Abstract
Cutaneous melanoma has the worst prognosis of all skin cancers. Although emerging targeted therapies, such as B-Raf kinase inhibitor vemurafenib, improve prognosis they require an accurate and sensitive means of detecting the pathogenic BRAF V600E mutation. We compared the sensitivity of four BRAF V600E detection methods in formalin-fixed, paraffin-embedded melanoma biopsies from 87 consecutive melanoma patients with Breslow stage I-V disease (staging based on the depth of tumor of invasion). The methods assessed were the widely used Cobas® 4800 system based on real-time PCR amplification, Sanger sequencing, allele-specific PCR (AS-PCR), and droplet digital PCR (ddPCR). The BRAF V600E mutation was found in 8 (9.2%), 23 (26.4%), 23 (26.4%) and 31 (35.6%) biopsies, respectively. The limit of detection (LoD) was determined by three different methods: Poisson confidence limits, calibration regression and Tzonev’s method. Pair-wise agreement between the methods was as follows: Cobas vs. Sanger, P = 0.33; Cobas® 4800 vs. AS-PCR, P = 0.33; Cobas® 4800 vs. ddPCR, P = 0.65; Sanger vs. AS-PCR, P = 1; Sanger vs. ddPCR, P = 0.08; AS-PCR vs. ddPCR, P = 0.06. Multinomial logistic regression was used for predictive modeling of the Breslow-Clark score; ddPCR emerged as the best predictor, the other predictors were mitotic activity, type of malignant melanoma and patient’s age. Our results demonstrate that ddPCR is the most sensitive method of detecting the BRAF V600E mutation.
Keywords: Melanoma, ddPCR, BRAF V600E, methods, diagnostic biomarker, treatment
Introduction
Melanoma is the most aggressive form of skin cancer and has the worst prognosis. Globally about 132,000 patients are diagnosed with melanoma every year, according to the World Health Organization [1], with the main population affected being relatively less pigmented Caucasians. Prognosis is largely determined by histological staging at diagnosis. Breslow staging is based on the depth of tumor of invasion and is the most widely used and best validated method. Improvements in understanding of the molecular basis of melanoma have resulted in development of targeted therapies and better prognoses. More than 60% of melanomas carry a BRAF mutation and 80% of mutations are the BRAF V600E mutation [2,3]. This mutation causes activation of the downstream Raf/MEK/ERK mitogen-activated protein kinase pathway [4,5]. Mutated BRAF has become an attractive therapeutic target; its inhibitor vemurafenib [6] has been shown to improve the survival of melanoma patients bearing the BRAF V600E mutation. Thus accurate identification of the BRAF V600E mutation is essential to the tailoring of patient management. Highly sensitive molecular techniques are needed to detect BRAF V600E mutation. Digital PCR is a cost-effective, sensitive method for detecting mutated DNA in tissue samples [7] and droplet digital PCR (ddPCR) is the latest, most sensitive iteration of this technology [8]. ddPCR employs water-oil emulsion microfluid droplet technology and uses the QX200TM droplet generator to split DNA molecules into approximately 20,000 droplets. Every droplet is analyzed individually using PCR in which the specific probe for the mutant sequence is labeled with a FAM fluorophore whilst the wild-type (WT) specific probe is labeled with a HEX fluorophore. Each droplet is scored individually by the QX200 droplet reader [8]. Thus ddPCR quantifies target and background DNAs which are randomly distributed among the droplets [9,10]; both mutated and WT DNA-containing droplets are counted to provide absolute quantification [11,12]. The data are then analyzed using Poisson statistics to assess the limit of detection (LoD) of the ddPCR assay [13].
The aim of this study was to assess the potential advantage of ddPCR as a method of detecting the BRAF V600E in patients with malignant melanoma relative to other widely used techniques-Cobas® 4800, Sanger sequencing and allele-specific PCR-through a side-by-side comparison. Our results indicate that ddPCR is the most sensitive and reliable method of analyzing formalin-fixed, paraffin-embedded (FFPE) biopsies.
