Abstract
Aims: Fibroblast growth factor 21 (FGF21) plays a critical role in protecting against myocardial ischemia/reperfusion (I/R) injury. However, the molecular mechanism is not completely understood. Here, we aimed to examine whether miRNA-145 (miR-145) is involved in FGF21 protection against myocardial I/R injury through angiopoietin-2 (Angpt2) and autophagy. Methods: We established a rat myocardial I/R model and H9c2 hypoxia/reoxygenation (H/R) model. After administration of FGF21 in the rat I/R model, the infarct size, morphological changes and apoptosis in myocardium were determined by 2,3,5-triphenyltetrazolium chloride (TTC), hematoxylin and eosin (HE), and Masson’s trichrome staining, and TUNEL assay, respectively. The expression levels of miR-145 and Angpt2 were evaluated by quantitative real-time PCR (qRT-PCR), Western blotting and immunohistochemical (IHC) staining. The activity of lactate dehydrogenase (LDH), TNF-α and IL-6 were assayed. Using a dual-luciferase reporter system, the targeted role of miR-145 on Angpt2 was studied. After transfection with miR-145 inhibitor, H9c2 cells were subjected to stimulated H/R with or without FGF21 treatment. The expression of Angpt2 was assessed while cell apoptosis and cell migration assays were performed. Results: FGF21 significantly decreased infarction after I/R, ameliorated I/R-induced cell apoptosis, and inhibited I/R-induced LDH, TNF-α and IL-6 in serum. FGF21 inhibited I/R-induced decrease in miR-145 level, increase in Angpt2 expression and decrease in autophagy; FGF21 also upregulated LC3-B and Beclin1 levels. miR-145 directly targeted Angpt2. The roles of FGF21 in expression of miR-145 and Angpt2 and activation of autophagy after H/R were reversed by miR-145 inhibitor. In addition, the FGF21-inhibited cell apoptosis and FGF21-promoted migration after H/R were restored by miR-145 inhibitor. Conclusion: FGF21 protects myocardial cells against I/R injury by promoting an increase in miR-145 levels and autophagy while inhibiting Angpt2 expression, suggesting a novel therapeutic strategy for protecting against myocardial I/R injury.
Keywords: FGF21, myocardial ischemia-reperfusion injury, miR-145, Angpt2, autophagy
Introduction
The restoration of blood supply to tissues or organs after ischemia can aggravate the injury of ischemic tissue, so as to lead to the ischemia-reperfusion (I/R) injury [1,2]. It well known that the tissues or organs that need high oxygen such as heart and brain tissues are prone to I/R injury [3,4]. A lot of factors exert protective effect in I/R-induced injury such as fibroblast growth factor 21 (FGF21) [5]. However, the molecular mechanism is still unclear.
Ischemia-reperfusion injury can induce apoptosis of tissues and organs, which is an important pathway and pathological manifestation of ischemia-reperfusion injury affecting tissues and organs [6]. Apoptosis is widely involved in the pathogenesis of many pathological conditions including myocardial I/R injury [7]. The classical apoptotic pathways include exogenous death receptor-mediated pathway and endogenous mitochondria-mediated pathway [6]. The death receptor is a member of the TNF-α receptor family, which is a transmembrane protein. In extracellular and cytoplasmic matrix, the regions rich of homologous cysteine exist that have proteolytic function and defined as death domain. It can bind with Fas-related protein (FADD), activate the Caspase cascade, and finally induce the occurrence of apoptosis. Apoptosis is only one form of cell death [6,8]. Cell death includes necrosis, apoptosis and autophagy [6]. In recent, autophagy was found to be involved in myocardial I/R injury. Post-conditioning of berbamine protects the heart from I/R injury via preventing I/R-induced impairment of autophagosome processing in cardiomyocytes, with increased LC3-II levels and accompanied increase in expression of Beclin1 [9]. Antioxidant N-acetylcysteine (NAC) reduces diabetic myocardial I/R injury via inhibiting excessive autophagy [10]. The hypoxia/reoxygenation (H/R)-induced high cell apoptosis in H9c2 cells were restored by ischemic post-conditioning, while inhibition of autophagy reversed these effects, suggesting autophagy was involved in the protection effect of ischemic post-conditioning against myocardial I/R injury [11]. I/R evokes inflammatory responses [12]. While autophagy is a survival mechanism for I/R cells, it also augments their production of interleukin (IL-6) [12]. To understand the mechanism of apoptosis and autophagy during I/R injury is of great significance for the prevention and treatment of I/R injury.
