Abstract
Berberine (BBR), an isoquinoline alkaloid that derived from the Chinese medicinal plant Coptis chinensis, has been identified with multiple pharmacological activities, including regulating glucose and cholesterol levels, anti-obesity effects and anti-diabetic effects. Due to its multiple activities, BBR and its metabolites have drawn great attention in biomedical research and clinical practices. After the recent re-discovery of brown adipose tissue (BAT) in adult humans, stimulating energy-dissipating via BAT activation and white-to-brown adipose tissue conversion have been regarded as potential therapeutic strategies for obesity and diabetes. Recent studies have demonstrated the activities of BBR in the activation of BAT and white-to-brown adipose tissue conversion, showing significant effectiveness in the treatment of diabetes. This review has summarized current studies that focused on the effect of BBR in the treatment of metabolic syndrome, especially in regulating BAT activities. Besides, the potential and molecular mechanisms of BBR in treating other risk factors of metabolic syndrome, including insulin resistance and dyslipidemia, are also reviewed, showing the great potential of BBR in treating the metabolic syndrome systematically.
Keywords: Berberine, brown adipose tissue, metabolic syndrome, obesity
Introduction
The metabolic syndrome is a group of risk factors that could significantly increase the likelihood of cardiovascular diseases, type II diabetes, and stroke. These risk factors in metabolic syndrome including obesity, insulin resistance, and dyslipidemia. The complexity of metabolic syndrome has raised a lot of difficulties in treating of this multifaceted health problem. Although the prevalence of metabolic syndrome has been continuously increasing for years, current therapeutic strategies still failed to provide effective and sufficient treatment.
Obesity is a status of chronic positive energy balance associated with excess fat storage that accumulates in adipose tissues [1]. After rediscovering functional brown adipose tissue (BAT) in adult human through several combined imaging techniques [2,3], increasing interests have been raised in treating obesity and diabetes via activation and recruitment of BAT, due to the particular function of BAT in dissipating chemical energy in the form of non-shivering thermogenesis. Therefore, activation of BAT and conversion of fat-accumulating white adipose tissue (WAT) into energy-dissipating BAT may serve as an effective and potential approach.
Berberine (BBR) is a natural product of quaternary ammonium salt from the group of isoquinoline alkaloids (2,3-methylenedioxy-9,10-dimethoxyprotoberberine chloride; C2OH18NO4 +) and has a molar mass of 336.36122 g/mol [4]. BBR can be isolated from a variety of plants, such as Coptis chinensis (Coptis or Goldthread), Hydrastis canadensis (goldenseal), Berberis aquifolium (Oregon grape), Berberis aristata (Tree Turmeric), Berberis vulgaris (Barberry), and Arcangelisia flava [5]. Recently, BBR was found to have anti-obesity activity through regulating BAT thermogenesis and inhibiting adipogenesis [6,7]. In addition, previous studies have reported the therapeutic effects of BBR in insulin resistance and dyslipidemia [8-11]. In this review, the roles of BBR in BAT activation, white-to-brown adipose tissue conversion, glucose metabolism, and lipid metabolism are reviewed, and its potential in treatment of metabolic syndrome is discussed.
Results
Drug metabolism and in vitro effects of BBR
There are four major metabolites of BBR: berberrubine (M1), thalifendine (M2), demethyleneberberine (M3) and jatrorrhizine (M4) [12]. The biodistribution and pharmacokinetics of BBR follows a classic pattern: oral intake, followed by the small intestine (the first-pass elimination), the liver (accumulation), the kidneys, the muscle, the heart and the pancreas [13,14]. After oral intake, the main metabolic pathways are oxidative demethylation (generating M1) and subsequent glucuronidation, whereas the pathways after intravenous dosing are oxidative demethylation (generating M3) and glucuronidation of M3 [15] (Figure 1).
Figure 1.
