysed in ice-cold cell lysis buffer (Beyotime, Nantong, China) with protease inhibitors (Thermo Fisher) and then proteins were separated in 10% polyacrylamide gel (Beyotime) and were transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, USA) by electrophoresis for 2 h at 310 mA. These membranes were incubated overnight at 4°C with the following primary antibodies: anti-phospho-ERK (ab201015, 1:1000, Abcam, Cambridge, UK), anti-ERK (ab184699, 1:10000, Abcam), anti-phospho-JNK (ab124956, 1:1000, Abcam), anti-JNK (ab179461, 1:1000, Abcam), anti-phospho-p38 (ab178867, 1:1000, Abcam), anti-p38 (ab170099, 1:1000, Abcam), anti-Wnt3a (ab219412), anti-Wnt5a (ab179824) and anti-β-actin (ab8226, 1:2500, Abcam). Membranes were then incubated for 1 h with HRP-conjugated secondary antibody (1:5000, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and protein bands were visualized using enhanced chemiluminescence method (Pierce, IL). The experiments were performed in triplicate and ImageJ software was used to quantify the intensity of western bands.
Total RNA extraction and real-time PCR
The expression of chondrogenic marker genes (Sox9, ACAN and COL2A1) and hypertrophic marker genes (Runx2, ALP and MMP13) during early (day 7) and late (day 21) chondrogenic differentiation were evaluated using real-time PCR. Total RNA was extracted using TRIzol® reagent (Invitrogen) and was reverse-transcribed using PrimeScript RT Reagent Kit (Takara, Otsu, Japan). Real-time PCR reaction was conducted in a final volume of 20 μL containing 10 μL of HieffTM qPCR SYBR® Green Master Mix (YEASEN) and 2 μL of DNA. PCR amplification was performed using a 7500 real-time PCR System (Applied Biosystems) based on the manufacturer’s instructions. β-actin served as the housekeeping gene. The primers sequences used are listed in Table 2. The experiments were performed in triplicate.
Osteogenic differentiation
Once the cells reached 90% confluency, the culture medium was switched to the osteogenic differentiation medium (Cyagen Biosciences) for 21 days before being assessed with an alkaline phosphatese (ALP) stain kit (Cyagen Biosciences) according to the manufacturer’s instructions. Real-time PCR was used to quantify the expression of ALP, the osteogenic marker gene. The primer sequences used are listed (Table 2). The experiments were conducted in triplicate.
Adipogenic differentiation
Once the cells reached 90% confluency, the culture medium was switched to the adipogenic induction medium (Cyagen Biosciences) for 21 days before being assessed with an Oil Red O (ORO) stain kit (Cyagen Biosciences) according to the manufacturer’s instructions. Real-time RT-PCR was used to quantify the expression of LPL and PPARγ, the adipogenic marker genes. The primer sequences used are listed (Table 2). The experiments were conducted in triplicate.
Statistical analysis
Statistical analysis was performed using SPSS 20.0 software (IBM, USA). All data were presented as the mean ± standard deviation (SD) and differences between groups were estimated using Student’s t-test or one-way ANOVA. A P value < 0.05 was considered statistically significant. ImageJ software was used to quantify the intensity of immunostaining and western bands.
Results
Characteristics of SDSCs and WJ-MSCs dECMs
The structure of dECMs deposited by both SDSCs and WJ-MSCs were both network-like, but the formation of WJ-MSCs dECM were more thick and compact. The fluorescence intensity of all three matrix proteins including type I collagen, type II collagen, and type III collagen were much stronger in WJ-MSCs dECM compared to those in SDSCs group (P < 0.05) (Figure 1B).
Proliferation of SDSCs and WJ-MSCs on dECMs
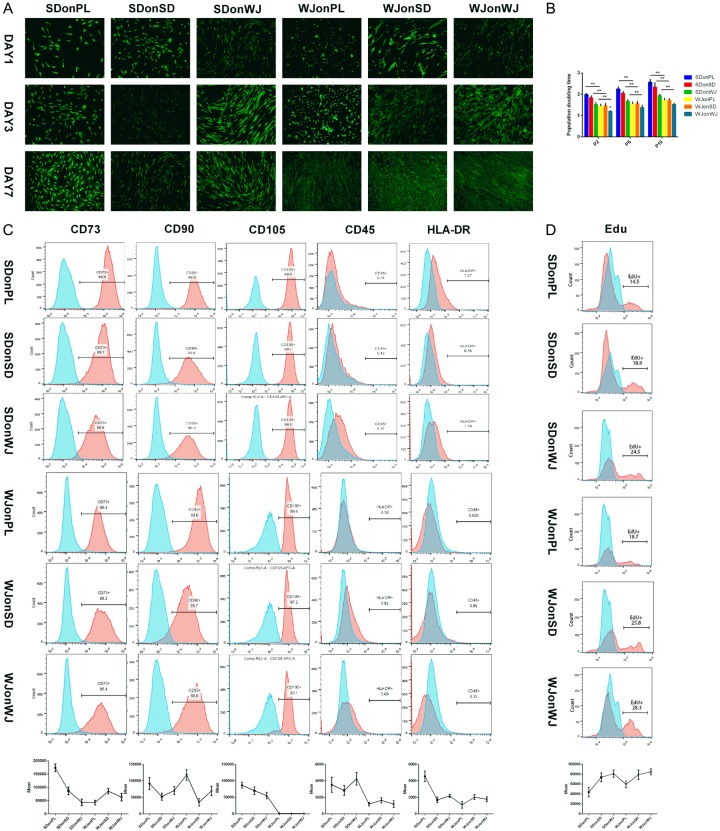
FDA staining showed remarkably increased viability, as well as more spindly shaped SDSCs and WJ-MSCs after dECM expansion (Figure 2A). A similar increase was observed in PDT evaluation and WJ-MSCs expanded on any substrate yielded less PDT than the corresponding SDSCs at passage 2, 6 and 10 (P < 0.05) (Figure 2B). These proliferation discrepancies were also found in Edu analysis which demonstrating the percentage of S phase-cells in cell cycle. PL expansion yielded 14.5% SDSCs and 18.7% WJ-MSCs at S phase while SDSCs dECM expansion increase the percentage to 18.9% and 24.5% respectively and WJ-MSCs dECM expansion further increase them to 25.8% and 28.3% respectively (Figure 2D).
