Abstract
Hair follicle stem cells (HFSCs) are an important source for skin tissue engineering studies and clinical applications. Here, we describe a differential enrichment approach to derive HFSCs from hair follicles of vibrissae and ear skin using the Rho-associated protein kinase (ROCK) inhibitor Y-27632. In the presence of Y-27632, primary cultured hair follicle cells grew in clustered colonies surrounded by keratinocyte-like cells and simultaneously expressed three HFSC markers: CD34, K15, and ITGB1. HFSCs cultured in medium containing Y-27632 were presented at a stable ratio of 30.7%, 34.1%, and 32.9% after passages 5, 10, and 15, respectively. By contrast, in medium containing epidermal growth factor, clustered HFSC colonies disappeared after 6 passages and lacked HFSC marker expression. After withdrawal of Y-27632 from the medium, HFSCs rapidly differentiated into keratinocyte-like cells. Furthermore, HFSCs derived with Y-27632 formed spherical clusters in collagen matrix in vitro, differentiated into keratinocytes and adipose cells under in vitro induction conditions, and cooperated with fetal dermal cells to regenerate hair follicles in vivo 6 weeks after their intracutaneous injection into immune-deficient mice. These findings suggest that Y-27632 maintains the self-renewal and stemness characteristics of HFSCs during primary skin tissue culture followed by enrichment passaging and that HFSCs derived with Y-27632 possess the differentiation potentials important for tissue engineering and other clinical applications.
Keywords: Hair follicular stem cells, ROCK inhibitor, differentiation, pluripotency, tissue engineering
Introduction
The skin and its appendages constitute the largest organ of the body. Stratified epithelia in the skin offer protection from environmental stresses including dehydration, irradiation, mechanical trauma, and pathogenic infection, whereas its appendages (e.g., hair, sebaceous glands) help regulate body temperature and influence animal interaction and social behaviors via camouflage and sexual signaling. The hair follicle comprises an intriguing sub-organ in the skin [1], as hair follicle growth and development involve many physiological phenomena such as the control of cell growth, mesenchymal-epithelial interactions, epithelial differentiation, and hormone action [2,3]. Hair follicles also play a central role in the processes of skin homeostasis, wound healing, and tumorigenesis [4-6].
Hair follicle stem cells (HFSCs) are recognized as outer root sheath cells in the bulge. HFSCs sustain an intact hair follicle structure during continuous hair cycling [7]. HFSCs also play a critical role in skin wound repair and tissue regeneration [8,9] and express markers such as CD34, keratin 15 (K15), integrin beta 1 (ITGB1), and CD200 [6,10,11]. Primary culture of HFSCs acquired from hair follicles in the presence of epidermal growth factor (EGF) is the most common approach to obtaining pluripotent cells [12,13], but cells generated under these conditions have poor proliferative ability and tend to differentiate after several passages in vitro [14]. Thus, new methods for generating and propagating HFSCs are needed.
Previous reports indicate that serine/threonine kinases, including Rho-associated protein kinase (ROCK), contribute to the regulation of keratinocyte differentiation [15,16]. In particular, the ROCK inhibitor Y-27632 plays a significant role in skin wound healing by enhancing cell proliferation, promoting epithelial differentiation, and negatively modulating cell migration and cell adhesion [17]. Y-27632 is widely used to protect cultured embryonic stem cells from death during cell passage [18]. Recently, Y-27632 was found to inhibit differentiation and maintain the stem cell phenotype of keratinocytes, greatly increasing their proliferative capacity and allowing efficient immortalization [15,16,19]. Here, we explore a new approach to isolating HFSCs by determining the impact of Y-2763 on HFSC maintenance, proliferation, and differentiation.
Materials and methods
Animals and materials
Animal maintenance, care, and use procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Nanjing Normal University. The experimental procedures in this study were in accordance with the Guiding Principles for the Care and Use of Laboratory Animals.
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise indicated. Basal medium consisted of Dulbecco’s Modified Eagle’s Medium (DMEM; 11995-065, Gibco) and 15% fetal bovine serum (FBS; SH30070.03, HyClone). As experimental treatments, basal medium was supplemented 10 μM Y-27632 or 10 ng/mL EGF.
Primary culture, differential enrichment, and maintenance of HFSCs
Skin and vibrissae tissue were micro-dissected for isolation of HFSCs from New Zealand White female rabbits at 5-8 months of age [20,21]. Briefly, for skin tissue, after shearing, sterile treatment, and removal of cartilage by forceps, dorsal ear skin was dissected into 2 mm2 pieces by scissors, transferred into 35-mm cell culture dishes with the dermis side down, and covered by cover slips for stabilization. For vibrissae tissue, individual hair follicles were carefully dissected from the upper-lip skin with forceps and 25 G syringe tips, transferred into 35-mm cell culture dishes, and covered by cover slips for stabilization. A volume of 3 mL of basal, Y-27632, or EGF medium was added to each culture dish. Tissues were cultured at 37°C in 5% CO2 humidified air, changing the medium every 2 days. After 7-10 days of culture, many primary cells had spread from the tissues, which were further cultured for 2-3 days under the same conditions and passaged into 60-mm dishes every 3-4 days. For cell passaging, upon reaching 80% confluency, cells were digested using 0.25% trypsin and centrifuged at 1000 rpm for 5 min. Cell pellets were suspended in the corresponding medium and plated into 100-mm cell culture dishes.
We developed a differential enrichment approach to collect a large number of HFSCs from a mixed population of HFSCs, keratinocytes, and other cell types. This differential enrichment approach takes advantage of the property of delayed adhesion of HFSCs to the surface of a culture dish. In detail, primary cultured and suspended cells were plated into 35-mm dishes after trypsin digestion and incubated for 15 min at 37°C to allow a portion of cells to attach to the dish (pre-attached cells). After 15 min of incubation, the culture medium containing unattached and suspended cells was collected and transferred to new 35-mm dishes until the cells attached to the culture dish (delay-attached cells). These two batches of differential enrichment-derived cell populations were cultured in Y-27632 medium. After 2 days of culture, more pre-attached cells grew as keratinocyte-like cells, whereas a large amount of delay-attached cells grew in clustered colonies with the properties of HFSCs. Enriched HFSCs were maintained by passaging every 3-4 days.