MiRNAs are a class of endogenous non-coding small RNA molecules that are widely present in the body and are 21-25 nucleotides in length. A large number of studies have shown that miRNAs participate in various processes of life, and their abnormalities in expression involved in the occurrence and development of various diseases including myocardial I/R injury [13,14]. Previous studies have shown that the expression of miRNA-145 (miR-145) in the rat/mouse heart transplantation-induced I/R injury and myocardial H/R cell model were significantly reduced [15,16], indicating that the abnormal expression of miR-145 is involved in myocardial I/R injury. In tumor, miR-145 serves as tumor suppressor via regulating angiopoietin-2 (Angpt2) [17,18]. A recent study reported that Angpt2 was downregulated in the FGF21 administration following simulated H/R, and inhibition of Angpt2 with FGF21 administration promoted the energy metabolism in cardiomyocytes, consequently leading to a more efficient cardioprotective effect [5]. This study is aimed to test whether miR-145 involved in FGF21 protected myocardial I/R injury through Angpt2 and autophagy.
Materials and methods
Establishment of Wistar rat myocardial I/R injury model
Adult Wistar rats (250-300 g) were purchased from the Laboratory Animal Center of Guangzhou University and fed with standard rat diet and water ad libitum. This study was approved by the Institutional Animal Care and Use Committee of Capital Medical University. The rats were randomly divided into three groups: sham control (n = 5), I/R (n = 8), and I/R+FGF21 (n = 8). In the I/R group, rats were anaesthetized with 3% pentobarbital sodium (intraperitoneal injection). The rats received the surgery beneath left anterior descending branch (LAD), and were injected with saline into jugular vein before the LAD was occluded for 10 min. The LAD of sham control was not ligated. For I/R and I/R+FGF21 rats, LAD was occluded for 30 min and reperfused for 120 min. For I/R+FGF21 rats, 0.1 mg/kg/day FGF21 was injected intraperitoneally for 4 weeks. After reperfusion, the hearts were harvested and cut into 1 mm-slices. The slices were stained with 1% 2,3,5-triphenyltetrazolium chloride (TTC, Sigma, USA) and fixed with 4% paraformaldehyde to determine the infarct size of myocardium. The infarct size (white-unstained necrotic tissue) was calculated as a percentage of left ventricular area based as previously described [19]. In brief, the tissue slice was placed under natural light and photographed using a Nikon COOLPIX A900 digital camera. The microscopic color image processing system (DpxView Pro, Korea) was used to calculate the infarct size (%, myocardial infarction area/left ventricular area ×100). For general histological analysis, routine hematoxylin and eosin (HE) staining and Masson’s trichrome staining were performed.
TUNEL assay
Cell apoptosis in heart slices (4 μm) was evaluated by Terminal deoxynucleotidyl transferase-mediated dUTP Nick End-Labeling (TUNEL) using the In-Situ Cell Death Detection Kit-POD (Roche, Germany). The slices were treated with TUNEL reaction mixture for 60 min after treatment with protease K (10 mmol/L) for 15 min, and were then incubated in converter-peroxidase (POD) for 30 min. Then, the images were evaluated for percentage of TUNEL-positive cells (apoptosis rate).
Immunohistochemical (IHC) staining
The heart slices (4 μm) were treated with 3% hydrogen peroxide for 20 min to block endogenous peroxidase, incubated with 1% bovine serum albumin and anti-Angpt2 (1:100, Santa Cruz, USA) for 2 h, and incubated with horseradish peroxidase (HRP)-conjugated anti-goat IgG for 60 min. Diaminobenzidine (DAB) was used as chromogen.