Materials and methods
Patients
Eighty-seven consecutive FFPE samples, histologically identified as melanomas, were selected for this study. Archived FFPE tissue from these 87 specimens was acquired from the Clinic of Dermatovenerology, Department of Plastic Surgery and Department of Pathology of Jessenius Faculty of Medicine and the University Hospital of Martin, Slovakia. Prior to biopsy all subjects provided written, informed consent to participation in the study. This prospective study was approved by the University Ethics Committee. Presence of the BRAF V600E mutation was analyzed with the Cobas® 4800 BRAF V600 Mutation test (Roche Molecular system), Sanger sequencing, allele-specific PCR and ddPCR; details of the techniques are given below.
DNA extraction
The black PREP FFPE DNA kit (Life Science-Analytik Jena, Germany) was used for fast isolation of genomic DNA from FFPE samples. This method is based on a newly patented chemical process combining lysis of FFPE tissue with subsequent binding of nucleic acids onto the surface of a spin filter membrane. After several washings the nucleic acids are eluted from the membrane with the elution buffer included in the kit.
Quantification of DNA
The concentration of DNA was measured using a Qubit Fluorometer 2.0 (Invitrogen California) dsDNA with Qubit DNA BR assay kit, (Life Technologies, California). Aliquots of 50-100 ng/μL of extracted DNA were used for ddPCR.
Mutation analysis
The V600E mutation was detected using the Cobas® 4800 BRAF V600 Mutation Test, according to the manufacturer’s protocol.
Sanger sequencing
For Sanger sequencing we PCR-amplified and sequenced exon 15 of BRAF gene. PCR was performed in a total volume of 25 μL. We used 50-100 ng of genomic DNA for PCR amplification, 50 umol/L each of dNTp, 20 pmol/L forward/reverse primer, 1.5 mmol/L MgCl2, 1 x PCR buffer and 2.5 U Taq polymerase. The PCR amplification was performed under the following conditions: 1 cycle at 95°C for 8 minutes; 40 cycles at 94°C for 20 s, 58°C for 20 s and 72°C for 7 minutes. Amplified products were purified using 1.5 μL of Exo-SAP-IT (Applied Biosystems, CA, USA) and sequenced in both directions using the BigDye Terminator Cycle Sequencing kit, version 3.1 (Applied Biosystems, CA, USA) with ABI PRISM DNA Sequence Analysis Software version 2.6. The results were analyzed using the Chromas software system.
Allele-specific PCR for detection of the BRAF V600E allele
To confirm the Sanger sequencing results we performed sensitive allele-specific PCR (AS-PCR). The PCR product was obtained using separate primer combinations for mutant and WT alleles. The mutation-specific primer: 5’-gtgattttggtctagctacaga-3 and WT-specific primer were separately combined with the same reverse primer: 5’-ggccaaaaatttaatcagtgga-3 in two different tubes. These primers are optimized for V600E BRAF mutation detection [14]. The thermal cycling conditions and the positive control bearing a heterogeneous V600E mutation were the same as described previously [14]. PCR products were separated by electrophoresis on 2% agarose gel stained with GelRed nucleic acid (Biotinum, Inc., USA) and visualized on an UV transilluminator.
Droplet digital PCR
To prepare the ddPCR reaction mixture we first created optimal amplification conditions for thermal cyclization for each test. Separation of mutation-negative and mutation-positive droplets was carried out using the BRAF V600E mutated RKO cell line (obtained from Dr. N.A.P. Franken, Academic Medical Center, University of Amsterdam, The Netherlands) diluted to 40 ng/μL (Table 1 and Figure 1); the WT DNA sample was also diluted to 40 ng/μL. The Taq polymerase reaction mixture consisted of 2 x ddPCR Supermix for Probes (BioRad), 20 x assay PrimePCR™ ddPCR™ Mutation Assay Kit BRAF WT for p.V600E, and BRAF p.V600E (Bio-Rad Laboratories). Subsequently we prepared seven different dilutions of DNA from the RKO line. A 1 μL aliquot of each dilution and 1 uL of WT DNA were mixed with MasterMix from the first step for a total volume of 20 μL. After this optimization the FFPE-derived DNA samples were analyzed. We prepared 20 μL aliquots of ddPCR reaction mixture consisting of 18 μL MasterMix (16 μL Supermix for probes [no deoxyuridine triphosphate], 1 μL FAM probe and 1 μL HEX probe) and 2 μL of DNA sample isolated from FFPE materials. The negative control (NTC) contained 2 μL of purified water. The WT-only samples contained 2 μL of DNA from FFPE samples. ddPCR was performed using the QX200TM ddPCR system according to the manufacturer’s instructions (Bio-Rad Laboratories).