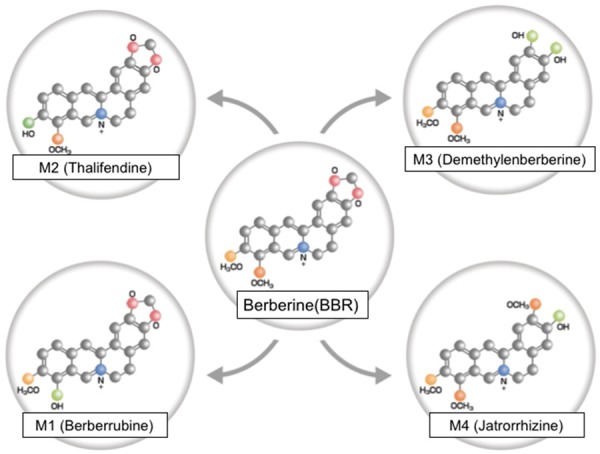
The chemical structures of berberine and its metabolites: M1 (Berberrubine), M2 (Thalifendine), M3 (Demethylenberberine), and M4 (Jatrorrhizine).
Several studies have identified the mechanisms of action of BBR at the cellular level, particularly in hepatic cells [16-18], vascular smooth muscle cells [19,20], pancreatic β-cells [21-23], adipocytes and myocytes [24,25]. Among the pathways through which BBR modulates the cellular processes, AMP-activated protein kinase (AMPK) plays a critical role [26]. AMPK functions as a cellular energy sensor that involves in stimulation of catabolic processes (such as fatty acid oxidation, glucose uptake, lipolysis) while inhibiting anabolic processes (such as gluconeogenesis, fatty acid synthesis and cholesterol synthesis) [26].
Effects of BBR on activation of BAT
White and brown adipose tissues are two different types of adipose tissue in human that have opposite physiological functions. White adipose tissue (WAT) stores energy in the form of large unilocular lipid-droplets within adipocytes, which is sensitive to the regulation of hormones, such as insulin and leptin [27]. Brown adipose tissue (BAT), on the other hand, is composed of multilocular lipid droplets and large numbers of mitochondria that contain uncoupling protein-1 (UCP1), which is histologically, morphologically and functionally distinct from WAT, with unique developmental patterns [28]. Previously, functional BAT was only observed in rodent and human neonates as a mechanism for adapting to the cold environment. However, recent studies have shown that adult humans also have functional BAT [2,29,30], which raises great research interests to BAT development in the past few years. Active BAT modulates heat generation by triglyceride hydrolysis and then oxidizes fatty acids, and it plays an important role in energy expenditure [31]. Several studies have found that changes in BAT activity can greatly affect body weight [32].
Berberine has been reported as a critical factor in promoting adaptive thermogenesis through activating BAT activity. Animal experiments showed that berberine (5 mg/kg body weight) administration increased whole-body energy expenditure by 20% without changes in physical activity. The increased expression of BAT thermogenic markers (such as Pgc1a, Cidea, and Ucp1) and the mitochondrial content in BAT after berberine administration confirmed the effect of berberine on BAT activation. Furthermore, the 18F-FDG-PET/CT results indicate the glucose uptake in BAT was enhanced [6]. Besides, the respiratory exchange ratio was significantly decreased in db/db mice, suggesting that berberine shifts the fuel preference toward fatty acid oxidation.
Effects of BBR on white-to-brown adipose tissue conversion
During the process of white to brown adipose tissue conversion, the expression of UCP1 increases in white adipocytes, resulting the accumulation of functional UCP1-rich cells within WAT. The development of clustering UCP1-expressing multilocular adipocytes with thermogenic capacity in WAT could be induced by various stimuli, such as cold exposure, and these adipocytes have been named beige or “brite” (brown in white) adipocytes [33,34] (Figure 2). These beige adipocytes have similar characteristics with brown adipocytes, including having abundant mitochondria within cell and expressing other key factors that associated with thermogenesis, including CIDEA and PGC1α [35,36].
Figure 2.
