Abstract
Cyclin-dependent kinase 7 (CDK7) is a member of the CDK family, which forms the CDK activating kinase complex with Cyclin H and RING finger protein Mat1 to control cell cycle progression and transcription by phosphorylating other CDKs and RNA polymerase II. In this study, we analyzed TCGA data and found that upregulation of CDK7 frequently occurred in human gastric cancer. A potent and selective irreversible CDK7 inhibitor THZ2 was able to induce cell growth inhibition, cell cycle arrest at G2/M phase and apoptosis with the increasing intracellular reactive oxidative species (ROS) levels in gastric cancer cells. Pretreatment with ROS scavenger N-acety-L-cysteine partially reversed cell apoptosis induced by THZ2. In the nude mice, THZ2 also suppressed the growth of xenograft tumors of gastric cancer. Overall, our data showed that inhibition of CDK7 with THZ2 in gastric cancer presented outstanding anticancer effect in vitro and in vivo, suggesting that CDK7 is a potential therapeutic target for gastric cancer patients.
Keywords: CDK7, THZ2, gastric cancer
Introduction
Gastric cancer is the fifth most common cancer and the third leading cause of cancer mortality in the world [1]. Due to difficult early detection and rapid progression, most patients diagnosed with gastric cancer were already in the advanced stage with lymph node invasion and metastasis, and the occurrence of gastric cancer with a 5-year survival rate just scope of 25% to 35% in advanced patients [1]. Treatment for gastric cancer includes surgery, chemotherapy, radiation therapy and immunotherapy [2]. The benefits of chemotherapy in gastric cancer are able to reduce tumor size, relieve symptoms and increase survival time [3-5]. However, drug resistance and side toxicity extremely limit the effectiveness of chemotherapy. Therefore, it is urgent to develop new therapeutic drugs against gastric cancer.
Recently, a potent and selective irreversible cyclin-dependent kinase 7 (CDK7) inhibitor THZ1 with IC50 of 3.2 nM showed the promising anticancer activity in various cancers [6-13]. Unfortunately, the short half-time (45 minutes) of THZ1 limits its application [9]. THZ2, an analog of THZ1 with a 5-fold improved halflife, inhibits CDK7 with IC50 of 13.9 nM and potently suppressed the growth of triple-negative breast cancer cells [9]. However, the anticancer effect of THZ2 in other cancers is still unknown. In the present study, we investigated the anticancer effects of THZ2 on gastric cancer cells.
Material and methods
Cell culture and reagents
Human gastric cancer cells (AGS, BGC-823, MGC-803, MKN-45, SGC-7901) and normal human gastric epithelium cells GES-1 were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/ml) and streptomycin (100 ng/ml) in a humidified incubator at 37°C with 5% CO2. THZ2 was from ApexBio, and N-acetly-L-cysteine (NAC) and dihydroethidium (DHE) were from Sigma-Aldrich. Anti-CCNB1 (RLT1169), Anti-CDK2 (RLT0832), Anti-EGFR (RLT1485), Anti-MCL-1 (RLT2679), Anti-MEK1/2 (RLT2714), Anti-N/H/K-RAS (RLT2960) and Anti-RAF-1 (RLT3979) antibodies were from Ruiying Biological. Anti-PARP (556494) and Anti-ERK1/2 (610123) antibodies were from BD Biosciences. Anti-p-CDK2 (T160) (2561), Anti-p-AKT (T308) (13038), Anti-AKT (4691), Anti-BCL-XL (2764), Anti-p-ERK1/2 (T202/Y204) (4370), Anti-p-JNK (T183/Y185) (9251), Anti-JNK (9258) and Anti-XIAP (2045) antibodies were from Cell Signaling Technologies. Anti-14-3-3 (SC-629) and Anti-CCNE (SC-481) antibodies were from Santa Cruz Biotechnology. Anti-CDK7 (D220401) antibodies were from Shanghai Sangon Biotech. Anti-GAPDH (LK-9002T) antibodies were from Tianjin Sungene Biotech.
Cell viability assay
Cells were firstly seeded into a 96-well plate at a density of 3000 cells per well, and incubated with THZ2 in three parallel wells for 72 h. Then MTT was added to each well at a final concentration of 0.5 mg/ml. After incubation for 4 h, formazan crystals were dissolved in 100 μl of DMSO, and absorbance at 570 nm was measured by plate reader (Bioteck). The concentrations required to inhibit growth by 50% (IC50) were calculated from survival curves using the Bliss method [14,15].
Cell cycle assay
Cells were harvested and washed twice with cold phosphate-buffered saline (PBS), then fixed with ice-cold 70% ethanol for 2 h at 4°C. After centrifugation at 200 × g for 10 minutes, cells were washed twice with PBS and resuspended with 0.5 ml PBS containing PI (50 μg/ml), 0.1% Triton X-100, 0.1% sodium citrate, and DNase-free RNase (100 μg/ml), and detected by FCM after 15 minutes incubation at room temperature in the dark. Fluorescence was measured at an excitation wavelength of 480 nm through a FL-2filter (585 nm). Data were analyzed using ModFit LT 3.0 software (Becton Dickinson) [16,17].