Table 1.
Mutation analysis was performed by the characterized RKO line in varying dilutions
| Sample | Type template | Concentration of RKO line in copies/μL, duplicate |
|---|---|---|
| NTC-negative control | MUTANT | 0 |
| WILD-TYPE | 0; 184 | |
| 50% MUT | MUTANT | 438; 421 |
| WILD-TYPE | 444; 421 | |
| 1:10 MUT | MUTANT | 34.1; 36.4 |
| WILD-TYPE | 234; 251 | |
| 1:100 MUT | MUTANT | 3.1; 3.82 |
| WILD-TYPE | 203; 227 | |
| 1:1000 MUT | MUTANT | 0.307; 0.182 |
| WILD-TYPE | 198; 21.2 | |
| 1:10000 MUT | MUTANT | 0; 0.185 |
| WILD-TYPE | 211; 175 | |
| 1:100000 MUT | MUTANT | 0; 0 |
| WILD-TYPE | 184; 207 | |
| 1:1000000 MUT | MUTANT | 0; 0 |
| WILD-TYPE | 198; 215 | |
| Positive control RKO line | MUTANT | 389 |
| WILD-TYPE | 193 |
The mutation was detected with 0.001% sensitivity using ddPCR.
Figure 1.
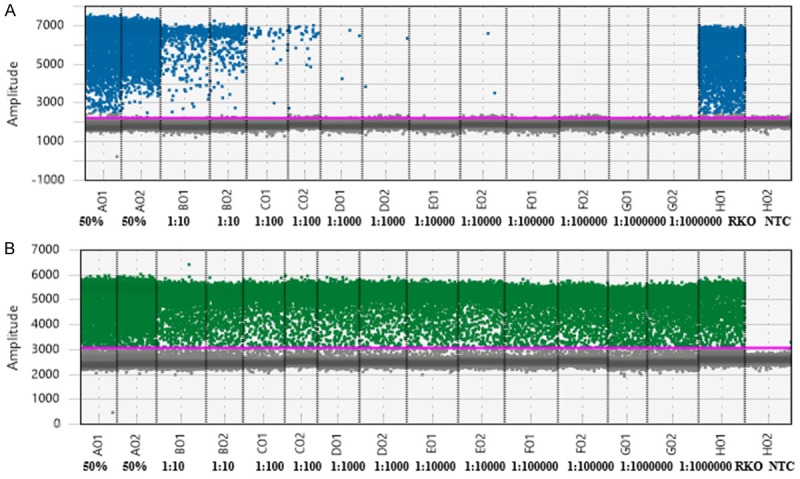
One-dimensional plot of ddPCR showing the sensitivity of this assay. Droplets contained serial dilutions of RKO (cell line homozygous for BRAF V600E) genomic DNA in human genomic DNA without BRAF V600E mutations. Channel A (A): FAM-BRAF V600E probe for detection of BRAF V600E-mutated amplicons in duplicates (50% MUT; 1:10; 1:100; 1:1000; 1:10000; 1:100000; 1:1000000). Chanel B (B): VIC-wild-type BRAF probe for detection of wild-type amplicons without BRAF V600E mutation in duplicates (50% MUT; 1:10; 1:100; 1:1000; 1:10000; 1:100000; 1:1000000). The samples are divided by dotted grey lines. The unbroken pink line is the no-amplification threshold: positive droplets (blue and green) are above and negative droplets (grey) below.
The creation of droplets in the QX200™ droplet generator
The DG8 (BioRad) cartridge was filled with 20 μL of MasterMix from the previous step and 70 μL of the oil droplet generator for each sample. After emulsification of samples to generate DNA droplets we transferred 40 μL of the resulting emulsion to a 96-well plate in accordance with the manufacturer’s instructions. The 96-well plate was secured on the support block and covered with 1 sheet of special pierceable foil seal.
ddPCR: the T100 ™ thermal cycler
Droplets are transferred to a 96-well plate for PCR in a thermal cycler. The reaction was run in each microbead according to the following protocol: activation of polymerase (95°C 10 min); 40 cycles consisting of the following sequence of steps: denaturation (94°C 30 sec), annealing/extension (58°C 60 sec) and final extension (98°C 10 min) with ramps of 2.5°C/sec. PCR products were held at 4°C until the next step of analysis.