Figure 2.
Cell viability, surface phenotypes and proliferation capacity of SDSCs and WJ-MSCs expanded on plastic flask (PL) and dECM. A. The viability of SDSCs and WJ-MSCs expanded on PL and dECM-deposited by SDSCs and WJ-MSCs at day 1, 3 and 7 were evaluated using FDA stain. Magnification: 100×. B. Population doubling time of SDSCs and WJ-MSCs (passage 2, 6 and 10) expanded on plastic flasks and dECM-deposited by SDSCs and WJ-MSCs were calculated. The experiments were conducted in triplicate. *P < 0.05 compare with other groups. C. Flow cytometry was used to measure the percentage of surface marker (CD73, CD90, CD105, CD45 and HLA-DR) of the expanded SDSCs and WJ-MSCs (passage 10) when cells reached 70% confluency. The experiments were conducted in triplicate and mean fluorescence intensity were demonstrated. D. Click-iT 5-ethynyl-2’-deoxyuridine (Edu) cell proliferation assay was used to detect the percentage of S phase-SDSCs and WJ-MSCs expanded on PL and dECM when cells reached 50% confluency. The experiments were conducted in triplicate.
SDSCs and WJ-MSCs surface phenotypes
All cells were CD45 negative, CD73 and CD90 positive. Additionally, HLA-DR percentage in SDSCs experienced a significant decrease after dECM expansion (0.76% for SDSC dECM and 1.18% for WJ-MSCs dECM) compared with PL expansion (7.27%), most of which were still relatively higher than WJ-MSCs expanded on any substance (0.10%, 0.92% and 0.69%). Meanwhile, dECM expansion yielded WJ-MSCs with a significant decrease in CD105 median fluorescence intensity of all WJ-MSCs groups compared to the correspodnding SDSCs groups (991.33 vs 86737.33, 1021.00 vs 70247.67, 961.00 vs 55446.00). Meanwhile, there was a decreased percentage (97.20% and 93.10%) of CD105-positive cells compared with PL expansion (99.80%), while it almost had no influence on SDSCs (99.70% and 99.50% vs 99.90%) (Figure 2C).
Chondrogenic differentiation of SDSCs and WJ-MSCs expanded on dECMs
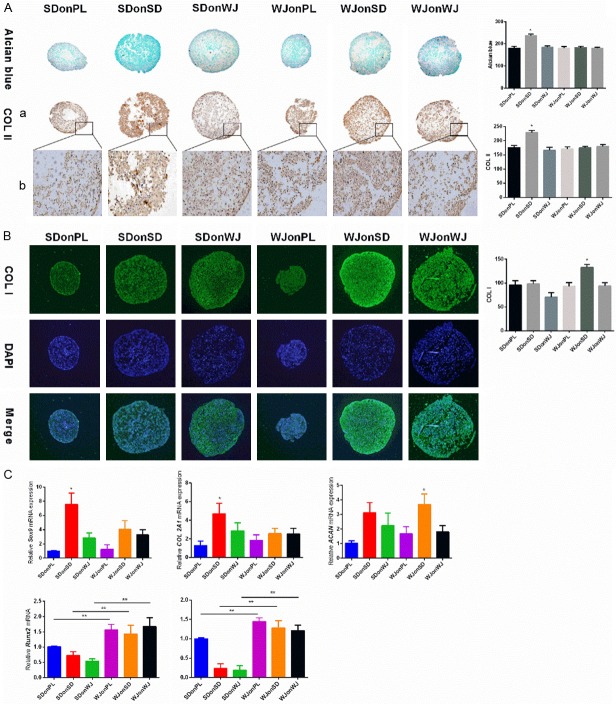
dECM expansion yielded the 7-day pellets with a larger sizes and higher expression of chondrogenic marker genes compared to PL groups. For histology and immunohistological evaluation, SDSCs dECM achieved SDSCs with the most intensive stain for Alcian blue and immunostain for type II collagen (P < 0.05) (Figure 3A), while pellets from WJonSD group were most intensively stained for type I collagen (Figure 3B). Consistent with the above data, RT-PCR results demonstrated the highest expression of Sox9 and COL2A1 in SDonSD group (P < 0.05) (Figure 3C). Meanwhile, the expression of hypertrophic genes including Runx2, and MMP13 were significantly higher in WJ-MSCs groups than the corresponding SDSCs groups (P < 0.05) (Figure 3C).
Figure 3.
Evaluation of early (7 days) chondrogenic differentiation. A. Alcian blue and immunostaining were used to detect sulfated glycosaminoglycan (sGAG) and type II collagen deposition of pellets from each group after 7 days of chondrogenic induction and ImageJ software was used to quantify the immunohistological intensity. Magnification: (a) 100× and (b) 200×. The experiments were conducted in triplicate. *P < 0.05 compare with other groups. B. Immunofluorescence staining was used to detect type I collagen deposition after 7 days of chondrogenic induction and 4,6-Diamino-2-phenylindole (DAPI) was used as a nuclear marker. ImageJ software was used to quantify the immunofluorescence intensity. Magnification: 100×. The experiments were conducted in triplicate. *P < 0.05 compare with other groups. C. Real-time polymerase chain reaction (PCR) was used to evaluate chondrogenic marker genes (Sox 9, COL2A1 and ACAN) and hypertrophic marker genes (RUNX2 and MMP13) expression. The experiments were conducted in triplicate. *P < 0.05 compare with other groups, **P < 0.05.