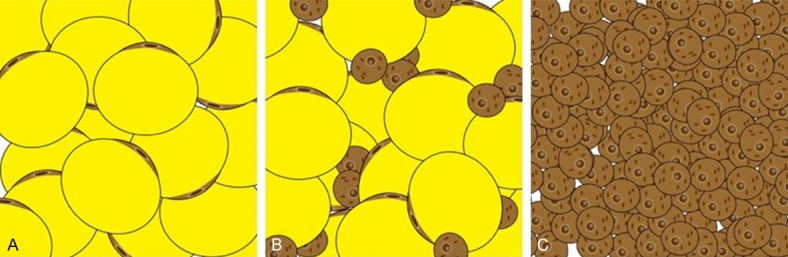
Different types of adipose tissue deposits. A. WAT (white adipose tissue) is the unilocular adipose tissue with sparse mitochondria, which could be found throughout the body, and is a storage site of energy surplus. B. Beige (brite) adipose tissue is a special type of adipose tissue with multilocular lipid droplets infiltrated in WAT. C. BAT (brown adipose tissue) is multilocular and highly vascularized tissue, and has dense mitochondria that could induce energy production in the form of heat.
Due to the specific function of BAT in energy expenditure, conversion of white into brown adipocytes is another potential methods to accelerate energy expenditure besides recruitment of brown fat [32]. The most effective sympathetic activator is chronic cold exposure [37], which could induce a massive thermogenic response in WAT. However, chronically exposing obese patients to cold temperatures cannot be a therapeutic method as it is unethical and uncomfortable. Therefore, other “browning agents” besides cold temperatures have been investigated for many years. In addition to or in combination with this system, various factors have been reported to be activators/recruiters of BAT and white-to-brown adipose tissue conversion [38-41]. Among them, BBR has attracted interests.
Berberine has shown effects in stimulating white-to-brown adipose conversion. However, this browning effect was only found in specific tissue: the development of brown-like adipocytes in the inguinal area is induced by berberine, but not in epididymal fat in mice. Meanwhile, the expression of Ucp1 and other thermogenic markers was up-regulated in inguinal WAT, and the mitochondrial biogenesis was also observed. AMPK and PGC1α activation are involved in the mechanism of thermogenic induction via berberine and its supplementation [6]. Thus, berberine has multiple regulations in brown adipocyte function through activating AMPK [42] (Figure 3). At least three distinct metabolic responses may occur upon thermogenic stimuli, including the following: 1) an increase in BAT activity in pre-existing classical brown adipocytes; 2) the metabolic switch of some, if not all, existing white adipocytes to beige adipocytes in subcutaneous fat (WAT browning); and 3) new beige adipocyte formation from adipogenic progenitor cells. Berberine can trigger thermogenic responses via AMPK/PGC1 signaling and lead to mitochondrial biogenesis as well as the anti-inflammatory function.
Figure 3.
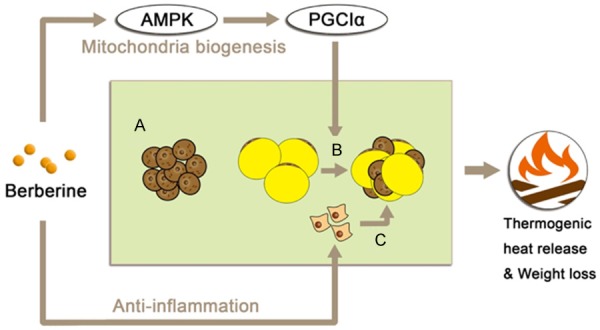
Three metabolic responses may occur upon thermogenic stimuli. A. Increase in BAT activity in pre-existing classical brown adipocytes; B. The metabolic switch of existing white adipocytes to beige adipocytes in subcutaneous fat (white-to-brown adipose tissue conversion); Berberine activate this conversion via AMPK/PGC1α signaling; C. New beige adipocyte formation from adipogenic progenitor cells.
Effect of BBR on lipid metabolism
High levels of low-density lipoprotein cholesterol (LDL-C) and oxidized LDL (oxLDL) in blood vessels are major risk factors for endothelial dysfunction and its progression in atherosclerosis [43]. Accumulation of LDL-C in blood vessels usually caused by inactivity of the LDL receptor (LDLR) or reduction in LDLR expression [44] (Figure 4).
Figure 4.
