Abstract
This study sought to determine the effect and explore the mechanism of the Chinese medicinal compound preparation Diwu Yanggan (DWYG) capsule on the occurrence and development of liver cancer using the Solt-Farber rat model. Sprague-Dawley rats were randomly distributed into a normal group, sham group, DWYG group, sorafenib group, and model group. The DWYG group and sorafenib group were given DWYG capsule and sorafenib tablet, respectively, with induction of the model. Hematoxylin-eosin (HE) staining was used to detect liver pathological changes. The content of nuclear DNA in the liver was detected by Feulgen staining, and the expression of PCNA was detected by immunohistochemical staining. Molecular biology methods were used to detect the expression of liver regeneration-related factors and Ras/Raf/Mek/Erk signaling pathway-related proteins and mRNAs. HE staining showed that compared with those in the model group, the liver pathological changes in the DWYG group were significantly reduced (P < 0.05). The nuclear DNA content in the liver based on Feulgen staining and the expression of PCNA in the DWYG group was lower than that in the model group (P < 0.05). The expression of regeneration-related factors and Ras/Raf/Mek/Erk signaling pathway-related proteins and mRNAs was significantly lower in the DWYG group than in the model group (P < 0.05). In conclusion, DWYG capsules to some degree inhibit the occurrence and development of liver cancer in the Solt-Farber rat model, and the effect is not inferior to that of sorafenib. DWYG capsules likely delay the occurrence and development of liver cancer and improve the liver regeneration microenvironment by regulating the Ras/Raf/Mek/Erk signaling pathway and regeneration-related factors.
Keywords: Liver cancer, Diwu Yanggan capsule, liver regeneration microenvironment, Ras/Raf/Mek/Erk signaling pathway
Introduction
Primary liver cancer is recognized as a major and difficult disease worldwide because of its occult pathogenesis, high morbidity, high mortality, high annual growth rate and high recurrence rate. At present, the pathogenesis of liver cancer is not fully clear, and prevention and control measures are very limited [1-4]. Surgical resection and liver transplantation are the primary methods of treatment for liver cancer patients, but their application is limited [5,6], and the long-term survival after surgical resection remains low [7,8], which may be related to the effect of the formed liver regeneration microenvironment on inducing residual cancer to disseminate and recur after surgical resection [9-11].
In recent years, an increasing number of reports have indicated and recognized the important role of the liver cancer microenvironment in the development and progression of liver cancer, and the concept of prevention and treatment of liver cancer is undergoing significant changes [12]. Therefore, the prevention and treatment of liver cancer by regulating the liver regeneration microenvironment may be a novel therapeutic strategy [13,14]. Our previous study has shown that the Chinese medicinal compound preparation Diwu Yanggan (DWYG) capsule could improve the liver tissue response rate of HBeAg-negative chronic hepatitis B and delay, prevent, or even reverse the progression of liver fibrosis. The results of a previous randomized controlled trial (RCT) has shown that DWYG capsule can significantly reduce the incidence of liver cirrhosis in patients with HBeAg-negative chronic hepatitis B and the risk of liver cancer [15]. By using the classic animal model of hepatic fibrosis induced by carbon tetrachloride and creating a model of monosodium L-glutamate (MSG) rat liver fibrosis induced by carbon tetrachloride, we found that DWYG exerts an antifibrotic effect likely by inhibiting the excessive activation of the Hh signaling pathway in the liver to regulate the imbalance between epithelial-mesenchymal transition (EMT) and mesenchymal-epithelial transition (MET) [16]. Other studies have shown that the DWYG capsule has an effect on preventing and treating liver cancer by affecting liver inflammatory damage, regulating immunity, improving the liver regeneration microenvironment, and regulating the Hh signaling pathway [16,17].
In this study, the Solt-Farber rat model was used to determine the effect of the DWYG capsule on the occurrence and development of liver cancer and to explore its mechanisms on the development and progression of liver cancer via the regulation of the Ras/Raf/Mek/extracellular signal-regulated kinase (ERK) signaling pathway to improve the liver regeneration microenvironment.
Materials and methods
Materials
2-Acetylaminofluorene (2-AAF) and N-nitrosodiethylamine (DEN) were purchased from Sigma-Aldrich (Saint Louis, MO, USA). Sorafenib tosylate tablets (trade name: Nexavar) were purchased from Bayer Schering Pharma AG (Berlin, Germany).