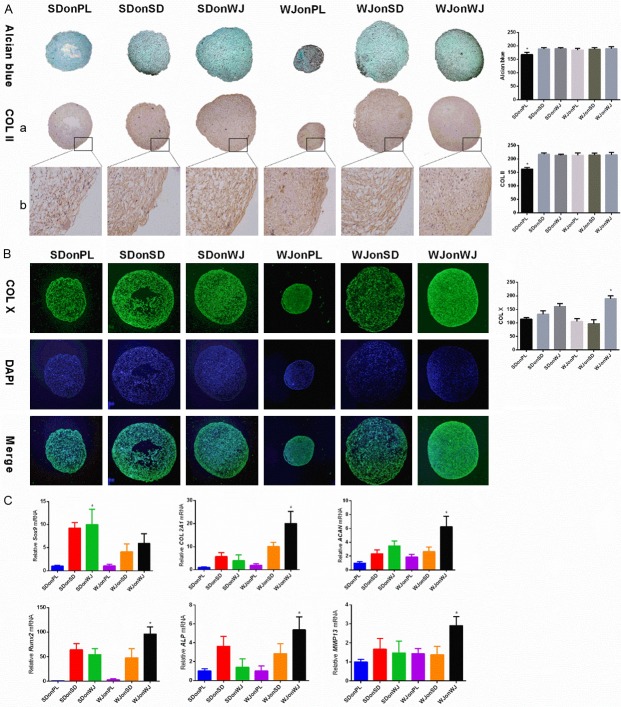
After a 21-day chondrogenic induction, pellets from dECM treated groups maintained their larger sizes and higher expressions of chondrogenic marker genes. While no significant difference was seen for the Alcian blue and immunostain intensity of type II collagen among dECM treated groups, pellets from WJonWJ group exhibited the most intensified type X collagen stain (P < 0.05) (Figure 4A and 4B). Meanwhile, RT-PCR results showed that pellets from WJonWJ group had a higher expression of COL 2A1 and ACAN than other groups, despite the relatively lower expression of Sox9 (P < 0.05) (Figure 4C). Interestingly, the expression of Runx2, ALP and MMP13 of late chondrogenic WJ-MSCs were significantly increased after WJ-MSCs dECM treatment (P < 0.05) (Figure 4C).
Figure 4.
Evaluation of late (21 days) chondrogenic differentiation. A. Alcian blue and immunostaining were used to detect sGAG and type II collagen deposition respectively in pellets from each group after 21 days of chondrogenic induction and ImageJ software was used to quantify the immunohistological intensity. Magnification: (a) 100× and (b) 200×. The experiments were conducted in triplicate. *P < 0.05 compare with other groups. B. Immunofluorescence staining was used to detect type X collagen deposition after 21 days of chondrogenic induction and DAPI was used as a nuclear marker. ImageJ software was used to quantify the immunofluorescence intensity. Magnification: 100×. The experiments were conducted in triplicate. *P < 0.05 compare with other groups. C. Real-time PCR was used to evaluate the chondrogenic marker genes (Sox 9, COL2A1 and ACAN) and hypertrophic marker genes (RUNX2, ALP and MMP13) expression. The experiments were conducted in triplicate. *P < 0.05 compare with other groups.
Osteogenic and adipogenic differentiations of SDSCs and WJ-MSCs expanded on dECMs
After a 21-day incubation in osteogenic induction medium, similar intensification for ALP staining was observed among PL and dECM groups, which was further confirmed by the similar ALP mRNA expression among all groups (Figure 5A and 5B). Similar phenomenon was observed in adipogenic induction groups as all SDSCs and WJ-MSCs displayed similar lipid droplet staining using Oil Red O staining kit (Figure 5C). However, the expression of LPL and PPARγ mRNA demonstrated significant increase in SDSCs groups after dECM treatment (Figure 5D).
Figure 5.
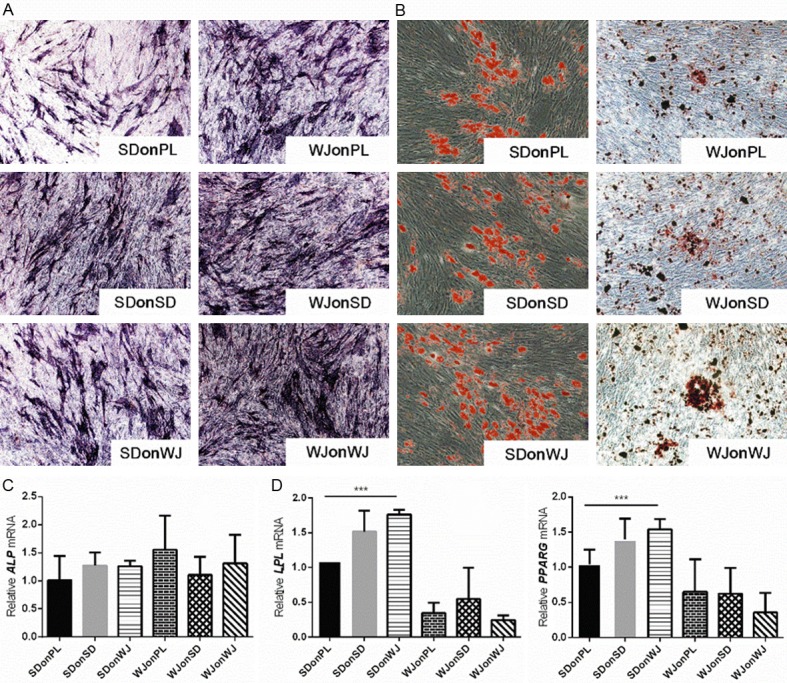
Evaluation of osteogenic and adipogenic differentiation. A. After a 21-day incubation in osteogenic medium, osteogenesis was evaluated using alkaline phosphatase (ALP) staining. Magnification: 100×. The experiments were conducted in triplicate. *P < 0.05. B. After a 21-day incubation in adipogenic medium, adipogenesis was evaluated using Oil Red O (ORO) staining. Magnification: 100×. C. Real-time PCR was used to evaluate the expression of ALP. The experiments were conducted in triplicate. D. Real-time PCR was used to evaluate the expression of adipogenic marker genes (LPL and PPARγ). The experiments were conducted in triplicate. ***P < 0.05 of any two groups.