Apoptosis assay
Cell apoptosis was evaluated with flow cytometry (FCM) assay. Briefly, cells were harvested and washed twice with PBS, stained with Annexin V-FITC and propidium iodide (PI) in the binding buffer, and detected by FACSCalibur FCM (BD, CA, USA) after 15 minutes incubation at room temperature in the dark. Fluorescence was measured at an excitation wave length of 480 nm through FL-1 (530 nm) and FL-2 filters (585 nm). The early apoptotic cells (Annexin V positive only) and late apoptotic cells (Annexin V and PI positive) were quantified [17,18].
Reactive oxygen species (ROS) assay
Cells were incubated with DHE (10 μM) for 30 minutes at 37°C in the dark. Five fields were observed randomly for each well. ROS activation were analyzed by calculate the percentage of positive cells [19,20].
Western blot analysis
Cells were harvested and washed twice with cold PBS, then resuspended and lysed in RIPA buffer (1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 10 ng/ml PMSF, 0.03% aprotinin, 1 μM sodium orthovanadate) at 4°C for 30 minutes. Lysates were centrifuged for 10 minutes at 14,000 × g and supernatants were stored at -80°C as whole cell extracts. Total protein concentrations were determined with Bradford assay. Thirty μg proteins of each sample were separated on 12% SDS-PAGE gels and transferred to polyvinylidene difluoride membranes. Membranes were blocked with 5% BSA and incubated with the indicated primary antibodies. Corresponding horseradish peroxidase-conjugated secondary antibodies were used against each primary antibody. Proteins were detected using the chemiluminescent detection reagents and Bio-Rad image system [21,22].
Nude mice xenograft tumor assay
Balb/c nude mice were obtained from the Guangdong Medical Laboratory Animal Center and feed on sterilized food and water. Six female nude mice with 5 weeks old were used for two groups. Each mouse was injected subcutaneously with BGC-823 cells (3 × 106 in 100 μl of medium) under the shoulder. When the subcutaneous tumors were approximately 0.3 × 0.3 cm2 (two perpendicular diameters) in size, mice were randomized into two groups, and were injected intraperitoneally with vehicle alone (0.9% saline) and THZ2 (10 mg/kg) once/day at first 16 days and twice/day at last 6 days. The body weights of mice and the two perpendicular diameters (A and B) of tumors were recorded. The tumor volume (V) was calculated according to the formula [23]: V = (π/6) × [(A+B)/2]3. The mice were anaesthetized after experiment, and tumor tissue was excised from the mice and weight. The rate of inhibition (IR) was calculated according to the formula [24]: IR = [1 - (Mean tumor weight of experimental group/Mean tumor weight of control group)] × 100%.
Statistical analysis
The CDK7 mRNA expressions in gastric cancer from The Cancer Genome Atlas (TCGA) data is analysis by Kolmogorov-Smirnov test and Chi-square test by using ks.test and chisq.test in the R language (version 3.4). A student’s t-test was used to compare individual data points among each group. A P-value of < 0.05 was set as the criterion for statistical significance.
Results
THZ2 inhibits the growth of gastric cancer cells in vitro
To explore the expression of CDK7 in gastric cancer, we firstly analyzed the TCGA data in 375 gastric cancer tissues and 32 gastric normal tissues. The results showed that the mRNA expression of CDK7 in gastric cancer tissues was significantly higher than that in gastric normal tissues (Figure 1A), which is consistent with previous reports [25,26]. We also detected the protein expression of CDK7 in five human gastric cancer cells AGS, BGC-823, MGC-803, MKN-45, SGC-7901 and normal human gastric epithelium cells GES-1. As shown in Figure 1B, the protein expression of CDK7 in MKN-45 and SGC-7901 were higher than those in other cell lines. Next, we investigate the effect of THZ2 (Figure 1C) on the growth of these cells with MTT assay. The survivals of all six cell lines were decreased in a dose-dependent manner after THZ2 treatment (Figure 1D). GES-1 cells were most insensitive to THZ2 with the highest IC50 values of 2.45 μM. The IC50 values of THZ2 in AGS, BGC-823, MGC-803, MKN-45 and SGC-7901 were 0.19 μM, 0.69 μM, 0.74 μM, 0.73 μM and 0.68 μM respectively, which were positively correlated with the protein expression of CDK7 in these cells (Figure 1E and 1F).
Figure 1.

THZ2 inhibits the growth of gastric cancer cells in vitro. A. CDK7 mRNA expression was analyzed between normal tissue and tumor tissue in gastric cancer of TCGA data. *P < 0.05 and **P < 0.01 vs. corresponding control. B. The protein expression of CDK7 in human gastric cancer cells was examined by Western blot, and 14-3-3 was used as loading control. The representative results of three independent experiments were shown. C. Chemical structure of THZ2. D. The representative growth curves of cells treated with THZ2 are shown. E. Summary of IC50 of THZ2 in the indicated human gastric cancer cells cells is shown. F. The correlation analysis of THZ2 IC50 values and relative CDK7 protein levels in five human gastric cancer cells is shown.