DWYG is a new drug authorized by the Hubei Food and Drug Administration (Grant No. Z20113160). The mixture includes five Chinese medicinal herbal extracts, whose proportions (w/w) are as follows: Rehmannia glutinosa (Gaertn.) DC. 20.0%; Artemisia scoparia Waldst. & Kitam. 33.3%; Curcuma longa L. 13.4%; Schisandra chinensis (Turcz.) Baill. 20.0%; and Glycyrrhiza uralensis Fisch. 13.4%. The DWYG capsules used in this study were provided by Traditional Chinese Medicine Preparation Room of Hubei Provincial Hospital of Traditional Chinese Medicine.
Animal model
Male Sprague-Dawley rats (SPF class, weighing 150-200 g) were purchased from Hubei Province Experimental Animal Research Center. All rats were housed with a 12-h light-dark cycle and with water and standard chow ad libitum.
The rats were randomly divided into five treatment groups including the normal group (N = 16), sham-operated group (sham group; N = 16), DWYG group (N = 16), sorafenib group (N = 16), and model group (N = 16). The liver cancer rat model was modified from previous reports [18]. In the sham group, the rat abdomens were opened, but no partial hepatectomy (PH) was performed. In the model, sorafenib and DWYG groups, the rats were treated with a one-time intraperitoneal injection of DEN (50 mg/kg), followed by PH at the end of the third week. Next, the three groups were orally administered 15 mg/kg 2-AAF once a day for 16 weeks. Meanwhile, the sham and normal groups were orally administered distilled water (10 ml/kg) once a day for 6 weeks, while the rats in the sorafenib and DWYG groups were orally administered sorafenib (80 mg/kg) and DWYG (750 mg/kg), respectively, once a day for 6 weeks. All rats were sacrificed, and liver pieces were fixed in 10% neutral buffered formalin or snap frozen in liquid nitrogen for further analyses.
Liver histology
Liver tissue samples from the different treatment groups were fixed in 10% neutral buffered formalin, paraffin-embedded, and sectioned at 5-μm thickness. For standard histology, liver sections were stained with hematoxylin-eosin (HE). Histological observations were performed using light microscopy.
Feulgen stain
Dewaxed tissue slices were subjected to warm (60°C) hydrochloric acid and then to Schiff’s reagent, followed by rinsing with distilled water after sulfurous acid, dehydrating, and sealing. The slices were observed by light microscopy (Nikon, Kyoto, Japan), and 3 visual fields were randomly chosen from each slice. The integral optical density (IOD) was analyzed with Image-Pro Plus 6.0 software (Media Cybernetics, USA).
Immunohistochemistry
Liver tissue sections were deparaffinized in xylene, rehydrated through graded ethanol and then boiled for 30 min in citrate buffer (10 mM, pH 6.0) for antigen retrieval. Endogenous peroxidase activity was inhibited by 3% hydrogen peroxide for 30 min; samples were then blocked with 5% bovine serum albumin (BSA; Boster Biological Technology, China) and incubated with diluted primary antibody against proliferating cell nuclear antigen (PCNA) (Santa Cruz, USA) for 12 h at 4°C followed by secondary antibody (Santa Cruz, USA) for 20 min at 37°C. Finally, the slides were visualized with 3,3’-diaminobenzidine (DAB; Boster Biological Technology, China) and counterstained with hematoxylin for microscopic observation. The mean optical density (OD) was analyzed with Image-Pro Plus 6.0 software.
Western blotting
Liver tissues were lysed in RIPA buffer supplemented with PMSF. Equal amounts of proteins were loaded and resolved on a 10% SDS-PAGE gel. The proteins were then transferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% nonfat milk and probed with primary anti-Raf-1, anti-MEK1, anti-ERK1, anti-vascular endothelial growth factor (VEGF), and anti-transforming growth factor (TGF)-α antibodies (Santa Cruz Biotechnology, USA). Following incubation with secondary antibodies (Santa Cruz Biotechnology, USA), the signals were visualized using a Bio-Rad Universal Hood Molecular Gel Imaging System (Bio-Rad, USA).
RNA isolation and quantitative real-time RT-PCR
Total RNA was isolated from liver tissues by TRIzol reagent (Invitrogen, USA) according to the manufacturer’s instructions. The first cDNA strand was synthesized using a ReverTra Ace qPCR RT Kit (Toyobo, Japan) and then amplified using THUNDERBIRD SYBR qPCR Mix (Toyobo, Japan) and specific primers on an ABI ViiATM 7 System (ABI, USA). The mRNA expression was normalized to the endogenous ‘housekeeping’ gene GAPDH. The primers were produced by Invitrogen. The sequences of specific primers are listed in Table 1.
Table 1.