Immunofluorescence identification of HFSCs
To determine the lineage of the derived cells, cells from passage 3 (P3), P5, P10, and P15 underwent immunofluorescent staining for the HFSC pluripotent markers CD34 (sc-7045, Santa Cruz Biotechnology, Dallas, TX, USA), K15 (ab80522, Abcam, Cambridge, MA, USA), and ITGB1 (sc-9970, Santa Cruz Biotechnology) [20]. Briefly, cells were fixed in 4% paraformaldehyde (PFA) and rinsed with phosphate-buffered saline (PBS) three times. Non-specific labeling was blocked by incubation with 3% bovine serum albumin (BSA) and 0.1% Triton-X 100 for 15 min at room temperature. Cells were incubated with primary antibodies at 4°C overnight with a dilution of 1:100 for CD34, 1:200 for K15, and 1:100 for ITGB1 in PBS containing 1% BSA, respectively. After three 5-min washes in PBS, treated cells were incubated in secondary goat anti-mouse IgG-FITC antibody (1:400, Santa Cruz Biotechnology) at 37°C for 1 h. After washing in PBS, cell nuclei were counterstained with 10 µg/mL 4,6-diamidine-2-phenylindole dihydrochloride (DAPI). An inverted fluorescence microscope was used for observation and imaging.
Effect of Y-27632 concentration on HFSC stemness
As we found that CD34, K15, and ITGB1 antibodies identically labeled HFSCs, only one marker was used to identify HFSCs in further experiments. To determine the effect of Y-27632 concentration on HFSC maintenance, enriched HFSCs were cultured in medium containing Y-27632 at a concentration of 0, 0.5, 1, 2.5, 5, 10, 20, or 50 μM. A total of 1 × 104 enriched cells at P5 containing HFSCs were plated in each well of a 24-well culture dish. After 3 days in culture, cells were stained with anti-K15 primary and secondary antibodies, and cell nuclei were stained with DAPI. The total number of cells and the number of K15-positive cells were counted under fluorescence microscopy.
Long-term passage of HFSCs in Y-27632 culture
To determine the long-term effects of Y-27632 on HFSCs derived from primary culture, HFSCs were cultured for up to 15 passages in medium containing 10 µM Y-27632. Cells were passaged every 3-4 days. Upon reaching 80% confluence, cells were digested with 0.25% trypsin and collected by centrifugation. Cells were re-suspended in Y-27632 medium and plated in a new culture dish. The medium was changed every other day. At P5, P10, and P15, anti-K15 was detected by fluorescence microscopy. The proportion of K15-positive HFSCs was calculated as the number of K15-positive cells divided by the total number of DAPI-stained nuclei.
Assessment of HFSC cell-autologous differentiation after Y-27632 removal
After passaging and culture for 12 h in medium containing 10 µM Y-27632, P5 HFSCs were allowed to reach 40% confluence, were washed three times with PBS, and fresh basal medium without Y-27632 was added. After 72 h, cells were labeled with anti-K15 and DAPI to determine HFSC differentiation. K15-labeled HFSCs and total cell number were counted.
Enrichment and construction of HFSC spheroidal clusters in in vitro 2-D and 3-D systems
For 2-D culture, P10 HFSCs were plated in collagen-coated 35-mm culture dishes. Briefly, collagen solution was prepared by dissolving 1 g freshly prepared rat tail collagen into 50 mL 0.1% acetic acid solution. A volume of 0.5 mL collagen solution was added to the culture dish overnight to form a thin collagen layer. Prior to use, the coated dish was rinsed with PBS three times. A volume of 2 mL containing a total of 1 × 105 HFSCs was plated into each coated dish, cultured in medium containing 10 µM Y-27632, and incubated at 37°C in 5% CO2 humidified air. The medium was replaced every 3 d. After 15 d, the morphology of cell clusters was examined. Cells were labeled with anti-CD34 to identify HFSCs.
For 3-D culture, P5 HFSCs were mixed with collagen solution to form a 3-D culture system [22]. Briefly, collagen solution was adjusted to pH 7.2 by 0.1 M NaOH. A total of 2 × 105/mL HFSCs were suspended in Y-27632 medium mixed 1:1 with 1 mL collagen solution (2 mL) and plated into 35-mm cell culture dishes. The cell mixture was allowed to solidify for 30 min, overlaid with 2 mL Y-27632 medium, and incubated at 37°C in 5% CO2 humidified air. Fresh Y-27632 medium was replaced the next day after initial seeding and every other day thereafter. After 10 d, spherical colonies were examined.
Differentiation of HFSCs into adipose cells in vitro
To investigate HFSC multi-lineage differentiation potential in vitro, P10 HFSCs were cultured in adipocyte induction medium (DMEM/F12, 10% FBS, 1 × ITS, 50 μg/mL 1-phosphate ascorbic acid, 100 nM dexamethasone, and 50 μg/mL indomethacin), and medium was replaced every 2 d. After 5-7 d in culture, cellular fat droplets were stained using Oil Red O [23]. Briefly, cells were fixed in 4% PFA for 30 min at room temperature and rinsed with PBS three times. After rinsing with 60% isopropanol, cells were stained with freshly prepared Oil Red O working solution for 5 min. Cells were rinsed with 60% isopropanol and distilled water. Cells were imaged using a phase contrast microscope.
Regeneration of hair follicles with HFSCs in vivo
To investigate the ability of HFSCs to generate hair follicles in vivo, four groups were arranged for this experiment as (1) a total of 5 × 106 P10 HFSCs, (2) an equal quantity of pre-prepared murine fetal dermal cells (C57BL/6, Charles River, Beijing, China), (3) a mixture of P10 HFSCs and dermal cells, and (4) PBS only (controls) in 100 µL medium, respectively, were injected intracutaneously into the hairless dorsal dermis of immune-deficient mice (8-week-old BALB/c immune-deficient nude mice, Charles River) [20]. After 6 weeks, the skin at the injection spot was dissected, and the growth of hair follicles was confirmed in frozen sections via hematoxylin and eosin (HE) staining. Briefly, after rinsing in PBS three times, dissected skin was fixed in 4% PFA for 30 min, embedded in OCT, and stored at -80°C until sectioning. The frozen tissue block was sectioned at 7-μm thickness using a Leica CM1950 cryostat. HE staining was performed according to the manufacturer’s instructions (HE staining kit, AR1180, Boster, China).