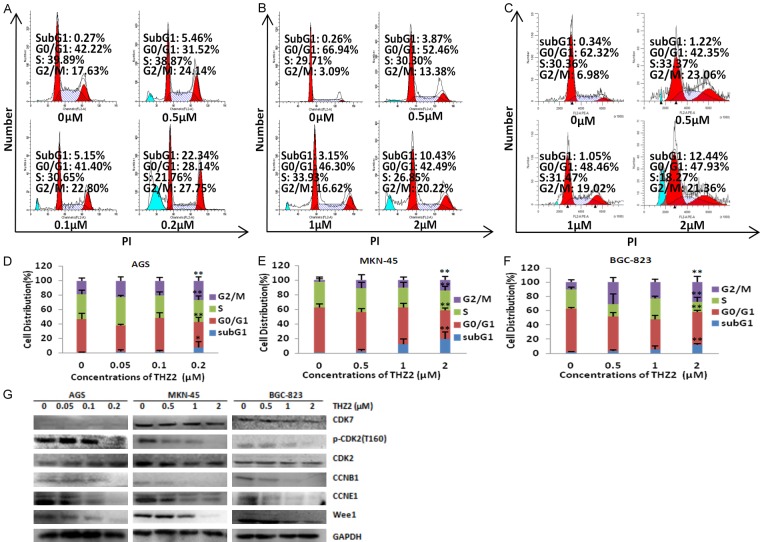
THZ2 induces cell cycle arrest at G2/M phase in gastric cancer cells
To determine whether the growth inhibition of THZ2 on gastric cancer cells is due to cell cycle arrest, AGS, MKN-45 and BGC-823 cells were treated with THZ2 at the indicated concentrations for 48 h, detected by FCM with PI staining and analysed with ModFit LT 3.0 software. As shown in Figure 2A-F, THZ2 induced the accumulation in Sub G1 and G2/M phase and reduction in G0/G1 and S phase in the dose-dependent manner in all three cell lines. To investigate the molecular mechanism of cell cycle arrest by THZ2, the cycle related proteins in these three cells were detected by Western blot. THZ2 treatment dose-dependently decreased the protein expressions of p-CDK2 (T160), CCNB1, CCNE1 and Wee1, but had no effect on the protein expressions of CDK7 and CDK2 (Figure 2G).
Figure 2.
THZ2 induces cell cycle arrest at G2/M phase in gastric cancer cells. AGS (A), MKN-45 (B) and BGC-823 (C) cells were treated with THZ2 at the indicated concentrations for 48 h. The distribution of cell cycle was detected by FCM with PI staining. The percentages of subG1, G1/G0, S, G2/M phase were calculated using ModFit LT 3.0 software. The protein expression was examined by Western blot, and GAPDH was used as loading control. The representative charts, quantified results (D-F) and Western blot results (G) of three independent experiments were shown. *P < 0.05 and **P < 0.01 vs. corresponding control.
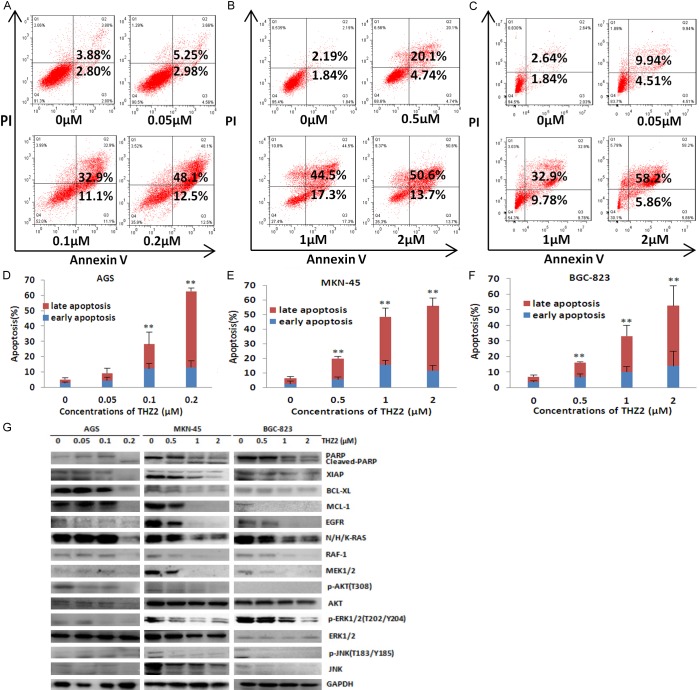
THZ2 induces apoptosis in gastric cancer cells
To further examine whether THZ2 is able to induce apoptosis in gastric cancer cells, AGS, MKN-45 and BGC-823 cells were treated with THZ2 at the indicated concentrations for 48 h, stained with Annexin V/PI and examined by FCM. As shown in Figure 3A-F, THZ2 induced apoptosis in a dose-dependent manner in all three cells. To detect the molecular mechanism of cell apoptosis by THZ2, the apoptosis and survival related proteins in these three cells are detected by Western blot. THZ2 treatment dose-dependently increased the protein expressions of apoptosis marker cleaved PARP, decreased the protein expressions of XIAP, BCL-XL, MCL-1, EGFR, N/H/K-RAS, RAF-1, MEK1/2, p-AKT (T308), p-ERK1/2 (T202/Y204), p-JNK (T183/Y185) and JNK, Wee1, but did not alter the protein expressions of AKT and ERK1/2 (Figure 3G).