Primer sequences
| Gene | Forward sequence (5’→3’) | Reverse sequence (5’→3’) | Product size (bp) |
|---|---|---|---|
| H-ras | CCATCAGTACAGGGAGCAGA | CGGGTCTTGGCTGATGTTTC | 168 |
| Raf-1 | AGCAATGGTTTCGGACTCAA | GCTTTCATAAGGCAGTCGTG | 221 |
| MEK1 | GTCCTACATGTCGCCTGAGA | GAGGTCGGCTATCCATTCCA | 239 |
| ERK1 | GGCTTTCTGACCGAGTATGTG | TTTAGGTCCTCTTGGGATGG | 215 |
| VEGF | CGTCTACCAGCGCAGCTATTG | CTCCAGGGCTTCATCATTGC | 145 |
| TGF-α | GCCCTGGCTGTCCTCATTATC | AGCAGGCAGTCCTTCCTTTCA | 133 |
| GAPDH | TGTTGCCATCAACGACCCCTT | CTCCACGACATACTCAGCA | 202 |
Statistical analysis
Data analysis was performed using SPSS 19.0 software (IBM Corp, USA). Data are shown as the mean ± standard deviation (SD). One-way ANOVA was performed to compare the differences between more than two groups. A probability value < 0.05 was considered to be statistically significant.
Results
DWYG treatment alleviates the degree of pathological changes in the Solt-Farber rat model
Gross morphology of the rat livers was visually observed, as shown in Figure 1A. The HE staining results (Figure 1B) showed no obvious lesions in the liver tissues of the normal and sham groups, and the morphology of liver tissues was normal. However, a series of pathological changes in liver tissues, such as hepatic cell cord disorder, abnormal cell morphology, increased nuclear and cytoplasmic content, deep nuclear staining, nuclear heterogeneity and inflammatory cell infiltration, were observed in the model group. Compared with those in the model group, the above pathological changes were obviously alleviated in the sorafenib and DWYG groups.
Figure 1.
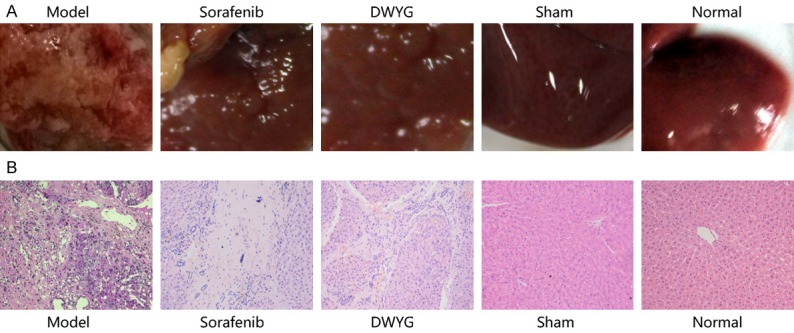
DWYG treatment inhibited tissue damage in the livers of Solt-Farber model rats. Normal SD rats (normal group), sham-operated rats (sham group), and model rats were orally administered distilled water, while the sorafenib group and DWYG group were orally administered sorafenib tablets (80 mg/kg) and DWYG capsules (750 mg/kg), respectively. Paraffin liver sections were dewaxed and rehydrated, and the sections were stained with hematoxylin-eosin. A. Liver images of the different groups. B. HE staining in the livers of different groups (100×).
DWYG treatment inhibits the proliferation of liver cancer cells
Feulgen staining showed that the nuclear DNA was purple red, and the cytoplasm and other components were pale green (Figure 2A). The IOD value of the model group was significantly higher than that of the normal group and the sham group (P < 0.05). Compared with that of the model group, the IOD value of the DWYG group and sorafenib group was decreased (P < 0.05). Additionally, the IOD value of the DWYG group was lower than that of the sorafenib group (P < 0.05) (Figure 2B).
Figure 2.