Statistical analyses
The proportion of HFSCs was calculated as the number of CD34- or K15-positive cells out of the total number of DAPI-stained cells and was analyzed using SPSS software (SPSS 18.0, IBM). All data in each replicate were arcsine transformed and subjected to one-way ANOVA. Means were compared using Fisher’s least significant difference test (LSD). Statistical significance was defined as P < 0.05.
Results
ROCK inhibitor Y-27632 promotes HFSC primary culture in vitro
Hair follicles obtained from vibrissae tissue were cultured in basal medium supplemented with 10 µM ROCK inhibitor Y-27632. At P0, cobblestone-like cells grew from the bulge region after 3 d (Figure 1A), on top of which many clustered colonies formed after P1 (Figure 1B).
Figure 1.
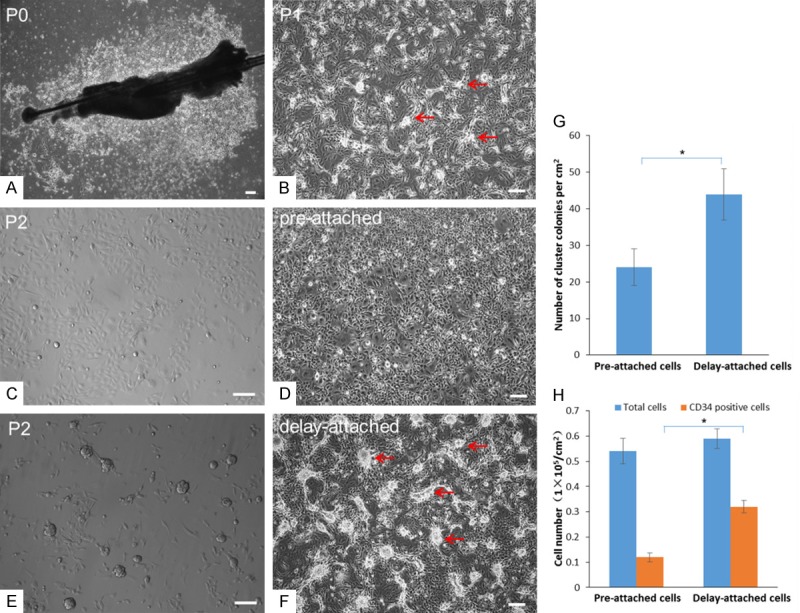
Primary culture of HFSCs using the differential enrichment approach. (A) Hair follicles were cultured in media containing Y-27632 for 7 d. At P0, cobblestone-like cells grew from the tissues. (B) After P1, clustered colonies formed on the layer of cobblestone-like cells (arrows). After P2, pre-attached cells (15 min after first plating) were cultured continuously in the original dish for 48 h, resulting in (C) many keratinocyte-type cells but (D) few HFSCs. (E) Cells still suspended in the medium 15 min after first plating were collected and re-plated in a new dish. (F) After 48 h, these delay-attached cells formed many clustered colonies (arrows). (G) The density of clustered colonies was significantly lower for pre-attached cells than for delay-attached cells after further culture. (H) There were significantly more CD34-labeled cells among delay-attached cells than among pre-attached cells. Scale bar = 10 µm. *P < 0.05.
We next attempted to enrich the clustered colony cells from the mixed cell population treated with Y-27632 using a differential enrichment approach. After trypsin digestion and 15 min of cell plating, P2 cells still suspended in medium were re-plated into new culture dishes. We found that pre-attached cells, which quickly adhered to the culture dishes during first 15 min of cell plating (Figure 1C), grew as keratinocyte-like cells within 48 h of culture (Figure 1D). By contrast, delay-attached cells, which did not adhere in the first 15 min and were later re-plated, grew in clustered colonies (Figure 1E). Within 48 h, cobblestone-like cells stretched out from the periphery of the clustered colonies (Figure 1F), and, after P3, the cultured cells appeared as clustered colonies comprised of cells with keratinocyte-like morphology. Compared with pre-attached cells, delay-attached cells showed significantly higher numbers of clustered colonies (24 per cm2 vs. 44 per cm2, P < 0.05, Figure 1G) and CD34-positive cells (1.2 × 104 vs. 3.2 × 104 per cm2, P < 0.05, Figure 1H).
Similar to our observations of vibrissae tissue cultures, many clustered colonies grew from hair follicles obtained from ear skin in Y-27632 medium after culturing for 7 days. These cells formed 35-42 clustered colonies with 2.4-3.5 × 104 CD34-positive cells per cm2 up to P15, and the morphology of HFSCs derived from ear skin was similar to that achieved from vibrissae tissue.
Clustered cells derived from Y-27632 culture possess HFSC characteristics
To identify cell types, cultured skin cells were detected by immunofluorescence staining for the HFSC markers CD34, K15, and ITGB1. CD34 and ITGB1 were mainly located on the cell membrane, whereas K15 was located in the cytoplasm. In Y-27632-treated cultures, clustered colonies after P3 expressed CD34, K15, and ITGB1 markers simultaneously (Figure 2A1-C4) and retained expression of HFSC markers up to P15 (Figure 3B1-B3). In basal medium without Y-27632 or EGF, only fibroblasts grew from the primary cultures after P3 (Figure 3A1-A3). Cells cultured in basal medium supplemented with 10 ng/mL EGF exhibited a polygon or fusiform shape with cobblestone-like morphology, forming a monolayer with fewer clustered colonies after P3 (Figure 3C1-C3). In addition, all EGF-treated cells transformed into cobblestone-like cells after P6 (Figure 3D1-D3).
Figure 2.