Evaluation of the results with the QX200™ droplet reader
After completion of the reaction the ddPCR plate was inserted into the droplet reader, which analyzed the droplets in each well. Analysis of the ddPCR data was performed with the analytical software QuantaSoftTM Analysis Pro (Bio-Rad Laboratories) using two probes: one which emitted an FAM fluorescence signal in the presence of the mutated target and a second which emitted a HEX fluorescence signal in the presence of the WT target. Serial dilutions of mutant genomic DNA labeled with FAM probe and WT DNA with a HEX-labeled probe were performed in duplicate. Each droplet in a sample is plotted on a graph of fluorescence intensity vs. droplet number.
Statistical analyses
Data on the following clinical variables were collected for the 87 melanoma specimens: gender, age, localization, type of melanoma, mitotic activity and TNM (Tumor-Node-Metastases classification); summary statistics can be found in Table 2. The presence of the BRAF V600E mutation in FFPE samples was detected using four methods: Sanger sequencing, Cobas® 4800, AS-PCR, and ddPCR, which also quantified the amount of mutated DNA present. Breslow-Clark scores were also available for all patients. Agreement between the methods was assessed with the McNemar test (Table 3). A multinomial logistic model was used to model the dependence of Breslow-Clark score on various potential predictors (gender; age; localization; type of melanoma; mitotic activity; TNM; ddPCR; Sanger; Cobas® 4800; AS-PCR). The Akaike information criterion (AIC) was used to remove irrelevant predictors were removed from the multinomial logit model. Type III ANOVA was used to assess the significance of the predictors in the reduced multinomial logit model [18]. The statistical analysis was performed with R ver. 3.2.2 [19], using the libraries net [20] and car [18]. Results with a p-value of less than 0.05 were considered statistically significant.
Table 2.
Descriptive statistics for the sample
| Statistic | N | Mean | St. Dev. |
|---|---|---|---|
| Age [yr] | 86 | 65.9 | 12.2 |
| Mitotic activity [mf/mm2] | 80 | 8.4 | 8.0 |
| ddPCR [copies/μL] | 87 | 39.0 | 141.7 |
Table 3.
Agreement between pairs of diagnostic methods: p-values for the McNemar test
| COBAS | SANGER | AS-PCR | ddPCR | |
|---|---|---|---|---|
| COBAS | P = 0.33 | P = 0.33 | P = 0.65 | |
| SANGER | P = 1 | P = 0.08 | ||
| AS-PCR | P = 0.06 |
Results
LoD of ddPCR
FFPE samples from all patients were analyzed using QuantaSoftTM Analysis Pro software (BioRad). Three different methods were used to determine the LoD of ddPCR: i) Poisson confidence intervals as described in the BioRad manual [15]; ii) Tzonev’s method [16]; iii) Calibration line with control of type I and type II errors [17]. Tzonev’s method [16] determines LoD in terms of number of events, whereas the other two methods determine LoD in terms of number of copies per microliter (copies/μL). The LoD for the second method [16] was 5 events per well. The other two methods had similar LoDs of approximately 0.2 copies/μL. The three methods corresponded closely as they all identified the same set of 31 positive samples. We investigated the analytical sensitivity of ddPCR using the RKO line containing BRAF V600E, which was serially diluted with WT DNA (range 50% to 0.000001%). The ddPCR was able to detect the mutation with 0.001% sensitivity in Supplementary Information.