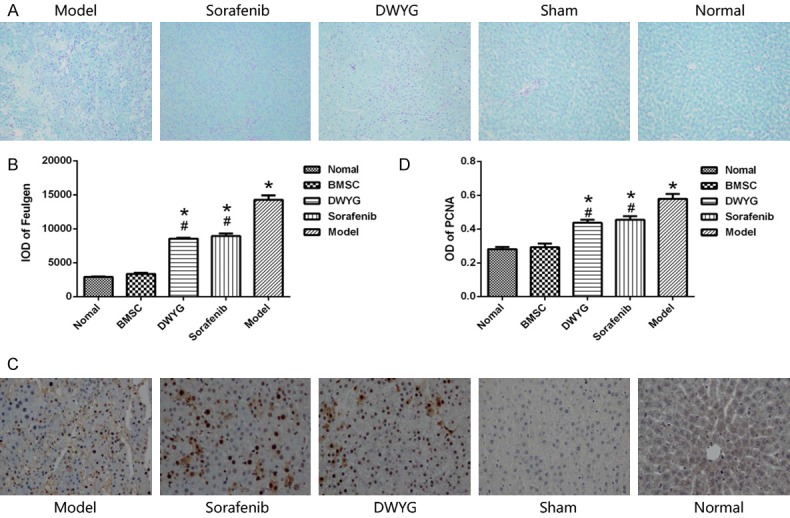
DWYG treatment affected the proliferation of liver cancer cells. A. Feulgen staining (100×); B. IOD values of the nuclear DNA in liver cells detected by Feulgen staining. *P < 0.05 vs the normal and sham groups, #P < 0.05 vs the model group. C. PCNA expression by immunohistochemical staining (100×). D. OD values of PCNA in the different treatment groups. Model: rats received a PH/2-AAF induction; Sorafenib: rats received a PH/2-AAF induction and sorafenib tablets at 80 mg/kg; DWYG: rats received a PH/2-AAF induction and DWYG capsules at 750 mg/kg; Sham: rats received a sham-operate but no PH/2-AAF induction; Normal: rats received no PH/2-AAF induction. *P < 0.05 vs the normal and sham groups, #P < 0.05 vs the model group.
Immunohistochemical staining for PCNA showed that the OD value of the model group was significantly higher than that of the normal group (P < 0.05) (Figure 2C, 2D). Compared with those of the model group, the OD values of the DWYG group and sorafenib group were decreased (P < 0.05). However, there was no significant difference between the DWYG and sorafenib groups.
DWYG treatment improves the liver regeneration microenvironment by regulating liver regeneration-related factors
Chronic inflammation and injury produce a large number of cytokines in the process of regeneration and healing. VEGF and TGF-α are not only related to liver regeneration but also closely related to the initiation and development of liver cancer. Moreover, VEGF and TGF-α also play an important role in activating the Ras/Raf/Mek/Erk pathway. The results showed that the mRNA expression of VEGF and TGF-α in the model group increased significantly, whereas the mRNA expression of VEGF and TGF-α was lower in the group treated with DWYG capsule than in the model group (Figure 3; P < 0.05). There was no significant difference between the DWYG groups and the sorafenib group. The mRNA results were consistent with the protein expression data (Figure 4). The results suggest that DWYG capsule can regulate the abnormal increase of liver regeneration-related factors to improve the liver regeneration microenvironment.
Figure 3.
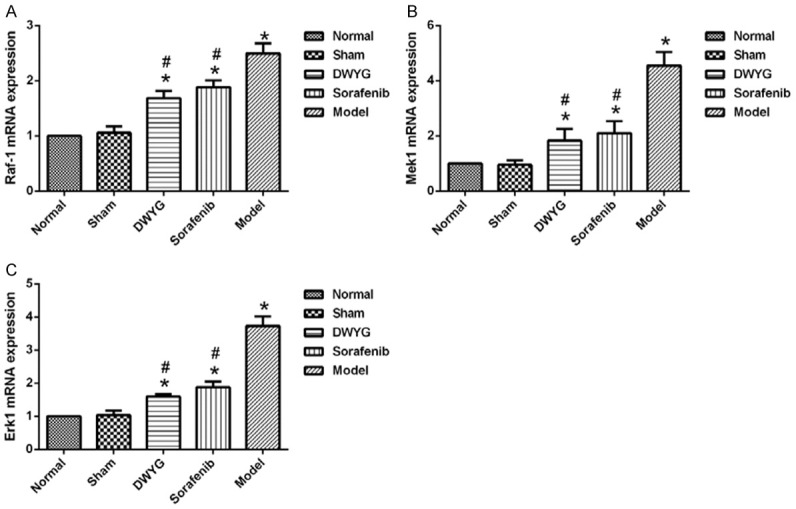
DWYG treatment affected the expression of mRNAs related to the Ras/Raf/Mek/Erk signaling pathway in the Solt-Farber model rats. A. The mRNA expression levels of Raf-1 in the liver tissues of different treated group were detected by quantitative real-time RT-PCR. Delta-delta-CT was calculated as described in Materials and methods, considering GAPDH as internal control. B. The mRNA expression levels of Mek1. C. The mRNA expression levels of Erk1. Data represent the means and standard deviation. *P < 0.05 vs the normal and sham groups. #P < 0.05 vs the model group.
Figure 4.