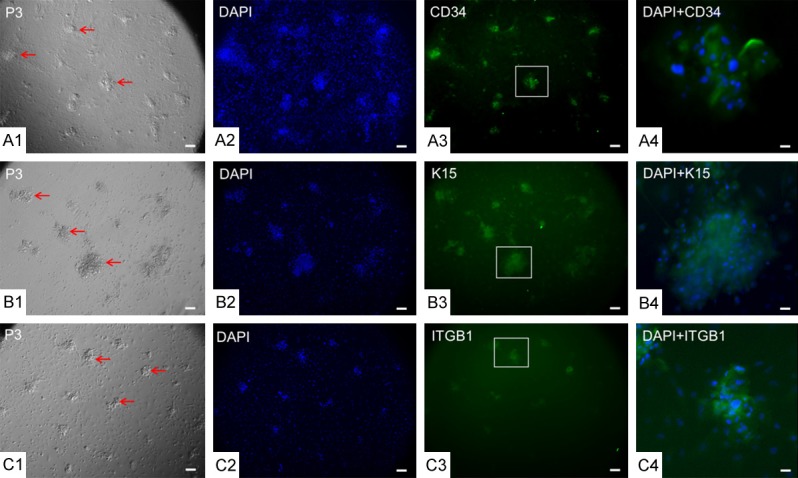
Clustered cells derived from Y-27632 represent HFSC characteristics. After P3, cells cultured with Y-27632 underwent immunofluorescence staining with HFSC-specific antibodies for (A1-A4) CD34, (B1-B4) K15, and (C1-C4) ITGB1. Cell nuclei were stained with DAPI. Colonies marked in a box in (A3, B3, and C3) were magnified 6 × in (A4, B4, and C4). CD34 and ITGB1 were located on cell membranes, whereas K15 was located in the cytoplasm. Scale bar = 10 µm in all panels except for (A4, B4, and C4), in which the scale bar = 1.67 µm.
Figure 3.
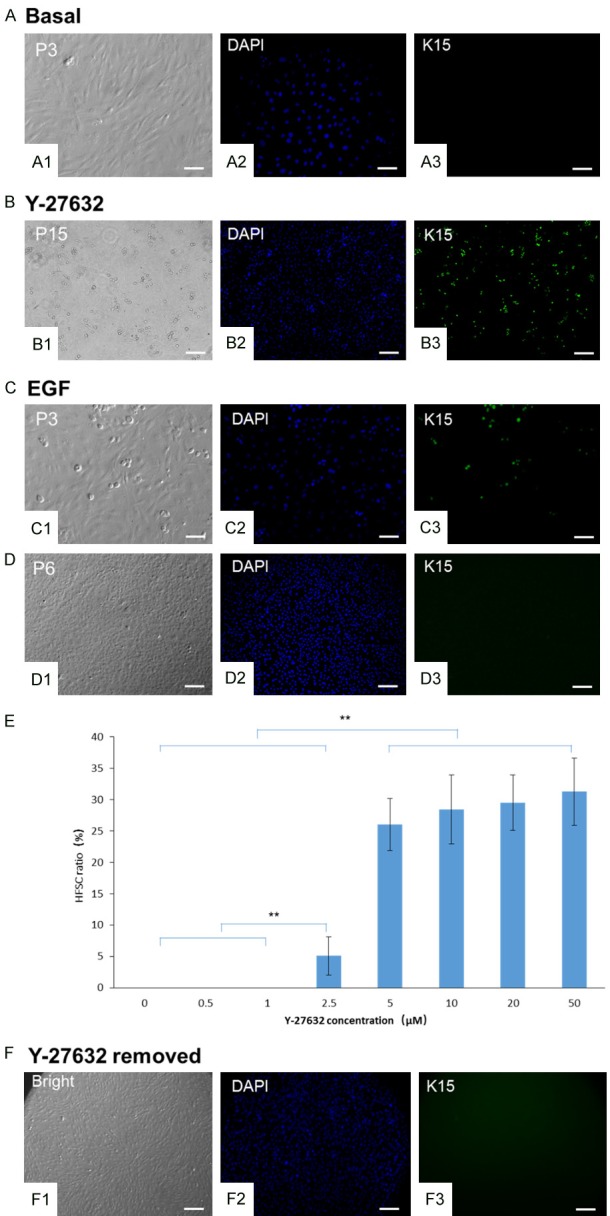
Dose-dependent effect of Y-27632 on HFSC enrichment. HFSCs were cultured in basal, Y-27632, or EGF medium. After P3, fibroblasts grew in basal medium from primary cultures (A1-A3) without the existence of HFSCs. Cells cultured in Y-27632 medium maintained clustered HFSC colonies labeled by anti-K15 until P15 (B1-B3). HFSCs cultured in EGF medium (C1-C3) differentiated into keratinocyte-like cells without K15 expression at P6 (D1-D3). (E) P5 HFSCs were maintained with increasing concentrations of Y-27632 (0, 0.5, 1, 2.5, 5, 10, 20, or 50 μM). After 3 d in culture, HFSCs were labeled by anti-K15. HFSC ratio was calculated as the number of HFSCs divided by the total cell number (**P < 0.01). When Y-27632 was removed from the culture medium, P10 HFSCs differentiated into cells that lacked K15 expression after 3 d (F1-F3). Scale bar = 10 µm.
Dose-dependent and long-term effects of Y-27632 on HFSC maintenance
P5 HFSCs were cultured with increasing concentrations of Y-27632 (0, 0.5, 1, 2.5, 5, 10, 20, and 50 μM). After 3 d in culture, no K15-labeled HFSCs were observed at Y-27632 concentrations of 0, 0.5, or 1 μM (Figure 3E). However, the ratio of K15-labeled HFSCs significantly increased at 2.5 μM Y-27632 (P < 0.01). Furthermore, the K15-labeled HFSC ratio was significantly higher at doses of 5, 10, 20, and 50 μM than at doses of 0, 0.5, 1, and 2.5 μM (P < 0.01). There were no significant differences in K15-labeled HFSC ratios among 5, 10, 20, and 50 μM doses except for a higher HFSC ratio at 10 μM than at 5 μM. At the highest concentration of Y-27532 (50 μM), HFSCs maintained a stable ratio of 31.3% of the total cell population with no reduction in cell viability. Thus, HFSCs were safely maintained at Y-27632 concentrations ranging from 10 to 50 μM, and the optimal concentration of Y-27632 for deriving and culturing HFSCs was determined to be 10 μM.