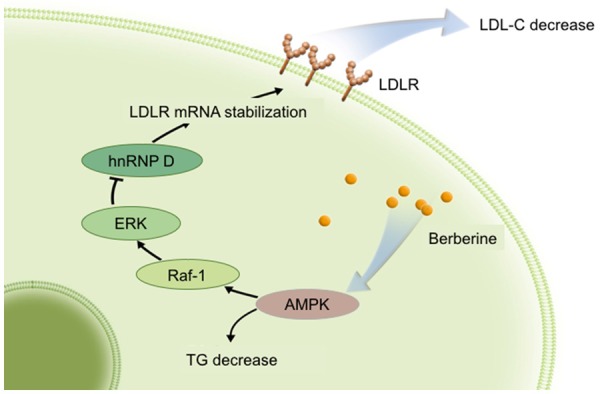
Main lipid-lowering effects of berberine through the APMK pathway. Briefly, LDLR mRNA can be stabilized by berberine through AMPK-dependent Raf-1 activation. Then, ERK is activated, and hnRNP D is down-regulated.
A great number of preclinical and clinical studies have suggested that BBR has multiple lipid-lowering activities, including facilitating LDLR mRNA expression and reducing. Previous studies have reported that BBR could increase the expression of the LDLR at the post-transcriptional level [16,45]. Briefly, BBR could up-regulate LDLR expression in liver cells by AMPK-dependent Raf-1 activation [46]. Then, the BBR-induced stabilization of LDLR mRNA is mediated by activation of extracellular signal-regulated kinase (ERK) signaling pathway [47] and down-regulation of mRNA decay-promoting factor heterogeneous nuclear ribonucleoprotein D (hnRNP D), a protein that responsible for rapid mRNA turnover [48]. Besides, in human macrophage-derived foam cells treated with oxLDL, BBR activates the AMPK-sirtuin 1-peroxisome proliferator-activated receptor λ (AMPK-SIRT1-PPARγ) pathway [49], therefore, inhibiting the expression of lectin-like ox-LDL-receptor 1 (LOX-1) [50] as well as reducing the uptake of oxLDL in macrophages, which could eventually impede the foam cell formation [51].
Beside of regulations in LDL, BBR and its metabolites could also reduce the triglyceride (TG) levels. BBR contributes to the reduction of TG and total cholesterol levels by modulating the expression of adipogenic transcription factors [52]. Furthermore, due to the critical role of AMPK in the regulation of fatty acids and TG biosynthesis, BBR and its metabolites could reduce the TG levels in hepatoma cells through AMPK activation [18,53].
BBR effects on glucose metabolism
Insulin resistance is another important risk factor of metabolic syndrome that induced by excess weight and physical inactivity [1]. As a potential agent for metabolic syndrome, BBR has shown its effect in the regulation of glucose metabolism, including preventing insulin resistance and reducing blood glucose level. It has been suggested that BBR could prevent insulin resistance by regulating the expression of insulin receptor, insulin receptor substrate-1, and glucagon in the high-fat diet-induced insulin resistance rat model [54].
Besides, BBR is also reported as a natural hypoglycemic agent due to its action on the AMPK signaling pathway with subsequent induction of glycolysis [24]. BBR could alleviate the reduction of glucose consumption and glucose uptake through stimulation of AMPK activity, which was observed in the H9c2 myoblast cell line treated with insulin to induce insulin resistance [55]. Activation of AMPK by BBR could not only induce glycolysis in the skeletal muscle L6, myoblast C2C12, and adipocyte 3T3-L1 cell lines [24], but also improve acute insulin-mediated glucose transporter type 4 (GLUT4) translocation, therefore facilitate the glucose transport into insulin-resistant myotubes via PI3K pathway [56] (Figure 5). In vivo experiments showed that activation of AMPK and AKT by BBR could restore fasting blood insulin and fasting blood glucose (FBG), as well as down-regulate the expression of glycogen synthase kinase 3 beta (GSK3β). This mechanism was confirmed in the palmitate-induced hypertrophy H9c2 myoblast cell line [57].
Figure 5.

Stimulation effect of berberine on glucose transport from plasma into cells Berberine could activate AMPK, increasing the translocation of GLUT4 from cytosolic vesicles to plasma membrane, which induces plasma glucose uptake and ultimately results in reduced insulin resistance.