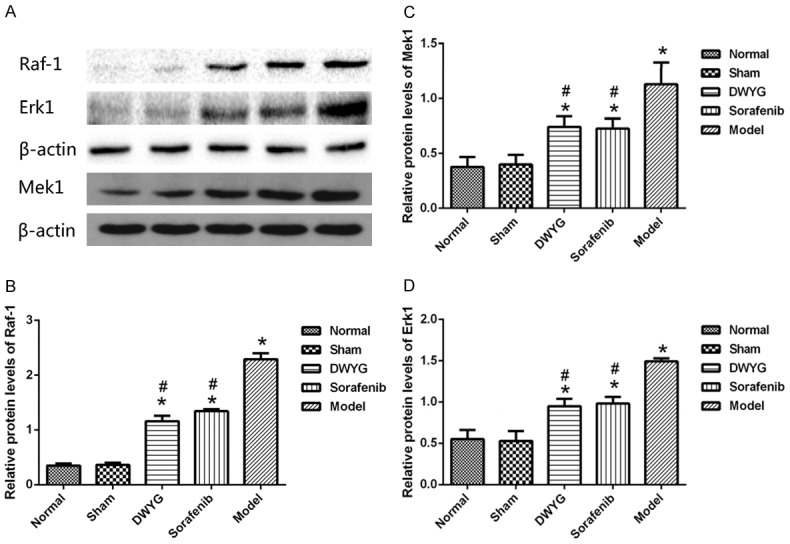
DWYG treatment affected the expression of proteins related to the Ras/Raf/Mek/Erk signaling pathway in the Solt-Farber model rats. A. Protein expression levels of Raf-1, Mek1 and Erk1 in liver tissues of the different treatment groups analyzed by Western blotting. Anti-β-actin blotting was used to control for equal protein loading. B-D. Relative quantifications of protein levels of Raf-1, Mek1, and Erk1 are shown in the different treatment groups. *P < 0.05 vs the normal and sham groups. #P < 0.05 vs the model group.
DWYG treatment inhibits the occurrence and development of liver cancer by regulating the Ras/Raf/Mek/Erk signaling pathway
The potential effects of DWYG on the expression levels of Ras/Raf/Mek/Erk signaling pathway-related factors in the liver cancer rat model were assessed by quantitative real-time RT-PCR and Western blotting. As indicated in Figures 3 and 4, compared with those in the normal and sham groups, Raf-1, Mek1, Erk1 mRNA and protein levels were increased in the model group. Western blotting revealed that changes in mRNA expression of the above molecules were accompanied by changes in protein expression, confirming that the Ras/Raf/Mek/Erk signaling pathway was activated during the evolution of liver cancer.
As shown in Figure 5, compared with that in the model group, the expression of Raf-1, Mek1 and Erk1 mRNA was reduced in the DWYG group (P < 0.05). Consistent changes were observed in protein expression (Figure 6). However, there was no significant difference in the mRNA and protein expression of Mek1, Raf-1 and Erk1 between the DWYG and sorafenib groups (P > 0.05). These findings suggested that DWYG treatment could inhibit the excessive activation of the Ras/Raf/Mek/Erk signaling pathway in the Solt-Farber rat model.
Figure 5.
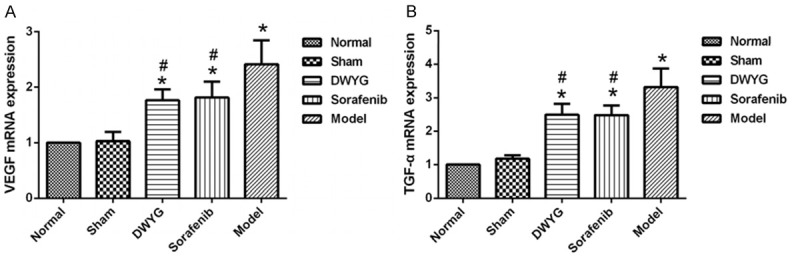
DWYG treatment regulated the mRNAs expression of liver regeneration-related factors. A. The mRNA expression levels of VEGF in the different treatment groups were detected by quantitative real-time RT-PCR. Delta-delta-CT was calculated as described in Materials and methods, considering GAPDH as internal control. B. The mRNA expression levels of TGF-α the different treatment groups. Model: rats received a PH/2-AAF induction; Sorafenib: rats received a PH/2-AAF induction and sorafenib tablets at 80 mg/kg; DWYG: rats received a PH/2-AAF induction and DWYG capsules at 750 mg/kg; Sham: rats received a sham-operate but no PH/2-AAF induction; Normal: rats received no PH/2-AAF induction. Data represent the means and standard deviation. *P < 0.05 vs the normal and sham groups. #P < 0.05 vs the model group.
Figure 6.