In this experiment, the initial 1 × 104 total cells at P5 were inoculated into one well of a 24-well dish (area of 2 cm2) at a cell concentration of 0.5 × 104/cm2. After 3 d of culture, total cell density was 6 × 104/cm2, and total cell number was 12 × 104 per well. K15 labeling showed that HFSC density was 1.9 × 104 cells/cm2, and the total HFSC number was 3.8 × 104/well. Therefore, the ratio of HFSCs in the total cell population was 31.7%. Cell number increased 12 times (12 × 104 vs. 1 × 104 cells/well) across 3 d in culture, representing 3.6 cell cycles and a HFSC doubling time of 20 h per cell cycle.
To confirm the necessity of Y-27632 for maintaining HFSCs in long-term culture, P10 HFSCs were cultured in basal medium without Y-27632. Expression of K15 disappeared in the clustered colonies 72 h after Y-27632 withdrawal (Figure 3F1-F3).
We further examined HFSC proliferation and self-renewal during long-term culture with 10 μM Y-27632. HFSCs continued to show K15 expression until P15 (Figure 3B1-B3) with a cell density of 1.8 × 104 cells/cm2 compared with 1.7 × 104 cells/cm2 at P5 and 1.9 × 104 cells/cm2 at P10. The ratio of K15-labeled cells was similar at P5, P10, and P15 (30.7%, 34.1%, and 32.9%, respectively, P > 0.05). We also found that 97.1% of P15 HFSCs in 15 metaphases exhibited a normal karyotype (n = 44, XX). These results suggest that Y-27632 supports the long-term propagation and maintenance of HFSCs in vitro.
We further plated P5 HFSCs on collagen-coated dishes in medium containing 10 µM Y-27632. Large clustered HFSC colonies labeled with CD34 were observed at a density of ~3600 colonies/cm2 after 15 d (Figure 4A, 4B). We also mixed HFSCs with collagen overlaid with medium containing 10 µM Y-27632 to generate a 3-D culture system. Spherical HFSC clusters were observed at a density of ~6400 colonies/cm3 after 10 d (Figure 4C).
Figure 4.
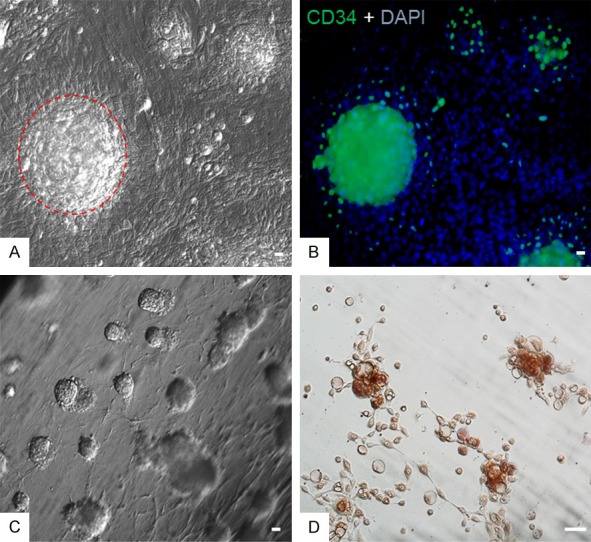
Enrichment and differentiation of HFSCs in vitro. P5 HFSCs were plated in collagen-coated dishes. After 15 d, cells formed as large spherical colonies (the dotted line circle in A) labeled with CD34 (B). P5 HFSCs were mixed with collagen to form a 3-D culture system. After 10 d, abundant spherical cell clusters had formed (C). P10 HFSCs were cultured under induction conditions. Adipose cells differentiated after 3 d of culture as identified by Oil Red O staining (D). Scale bar = 10 µm.
HFSC induction into adipose cells in vitro and generation of hair follicles in vivo
We further attempted to induce these HFSCs into adipose cells. After 2 d of culture under induction media containing ascorbic acid, dexamethasone, and indomethacin, HFSCs differentiated into adipose cells, with fat vesicles observed in cell cytoplasm by staining with Oil Red O (Figure 4D). HFSC marker expression was not observed in induced cells.
P10 HFSCs, dermal cells, or HFSCs mixed with dermal cells were injected intracutaneously into the hairless dorsal skin of immune-deficient nude mice, respectively. After 6 weeks, skin tissues were frozen, sectioned, and stained with HE. Injection of dermal cells alone did not change the thickness of the epidermis or subcutaneous tissue (Figure 5A) compared with injection of HFSCs alone (Figure 5B) or combined injection of dermal cells and HFSCs (Figure 5C). In addition, although injection of dermal cells alone increased the thickness of the dermis (120 ± 11 μm vs. 80 ± 9 μm in PBS injection only, n = 5, P < 0.05), injection of HFSCs alone did not change the thickness of the dermis (87 ± 12 μm vs. 80 ± 9 μm in PBS injection only, n = 5, P > 0.05). No hair bulbs or shafts were observed in the dermis or subcutaneous tissue after injection of either dermal cells alone or HFSCs alone. However, combined injection of dermal cells and HFSCs markedly increased the thickness of the dermis (150 ± 17 μm, n = 6) and resulted in the appearance of hair bulbs and shaft structures aligned in the subcutaneous tissue and distributed in the dermis with a density of 121 ± 23 per cm2 (n = 6).
Figure 5.