Effect of BBR on obesity-associated inflammation
In addition, the expression of pro-inflammatory genes in the adipose tissue of obese mice is inhibited by BBR, which indicates that BBR may moderate both the acute and low-grade inflammatory response in obesity [25]. BBR has a long-term role in the gastrointestinal tract and plays a role in the modification of the gut microbiota, therefore the treatment of BBR modulates the development of insulin resistance and prevent obese rats from having an increase in body weight compared to untreated animals [58]. BBR also reduces the adiposity index and relieves systemic inflammation [59]. Gut microbiota research has revealed that BBR dramatically changes its composition and that selectively eliminated or facilitate the growth of several intestinal microbes [60], contributing to the alleviation of systemic inflammation and the beneficial effects of BBR against insulin resistance, obesity, and diabetes.
Discussion
Berberine is a natural product that has already been used in bacterial infection for years. However, recent studies have unveiled the great potential of BBR in the treatment of metabolic syndrome due to its noticeable anti-obesity activity through BAT activation and white-to-brown adipocytes conversion. Besides, the regulatory function of BBR in insulin resistance and dyslipidemia enable BBR to provide symphonic therapy in metabolic syndrome. Results from current studies, both in vitro and in vivo, suggest that BBR could be a powerful agent for metabolic syndrome. However, the overall effect of BBR in metabolic syndrome was not systemically tested, partly because the preclinical models for metabolic syndrome are limited. Once the effect of BBR was confirmed in animal models, clinical trials need to be done for establishing the therapeutic effectiveness of BBR.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [grant numbers 81701664], the Technology Innovation Program in Southwest Hospital (SWH2016JCYB-30).
Disclosure of conflict of interest
None.
References
- 1.Gupta D, Krueger CB, Lastra G. Over-nutrition, obesity and insulin resistance in the development of β-cell dysfunction. Curr Diabetes Rev. 2012;8:76–83. doi: 10.2174/157339912799424564. [DOI] [PubMed] [Google Scholar]
- 2.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerbäck S. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 3.Gifford A, Towse TF, Walker RC, Avison MJ, Welch EB. Characterizing active and inactive brown adipose tissue in adult humans using PET-CT and MR imaging. Am J Physiol Endocrinol Metab. 2016;311:E95–E104. doi: 10.1152/ajpendo.00482.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derosa G, Maffioli P, Cicero AF. Berberine on metabolic and cardiovascular risk factors: an analysis from preclinical evidences to clinical trials. Expert Opin Biol Ther. 2012;12:1113–1124. doi: 10.1517/14712598.2012.704014. [DOI] [PubMed] [Google Scholar]
- 5.Singh IP, Mahajan S. Berberine and its derivatives: a patent review (2009-2012) Expert Opin Ther Pat. 2013;23:215–231. doi: 10.1517/13543776.2013.746314. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z, Zhang H, Li B, Meng X, Wang J, Zhang Y, Yao S, Ma Q, Jin L, Yang J. Berberine activates thermogenesis in white and brown adipose tissue. Nat Commun. 2014;5:5493. doi: 10.1038/ncomms6493. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Tang H, Deng R, Wang N, Zhang Y, Wang Y, Liu Y, Li F, Wang X, Zhou L. Berberine suppresses adipocyte differentiation via decreasing CREB transcriptional activity. PLoS One. 2015;10:e0125667. doi: 10.1371/journal.pone.0125667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, Ye JM, Lee CH, Oh WK, Kim CT. Berberine, a natural plant product, activates AMPactivated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256–2264. doi: 10.2337/db06-0006. [DOI] [PubMed] [Google Scholar]