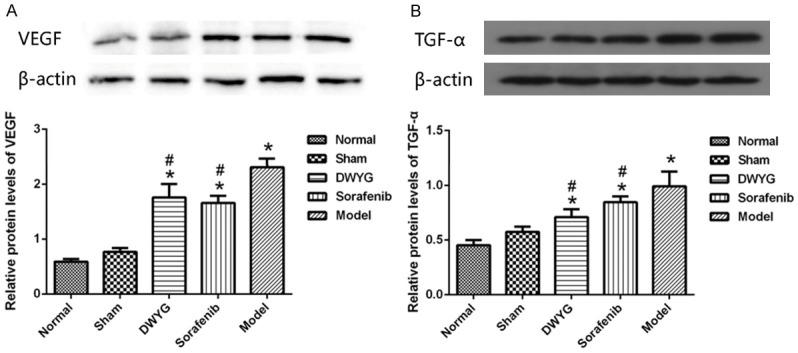
DWYG treatment regulated the proteins expression of liver regeneration-related factors. A. Relative proteins expression levels of VEGF in the different treatment groups. Anti-β-actin blotting was used to control for equal protein loading. B. Relative proteins expression levels of TGF-α in the different treatment groups. *P < 0.05 vs the normal and sham groups. #P < 0.05 vs the model group.
Discussion
The occurrence and development of liver cancer is essentially one of the serious outcomes of uncontrolled liver regeneration. Liver cancer progression is closely related to the abnormal liver regeneration microenvironment. Liver regeneration is an inevitable repair mechanism after liver injury. The abnormal liver regeneration microenvironment is one of the important conditions for the occurrence and development of liver cancer. Based on the study of regulation of the liver regeneration microenvironment in liver cancer, we proposed a new strategy to control the occurrence and development of liver cancer by modulating liver regeneration [13,17,19-22].
In a previous study, the 2-AAF/PH rat model of hepatic precancerous lesion was established to investigate the effects of the DWYG capsule on the occurrence and development of liver cancer. Using this model, we found that DWYG can improve the survival rate of the rats, promote the regeneration of hepatic oval cells (HOCs), inhibit hepatic precancerous lesion formation and restore the structure and function of the liver. The related mechanism may be the promotion of the proliferation and differentiation of bone marrow stem cells (BMSCs) and HOCs in the early and intermediate stages (8-14 days after hepatectomy) by DWYG, which favors liver regeneration and repair. In the late stage (17-22 days after hepatectomy), DWYG can inhibit the overproliferation and abnormal differentiation of HOCs, which can promote the prevention and treatment of liver cancer [23]. DWYG can likely inhibit HOC proliferation and abnormal differentiation by inhibiting the overactivation of the Wnt/β-catenin pathway. Furthermore, DWYG can regulate the expression of liver regeneration-related cytokines (TNF-α, IL-1, GRO/KC, vascular endothelial growth factor (VEGF), IFN-γ) in the Solt-Farber rat model, improving the liver regeneration microenvironment and preventing and ameliorating the occurrence and development of hepatic precancerous lesions [13,14].
In this study, HE staining showed that the pathological changes in rats in the model group were more obvious than those in the DWYG group, indicating that DWYG decreased the degree of lesion formation and delayed the progression of liver cancer. The proliferation of cancer cells is proportional to the content of DNA in the nucleus. The higher the DNA content, the more cells proliferate, the more abundant the genetic material and the higher the degree of malignancy. PCNA, which is expressed only in normal proliferating cells and tumor cells, changes in amount with DNA synthesis and cell proliferation. The results showed that DWYG treatment can downregulate the expression of PCNA, consistent with the results of Feulgen staining. These results indicated that DWYG treatment inhibited the proliferation of liver cancer cells, thereby delaying the progression of liver cancer.
Liver regeneration during chronic liver diseases is often disturbed by various factors. Thus, the organ cannot be completely regenerated or repaired, thereby forming an abnormal liver regeneration microenvironment (with inflammation-induced fibrosis, the disorder of cytokine network, etc.). The deterioration of the liver regeneration microenvironment provides the necessary conditions for liver cancer initiation and progression [24]. The liver regeneration microenvironment is a complex system that can be regulated by a variety of cytokines and signal pathways, including the Ras/Raf/Mek/Erk signaling pathway. The Ras/Raf/Mek/Erk signaling pathway is a classical form of the MAPK pathway, which is continuously activated in liver cancer and regulates cell proliferation, differentiation, apoptosis and angiogenesis [25]. A large number of studies have shown that liver cancer occurrence is associated with abnormal activation of the Ras/Raf/MEK/ERK signaling pathway [26-29]. In addition, many upstream growth factors in liver cancer, such as VEGF and TGF-α, activate the Ras/Raf/MEK/ERK pathway, which can contribute to the occurrence, development and metastasis of liver cancer [30].