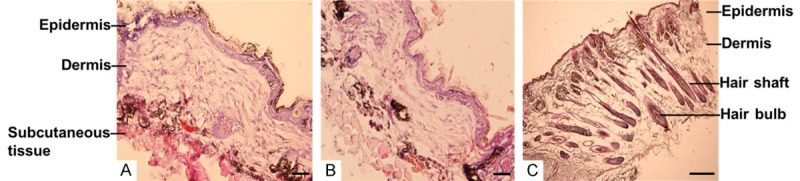
In vivo generation of hair follicles by HFSCs in immune-deficient nude mice. P10 HFSCs mixed with dermal cells were injected intracutaneously into immune-deficient nude mice. After 6 weeks, tissues were biopsied, frozen, sectioned, and assessed by HE staining. No hair structures were observed after injection of dermal cells (A) or HFSCs (B) alone, whereas hair follicles were clearly observed with complete hair bulb and hair shaft structures throughout the subcutaneous tissue, dermis, and epidermis when dermal cells and HFSCs were injected together (C). Scale bar = 30 µm.
Discussion
Our study demonstrates that the ROCK inhibitor Y-27632 facilitates the enrichment and isolation of HFSCs during primary skin tissue culture and subsequent passaging over a long duration. Y-27632 maintained the HFSC characteristics of self-renewal and stemness until P15, with these HFSCs possessing further differentiation properties into defined skin cell types and hair structures.
HFSCs provide an important source for the study of skin tissue engineering and other clinical applications [24,25]. The culture of primary hair follicle cells in the presence of EGF, such as in keratinocyte growth medium or FAD medium, is the main approach used to obtain HFSCs [10,26]. However, HFSCs have poor proliferative capacity and rapidly lose many skin stem cell characteristics, such as expression of HFSC markers, after several passages. Here, we report a differential enrichment procedure for deriving HFSCs from hair follicles of vibrissae and ear skin of rabbits using Y-27632. We found that both Y-27632 and EGF supported the growth of HFSCs from hair follicles, which exhibited a phenotype of clustered colonies in association with keratinocyte-like cells and expressed the HFSC markers CD34, K15, and ITGB1 [25,27]. HFSCs cultured in Y-27632 and EGF medium exhibited similar characteristics after initial passages. However, HFSCs cultured in Y-27632 grew in clustered colonies for up to 15 passages with a stable ratio of K15-labeled cells, whereas HFSCs cultured in EGF grew in clustered colonies only until P5 and then rapidly differentiated into keratinocytes lacking K15 expression.
Recently, Chacon-Martinez et al. have reported that Matrigel or collagen 1 and Y-27632 together with FGF2 and VEGF-A in keratinocyte growth medium (referred to as the 3C system) are sufficient to maintain and expand HFSCs in spheroid cell clusters, showing the robustness of the 3C system in conserving hair follicle-generating ability after 10 passages [14,28]. In the 3C system, 3-D configuration is essential, as there is no enrichment of CD34-positive cells when cells are cultured in 3C medium under standard 2-D conditions. However, we found that HFSCs can be expanded and maintained long-term in Y-27632 medium without other growth factors. Under our conditions, HFSCs formed clustered colonies not only in a 2-D condition (i.e., collagen-coated culture dish) but also in a 3-D condition, in which HFSCs grew into spherical clusters with a morphology similar to that observed by Chacon-Martinez et al. We noted that clustered colonies were inevitably surrounded by keratinocyte-like cells and that when these were removed, new keratinocyte-like cells differentiated from the clustered colonies (data not shown). From our study, HFSCs represented 3.6 cell cycles through 72 h culture and with a HFSCs doubling time of 20 h per cell cycle. Likewise, in accordance with a previous report [28], we found that the ratio of HFSCs expressing K15 was stable at 30.7%, 34.1%, and 32.9% at P5, P10, and P15, respectively, suggesting that HFSCs and non-HFSCs achieve equilibrium in the process of cell propagation.
Our results show that the ROCK inhibitor Y-27632 plays a vital role in HFSC self-renewal and growth. The maintenance of clustered colonies was dependent on a sufficient concentration of Y-27632 (> 5 µM), with a stemness phenotype achieved after supplying a threshold Y-27632 concentration. After withdrawal of Y-27632 from the medium, clustered colonies rapidly differentiated into keratinocyte-like cells, although the underlying mechanism of this process is not fully understood. In fact, previous studies report that Y-27632 plays an important role in a wide range of cell types, including stem cells [29-31]. For example, a Y-27632 inhibitor protects embryonic stem cells from damage during cell passage and prevent cells from undergoing cryo-damage during cryopreservation [18,32]. In addition, Omelchenko et al. report that Y-27632 promotes epithelial cell transformation into “lander cells” that facilitate skin wound healing [17]. McMullan et al. further demonstrate that keratinocyte differentiation is regulated by the ROCK signaling pathway and that the blockade of ROCK function inhibits keratinocyte terminal differentiation and increases cell proliferation [16]. Y-27632 has been confirmed to mediate primary human keratinocyte immortalization [19]. Theoretically, it is demonstrated that ROCK1 and ROCK2 play distinct roles in the regulation of keratinocyte differentiation [33]. ROCK1 depletion decreases keratinocyte adhesion to fibronectin and increases terminal differentiation, whereas ROCK2 depletion increases keratinocyte adhesion to fibronectin and decreases terminal differentiation. Whether these signaling molecules exert a similar role in other types of skin cells, especially in HFSCs, which play an important role in wound healing and hair regeneration, had not been previously explored. In our study, as primary cultured HFSCs grew in clustered colonies, we believe that Y-27632 enriches and maintains HFSCs by blocking ROCK2 activity and thereby reducing HFSC differentiation and promoting their proliferation and stemness. As HFSCs are usually accompanied by keratinocytes in primary culture and passage [28], we believe that an interaction between keratinocytes and HFSCs through the blockade of ROCK2 activity may occur during culture and passaging, and keratinocyte adhesion to fibronectin may help HFSCs grow at a stable ratio (30.7-34.1% from P5 to P15) with keratinocytes during long-term passaging. In future studies, we will investigate the detailed molecular mechanisms of the effect of Y-27632 on the ROCK signaling pathway and its influence on HFSC proliferation and maintenance.