Figure 3.
THZ2 induces apoptosis in gastric cancer cells. AGS (A), MKN-45 (B) and BGC-823 (C) cells were treated with THZ2 at the indicated concentrations for 48 h. The apoptosis was detected by FCM with Annexin V/PI staining. The proportions of Annexin V+/PI- and Annexin V+/PI+ cells indicated the early and late stage of apoptosis. The protein expression was examined by Western blot, and GAPDH was used as loading control. The representative charts, quantified results (D-F) and Western blot results (G) of three independent experiments were shown. *P < 0.05 and **P < 0.01 vs. corresponding control.
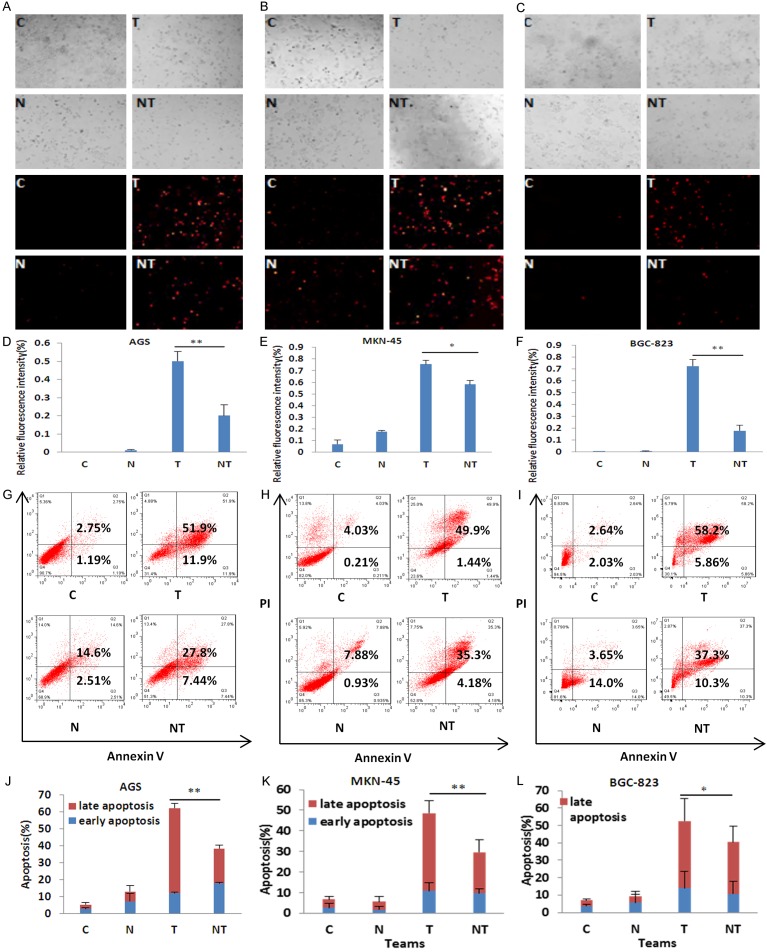
ROS is critical for THZ2-induced apoptosis in gastric cancer cells
ROS plays a critical role in mediating numerous anticancer agents executing anticancer effects [27]. To examine the role of ROS on the effect of THZ2 in gastric cancer cells, the ROS fluorescent probe dihydroethidium (DHE) was used to stain AGS, MKN-45 and BGC-823 cells after THZ2 treatment for 48 h with or without the ROS scavenger NAC at 5 mM pretreated for 1 h. As shown in Figure 4, THZ2 enhanced the fluorescent intensity of DHE, while NAC partially rescued THZ2-induced DHE fluorescent signals and apoptosis in all three cells.
Figure 4.
ROS is critical for THZ2-induced apoptosis in gastric cancer cells. AGS (A), MKN-45 (B) and BGC-823 (C) cells were treated with THZ2 at the concentrations of 0.2 μM, 2 μM and 2 μM for 48 h respectively in the present or absent of 5 mM NAC pretreatment for 1 h, stained with DHE and photographed under florescent microscope. The representative micrographs and quantified results (D-F) of three independent experiments were shown. The apoptosis was detected by FCM with Annexin V/PI staining. The representative charts (G-I) and quantified results (J-L) of three independent experiments were shown. C: Control. N: NAC. T: THZ2. NT: NAC+THZ2. *P < 0.05 and **P < 0.01 vs. corresponding control.