- 9.Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, Wang Y, Wang Z, Si S, Pan H. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–51. doi: 10.1038/nm1135. [DOI] [PubMed] [Google Scholar]
- 10.Turner N, Li JY, Gosby A, To SW, Cheng Z, Miyoshi H, Taketo MM, Cooney GJ, Kraegen EW, James DE. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I. Diabetes. 2008;57:1414–1418. doi: 10.2337/db07-1552. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Li X, Zou D, Liu W, Yang J, Zhu N, Huo L, Wang M, Hong J, Wu P. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J Clin Endocrinol Metab. 2008;93:2559–2565. doi: 10.1210/jc.2007-2404. [DOI] [PubMed] [Google Scholar]
- 12.Qiu F, Zhu Z, Kang N, Piao S, Qin G, Yao X. Isolation and identification of urinary metabolites of berberine in rats and humans. Drug Metab Dispos. 2008;36:2159–2165. doi: 10.1124/dmd.108.021659. [DOI] [PubMed] [Google Scholar]
- 13.Liu YT, Hao HP, Xie HG, Lai L, Wang Q, Liu CX, Wang GJ. Extensive intestinal first-pass elimination and predominant hepatic distribution of berberine explain its low plasma levels in rats. Drug Metab Dispos. 2010;38:1779–1784. doi: 10.1124/dmd.110.033936. [DOI] [PubMed] [Google Scholar]
- 14.Tan XS, Ma JY, Feng R, Ma C, Chen WJ, Sun YP, Fu J, Huang M, He CY, Shou JW. Tissue distribution of berberine and its metabolites after oral administration in rats. PLoS One. 2013;8:e77969. doi: 10.1371/journal.pone.0077969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Hao H, Xie H, Lv H, Liu C, Wang G. Oxidative demethylenation and subsequent glucuronidation are the major metabolic pathways of berberine in rats. J Pharm Sci. 2009;98:4391–4401. doi: 10.1002/jps.21721. [DOI] [PubMed] [Google Scholar]
- 16.Cameron J, Ranheim T, Kulseth MA, Leren TP, Berge KE. Berberine decreases PCSK9 expression in HepG2 cells. Atherosclerosis. 2008;201:266–273. doi: 10.1016/j.atherosclerosis.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Dong B, Park SW, Lee HS, Chen W, Liu J. Hepatocyte nuclear factor 1α plays a critical role in PCSK9 gene transcription and regulation by the natural hypocholesterolemic compound berberine. J Biol Chem. 2009;284:28885–28895. doi: 10.1074/jbc.M109.052407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao S, Zhou Y, Xu P, Wang Y, Yan J, Bin W, Qiu F, Kang N. Berberine metabolites exhibit triglyceride-lowering effects via activation of AMP-activated protein kinase in Hep G2 cells. J Ethnopharmacol. 2013;149:576–582. doi: 10.1016/j.jep.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 19.Cho BJ, Im EK, Kwon JH, Lee KH, Shin HJ, Oh J, Kang SM, Chung JH, Jang Y. Berberine inhibits the production of lysophosphatidylcholine-induced reactive oxygen species and the ERK1/2 pathway in vascular smooth muscle cells. Mol Cells. 2005;20:429–34. [PubMed] [Google Scholar]
- 20.Lee S, Lim HJ, Park HY, Lee KS, Park JH, Jang Y. Berberine inhibits rat vascular smooth muscle cell proliferation and migration in vitro and improves neointima formation after balloon injury in vivo: berberine improves neointima formation in a rat model. Atherosclerosis. 2006;186:29–37. doi: 10.1016/j.atherosclerosis.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Geng YN, Jiang JD, Kong WJ. Antioxidant and anti-inflammatory activities of berberine in the treatment of diabetes mellitus. Evid Based Complement Alternat Med. 2014;2014:289264. doi: 10.1155/2014/289264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Wei J, Xue R, Wu JD, Zhao W, Wang ZZ, Wang SK, Zhou ZX, Song DQ, Wang YM. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism. 2010;59:285–292. doi: 10.1016/j.metabol.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 23.Shen N, Huan Y, Shen ZF. Berberine inhibits mouse insulin gene promoter through activation of AMP activated protein kinase and may exert beneficial effect on pancreatic β-cell. Eur J Pharmacol. 2012;694:120–126. doi: 10.1016/j.ejphar.2012.07.052. [DOI] [PubMed] [Google Scholar]