The results of this study showed that DWYG treatment could improve the liver regeneration microenvironment by regulating the RAF/MEK/ERK pathway, thereby inhibiting the occurrence and development of liver cancer. Furthermore, we used sorafenib as a control drug and found that sorafenib has some inhibitory effect on tumor development, but its long-term use can lead to a toxicity. In contrast, long-term DWYG use can improve the in vivo environment and liver regeneration microenvironment without obvious adverse reactions, thereby contributing to the prevention and treatment of liver cancer.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (No. 81373513, No. 90709041, No. 30672590, No. 30271562, No. 30371787, No. 81102531, No. 81274147, No. 81373513, No. 81573815, No. 81603484, and No. 81703912), the National TCM Clinical Project (JDZX2012054 and JDZX2015172), Natural Science Foundation of Hubei Province (No. 2017CFB380), National Nonprofit Institute Research Grant for Institute of Basic Theory for Chinese Medicine, CACMS (YZ-1615) and Hanmin Li National Famous Old Chinese Medicine Experts Inheritance Studio.
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Chen JG, Zhang SW. Liver cancer epidemic in China: past, present and future. Semin Cancer Biol. 2011;21:59–69. doi: 10.1016/j.semcancer.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 3.He W, Zeng Q, Zheng Y, Chen M, Shen J, Qiu J, Chen M, Zou R, Liao Y, Li Q, Wu X, Li B, Yuan Y. The role of clinically significant portal hypertension in hepatic resection for hepatocellular carcinoma patients: a propensity score matching analysis. BMC Cancer. 2015;15:263. doi: 10.1186/s12885-015-1280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Bruix J, Gores GJ. Surgical resection versus transplantation for early hepatocellular carcinoma: clues for the best strategy. Hepatology. 2000;31:1019–1021. doi: 10.1053/he.2000.6959. [DOI] [PubMed] [Google Scholar]
- 6.GK AA. American Society of Clinical Oncology educational book. Alexandria, Va.: American Society of Clinical Oncology; 2004. Current and novel therapeutics for hepatocellular carcinoma; pp. 192–197. [DOI] [PubMed] [Google Scholar]
- 7.Baek YH, Kim KT, Lee SW, Jeong JS, Park BH, Nam KJ, Cho JH, Kim YH, Roh YH, Lee HS, Choi YM, Han SY. Efficacy of hepatic arterial infusion chemotherapy in advanced hepatocellular carcinoma. World J Gastroenterol. 2012;18:3426–3434. doi: 10.3748/wjg.v18.i26.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JY, Chung SM, Choi BO, Kay CS. Hepatocellular carcinoma with portal vein tumor thrombosis: improved treatment outcomes with external beam radiation therapy. Hepatol Res. 2011;41:813–824. doi: 10.1111/j.1872-034X.2011.00826.x. [DOI] [PubMed] [Google Scholar]
- 9.Ding T, Xu J, Zhang Y, Guo RP, Wu WC, Zhang SD, Qian CN, Zheng L. Endothelium-coated tumor clusters are associated with poor prognosis and micrometastasis of hepatocellular carcinoma after resection. Cancer. 2011;117:4878–4889. doi: 10.1002/cncr.26137. [DOI] [PubMed] [Google Scholar]
- 10.Zhong C, Wei W, Su XK, Li HD, Xu FB, Guo RP. Serum and tissue vascular endothelial growth factor predicts prognosis in hepatocellular carcinoma patients after partial liver resection. Hepatogastroenterology. 2012;59:93–97. doi: 10.5754/hge10638. [DOI] [PubMed] [Google Scholar]
- 11.Shi JH, Liu SZ, Wierod L, Scholz H, Anmarkrud JA, Huitfeldt HS, Zhang SJ, Line PD. RAF-targeted therapy for hepatocellular carcinoma in the regenerating liver. J Surg Oncol. 2013;107:393–401. doi: 10.1002/jso.23224. [DOI] [PubMed] [Google Scholar]
- 12.Leonardi GC, Candido S, Cervello M, Nicolosi D, Raiti F, Travali S, Spandidos DA, Libra M. The tumor microenvironment in hepatocellular carcinoma (review) Int J Oncol. 2012;40:1733–1747. doi: 10.3892/ijo.2012.1408. [DOI] [PubMed] [Google Scholar]
- 13.Li HM. Progress and prospect of regulation of liver regeneration by traditional Chinese medicine. World Chin J Digestol. 2017;25:1338–1344. [Google Scholar]