Cell culture, transfection, and H/R and FGF21 treatment
Rat ventricular cell line H9c2 and human embryonic kidney cell line HEK293 cells (American Type Culture Collection, ATCC, USA) were cultured in Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS, Gibco, USA) and 100 µg/ml penicillin/streptomycin at 37°C, 5% CO2. The miR-145 mimics/inhibitor was purchased from Genepharma, China. For transfection, H9c2 cells were seeded in 100-mm plate at 1×106 until 50% confluence. Then, 0.5 nmol miR-145 mimics/inhibitor was transfected into cells using the Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s manual. After 48 h, the expression of miR-145 was determined and cells were used in further experiments. For the establishment of I/R, cells were kept in a hypoxic chamber at 37°C for 6 h with serum- and glucose-deficient DMEM. They were then reoxygenated for 12 h with DMEM containing 10% FBS for H/R. To determine the role of FGF21 in miR-145 effects during H/R-injury in cells, 1 μg/ml FGF21 were added in DMEM, as described previously [5].
qRT-PCR
Total RNA was isolated from heart tissue or cells. Quantitative real-time PCR was performed with an ABI 7500 system (Applied Biosystems, USA) using a reverse transcript kit (Promega, USA) and SYBR Green Master Mix kit (Takara, Japan). The primers were used in the study were as follows for (5’-3’): rno-miR-145-5p (reverse transcript) CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGGAATCCCT; rno-miR-145-5p (forward) ACACTCCAGCTGGGGTCCAGTTTTCCCAGGAAT; U6 (forward) CTCGCTTCGGCAGCACA, U6 (Reverse) AACGCTTCACGAATTTGCGT; Angpt2 (Forward) AGTATTGGCTGGGCAACGAG, Angpt2 (Reverse) TTTGTCATTGTCCGAATCCTTT; GAPDH (Forward) CCTCGTCTCATAGACAAGATGGT, GAPDH (Reverse) GGGTAGAGTCATACTGGAACATG. The relative gene expression levels were normalized to U6 or GAPDH and calculated using the 2-ΔΔCt method.
Determination of LDH, TNF-α and IL-6 levels
Following reperfusion of rats and treatment of cells, plasma samples and culture media were collected from the coronary effluent and cell cultures, respectively. The activity of lactate dehydrogenase (LDH), and levels of TNF-α and IL-6 were assayed using LDH (Nanjing Jiancheng Bioengineering, China) and enzyme-linked immunosorbent assay (ELISA; Roche, Germany) kits according to the manufacturer’s instruction.
Cell apoptosis
Cell apoptosis was evaluated by flow cytometry and Hoechst 33258 staining. For flow cytometry analysis, after H/R, H/R+FGF21 or H/R+FGF21 inhibitor treatment, cell apoptosis was assessed by Annexin V-FITC and propiduim iodide (BD Biosciences, USA) according to the manufacturer’s instructions. The stained cells were analyzed by flow cytometry (BD, USA). After treatment, cells were also stained with Hoechst 33258.
Luciferase reporter system
After miR-145 mimics were transfected into HEK293 cells, vectors containing wide type (WT) or mutant type (MUT) GV126-Angpt2-3’UTR and expressing firefly luciferase, along with pRL-cmv vectors expressing renilla luciferase (Geneparam, USA) were transfected using Lipofectamine 2000. Luciferase activity was measured using the Dual-Luciferase Reporter System according to manufacturer’s instruction (Promega, USA). The renilla luciferase activity was designated as the internal control.
Western blot analysis
Proteins were isolated from homogenized hearts and cultured H9c2 cells, and quantified by BCA assay (Beyotime, China). A total of 50 μg of protein was separated by 10% SDS-PAGE, and transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore, USA). The blots were incubated with anti-Angpt2, anti-Beclin1, anti-LC3B, and anti-GAPDH (all 1:1000; Cell signaling Technology, USA) overnight at 4°C and then incubated with horseradish peroxidase (HRP)-conjugated anti-IgG secondary antibody (1:2000) for 1 h. The blots were visualized with an enhanced chemoluminescence kit (AmershamParmacis, USA).
Immunofluorescence staining
After H/R with or without FGF21 and/or miR-145 inhibitor treatment, cell slides were fixed with 4% paraformaldehyde for 15 min, incubated with 2% bovine serum albumin for 30 min, and incubated with primary anti-Angpt2 (1:100) overnight at 4°C. Cells were then incubated with fluorescein isothiocyanate (FITC)-conjugated secondary antibody (1:1000) for 60 min. The staining was evaluated by LSM50 confocal microscopy [20].
Autophagy assays
Autophagic activity was analyzed by assessing GFP-LC3 puncta. H9c2 cells were transduced by a lentiviral vector to stably express GFP-LC3. Numbers of GFP-LC3 puncta per cell in cells transfected with GFP-LC3 were quantified by fluorescence microscopy. All GFP-LC3 puncta quantitation was performed by an observer blinded to experimental conditions.