Quantification of BRAF V600E
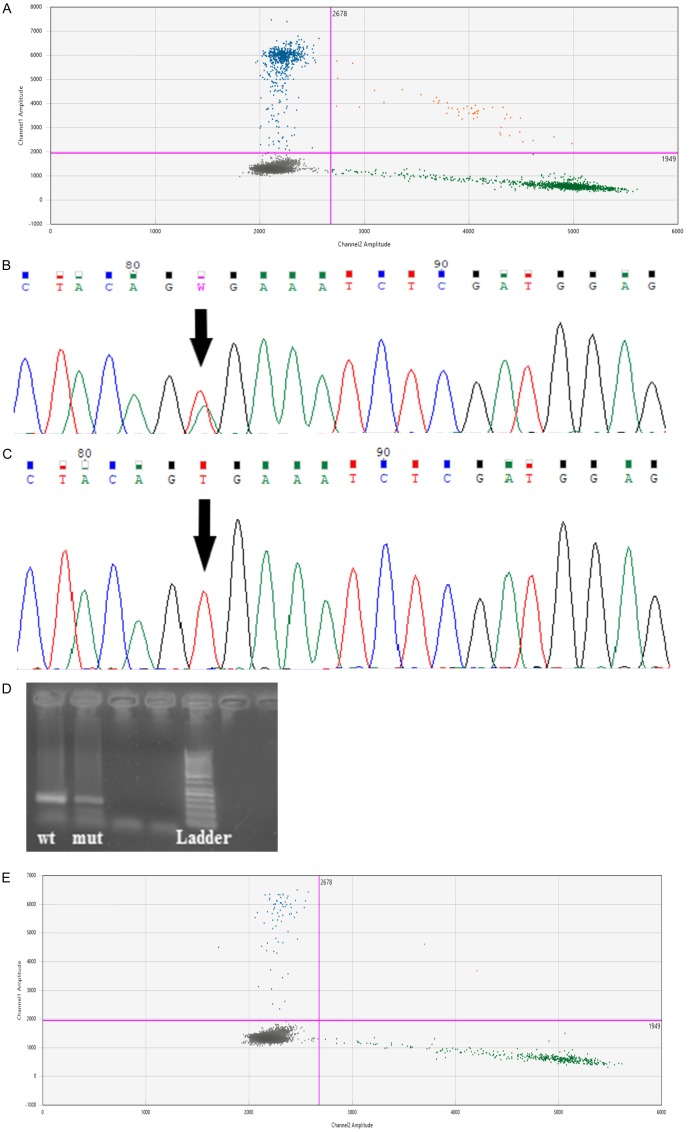
BRAF V600E mutation status was determined by Cobas® 4800, Sanger sequencing, allele-specific PCR and ddPCR. BRAF V600E mutations were found in 31 (35.6%), 23 (26.4%), 23 (26.4%) and 8 (9.2%) samples using ddPCR, allele-specific PCR, Sanger sequencing and Cobas® 4800 respectively (Figure 2). For ddPCR analysis see Figures 2 and 3. The concentration of target DNA in copies/μL was calculated by comparing the numbers of positive and negative droplets in the sample (Figure 3).
Figure 2.
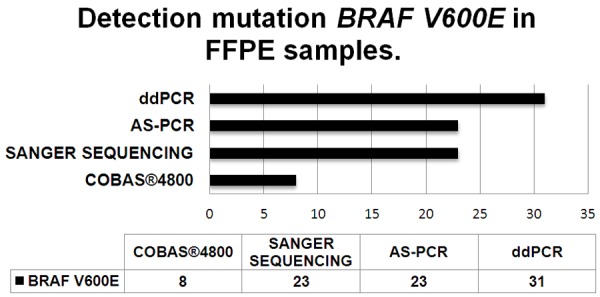
Number of BRAF V600E-positive patients (x-axis) detected with each of four mutation detection methods (ddPCR, AS-Allele-specific PCR, Sanger sequencing, Cobas® 4800).
Figure 3.
These figures shows samples from one patient with malignant melanoma (primary biopsy: A; C versus distant metastases B; D; E). Sample (A) was positive for the BRAF V600E mutation only with droplet digital PCR. The levels of positive and negative droplets in sample (A) were 62.6 and 111 copies/μL respectively and these figures were used to calculate the concentration of target DNA. Sample (C): was negative for the BRAF V600E mutation using Sanger sequencing. Sample (B) was positive for the BRAF V600E mutation by Sanger sequencing (B), allele-specific PCR (D) and droplet digital PCR (E). The levels of positive and negative droplets in sample (D) were 6.21 and 36.6 copies/μL respectively. The blue cluster (FAM-fluorescence signal) represents droplets that were positive for the BRAF V600E mutation and the green cluster (HEX-fluorescence signal) represents droplets that were negative for BRAF V600E. The brown cluster represents double-positive droplets containing both wild-type and mutated DNA and the grey cluster represents droplets containing neither template.