Our study further demonstrates that the culture of HFSCs in Y-27632 medium did not compromise their differentiation potential in vitro or in vivo. We found that HFSCs could be induced into keratinocyte-like cells and adipose cells under appropriate induction and differentiation conditions. More interestingly, HFSCs injected together with fetal dermal cells differentiated into hair bulbs and shafts in the skin dermis of immune-deficient nude mice. In control conditions, injected dermal cells or HFSCs alone did not form hair structures, indicating that skin stem cells may utilize dermal cells as a network or frame for hair structures [34]. Consistently, we found that injection of dermal cells alone increased the thickness of the dermis, implying that dermal cells provide a prerequisite environment for the growth of hair structures [35]. It is necessary to point out that nude mice are not completely bald but show an “abortive” reduced hair growth at different sites [36]. Nevertheless, they remain a useful model for studying epithelial-mesenchymal interactions in hair growth and regeneration [37,38]. In this study, we selected albino nude mice on which some skin areas completely lacked hair structures (as shown in Figure 5A-C) for injection of dermal cells and HFSCs. The growth of hair structures was clearly observed in the area in which both dermal and skin stem cells were injected. To completely eliminate this mixture of inherent hair development, it would be necessary to use genetically labeled HFSCs (e.g., with GFP) for the in vivo regeneration of hair structures. Recently, we established a transgenic rabbit line expressing GFP protein (data not shown), which we are using to investigate the isolation and enrichment of HFSCs from skin and the subsequent regeneration of new hair structures carrying GFP markers.
In conclusion, we describe a differential enrichment procedure to derive skin stem cells from hair follicles of vibrissae and ear skin of rabbits in vitro by supplementing the culture medium with the ROCK inhibitor Y-27632. Unlike EGF-based HFSC derivation, which is used to generate HFSCs with few passages (i.e., < 6 passages), our approach enables long-term culture of HFSCs that are able to self-renew and proliferate for up to 15 passages and can differentiate into adipose cells and keratinocytes in vitro and into hair bulb and shaft structures in vivo. This unique HFSC isolation and enrichment procedure could serve as an effective, alternative resource for the large-scale generation of HFSCs for research on skin tissue engineering and related medical applications.
Acknowledgements
This study was supported in part by grants from the National Natural Science Foundation of China (31701285 and 31471388), China Postdoctoral Science Foundation (2018M632330), and Priority Academic Program Development of Jiangsu Higher Education Institutions.
Disclosure of conflict of interest
None.
References
- 1.Ohyama M. Hair follicle bulge: a fascinating reservoir of epithelial stem cells. J Dermatol Sci. 2007;46:81–89. doi: 10.1016/j.jdermsci.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Rompolas P, Greco V. Stem cell dynamics in the hair follicle niche. Semin Cell Dev Biol. 2014;25:34–42. doi: 10.1016/j.semcdb.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yi R. Concise review: mechanisms of quiescent hair follicle stem cell regulation. Stem Cells. 2017;35:2323–2330. doi: 10.1002/stem.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Legue E, Sequeira I, Nicolas JF. Hair follicle renewal: authentic morphogenesis that depends on a complex progression of stem cell lineages. Development. 2010;137:569–577. doi: 10.1242/dev.044123. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Tredget EE, Wu Y. Dynamic signals for hair follicle development and regeneration. Stem Cells Dev. 2012;21:7–18. doi: 10.1089/scd.2011.0230. [DOI] [PubMed] [Google Scholar]
- 6.Yang L, Peng R. Unveiling hair follicle stem cells. Stem Cell Rev Rep. 2010;6:658–664. doi: 10.1007/s12015-010-9172-z. [DOI] [PubMed] [Google Scholar]
- 7.Myung P, Ito M. Dissecting the bulge in hair regeneration. J Clin Invest. 2012;122:448–454. doi: 10.1172/JCI57414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leiros GJ, Kusinsky AG, Drago H, Bossi S, Sturla F, Castellanos ML, Stella IY, Balana ME. Dermal papilla cells improve the wound healing process and generate hair bud-like structures in grafted skin substitutes using hair follicle stem cells. Stem Cells Transl Med. 2014;3:1209–1219. doi: 10.5966/sctm.2013-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcin CL, Ansell DM, Headon DJ, Paus R, Hardman MJ. Hair follicle bulge stem cells appear dispensable for the acute phase of wound re-epithelialization. Stem Cells. 2016;34:1377–1385. doi: 10.1002/stem.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgård R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- 11.Petersson M, Frances D, Niemann C. Lineage tracing of hair follicle stem cells in epidermal whole mounts. Methods Mol Biol. 2013;989:45–60. doi: 10.1007/978-1-62703-330-5_5. [DOI] [PubMed] [Google Scholar]
- 12.Mak KK, Chan SY. Epidermal growth factor as a biologic switch in hair growth cycle. J Biol Chem. 2003;278:26120–26126. doi: 10.1074/jbc.M212082200. [DOI] [PubMed] [Google Scholar]
- 13.Bai TT, Liu FL, Zou F, Zhao GF, Jiang YX, Liu L, Shi JH, Hao DS, Zhang Q, Zheng T, Zhang YY, Liu MS, Li SL, Qi LC, Liu JY. Epidermal growth factor induces proliferation of hair follicle-derived mesenchymal stem cells through epidermal growth factor receptor-mediated activation of ERK and AKT signaling pathways associated with upregulation of cyclin D1 and downregulation of p16. Stem Cells Dev. 2017;26:113–122. doi: 10.1089/scd.2016.0234. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez-Danes A, Blanpain C. Maintaining hair follicle stem cell identity in a dish. EMBO J. 2017;36:132–134. doi: 10.15252/embj.201696051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terunuma A, Limgala RP, Park CJ, Choudhary I, Vogel JC. Efficient procurement of epithelial stem cells from human tissue specimens using a Rho-associated protein kinase inhibitor Y-27632. Tissue Engineering Part A. 2010;16:1363–1368. doi: 10.1089/ten.tea.2009.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMullan R, Lax S, Robertson VH, Radford DJ, Broad S, Watt FM, Rowles A, Croft DR, Olson MF, Hotchin NA. Keratinocyte differentiation is regulated by the Rho and ROCK signaling pathway. Curr Biol. 2003;13:2185–2189. doi: 10.1016/j.cub.2003.11.050. [DOI] [PubMed] [Google Scholar]