Cell migration assay
After transfection, cells were seeded in chambers with 8-μm pores for the Transwell assay (Millipore, USA) with serum-free medium. FBS (10%) was added into bottom chamber as a chemoattractant. Then, cells were treated with H/R with or without FGF21 and/or miR-145 inhibitor. After treatment, the non-migrated cells in the upper chamber were scrubbed with a sterile cotton swab. The migrated cells were stained with 0.1% crystal violet and counted using Image-Pro Plus v7.0 software (Media Cybernetics, Inc. USA).
Statistical analysis
All data were expressed as means ± standard deviation (SD) from three or more independent experiments. Statistical analysis was carried out by a two-tailed Student’s t-test or ANOVA using SPSS 15.0, and statistical significance was considered at P<0.05.
Results
FGF21 inhibits myocardial I/R injury
To determine the role of FGF21 in myocardial I/R injury, 0.1 mg/kg/day FGF21 was injected intraperitoneally into the rats for 4 weeks. Morphological alterations, myocardial damage, and inflammation response were then evaluated (Figure 1). The infarct size was significantly increased in the I/R group compared with the sham group (P<0.01) (Figure 1A and 1B). FGF21 significantly decreased the infarction after I/R (P<0.05) (Figure 1A and 1B). TUNEL assay revealed that FGF21 significantly ameliorated I/R-induced cell apoptosis (P<0.01) (Figure 1C and 1D), indicating that FGF21 reduced impairment of cardiac function. Microscopic evaluation showed orderly arrangement of myocardial fibers in the sham group (Figure 1E). In the I/R group, the myocardial fibers became loosely and irregularly arranged, and the fibrosis area was significantly increased compared with the sham group (P<0.01). However, the percentage fibrosis area was significantly decreased in I/R+FGF21 group compared with the I/R group, indicating that FGF21 reduced the morphological change after I/R (P<0.05) (Figure 1E and 1F). Myocardial damage was evaluated by the measuring LDH release into the coronary effluent (Figure 1G). The activity of LDH was significantly increased after I/R, and the level of LDH induced by I/R was significantly inhibited by FGF21 treatment (Figure 1G). Moreover, increases in levels of inflammation factors including TNF-α (Figure 1H) and IL-6 (Figure 1I) induced by I/R were significantly inhibited by FGF21 treatment. These results provided further support that FGF21 protects against myocardial I/R injury.