THZ2 inhibits the subcutaneous xenograft growth of gastric cancer in nude mice
To further estimate the anti-gastric cancer effects of THZ2 in vivo, we generated the xenograft tumor models by transplanting BGC-823 cells into nude mice. As shown in (Figure 5A-D), compared with the control group, treatment with THZ2 significantly inhibited the growth of BGC-823 tumors by diminishing the volume and weight of tumors, but did not change the the weight of nude mice. The inhibition rate of tumor growth in THZ2 treatment group was 44.00% (Figure 5E).
Figure 5.
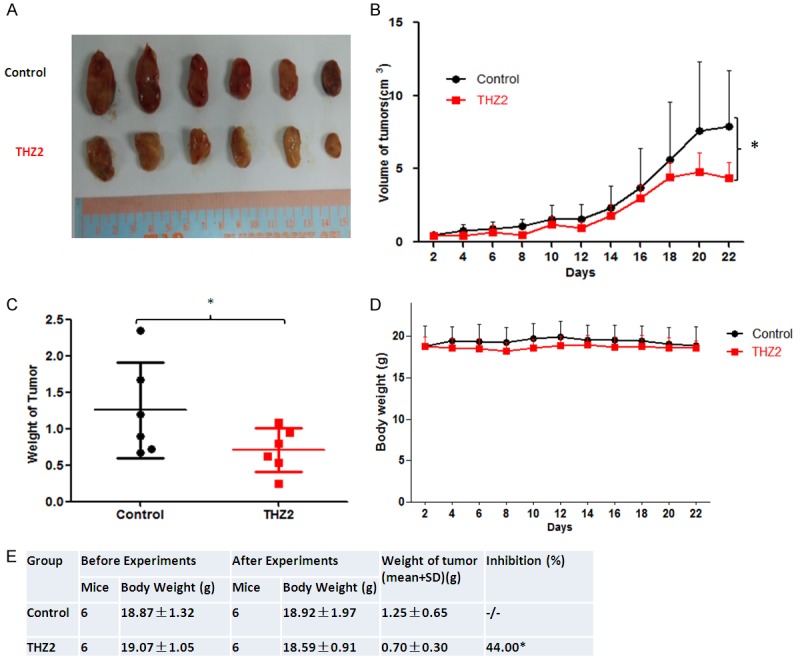
THZ2 inhibits the subcutaneous xenograft growth of gastric cancer in nude mice. Each mouse was injected subcutaneously with BGC-823 cells (3 × 106 in 100 μl of medium) under the shoulder. When the subcutaneous tumors were approximately 0.3 × 0.3 cm2 (two perpendicular diameters) in size, mice were randomized into two groups, and were injected intraperitoneally with vehicle alone (0.9% saline) and THZ2 (10 mg/kg) once/day at first 16 days and twice/day at last 6 days. The body weights of mice and the two perpendicular diameters of the tumor were recorded. The mice were anaesthetized after experiment, and tumor tissue was excised from the mice and weighed. The original tumors (A), tumor volume (B), tumor weight (C), body weight (D) and summary data (E) were shown. The values presented are the means ± SD for each group. *P < 0.05 and **P < 0.01 vs. corresponding control.
Discussion
Cyclin-dependent kinase 7 (CDK7), a member of the CDK family, forms a trimeric complex with cyclin H and RING finger protein MAT1, which functions as a Cdk-activating kinase (CAK) to phosphorylate and activate CDK1, CDK2, CDK4 and CDK6 to ensure the cell cycle transition. Additionally, CDK7 is an essential component of the transcription factor TFIIH, which controls transcription initiation through phosphorylating and activating RNA polymerase II [28]. Therefore, CDK7 is a critical connection between the regulation of cell cycle and transcription. Upregulation of CDK7 has been reported in multiple types of cancers, including breast cancer [29,30], esophageal squamous cell carcinoma [31], gastric cancer [25,26], hepatocellular carcinoma [32] and glioma [8], etc. We analysed TCGA data and found that upregulation of CDK7 frequently occured in human gastric cancer. Targeting CDK7 offers an attractive opportunity to inhibit two major drivers of tumorigenesis: cell cycle deregulation and transcriptional diversity.