Agreement between the diagnostic methods
The McNemar test was used to assess agreement of diagnostic methods. In none of the pairs did the result provide grounds to reject the null hypothesis of agreement (i.e., marginal homogeneity); the data are provided in Supplementary Table 1, and the p-values are presented in Table 2. Sanger sequencing and ddPCR produced the same result in 82.6% of BRAF patients and in 81.3% of WT samples (N = 87). Agreement between AS-PCR and ddPCR was 86.9% agreement for BRAF samples and 76.8% agreement for WT samples (N = 87). Agreement between AS-PCR and Sanger sequencing was 91.0% for BRAF and 96.9% for WT (N = 87). Agreement between Sanger sequencing and Cobas® 4800 was 85.7% for BRAF and 84.6% for WT (N = 20). Agreement between AS-PCR and Cobas was also 85.7% for BRAF and 84.6% for WT (N = 20). Agreement between ddPCR and Cobas® 4800 was 88.9% for BRAF and 100% for WT (N = 20).
Predicting the Breslow-Clark grade by a multinomial logistic regression
Age, type of melanoma, TNM, mitotic activity, ddPCR, Sanger, Cobas® 4800 and AS-PCR were entered in a multinomial logistic regression model as predictors of Breslow-Clark grade. Model simplification based on the AIC [18] indicated that age, type of melanoma, mitotic activity and ddPCR were the important predictors. A Type III ANOVA [18] yielded the following p-values, age: P = 0.01517; type of melanoma: P = 0.0000629; mitotic activity: P < 0.000001; ddPCR: P = 0.02266.
Discussion
Advances in our understanding of molecular biology of melanoma, the most aggressive form of skin cancer, are now being transferred to our therapeutic arsenal. The most prevalent mutations in melanoma are BRAF mutations, of which the BRAF V600E mutation (valine is replaced by glutamic acid at amino acid position 600) is the most common and increases MAP/ERK kinase activity [4,5].
Selective BRAF V600E inhibitors like vemurafenib promote tumor regression. Vemurafenib (Zelboraf®) is a tyrosine kinase inhibitor with activity against BRAF kinase. Treatment with vemurafenib was shown to improve survival in patients with BRAF V600E-positive melanoma [6,21] and so molecular testing for the presence of the BRAF V600E mutation should be carried out prior to making decisions about the treatment of melanoma patients Several methods of testing for the BRAF V600E mutation are available, including AS-PCR, Sanger sequencing, Cobas® 4800 and ddPCR [14,22,23]. Real-time PCR on the Rotor-Gene 5plex HRM Instrument [24] can also be used to detect low percentages of somatic BRAF mutations.
However, only a few studies have used these methods in FFPE specimens for which a definitive histological diagnosis is available. Here we compared the sensitivity of ddPCR and all commonly used molecular analytical methods [25] for BRAF V600E mutation detection in from the largest consecutive cohort of malignant melanoma specimens investigated to date. Although several recent studies had concluded that ddPCR is a reliable, fast and sensitive technique for detection of BRAF V600E mutations it had not been compared directly with other detection methods in FFPE melanoma specimens and this is what we have done. We calculated the pair-wise agreement between four widely used methods for detecting the BRAF V600E mutation using the McNemar test. The results indicated good agreement between all four methods about the presence of the BRAF V600E mutation (all p-values for the McNemar test above 0.05). Nevertheless we found that Sanger sequencing, Cobas® 4800 instrument, and AS-PCR methods could miss low allelic BRAF V600E burdens in tumor cells. In our sample there were 8 patients with melanomas in which the BRAF V600E mutation was only detected with ddPCR. These patients could have benefited from vemurafenib therapy. In two of these eight patients BRAF V600E mutations were detected in the primary tumor but the distant metastases had a greater allelic BRAF V600E mutation burden which was detected with the other methods as well as with ddPCR. It has been shown that melanomas can be polyclonal for the BRAF V600E mutation [26]; hence the low concentration of BRAF V600E-positive tumor cells in the primary tumors of the two above-mentioned patients which, nevertheless, accounted for the aggressive behavior of these tumors and the distant metastases. Our sample also contained one patient with a primary tumor in which BRAF V600E was undetectable who went on to develop metastases with BRAF V600E-positive tumor cells. In this patient the BRAF V600E mutation was detected only with ddPCR and only in the metastases, so in this instance we could not detect extremely low number of BRAF V600E positive tumor cells in the primary lesion and subsequent clonal evolution of BRAF V600E in the metastatic lesion. Furthermore, five of the eight melanoma patients