Figure 1.
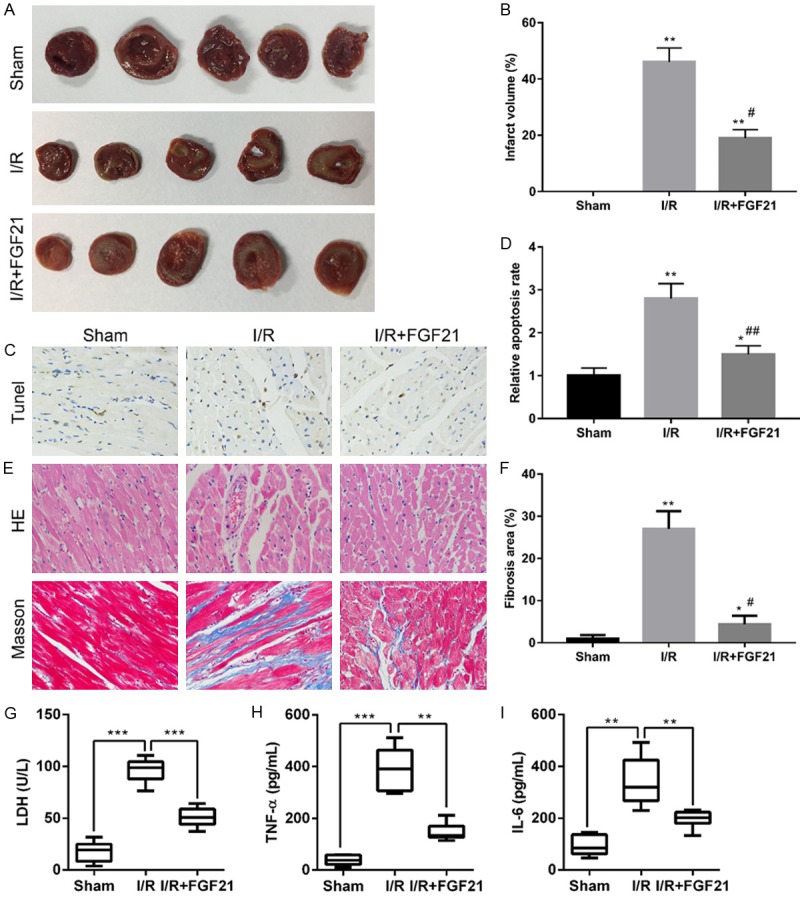
The protective effect of FGF21 on ischemia-reperfusion (I/R) injury in rats. I/R rats were injected intraperitoneally with 0. 1 mg/kg/day FGF21 for 4 weeks (I/R+FGF21). The sham control (sham) was not LAD ligated. (A, B) After reperfusion, hearts were harvested, sliced and stained with TTC (A) to examine the infarct size of the myocardium (B). (C) Cell apoptosis of myocardial tissue was evaluated by TUNEL assay. Brown staining of the nucleus indicated apoptosis. Magnification = 400×. (D) Comparison of apoptotic rates by TUNEL analysis. *P<0.05; **P<0.01 vs. sham. #P<0.05 vs. I/R. (E) HE (upper panel) and Masson trichrome staining (lower panel). Unaffected areas were red, while infarction areas were gray and white. Magnification = 400×. (F) Fibrosis area as percentage. *P<0.05; **P<0.01 vs. sham. #P<0.05 vs. I/R. (G) LDH activity, (H) TNF-α and (I) IL-6 in serum levels were measured by ELISA. **P<0.01, ***P<0.001.
FGF21 inhibits I/R-induced increase in miR-145 level and decrease in Angpt2 expression
To determine the molecular mechanism of FGF21 protection against myocardial I/R injury, we evaluated levels of miR-145 and Angpt2 in heart tissue (Figure 2). There was a significant decrease in miR-145 level in the I/R group compared with the sham group (P<0.01). The miR-145 level was significantly higher in the I/R+FGF21 group compared with the I/R group (P<0.01) (Figure 2A). In contrast, the Angpt2 mRNA expression in I/R group was significantly increased compared with the sham group, while the level in the I/R+FGF21 group was significantly decreased (P<0.01) (Figure 2B). The expression of Angpt2 protein was measured by IHC staining and Western blotting (Figure 2C, 2D). Both IHC staining and Western blotting analysis showed that I/R significantly increased the expression of Angpt2 and that FGF21 significantly inhibited the I/R-induced expression of Angpt2 (P<0.05). These results supported a relationship between miR-145 and Angpt2. Additionally, there was significant decrease in LC3B (LC3B II/I) and Beclin1 expression in the I/R group compared with the sham group, and the levels were significantly elevated in I/R+FGF21 group compared with the I/R group, indicating that FGF21 protected the myocardiocytes from the I/R injury via the promotion of autophagy (P<0.05).
Figure 2.