Involvement of MAPKs and Wnts pathways in dECM-mediated expansion and chondrogenesis
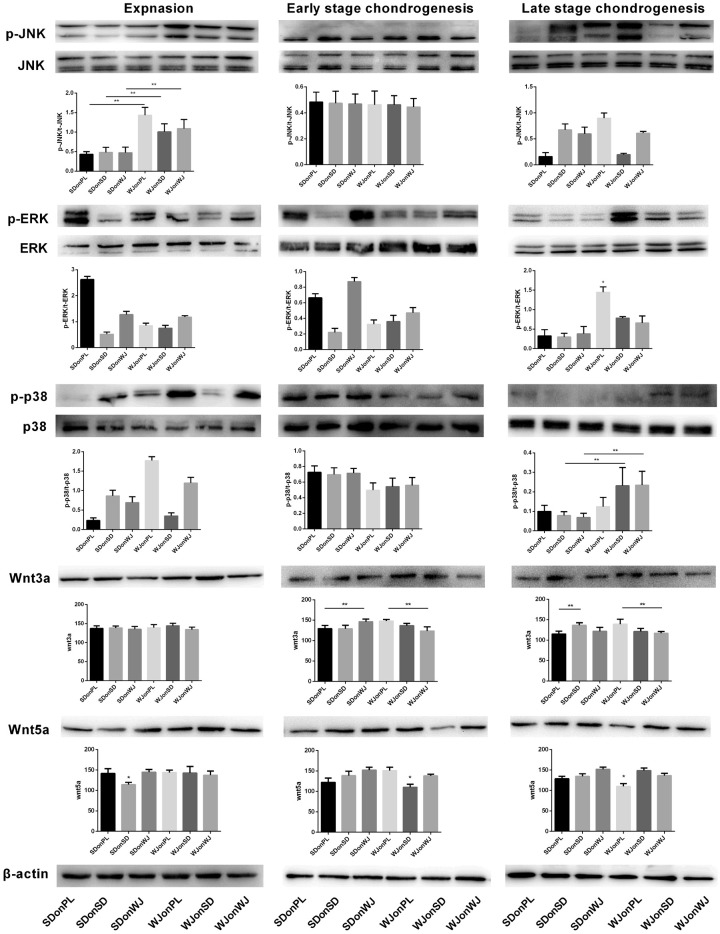
Western blot was used to evaluate the expression of MAPK signaling pathway (ERK, p38 and JNK) and Wnt signaling pathway (Wnt3a and Wnt5a) during expansion and chondrogenic induction of SDSCs and WJ-MSCs. Phosphorylation (p-) JNK and p-p38 were actively involved in expansion and late stage chondrogenesis (Figure 7). ImageJ analysis revealed that during the expansion stage, the intensity of p-JNK was almost doubled in all WJ-MSCs groups compared to SDSCs groups (P < 0.05). Moreover, p-p38 expression yielded a nearly 2-fold increase during late chondrogenesis in WJ-MSCs groups after dECM treatment compared to the corresponding SDSCs groups (P < 0.05) (Figure 6). No significant difference of the expression of t-p38, t-ERK and t-JNK was observed among all groups (Figure 6).
Figure 7.
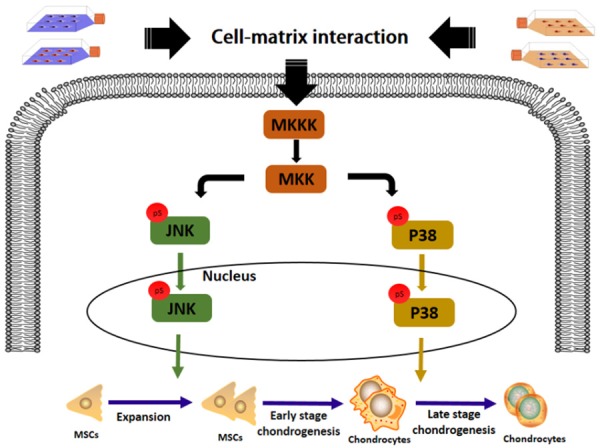
The schematic diagram of the potential involvement of JNK and p38 during the proliferation and late chondrogenic differentiation of SDSCs and WJ-MSCs expanded on PL and dECM.
Figure 6.
Involvement of MAPKs and Wnts in expansion, early (7 days) and late (21 days) chondrogenic differentiation of SDSCs and WJ-MSCs expanded on PL and dECM. Western blot was used to detect the expressions of MAPK signals (JNK, ERK and p38 at both phosphorylation and total protein levels) and Wnts signals (Wnt3a and Wnt5a). p-JNK and p-38 were actively involved in the expansion and late chondrogenesis, respectively. β-actin was used as the internal control and ImageJ software was used to quantify the bands. The experiments were conducted in triplicate. *P < 0.05 compare with other groups. **P < 0.05.