whose tumors were only identified as BRAF V600E-positive using ddPCR later developed sentinel lymph nodes metastases, attesting to the greater aggression of BRAF V600E-positive melanoma clones. On the basis of our results, we recommend that ddPCR should be the primary method of detecting and monitoring BRAF V600E-mutated melanomas. Finally, we have shown that the allelic burden of mutated BRAF V600E as measured by ddPCR is a significant predictor of Breslow-Clark score in melanoma, along with age, mitotic activity and histological type. We also showed that quantitative allelic burden as assessed by other methods (i.e. Sanger sequencing, Cobas® 4800 and AS-PCR) does not predict Breslow-Clark score. ddPCR is suitable for routine laboratory detection of BRAF V600E mutation not only in fresh biopsy specimens archived diagnostic material such as FFPE specimens. ddPCR is thus a valuable tool not only for initial diagnosis and patient follow-up, but also for retrospective analyses.
Acknowledgements
We are sincerely grateful to all the patients who participated in this research. This work was supported by the “Biomedical Center Martin (BioMed Martin)” ITMS code 26220220187 project which is co-financed by EU sources and the Slovak Research and Development Agency under contracts no. APVV-16-0066 and no. APVV-14-0273.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.World Health Organization. Ultraviolet radiation, Skin cancer. Geneva: 2018. [Google Scholar]
- 2.Davies H, Bignell GR, COX C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks N, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JWC, Leung SY, Yuen ST, Weber BL, Siegler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 3.Smalley KS. Understanding melanoma signaling networks as the basis for molecular targeted therapy. J Invest Dermatol. 2010;130:28–37. doi: 10.1038/jid.2009.177. [DOI] [PubMed] [Google Scholar]
- 4.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O’Dwyer PJ, Lee RJ, Grippo JF, Nolop K, Chapman PB. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satyamoorthy K, Li G, Gerrero MR, Brose MS, Volpe P, Webe B, Van Belle P, Elder DE, Herly M. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res. 2003;63:756–759. [PubMed] [Google Scholar]
- 6.Chapman PB, Hauschild A, Robert C, Haane JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorrigan O, Lebbe C, Jouary T, Schadendorf D, Ribas A, O’Day SJ, Sosman JA, Kirkwood JM, Eggermont AM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur GA BRIM-3 Study Group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudecova I. Digital PCR analysis of circulating nucleic acids. Clin Biochem. 2015;48:948–956. doi: 10.1016/j.clinbiochem.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Day E, Dear PH, McCaughan F. Digital PCR strategies in the development and analysis of molecular biomarkers for personalized medicine. Methods. 2013;59:101–107. doi: 10.1016/j.ymeth.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Dube S, Qin J, Ramakrishnan R. Mathematical analysis of copy number variation in a DNA sample using digital PCR on a nanofluidic device. PLoS One. 2008;3:e2876. doi: 10.1371/journal.pone.0002876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weaver S, Dube S, Mir A, Qin J, Sun G, Ramakrishnan R, Jones RC, Lival KJ. Taking qPCR to a higher level: analysis of CNV reveals the power of high throughput qPCR to enhance quantitative resolution. Methods. 2010;50:271–276. doi: 10.1016/j.ymeth.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Hindson BJ, Ness KD, Masquelier DS, Belgrader P, Heredia NJ, Makarewicz AJ, Bright IJ, Lucero MY, Hiddessen AL, Legler TC, Kitano TK, Hodel MR, Petersen JF, Wyatt PW, Steenblock ER, Shah PH, Bousse LJ, Troup CB, Mellen JC, Wittmann DK, Erndt NG, Cauley TH, Koehler RT, So AP, Dube S, Rose KA, Montesclaros L, Wang S, Stumbo DP, Hodges SP, Romine S, Milanovich FP, White HE, Regan JF, Karlin-Neumann GA, Hindson CM, Saxonov S, Colston BW. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem. 2011;83:8604–8610. doi: 10.1021/ac202028g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogelstein B, Kinzler KW. Digital PCR. Proc Natl Acad Sci U S A. 1999;96:9236–9241. doi: 10.1073/pnas.96.16.9236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker Digital PCR hits its stride. Nat Methods. 2012;9:541–544. [Google Scholar]
- 14.Jasek K, Buzalkova V, Minarik G, Stanclova A, Szepe P, Plank L, Lasabova Z. Detection of mutations in the BRAF gene in patients with KIT and PDGFRA wild-type gastrointestinal stromal tumors. Virchows Arch. 2017;470:29–36. doi: 10.1007/s00428-016-2044-4. [DOI] [PubMed] [Google Scholar]
- 15.Biorad: Droplet Digital PCR (Applications Guide) Accessible at http://www.biorad.com/webroot/web/pdf/lsr/literature/Bulletin_6407.pdf; accessed 25.1.2018.