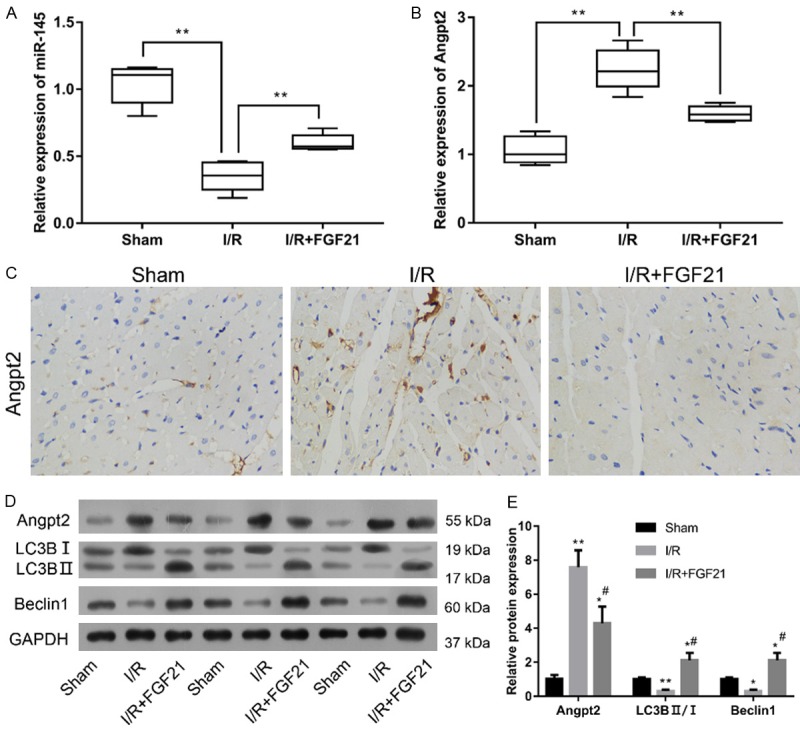
The effect of FGF21 on expression of miR-145 and Angpt2 and on autophagy after I/R. I/R rats were injected intraperitoneally with 0. 1 mg/kg/day FGF21 for 4 weeks (I/R+FGF21). (A, B) Expression levels of miR-145 (A) and Angpt2 mRNA (B) in myocardial tissue were measured by qRT-PCR. **P<0.01 vs. I/R. (C) IHC staining of Angpt2. Magnification = 400×. (D) Western blot analysis of Angpt2 and autophagy markers including LC3-B I, LC3-B II and Beclin1. (E) Quantification of protein expression. *P<0.05; **P<0.01 vs. sham. #P<0.05 vs. I/R.
miR-145 directly targets Angpt2
To determine whether miR-145 is involved in myocardial damage via the direct targeting of Angpt2, the dual-luciferase reporter system was used in HEK293 cells (Figure 3A). miR-145 mimics significantly decreased the luciferase activity in the Angpt2-3’UTR-wild type group (P<0.05), but there was no change in the Angpt2-3’UTR-mutant type group (Figure 3B).
Figure 3.
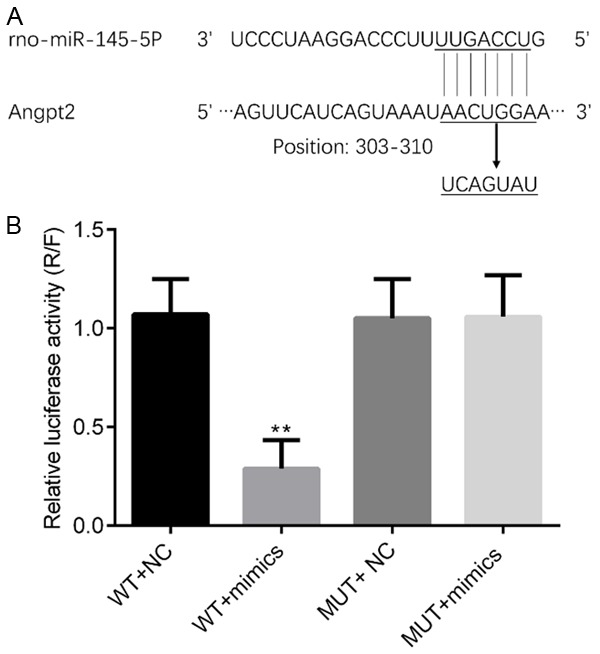
miR-145 directly targets the 3’UTR of Angpt2 from 303-310. A. The pluc-Reporter luciferase vector, wild type (WT)-3’UTR of Angpt2 containing the miR-145 binding site, and the corresponding mutated vectors (MUT) was constructed. B. After transfection into HEK-293T cells, relative luciferase activity was measured. **P<0.01.