In this report, we firstly show that THZ2 had anti-growth effect on human gastric cancer cells in vitro and in vivo. Treatment with THZ2 induces cell arrest at G2/M phase and apoptosis in the concentration-dependent manner. CDK7 inhibition by THZ2 leads to a reduction in its substrate CDK2 T160 phosphorylation and inactivation of survival signaling pathway EGFR/Ras/AKT/ERK. These evidences implicate that targeting CDK7-dependent transcriptional addictions in human gastric cancer may be equally effective with other cancers such as small cell lung cancer, triple-negative breast cancer, ovarian cancer, nasopharyngeal carcinoma, oesophageal squamous cell carcinoma, etc [6,7,10,11,29]. Furthermore, THZ2 also increases the intracellular ROS levels, and pretreated with ROS scavenger NAC partially reverses THZ2-induced apoptosis, suggesting that ROS plays an important role in cancer treatment with THZ2. Another selective CDK7 inhibitor BS-181 inhibited CDK7 activity with an IC50 of 21 nM, promoted cell cycle arrest and apoptosis in cancer cells, and showed antitumor effects in vivo with a plasma elimination half-life of 405 minutes after i.p. administration of 10 mg/kg in mice [33]. BS-181 also suppressed cell proliferation and induced cell cycle arrest and apoptosis in gastric cancer [34] and ameliorated experimental arthritis in mice [35].
In conclusion, our analysis of TCGA data shows that upregulation of CDK7 frequently occurred in human gastric cancer. Inhibition of CDK7 with THZ2 can potently induce cell growth inhibition, cell cycle arrest and apoptosis in gastric cancer cells in vitro and in vivo. The beneficial therapy of THZ2 appears to be potential treatment strategy in patients with gastric cancer.
Acknowledgements
This work was supported by funds from the National Key Research and Development Program of China No. 2017YFA0505104 (Z. S.), the National Natural Science Foundation of China Nos. 81661148049 and 81772540 (Z. S.), the Guangdong Natural Science Funds for Distinguished Young Scholar No. 2014A030306001 (Z. S.), the Guangdong Special Support Program for Young Talent No. 2015TQ01R350 (Z. S.), the Science and Technology Program of Guangdong No. 2016A050502027 (Z. S.) and the Science and Technology Program of Guangzhou No. 201704030058 (Z. S.).
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Yoong J, Michael M, Leong T. Targeted therapies for gastric cancer: current status. Drugs. 2011;71:1367–1384. doi: 10.2165/11592530-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Ilson DH. Adjuvant treatment for gastric cancer: too much is not enough. Lancet Oncol. 2014;15:788–789. doi: 10.1016/S1470-2045(14)70293-1. [DOI] [PubMed] [Google Scholar]
- 4.Group G, Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M, Van Cutsem E, Buyse M. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729–1737. doi: 10.1001/jama.2010.534. [DOI] [PubMed] [Google Scholar]
- 5.Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, Higashino M, Yamamura Y, Kurita A, Arai K ACTS-GC Group. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–1820. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 6.Yuan J, Jiang YY, Mayakonda A, Huang M, Ding LW, Lin H, Yu F, Lu Y, Loh TKS, Chow M, Savage S, Tyner JW, Lin DC, Koeffler HP. Superenhancers promote transcriptional dysregulation in nasopharyngeal carcinoma. Cancer Res. 2017;77:6614–6626. doi: 10.1158/0008-5472.CAN-17-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Z, Peng H, Wang X, Yin X, Ma P, Jing Y, Cai MC, Liu J, Zhang M, Zhang S, Shi K, Gao WQ, Di W, Zhuang G. Preclinical efficacy and molecular mechanism of targeting CDK7-dependent transcriptional addiction in ovarian cancer. Mol Cancer Ther. 2017;16:1739–1750. doi: 10.1158/1535-7163.MCT-17-0078. [DOI] [PubMed] [Google Scholar]
- 8.Greenall SA, Lim YC, Mitchell CB, Ensbey KS, Stringer BW, Wilding AL, O’Neill GM, McDonald KL, Gough DJ, Day BW, Johns TG. Cyclindependent kinase 7 is a therapeutic target in high-grade glioma. Oncogenesis. 2017;6:e336. doi: 10.1038/oncsis.2017.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li B, Ni Chonghaile T, Fan Y, Madden SF, Klinger R, O’Connor AE, Walsh L, O’Hurley G, Mallya Udupi G, Joseph J, Tarrant F, Conroy E, Gaber A, Chin SF, Bardwell HA, Provenzano E, Crown J, Dubois T, Linn S, Jirstrom K, Caldas C, O’Connor DP, Gallagher WM. Therapeutic rationale to target highly expressed CDK7 conferring poor outcomes in triple-negative breast cancer. Cancer Res. 2017;77:3834–3845. doi: 10.1158/0008-5472.CAN-16-2546. [DOI] [PubMed] [Google Scholar]