- 24.Yin J, Gao Z, Liu D, Liu Z, Ye J. Berberine improves glucose metabolism through induction of glycolysis. Am J Physiol Endocrinol Metab. 2008;294:E148–E156. doi: 10.1152/ajpendo.00211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeong HW, Hsu KC, Lee JW, Ham M, Huh JY, Shin HJ, Kim WS, Kim JB. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab. 2009;296:E955–E964. doi: 10.1152/ajpendo.90599.2008. [DOI] [PubMed] [Google Scholar]
- 26.Krishan S, Richardson DR, Sahni S. Adenosine monophosphate-activated kinase and its key role in catabolism: structure, regulation, biological activity, and pharmacological activation. Mol Pharmacol. 2015;87:363–377. doi: 10.1124/mol.114.095810. [DOI] [PubMed] [Google Scholar]
- 27.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–77. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Coldactivated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 31.Lazar MA. How now, brown fat? Science. 2008;321:1048–1049. doi: 10.1126/science.1164094. [DOI] [PubMed] [Google Scholar]
- 32.Langin D. Recruitment of brown fat and conversion of white into brown adipocytes: strategies to fight the metabolic complications of obesity? Biochim Biophys Acta. 2010;1801:372–376. doi: 10.1016/j.bbalip.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Rosenwald M, Perdikari A, Rülicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol. 2013;15:659–67. doi: 10.1038/ncb2740. [DOI] [PubMed] [Google Scholar]
- 34.Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10:24–36. doi: 10.1038/nrendo.2013.204. [DOI] [PubMed] [Google Scholar]
- 35.Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 37.Nedergaard J, Cannon B. The browning of white adipose tissue: some burning issues. Cell Metab. 2014;20:396–407. doi: 10.1016/j.cmet.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Bonet ML, Oliver P, Palou A. Pharmacological and nutritional agents promoting browning of white adipose tissue. Biochim Biophys Acta. 2013;1831:969–985. doi: 10.1016/j.bbalip.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ. A PGC1-[agr] -dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng XR, Gennemark P, O’Mahony G, Bartesaghi S. Unlock the thermogenic potential of adipose tissue: pharmacological modulation and implications for treatment of diabetes and obesity. Front Endocrinol (Lausanne) 2015;6:174. doi: 10.3389/fendo.2015.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baskaran P, Krishnan V, Ren J, Thyagarajan B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br J Pharmacol. 2016;173:2369–2389. doi: 10.1111/bph.13514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Dam AD, Kooijman S, Schilperoort M, Rensen PC, Boon MR. Regulation of brown fat by AMP-activated protein kinase. Trends Mol Med. 2015;21:571–579. doi: 10.1016/j.molmed.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Lubrano V, Balzan S. LOX-1 and ROS, inseparable factors in the process of endothelial damage. Free Radic Res. 2014;48:841–848. doi: 10.3109/10715762.2014.929122. [DOI] [PubMed] [Google Scholar]
- 44.Kong WJ, Liu J, Jiang JD. Human low-density lipoprotein receptor gene and its regulation. J Mol Med. 2006;84:29–36. doi: 10.1007/s00109-005-0717-6. [DOI] [PubMed] [Google Scholar]
- 45.Lee S, Lim HJ, Park JH, Lee KS, Jang Y, Park HY. Berberine-induced LDLR up-regulation involves JNK pathway. Biochem Biophys Res Commun. 2007;362:853–857. doi: 10.1016/j.bbrc.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 46.Li Z, Jiang JD, Kong WJ. Berberine up-regulates hepatic low-density lipoprotein receptor through ras-independent but AMP-activated protein kinase-dependent Raf-1 activation. Biol Pharm Bull. 2014;37:1766–1775. doi: 10.1248/bpb.b14-00412. [DOI] [PubMed] [Google Scholar]