- 16.Tzonev S. Sensitivity, specificity and limit of detection in dPCRBIO-RAD; (Powerpoint slides) 2016, Retrieved from Eddie van Collenburg, DBC Summit. [Google Scholar]
- 17.Massart DL, Vandenginste BGM, Buydens LMC, De Jong S, Lewi PJ, Smeyers-Verbeke J. Chapter title. Handbook of chemometrics and qualimetrics: Part A. Amsterdam: Elsevier; 1997. [Google Scholar]
- 18.Fox J, Weisberg S. An {R} companion to applied regression. 2nd ed. Thousand Oaks CA: Sage; 2011. URL: http://socserv.socsci.mcmaster.ca/jfox/Books/Companion. [Google Scholar]
- 19.R Core Team R. A language and environment for statistic al computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. URL https://www.R-project.org/ [Google Scholar]
- 20.Venables WN, Ripley BD. Modern Applied Statistics with S. 4th edition. NewYork: Springer; 2002. [Google Scholar]
- 21.Miller DM, Flaherty KT. Cyclin-dependent kinases as therapeutic targets in melanoma. Pigment Cell Melanoma Res. 2014;26:351–365. doi: 10.1111/pcmr.12211. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Rios F, Angulo B, Gomez B, Mair D, Martinez R, Conde E, Shieh F, Vaks J, Langland R, Lawrence HJ, de Castro DG. Comparison of testing methods for the detection of BRAF V600E mutations in malignant melanoma: pre-approval validation study of the companion diagnostic test for vemurafenib. PLoS One. 2012;8:e53733. doi: 10.1371/journal.pone.0053733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanmamed MF, Fernández-Landázuri S, Rodriguéz C, Zárate R, Lozano MD, Zubiri L, Perez-Gracia JL, Martín-Algarra S, González A. Quantitative cell-free circulating BRAFV600E mutation analysis by use of droplet digital PCR in the follow-up of patients with melanoma being treated with BRAF inhibitors. Clin Chem. 2015;61:297–304. doi: 10.1373/clinchem.2014.230235. [DOI] [PubMed] [Google Scholar]
- 24.Hugdahl E, Kalvenes MB, Puntervoll HE, Ladstein RG, Akslen LA. BRAF-V600E expression in primary nodular melanoma is associated with aggressive tumour features and reduced survival. Br J Cancer. 2016;114:801–808. doi: 10.1038/bjc.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McEvoy AC, WooD BA, Ardakani NM, Pereira M, Pearce R, Cowell L, Grieu-lacopetta F, Spicer AJ, Amanuel B, Ziman M, Gray AS. Droplet digital pcr for mutation detection in formalin-fixed, paraffin-embedded melanoma tissues: a comparison with sanger sequencing and pyrosequencing. J Mol Diag. 2018;20:240–252. doi: 10.1016/j.jmoldx.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Yancovitz M, Litterman A, Yoon J, Ng E, Shapiro RL, Berman RS, Pavlick AC, Darvishian F, Christos P, Mazumdar M, Osman I, Polsky D. Intraand inter-tumor heterogeneity of BRAF (V600E) mutations in primary and metastatic melanoma. PLoS One. 2012;7:e29336. doi: 10.1371/journal.pone.0029336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.