Discussion
Monolayer expansion leads to MSCs senescence and this senescence-related proliferation arrestment hinders the acquisition of large amounts of MSCs for further usage [23]. Compared with embryonic stem cells, adult MSCs, especially those acquired from elder donors, have significantly inferior self-refreshing capacity and increased vulnerability to cell senescence. As fetus tissue-derived cells, WJ-MSCs share some characteristics unique to fetal MSCs, including the greater in vitro expansion capacity than adult MSCs. While bone marrow-derived mesenchymal stem cells (BMSCs) stop proliferation only around 30 total PDs, UC-derived MSCs (UC-MSCs) achieve about 60 to 80 total PDs [9,10]. This phenomenon was confirmed in our study as WJ-MSCs expanded on both PL and dECM yielded more PDs than the corresponding SDSCs at any passages exhibited. This stronger population doubling ability can be attributed to the superior DNA duplication ability as relatively more S phase-WJ-MSCs were found in Edu analysis compared to the corresponding SDSCs. Meanwhile, the onset of cell senescence is known to cause a significant reduction in telomere length [24]. UC-MSC telomere length (54-79 PDs) is longer than that of BMSCs (27-33 PDs) at senescence [24]. Thus, the relatively longer telomere may be another reason for the relatively stronger proliferation activity of WJ-MSCs [9].
Despite the usage of more energetic cells, the optimization of the expansion environment is another strategy to improve the quality and quantity of the expanded cells. dECM was found to be an outstanding expansion system for cell senescence alleviation. dECM cultures enhance the antioxidant properties and proliferation ability of UC-MSCs, ADSCs and SDSCs [16,25,26]. Similarly, our study demonstrated a significant increase in the number of both SDSCs and WJ-MSCs after dECM expansion. This phenomenon is not astonishing as the main function of ECM is to reserve growth factors and provide cues to regulate cell proliferation and differentiation [13]. Surprisingly, a decrease in CD105 positive percentage of WJ-MSCs was observed after dECM expansion. CD105, also known as endoglin, is a marker for neovascularization and is strongly expressed in blood vessel of tumor tissues [27]. Cartilage is avascular tissue and the significant loss of CD105 intensity in WJ-MSCs may indicate the improved chondrogenic potential. Meanwhile, CD105 is also a type I membrane glycoprotein located on the cell surface and is an auxiliary receptor for the TGF-β receptor complex that modulates the response to the binding of TGF-β1 and TGF-β3 [28]. TGF-β1 and TGF-β3 are two isotypes that are generally used for the induction of adult stem cells toward chondrogenic differentiation [29]. However, TGF-β1 inhibits the proliferation of most cell types, such as epithelial and endothelial cells, and the suppression of TGF-β receptor promotes the expansion of human endometrial MSCs [30,31]. Moreover, low dose (0.1 ng/mL) instead of high dose (5, 10 or 20 ng/mL) TGF-β1 was found to enhance the proliferation ability of bone marrow stromal fibroblasts and UC-MSCs [32,33]. In this scenario, the decreased expression of CD105 in WJ-MSCs may be responsible for the increased proliferation ability after dECM expression. Meanwhile, we further found that WJ-MSCs dECM was superior to that produced by SDSCs in terms of enhancing proliferation. In accordance with our finding, Pei et al. revealed that fetal SDSCs-generated dECM is better than adult SDSCs on promoting proliferation and chondrogenic differentiation [17]. Moreover, Choi et al. found that ECM generation by younger cells recharges senescent human diploid fibroblasts to a more energetic status [34]. According to the proteomics analysis of both adult and fetal dECM, fetal dECM has more matrix components that favoring cell expansion, while adult dECM is more inclined to play a role during differentiation [17]. In addition, the mechanically more compact construction of WJ-MSCs dECM may delay the diffusion and elevate the local concentration of beneficial factors that facilitate the signal process [35].
Immunological rejection is another problem after MSC transplantation. HLA-DR is a well-acknowledged marker for graft rejection and other adverse immunological problems. In this study, HLA-DR positive WJ-MSCs remained under 1% regardless of expansion substrates, which is in accordance with its low immunogenicity characteristics [36]. By contrast, a more than 7-fold increase in HLA-DR positive SDSCs were observed for PL culture. To further confirm this result, the expression of CD45 was also examined in order to rule out the possible contamination of immune cells, which may cause false HLA-DR+ expression. Less than 1% of CD45 positive cells were present in all groups, which further verified the fact that there was a significant population of HLA-DR+ SDSCs when expanded on PL. This phenomenon could be bothersome for further clinical usage due to their potential to cause T-cell activation [37]. Surprisingly, this undesired high HLA-DR expression was successfully reversed by dECM preconditioning, which lower the risk of potential immunological rejection for SDSCs after transplantation.