- 17.Omelchenko T, Vasiliev JM, Gelfand IM, Feder HH, Bonder EM. Rho-dependent formation of epithelial “leader” cells during wound healing. Proc Natl Acad Sci U S A. 2003;100:10788–10793. doi: 10.1073/pnas.1834401100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa SI, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 19.Chapman S, Liu X, Meyers C, Schlegel R, McBride AA. Human keratinocytes are efficiently immortalized by a Rho kinase inhibitor. J Clin Invest. 2010;120:2619–2626. doi: 10.1172/JCI42297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, Zhang S, Zhao H, Qiao J, Liu S, Deng Z, Lei X, Ning L, Cao Y, Zhao Y, Duan E. Ovine hair follicle stem cells derived from single vibrissae reconstitute haired skin. Int J Mol Sci. 2015;16:17779–17797. doi: 10.3390/ijms160817779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He N, Dong Z, Tao L, Zhao S, Bou S, Liu D. Isolation and characterization of hair follicle stem cells from Arbas Cashmere goat. Cytotechnology. 2016;68:2579–2588. doi: 10.1007/s10616-016-9981-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miao Y, Sun YB, Liu BC, Jiang JD, Hu ZQ. Controllable production of transplantable adult human high-passage dermal papilla spheroids using 3D matrigel culture. Tissue Eng Part A. 2014;20:2329–2338. doi: 10.1089/ten.tea.2013.0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma D, Kua JEH, Lim WK, Lee ST, Chua AW. In vitro characterization of human hair follicle dermal sheath mesenchymal stromal cells and their potential in enhancing diabetic wound healing. Cytotherapy. 2015;17:1036–1051. doi: 10.1016/j.jcyt.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Garcin CL, Ansell DM, Headon DJ, Paus R, Hardman MJ. Hair follicle bulge stem cells appear dispensable for the acute phase of wound re-epithelialisation. Stem Cells. 2016;34:1377–85. doi: 10.1002/stem.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoo BY, Shin YH, Yoon HH, Seo YK, Park JK. Hair follicular cell/organ culture in tissue engineering and regenerative medicine. Biochemical Engineering Journal. 2010;48:323–331. [Google Scholar]
- 26.Morgner J, Ghatak S, Jakobi T, Dieterich C, Aumailley M, Wickstrom SA. Integrin-linked kinase regulates the niche of quiescent epidermal stem cells. Nat Commun. 2015;6:8198. doi: 10.1038/ncomms9198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roh C, Tao Q, Photopoulos C, Lyle S. In vitro differences between keratinocyte stem cells and transit-amplifying cells of the human hair follicle. J Invest Dermatol. 2005;125:1099–1105. doi: 10.1111/j.0022-202X.2005.23958.x. [DOI] [PubMed] [Google Scholar]
- 28.Chacon-Martinez CA, Klose M, Niemann C, Glauche I, Wickstrom SA. Hair follicle stem cell cultures reveal self-organizing plasticity of stem cells and their progeny. EMBO J. 2017;36:151–164. doi: 10.15252/embj.201694902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tong J, Wang Y, Chang B, Zhang D, Wang B. Y-27632 inhibits ethanol-induced increase in intestinal epithelial barrier permeability. Mol Med Rep. 2014;9:2357–2361. doi: 10.3892/mmr.2014.2060. [DOI] [PubMed] [Google Scholar]
- 30.Li Z, Han S, Wang X, Han F, Zhu X, Zheng Z, Wang H, Zhou Q, Wang Y, Su L, Shi J, Tang C, Hu D. Rho kinase inhibitor Y-27632 promotes the differentiation of human bone marrow mesenchymal stem cells into keratinocytelike cells in xeno-free conditioned medium. Stem Cell Res Ther. 2015;6:17. doi: 10.1186/s13287-015-0008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J, Park S, Roh S. Y-27632, a ROCK inhibitor, delays senescence of putative murine salivary gland stem cells in culture. Arch Oral Biol. 2015;60:875–882. doi: 10.1016/j.archoralbio.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Kurosawa H. Application of Rho-associated protein kinase (ROCK) inhibitor to human pluripotent stem cells. J Biosci Bioeng. 2012;114:577–581. doi: 10.1016/j.jbiosc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 33.Lock FE, Hotchin NA. Distinct roles for ROCK1 and ROCK2 in the regulation of keratinocyte differentiation. PLoS One. 2009;4:e8190. doi: 10.1371/journal.pone.0008190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rezza A, Wang ZC, Sennett R, Qiao WL, Wang DM, Heitman N, Mok KW, Clavel C, Yi R, Zandstra P, Ma’ayan A, Rendl M. Signaling networks among stem cell precursors, transit-amplifying progenitors, and their niche in developing hair follicles. Cell Rep. 2016;14:3001–3018. doi: 10.1016/j.celrep.2016.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinberg WC, Goodman LV, George C, Morgan DL, Ledbetter S, Yuspa SH, Lichti U. Reconstitution of hair follicle development in vivo: determination of follicle formation, hair growth, and hair quality by dermal cells. J Invest Dermatol. 1993;100:229–236. doi: 10.1111/1523-1747.ep12468971. [DOI] [PubMed] [Google Scholar]
- 36.Militzer K. Hair growth pattern in nude mice. Cells Tissues Organs. 2001;168:285–294. doi: 10.1159/000047845. [DOI] [PubMed] [Google Scholar]
- 37.Zhang D, Gu L, Li J, Li Z, Wang C, Wang Z, Liu L, Lee M, Sung C. Exogenous stimulations change nude mouse hair cycle pattern. J Dermatolog Treat. 2012;23:90–96. doi: 10.3109/09546634.2010.495378. [DOI] [PubMed] [Google Scholar]
- 38.Ohyama M, Veraitch O. Strategies to enhance epithelial-mesenchymal interactions for human hair follicle bioengineering. J Dermatol Sci. 2013;70:78–87. doi: 10.1016/j.jdermsci.2013.02.004. [DOI] [PubMed] [Google Scholar]