- 10.Jiang YY, Lin DC, Mayakonda A, Hazawa M, Ding LW, Chien WW, Xu L, Chen Y, Xiao JF, Senapedis W, Baloglu E, Kanojia D, Shang L, Xu X, Yang H, Tyner JW, Wang MR, Koeffler HP. Targeting super-enhancer-associated oncogenes in oesophageal squamous cell carcinoma. Gut. 2017;66:1358–1368. doi: 10.1136/gutjnl-2016-311818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen CL, Kwiatkowski N, Abraham BJ, Carretero J, Al-Shahrour F, Zhang T, Chipumuro E, Herter-Sprie GS, Akbay EA, Altabef A, Zhang J, Shimamura T, Capelletti M, Reibel JB, Cavanaugh JD, Gao P, Liu Y, Michaelsen SR, Poulsen HS, Aref AR, Barbie DA, Bradner JE, George RE, Gray NS, Young RA, Wong KK. Targeting transcriptional addictions in small cell lung cancer with a covalent CDK7 inhibitor. Cancer Cell. 2014;26:909–922. doi: 10.1016/j.ccell.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chipumuro E, Marco E, Christensen CL, Kwiatkowski N, Zhang T, Hatheway CM, Abraham BJ, Sharma B, Yeung C, Altabef A, Perez-Atayde A, Wong KK, Yuan GC, Gray NS, Young RA, George RE. CDK7 inhibition suppresses superenhancer-linked oncogenic transcription in MYCN-driven cancer. Cell. 2014;159:1126–1139. doi: 10.1016/j.cell.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwiatkowski N, Zhang T, Rahl PB, Abraham BJ, Reddy J, Ficarro SB, Dastur A, Amzallag A, Ramaswamy S, Tesar B, Jenkins CE, Hannett NM, McMillin D, Sanda T, Sim T, Kim ND, Look T, Mitsiades CS, Weng AP, Brown JR, Benes CH, Marto JA, Young RA, Gray NS. Targeting transcription regulation in cancer with a covalent CDK7 inhibitor. Nature. 2014;511:616–620. doi: 10.1038/nature13393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang WJ, Li Y, Wei MN, Chen Y, Qiu JG, Jiang QW, Yang Y, Zheng DW, Qin WM, Huang JR, Wang K, Zhang WJ, Wang YJ, Yang DH, Chen ZS, Shi Z. Synergistic antitumor activity of regorafenib and lapatinib in preclinical models of human colorectal cancer. Cancer Lett. 2017;386:100–109. doi: 10.1016/j.canlet.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Mei XL, Yang Y, Zhang YJ, Li Y, Zhao JM, Qiu JG, Zhang WJ, Jiang QW, Xue YQ, Zheng DW, Chen Y, Qin WM, Wei MN, Shi Z. Sildenafil inhibits the growth of human colorectal cancer in vitro and in vivo. Am J Cancer Res. 2015;5:3311–3324. [PMC free article] [PubMed] [Google Scholar]
- 16.Peng QQ, Wang K, Cheng KJ, Yang HG, Qiu JG, Zhang WJ, Jiang QW, Yang Y, Zheng DW, Huang JR, Wei MN, Shi Z, Wang W. Caragaphenol a induces reactive oxygen species related apoptosis in human gastric cancer cells. Am J Transl Res. 2017;9:3804–3815. [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng DW, Xue YQ, Li Y, Di JM, Qiu JG, Zhang WJ, Jiang QW, Yang Y, Chen Y, Wei MN, Huang JR, Wang K, Wei X, Shi Z. Volasertib suppresses the growth of human hepatocellular carcinoma in vitro and in vivo. Am J Cancer Res. 2016;6:2476–2488. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Lv M, Qiu JG, Zhang WJ, Jiang QW, Qin WM, Yang Y, Zheng DW, Chen Y, Huang JR, Wang K, Wei MN, Cheng KJ, Shi Z. Wallichinine reverses ABCB1-mediated cancer multidrug resistance. Am J Transl Res. 2016;8:2969–2980. [PMC free article] [PubMed] [Google Scholar]
- 19.Xie FF, Pan SS, Ou RY, Zheng ZZ, Huang XX, Jian MT, Qiu JG, Zhang WJ, Jiang QW, Yang Y, Li WF, Shi Z, Yan XJ. Volasertib suppresses tumor growth and potentiates the activity of cisplatin in cervical cancer. Am J Cancer Res. 2015;5:3548–3559. [PMC free article] [PubMed] [Google Scholar]