- 14.Li HM. Basic and clinical research of traditional Chinese medicine regulating and controlling liver regeneration. Chin Arch Tradit Chin. 2017;35:1927–1931. [Google Scholar]
- 15.Li H, Ye Z, Gao X, Zhang L, Yao X, Gu J, Lu D, Wan M, Xiao L, Cai W, Yan X, Zhao B, Wu Y, Zhang J. Diwu Yanggan capsule improving liver histological response for patients with HBeAg-negative chronic hepatitis B: a randomized controlled clinical trial. Am J Transl Res. 2018;10:1511–1521. [PMC free article] [PubMed] [Google Scholar]
- 16.Shen X, Peng Y, Cheng SS, Li HM. Regulation effect of Diwu Yanggan capsule on the hedgehog signal pathway in the liver tissues of fibrotie rats. China J Tradit Chin Med Pharm. 2015;30:2955–2957. [Google Scholar]
- 17.Li H, Zhang L. Liver regeneration microenvironment of hepatocellular carcinoma for prevention and therapy. Oncotarget. 2017;8:1805–1813. doi: 10.18632/oncotarget.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solt DB, Medline A, Farber E. Rapid emergence of carcinogen-induced hyperplastic lesions in a new model for the sequential analysis of liver carcinogenesis. Am J Pathol. 1977;88:595–618. [PMC free article] [PubMed] [Google Scholar]
- 19.Li HM. Microcirculation of liver cancer, microenvironment of liver regeneration, and the strategy of Chinese medicine. Chin J Integr Med. 2016;22:163–167. doi: 10.1007/s11655-016-2460-y. [DOI] [PubMed] [Google Scholar]
- 20.Li HM, Zhao B, Gao X, Shen X, Wu Y, Zhang JR, Song HL, Ye ZH, Cheng SS. Tonifying kidney network to modulate liver regeneration microenvironment to prevent and cure liver cancer. Hubei Zhongyiyao Daxue Xuebao. 2015;17:5–8. [Google Scholar]
- 21.Li HM. Construction and application of tertiary prevention program for liver cancer based on “tonifying the kidney to promote liver regeneration and repair by affecting stem cells and their microenvironment”. Zhongxiyi Jiehe Ganbing Za Zhi. 2015;25:369–372. [Google Scholar]
- 22.Li HM. Regulation of liver regeneration: research progress and prospect. World Chin J Digestol. 2015;23:3337–3343. [Google Scholar]
- 23.Zhao BB, Li HM, Gao X, Ye ZH, Cheng SS. The herbal compound “diwu yanggan” modulates liver regeneration by affecting the hepatic stem cell microenvironment in 2-acetylaminofluorene/partial hepatectomy rats. Evid Based Complement Alternat Med. 2015;2015:468303. doi: 10.1155/2015/468303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li HM, Ye ZH. Microenvironment of liver regeneration in liver cancer. Chin J Integr Med. 2017;23:555–560. doi: 10.1007/s11655-017-2806-0. [DOI] [PubMed] [Google Scholar]
- 25.Yang S, Liu G. Targeting the Ras/Raf/MEK/ERK pathway in hepatocellular carcinoma. Oncol Lett. 2017;13:1041–1047. doi: 10.3892/ol.2017.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito Y, Sasaki Y, Horimoto M, Wada S, Tanaka Y, Kasahara A, Ueki T, Hirano T, Yamamoto H, Fujimoto J, Okamoto E, Hayashi N, Hori M. Activation of mitogen-activated protein kinases/extracellular signal-regulated kinases in human hepatocellular carcinoma. Hepatology. 1998;27:951–958. doi: 10.1002/hep.510270409. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann K, Shibo L, Xiao Z, Longerich T, Buchler MW, Schemmer P. Correlation of gene expression of ATP-binding cassette protein and tyrosine kinase signaling pathway in patients with hepatocellular carcinoma. Anticancer Res. 2011;31:3883–3890. [PubMed] [Google Scholar]
- 28.Hoshino R, Chatani Y, Yamori T, Tsuruo T, Oka H, Yoshida O, Shimada Y, Ari-i S, Wada H, Fujimoto J, Kohno M. Constitutive activation of the 41-/43-kDa mitogen-activated protein kinase signaling pathway in human tumors. Oncogene. 1999;18:813–822. doi: 10.1038/sj.onc.1202367. [DOI] [PubMed] [Google Scholar]
- 29.Oka H, Chatani Y, Hoshino R, Ogawa O, Kakehi Y, Terachi T, Okada Y, Kawaichi M, Kohno M, Yoshida O. Constitutive activation of mitogen-activated protein (MAP) kinases in human renal cell carcinoma. Cancer Res. 1995;55:4182–4187. [PubMed] [Google Scholar]
- 30.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]