Cartilage matrix is composed of a network of specific molecules that give the tissue its functional characteristics and its ability to resist mechanical stress [38]. The matrix synthesis of chondrogenic pellets was evaluated at both transcriptional and protein levels. Similar to the proliferation results, dECM expansion yielded both SDSCs and WJ-MSCs with increased matrix deposition as evidenced by the obviously larger pellet sizes, as well as the higher chondrogenic genes expression after both 7 and 21 days of chondrogenic induction. Alcian blue stain demonstrated that SDSC dECM achieved SDSCs with more sGAG deposition than other groups after 7 days chondrogenesis. This increase can be attributed to the significantly elevated expression of Sox9, which is the critical transcription factor for the production of cartilage-specific protein including ACAN and type II collagen [39]. Meanwhile, the better chondrogenesis of SDonSD group further confirms the previous conclusion that SDSCs have better chondrogenic potential, and SDSCs dECM can direct expanded stem cells to chondrogenesis [25,40,41]. Meanwhile, after 21 days of chondrogenic induction, there was no significant difference on sGAG deposition among pellets in all dECM-treated groups. This phenomenon is not surprising, as WJ-MSCs are known to be abundant in sGAG and its amount can be further increased by chondrogenic induction [12]. Meanwhile, the expression of cartilage specific genes including Sox9 and COL2A1 were detected in WJ-MSCs, which may further contribute to the matrix deposition [12]. For collagen synthesis, SDSCs dECM achieved SDSCs with more type II collagen deposition during early stage chondrogenesis. Type II collagen is the leading type of collagen in physiological hyaline cartilage and its increase results in the enhanced tensile strength of cartilage. The differentiation profile of WJ-MSCs tend to be composed of a larger amount of type I collagen and a smaller amount of type II collagen [42]. Type I collagen is encoded by COL1a1 gene, the over-expression of which is associated with the formation of fibrocartilage instead of hyaline cartilage [43]. However, WJ-MSCs dECM treatment seemed to change this undesired profile as it enhanced type II collagen synthesis. This phenomenon is explicable as WJ-MSCs are known to be abundant in hyaluronic acid (HA), which is a powerful biomechanical factor that enhancing the chondrogenesis and matrix deposition of MSCs, including increasing type II collagen synthesis [44-46]. Meanwhile, there was an undesirable increase in type X collagen in WJonWJ group after 21 days of chondrogenic culture. Type X collagen is a typical feature of hypertrophic chondrocytes during in vitro chondrogenesis and it suggests an initiation of a less favorable transient differentiation [47]. So the increased type X in WJonWJ group is indicative of a tendency of hypertrophic differentiation. Interestingly, SDSCs expanded on dECM were reported to have the similar phenomenon [25]. However, when transplanting MSCs into the cartilage defect of minipigs, an ideal type II collagen-positive type X collagen negative chondrocyte is achieved [47]. In addition, the application of hydrostatic pressure induces a reduction in type X collagen, while maintaining its pro-chondrogenic ability [48]. The joint microenvironment consists of a complex interaction of different biochemical and mechanical factors, thus further in vivo studies are needed to provide more evidence for chondrogenic hypertrophy prevention.
Regarding the potential mechanisms, significant phosphorylation of JNK was observed in WJ-MSCs during expansion, which may contribute to its relatively stronger proliferation ability [49]. p38 has been reported to be negatively associated with chondrogenesis, as p38 inhibitor (sb203580) preconditioning recharges dECM expanded SDSCs chondrogenesis in an inflammatory environment [50]. Moreover, p38 inhibition leads to the inhibition of type X collagen expression and the reversion back to a prehypertrophic phenotype during early stage hypertrophic chondrocyte [18]. Hence, the up-regulation of p38 level in WJ-MSCs after dECM pretreatment is indicative of the increased chondrogenic hypertrophy, the inhibition of which should be considered to improve the quality of cartilage.
In summary, SDSCs and WJ-MSCs dECMs are promising expansion system for enhancing the proliferation and chondrogenic differentiation potentials of the expanded stem cells. WJ-MSCs-deposited dECM is superior to dECM produced by SDSCs on enhancing proliferation and lowering immunogenicity, but is inferior to SDSCs upon early chondrogenic differentiation, which is compensated during late stage chondrogenesis. JNK and p38 signals are actively involved in these processes. WJ-MSCs dECM can be a promising expansion system to recharge stem cells for cartilage regeneration and transplantation.
Acknowledgements
This work is funded by the National Natural Science Foundation of China (No. 81672157).
Disclosure of conflict of interest
None.
References
- 1.Reppel L, Schiavi J, Charif N, Leger L, Yu H, Pinzano A, Henrionnet C, Stoltz JF, Bensoussan D, Huselstein C. Chondrogenic induction of mesenchymal stromal/stem cells from Wharton’s jelly embedded in alginate hydrogel and without added growth factor: an alternative stem cell source for cartilage tissue engineering. Stem Cell Res Ther. 2015;6:260. doi: 10.1186/s13287-015-0263-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redman SN, Oldfield SF, Archer CW. Current strategies for articular cartilage repair. Eur Cell Mater. 2005;9:23–32. doi: 10.22203/ecm.v009a04. discussion 23-32. [DOI] [PubMed] [Google Scholar]
- 3.Kuo CK, Li WJ, Mauck RL, Tuan RS. Cartilage tissue engineering: its potential and uses. Curr Opin Rheumatol. 2006;18:64–73. doi: 10.1097/01.bor.0000198005.88568.df. [DOI] [PubMed] [Google Scholar]