- 20.Gong LH, Chen XX, Wang H, Jiang QW, Pan SS, Qiu JG, Mei XL, Xue YQ, Qin WM, Zheng FY, Shi Z, Yan XJ. Piperlongumine induces apoptosis and synergizes with cisplatin or paclitaxel in human ovarian cancer cells. Oxid Med Cell Longev. 2014;2014:906804. doi: 10.1155/2014/906804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi Z, Park HR, Du Y, Li Z, Cheng K, Sun SY, Li Z, Fu H, Khuri FR. Cables1 complex couples survival signaling to the cell death machinery. Cancer Res. 2015;75:147–158. doi: 10.1158/0008-5472.CAN-14-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi Z, Li Z, Li ZJ, Cheng K, Du Y, Fu H, Khuri FR. Cables1 controls p21/Cip1 protein stability by antagonizing proteasome subunit alpha type 3. Oncogene. 2015;34:2538–2545. doi: 10.1038/onc.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu JG, Zhang YJ, Li Y, Zhao JM, Zhang WJ, Jiang QW, Mei XL, Xue YQ, Qin WM, Yang Y, Zheng DW, Chen Y, Wei MN, Shi Z. Trametinib modulates cancer multidrug resistance by targeting ABCB1 transporter. Oncotarget. 2015;6:15494–15509. doi: 10.18632/oncotarget.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang QW, Cheng KJ, Mei XL, Qiu JG, Zhang WJ, Xue YQ, Qin WM, Yang Y, Zheng DW, Chen Y, Wei MN, Zhang X, Lv M, Chen MW, Wei X, Shi Z. Synergistic anticancer effects of triptolide and celastrol, two main compounds from thunder god vine. Oncotarget. 2015;6:32790–32804. doi: 10.18632/oncotarget.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naseh G, Mohammadifard M, Mohammadifard M. Upregulation of cyclin-dependent kinase 7 and matrix metalloproteinase-14 expression contribute to metastatic properties of gastric cancer. IUBMB Life. 2016;68:799–805. doi: 10.1002/iub.1543. [DOI] [PubMed] [Google Scholar]
- 26.Wang Q, Li M, Zhang X, Huang H, Huang J, Ke J, Ding H, Xiao J, Shan X, Liu Q, Bao B, Yang L. Upregulation of CDK7 in gastric cancer cell promotes tumor cell proliferation and predicts poor prognosis. Exp Mol Pathol. 2016;100:514–521. doi: 10.1016/j.yexmp.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Xue YQ, Di JM, Luo Y, Cheng KJ, Wei X, Shi Z. Resveratrol oligomers for the prevention and treatment of cancers. Oxid Med Cell Longev. 2014;2014:765832. doi: 10.1155/2014/765832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lolli G, Johnson LN. CAK-cyclin-dependent activating kinase: a key kinase in cell cycle control and a target for drugs? Cell Cycle. 2005;4:572–577. [PubMed] [Google Scholar]
- 29.Wang Y, Zhang T, Kwiatkowski N, Abraham BJ, Lee TI, Xie S, Yuzugullu H, Von T, Li H, Lin Z, Stover DG, Lim E, Wang ZC, Iglehart JD, Young RA, Gray NS, Zhao JJ. CDK7-dependent transcriptional addiction in triple-negative breast cancer. Cell. 2015;163:174–186. doi: 10.1016/j.cell.2015.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel H, Abduljabbar R, Lai CF, Periyasamy M, Harrod A, Gemma C, Steel JH, Patel N, Busonero C, Jerjees D, Remenyi J, Smith S, Gomm JJ, Magnani L, Gyorffy B, Jones LJ, Fuller-Pace F, Shousha S, Buluwela L, Rakha EA, Ellis IO, Coombes RC, Ali S. Expression of CDK7, Cyclin H, and MAT1 is elevated in breast cancer and is prognostic in estrogen receptor-positive breast cancer. Clin Cancer Res. 2016;22:5929–5938. doi: 10.1158/1078-0432.CCR-15-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Zhu J, Yang L, Guan C, Ni R, Wang Y, Ji L, Tian Y. Interaction with CCNH/CDK7 facilitates CtBP2 promoting esophageal squamous cell carcinoma (ESCC) metastasis via upregulating epithelial-mesenchymal transition (EMT) progression. Tumour Biol. 2015;36:6701–6714. doi: 10.1007/s13277-015-3354-x. [DOI] [PubMed] [Google Scholar]
- 32.Wang C, Jin H, Gao D, Wang L, Evers B, Xue Z, Jin G, Lieftink C, Beijersbergen RL, Qin W, Bernards R. A CRISPR screen identifies CDK7 as a therapeutic target in hepatocellular carcinoma. Cell Res. 2018;28:690–692. doi: 10.1038/s41422-018-0020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ali S, Heathcote DA, Kroll SH, Jogalekar AS, Scheiper B, Patel H, Brackow J, Siwicka A, Fuchter MJ, Periyasamy M, Tolhurst RS, Kanneganti SK, Snyder JP, Liotta DC, Aboagye EO, Barrett AG, Coombes RC. The development of a selective cyclin-dependent kinase inhibitor that shows antitumor activity. Cancer Res. 2009;69:6208–6215. doi: 10.1158/0008-5472.CAN-09-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang BY, Liu QY, Cao J, Chen JW, Liu ZS. Selective CDK7 inhibition with BS-181 suppresses cell proliferation and induces cell cycle arrest and apoptosis in gastric cancer. Drug Des Devel Ther. 2016;10:1181–1189. doi: 10.2147/DDDT.S86317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xia Y, Lin LY, Liu ML, Wang Z, Hong HH, Guo XG, Gao GQ. Selective inhibition of CDK7 ameliorates experimental arthritis in mice. Clin Exp Med. 2015;15:269–275. doi: 10.1007/s10238-014-0305-6. [DOI] [PubMed] [Google Scholar]