- 47.Abidi P, Zhou Y, Jiang JD, Liu J. Extracellular signal-regulated kinase-dependent stabilization of hepatic low-density lipoprotein receptor mRNA by herbal medicine berberine. Arterioscler Thromb Vasc Biol. 2005;25:2170–6. doi: 10.1161/01.ATV.0000181761.16341.2b. [DOI] [PubMed] [Google Scholar]
- 48.Singh AB, Li H, Kan CF, Dong B, Nicolls MR, Liu J. The critical role of mrna destabilizing protein heterogeneous nuclear ribonucleoprotein D in 3’ untranslated region-mediated decay of low-density lipoprotein receptor mRNA in liver tissue. Arterioscler Thromb Vasc Biol. 2014;34:8–16. doi: 10.1161/ATVBAHA.112.301131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chi L, Peng L, Pan N, Hu X, Zhang Y. The anti-atherogenic effects of berberine on foam cell formation are mediated through the upregulation of sirtuin 1. Int J Mol Med. 2014;34:1087–1093. doi: 10.3892/ijmm.2014.1868. [DOI] [PubMed] [Google Scholar]
- 50.Guan S, Wang B, Li W, Guan J, Fang X. Effects of berberine on expression of LOX-1 and SR-BI in human macrophage-derived foam cells induced by ox-LDL. Am J Chin Med. 2010;38:1161–1169. doi: 10.1142/S0192415X10008548. [DOI] [PubMed] [Google Scholar]
- 51.Huang Z, Dong F, Li S, Chu M, Zhou H, Lu Z, Huang W. Berberine-induced inhibition of adipocyte enhancer-binding protein 1 attenuates oxidized low-density lipoprotein accumulation and foam cell formation in phorbol 12-myristate 13-acetate-induced macrophages. Eur J Pharmacol. 2012;690:164–169. doi: 10.1016/j.ejphar.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 52.Hu Y, Davies GE. Berberine inhibits adipogenesis in high-fat diet-induced obesity mice. Fitoterapia. 2010;81:358–366. doi: 10.1016/j.fitote.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Q, Xiao X, Feng K, Wang T, Li W, Yuan T, Sun X, Sun Q, Xiang H, Wang H. Berberine moderates glucose and lipid metabolism through multipathway mechanism. Evid Based Complement Alternat Med. 2010:2011. doi: 10.1155/2011/924851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gu J, Gao F, Zhao T. A preliminary investigation of the mechanisms underlying the effect of berberine in preventing high-fat diet-induced insulin resistance in rats. J Physiol Pharmacol. 2012;63:505–513. [PubMed] [Google Scholar]
- 55.Chang W, Zhang M, Li J, Meng Z, Wei S, Du H, Chen L, Hatch GM. Berberine improves insulin resistance in cardiomyocytes via activation of 5’-adenosine monophosphate-activated protein kinase. Metabolism. 2013;62:1159–1167. doi: 10.1016/j.metabol.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 56.Liu LZ, Cheung SC, Lan LL, Ho SK, Xu HX, Chan JC, Tong PC. Berberine modulates insulin signaling transduction in insulin-resistant cells. Mol Cell Endocrinol. 2010;317:148–153. doi: 10.1016/j.mce.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 57.Chang W, Zhang M, Meng Z, Yu Y, Yao F, Hatch GM, Chen L. Berberine treatment prevents cardiac dysfunction and remodeling through activation of 5’-adenosine monophosphateactivated protein kinase in type 2 diabetic rats and in palmitate-induced hypertrophic H9c2 cells. Eur J Pharmacol. 2015;769:55–63. doi: 10.1016/j.ejphar.2015.10.043. [DOI] [PubMed] [Google Scholar]
- 58.Han J, Lin H, Huang W. Modulating gut microbiota as an anti-diabetic mechanism of berberine. Med Sci Monit. 2011;17:RA164–7. doi: 10.12659/MSM.881842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang X, Zhao Y, Zhang M, Pang X, Xu J, Kang C, Li M, Zhang C, Zhang Z, Zhang Y. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS One. 2012;7:e42529. doi: 10.1371/journal.pone.0042529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xie W, Gu D, Li J, Cui K, Zhang Y. Effects and action mechanisms of berberine and Rhizoma coptidis on gut microbes and obesity in high-fat diet-fed C57BL/6J mice. PLoS One. 2011;6:e24520. doi: 10.1371/journal.pone.0024520. [DOI] [PMC free article] [PubMed] [Google Scholar]