- 4.Jones BA, Pei M. Synovium-derived stem cells: a tissue-specific stem cell for cartilage engineering and regeneration. Tissue Eng Part B Rev. 2012;18:301–311. doi: 10.1089/ten.TEB.2012.0002. [DOI] [PubMed] [Google Scholar]
- 5.Pizzute T, Lynch K, Pei M. Impact of tissue-specific stem cells on lineage-specific differentiation: a focus on the musculoskeletal system. Stem Cell Rev. 2015;11:119–132. doi: 10.1007/s12015-014-9546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Pei M. Cell senescence: a challenge in cartilage engineering and regeneration. Tissue Eng Part B Rev. 2012;18:270–287. doi: 10.1089/ten.TEB.2011.0583. [DOI] [PubMed] [Google Scholar]
- 7.Benirschke K, Kaufmann P. Pathology of the human placenta. New York: Springer-Verlag; 1995. [Google Scholar]
- 8.Nekanti U, Rao VB, Bahirvani AG, Jan M, Totey S, Ta M. Long-term expansion and pluripotent marker array analysis of Wharton’s jelly-derived mesenchymal stem cells. Stem Cells Dev. 2010;19:117–130. doi: 10.1089/scd.2009.0177. [DOI] [PubMed] [Google Scholar]
- 9.Guillot PV, Gotherstrom C, Chan J, Kurata H, Fisk NM. Human first-trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC. Stem Cells. 2007;25:646–654. doi: 10.1634/stemcells.2006-0208. [DOI] [PubMed] [Google Scholar]
- 10.Troyer DL, Weiss ML. Wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells. 2008;26:591–599. doi: 10.1634/stemcells.2007-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nirmal RS, Nair PD. Significance of soluble growth factors in the chondrogenic response of human umbilical cord matrix stem cells in a porous three dimensional scaffold. Eur Cell Mater. 2013;26:234–251. doi: 10.22203/ecm.v026a17. [DOI] [PubMed] [Google Scholar]
- 12.Liu S, Hou KD, Yuan M, Peng J, Zhang L, Sui X, Zhao B, Xu W, Wang A, Lu S, Guo Q. Characteristics of mesenchymal stem cells derived from Wharton’s jelly of human umbilical cord and for fabrication of non-scaffold tissue-engineered cartilage. J Biosci Bioeng. 2014;117:229–235. doi: 10.1016/j.jbiosc.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Brown B, Lindberg K, Reing J, Stolz DB, Badylak SF. The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng. 2006;12:519–526. doi: 10.1089/ten.2006.12.519. [DOI] [PubMed] [Google Scholar]
- 14.Bhrany AD, Beckstead BL, Lang TC, Farwell DG, Giachelli CM, Ratner BD. Development of an esophagus acellular matrix tissue scaffold. Tissue Eng. 2006;12:319–330. doi: 10.1089/ten.2006.12.319. [DOI] [PubMed] [Google Scholar]
- 15.He F, Chen X, Pei M. Reconstruction of an in vitro tissue-specific microenvironment to rejuvenate synovium-derived stem cells for cartilage tissue engineering. Tissue Eng Part A. 2009;15:3809–3821. doi: 10.1089/ten.TEA.2009.0188. [DOI] [PubMed] [Google Scholar]
- 16.He F, Pei M. Extracellular matrix enhances differentiation of adipose stem cells from infrapatellar fat pad toward chondrogenesis. J Tissue Eng Regen Med. 2013;7:73–84. doi: 10.1002/term.505. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Hansen KC, Zhang Y, Dong C, Dinu CZ, Dzieciatkowska M, Pei M. Rejuvenation of chondrogenic potential in a young stem cell microenvironment. Biomaterials. 2014;35:642–653. doi: 10.1016/j.biomaterials.2013.09.099. [DOI] [PubMed] [Google Scholar]
- 18.Mwale F, Yao G, Ouellet JA, Petit A, Antoniou J. Effect of parathyroid hormone on type X and type II collagen expression in mesenchymal stem cells from osteoarthritic patients. Tissue Eng Part A. 2010;16:3449–3455. doi: 10.1089/ten.TEA.2010.0091. [DOI] [PubMed] [Google Scholar]
- 19.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8:727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Hartmann C. A Wnt canon orchestrating osteoblastogenesis. Trends Cell Biol. 2006;16:151–158. doi: 10.1016/j.tcb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Greenwood SK, Hill RB, Sun JT, Armstrong MJ, Johnson TE, Gara JP, Galloway SM. Population doubling: a simple and more accurate estimation of cell growth suppression in the in vitro assay for chromosomal aberrations that reduced irrelevant positive results (vol 43, pg 36, 2004) Environmental And Molecular Mutagenesis. 2004;44:90–90. doi: 10.1002/em.10207. [DOI] [PubMed] [Google Scholar]
- 23.Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V, Ho AD. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One. 2008;3:e2213. doi: 10.1371/journal.pone.0002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vidal MA, Walker NJ, Napoli E, Borjesson DL. Evaluation of senescence in mesenchymal stem cells isolated from equine bone marrow, adipose tissue, and umbilical cord tissue. Stem Cells Dev. 2012;21:273–283. doi: 10.1089/scd.2010.0589. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Li J, Davis ME, Pei M. Delineation of in vitro chondrogenesis of human synovial stem cells following preconditioning using decellularized matrix. Acta Biomater. 2015;20:39–50. doi: 10.1016/j.actbio.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Zhou L, Chen X, Liu T, Pan G, Cui W, Li M, Luo ZP, Pei M, Yang H, Gong Y, He F. Culturing on decellularized extracellular matrix enhances antioxidant properties of human umbilical cord-derived mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl. 2016;61:437–448. doi: 10.1016/j.msec.2015.12.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duff SE, Li C, Garland JM, Kumar S. CD105 is important for angiogenesis: evidence and potential applications. FASEB J. 2003;17:984–992. doi: 10.1096/fj.02-0634rev. [DOI] [PubMed] [Google Scholar]
- 28.Guerrero-Esteo M, Sanchez-Elsner T, Letamendia A, Bernabeu C. Extracellular and cytoplasmic domains of endoglin interact with the transforming growth factor-beta receptors I and II. J Biol Chem. 2002;277:29197–29209. doi: 10.1074/jbc.M111991200. [DOI] [PubMed] [Google Scholar]
- 29.Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- 30.Huang SS, Huang JS. TGF-beta control of cell proliferation. J Cell Biochem. 2005;96:447–462. doi: 10.1002/jcb.20558. [DOI] [PubMed] [Google Scholar]
- 31.Gurung S, Werkmeister JA, Gargett CE. Inhibition of transforming growth factor-beta Receptor signaling promotes culture expansion of undifferentiated human endometrial mesenchymal stem/stromal cells. Sci Rep. 2015;5:15042. doi: 10.1038/srep15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li D, Liu Q, Qi L, Dai X, Liu H, Wang Y. Low levels of TGF-beta1 enhance human umbilical cord-derived mesenchymal stem cell fibronectin production and extend survival time in a rat model of lipopolysaccharide-induced acute lung injury. Mol Med Rep. 2016;14:1681–1692. doi: 10.3892/mmr.2016.5416. [DOI] [PubMed] [Google Scholar]
- 33.Locklin RM, Oreffo RO, Triffitt JT. Effects of TGFbeta and bFGF on the differentiation of human bone marrow stromal fibroblasts. Cell Biol Int. 1999;23:185–194. doi: 10.1006/cbir.1998.0338. [DOI] [PubMed] [Google Scholar]
- 34.Choi HR, Cho KA, Kang HT, Lee JB, Kaeberlein M, Suh Y, Chung IK, Park SC. Restoration of senescent human diploid fibroblasts by modulation of the extracellular matrix. Aging Cell. 2011;10:148–157. doi: 10.1111/j.1474-9726.2010.00654.x. [DOI] [PubMed] [Google Scholar]
- 35.Tschumperlin DJ, Dai G, Maly IV, Kikuchi T, Laiho LH, McVittie AK, Haley KJ, Lilly CM, So PT, Lauffenburger DA, Kamm RD, Drazen JM. Mechanotransduction through growth-factor shedding into the extracellular space. Nature. 2004;429:83–86. doi: 10.1038/nature02543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stubbendorff M, Deuse T, Hua X, Phan TT, Bieback K, Atkinson K, Eiermann TH, Velden J, Schroder C, Reichenspurner H, Robbins RC, Volk HD, Schrepfer S. Immunological properties of extraembryonic human mesenchymal stromal cells derived from gestational tissue. Stem Cells Dev. 2013;22:2619–2629. doi: 10.1089/scd.2013.0043. [DOI] [PubMed] [Google Scholar]
- 37.Kraft M, Filsinger S, Kramer KL, Kabelitz D, Hansch GM, Schoels M. Synovial fibroblasts as accessory cells for staphylococcal enterotoxin-mediated T-cell activation. Immunology. 1995;85:461–466. [PMC free article] [PubMed] [Google Scholar]
- 38.Poole AR, Kojima T, Yasuda T, Mwale F, Kobayashi M, Laverty S. Composition and structure of articular cartilage: a template for tissue repair. Clin Orthop Relat Res. 2001;(Suppl):S26–33. doi: 10.1097/00003086-200110001-00004. [DOI] [PubMed] [Google Scholar]
- 39.Studer D, Millan C, Ozturk E, Maniura-Weber K, Zenobi-Wong M. Molecular and biophysical mechanisms regulating hypertrophic differentiation in chondrocytes and mesenchymal stem cells. Eur Cell Mater. 2012;24:118–135. doi: 10.22203/ecm.v024a09. discussion 135. [DOI] [PubMed] [Google Scholar]
- 40.Pei M, He F, Li J, Tidwell JE, Jones AC, McDonough EB. Repair of large animal partial-thickness cartilage defects through intraarticular injection of matrix-rejuvenated synovium-derived stem cells. Tissue Eng Part A. 2013;19:1144–1154. doi: 10.1089/ten.TEA.2012.0351. [DOI] [PubMed] [Google Scholar]
- 41.Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327:449–462. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Tran I, Seshareddy K, Weiss ML, Detamore MS. A comparison of human bone marrow-derived mesenchymal stem cells and human umbilical cord-derived mesenchymal stromal cells for cartilage tissue engineering. Tissue Eng Part A. 2009;15:2259–2266. doi: 10.1089/ten.tea.2008.0393. [DOI] [PubMed] [Google Scholar]
- 43.Diaz-Romero J, Nesic D, Grogan SP, Heini P, Mainil-Varlet P. Immunophenotypic changes of human articular chondrocytes during monolayer culture reflect bona fide dedifferentiation rather than amplification of progenitor cells. J Cell Physiol. 2008;214:75–83. doi: 10.1002/jcp.21161. [DOI] [PubMed] [Google Scholar]
- 44.Amann E, Wolff P, Breel E, van Griensven M, Balmayor ER. Hyaluronic acid facilitates chondrogenesis and matrix deposition of human adipose derived mesenchymal stem cells and human chondrocytes co-cultures. Acta Biomater. 2017;52:130–144. doi: 10.1016/j.actbio.2017.01.064. [DOI] [PubMed] [Google Scholar]
- 45.Yoo HS, Lee EA, Yoon JJ, Park TG. Hyaluronic acid modified biodegradable scaffolds for cartilage tissue engineering. Biomaterials. 2005;26:1925–1933. doi: 10.1016/j.biomaterials.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 46.Wu SC, Chang JK, Wang CK, Wang GJ, Ho ML. Enhancement of chondrogenesis of human adipose derived stem cells in a hyaluronan-enriched microenvironment. Biomaterials. 2010;31:631–640. doi: 10.1016/j.biomaterials.2009.09.089. [DOI] [PubMed] [Google Scholar]
- 47.Steck E, Fischer J, Lorenz H, Gotterbarm T, Jung M, Richter W. Mesenchymal stem cell differentiation in an experimental cartilage defect: restriction of hypertrophy to bone-close neocartilage. Stem Cells Dev. 2009;18:969–978. doi: 10.1089/scd.2008.0213. [DOI] [PubMed] [Google Scholar]
- 48.Freeman FE, Schiavi J, Brennan MA, Owens P, Layrolle P, McNamara LM. (*) Mimicking the biochemical and mechanical extracellular environment of the endochondral ossification process to enhance the in vitro mineralization potential of human mesenchymal stem cells. Tissue Eng Part A. 2017;23:1466–1478. doi: 10.1089/ten.TEA.2017.0052. [DOI] [PubMed] [Google Scholar]
- 49.Cao Y, Xia DS, Qi SR, Du J, Ma P, Wang SL, Fan ZP. Epiregulin can promote proliferation of stem cells from the dental apical papilla via MEK/Erk and JNK signalling pathways. Cell Prolif. 2013;46:447–456. doi: 10.1111/cpr.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y, Pizzute T, Li J, He F, Pei M. sb203580 preconditioning recharges matrix-expanded human adult stem cells for chondrogenesis in an inflammatory environment - a feasible approach for autologous stem cell based osteoarthritic cartilage repair. Biomaterials. 2015;64:88–97. doi: 10.1016/j.biomaterials.2015.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]