miR-145 inhibitor attenuated the inhibitory effect of FGF21 on I/R-induced Angpt2 expression and autophagy
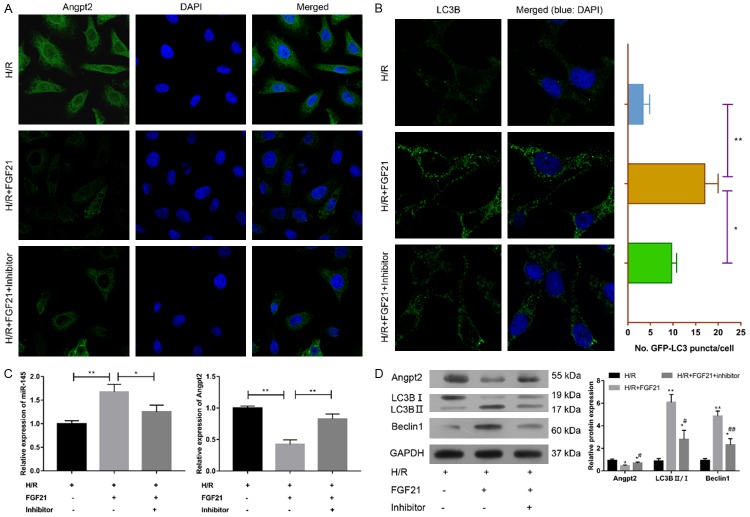
To confirm the protective role of miR-145 onFGF21 in myocardial H/R injury, H9c2 cells was transfected with miR-145 inhibitor. After transfection, the FGF21-induced miR-145 expression was significantly decreased after H/R (P<0.05) (Figure 4C). The qRT-PCR, immunofluorescence staining, Western blotting assays revealed that FGF21-inhibited Angpt2 expression was significantly rescued at both mRNA and protein levels (Figure 4A, 4C, 4D). These results suggested that downregulation of miR-145 attenuated the inhibitory effect of FGF21 on H/R-induced Angpt2 expression.
Figure 4.
Downregulation of miR-145 restored FGF21-inhibited Angpt2 expression and autophagy. H9c2 cells were transfected with miR-145 inhibitor and underwent H/R injury and FGF21 treatment. A. The distribution of Angpt2 was determined by immunofluorescence staining. LSM magnification = 120×. B. The distribution of LC3B was determineed by immunofluorescence staining. LSM magnification = 120×. *P<0.05; **P<0.01 vs. H/R+FGF21. The average number of LC3B puncta/cell was determined. C. Expression levels of miR-145 and Angpt2 were measured by qRT-PCR. *P<0.05; **P<0.01 vs. H/R+FGF21. D. Western blotting analysis of Angpt2, LC3-B I, LC3-B II and Beclin1. *P<0.05; **P<0.01 vs. H/R. #P<0.05; ##P<0.01 vs. H/R+FGF21.
In addition, the expression levels of autophagy markers including LC3B (LC3B II/I) and Beclin1 were substantially inhibited in the H/R model after the treatment with miR-145 inhibitor (Figure 4B, 4D), suggesting miR-145 inhibitor rescued FGF21-inhibited autophagy in myocardiocytes.
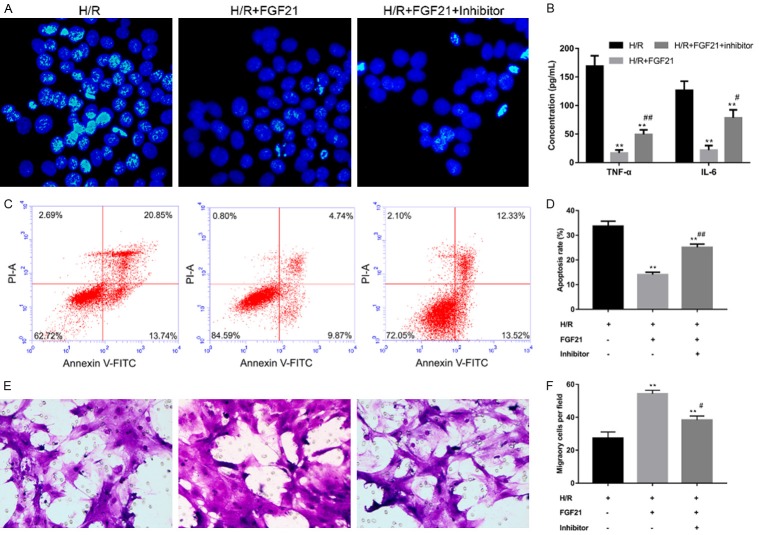
miR-145 inhibitor rescued FGF21-ihbited cell apoptosis and inhibited cell migration after I/R
To examine the role of miR-145 in myocardial I/R injury, cell apoptosis was assessed by Hoechst 33258 and flow cytometry, and migration was evaluated by Transwell assay. FGF21 inhibited cell apoptosis after H/R, but this effect was reversed by miR-145 inhibitor (Figure 5A, 5C, 5D), suggesting miR-145 inhibitor rescued the inhibitory effect of FGF21 on H/R-induced cell apoptosis. After the level of miR-145 was suppressed, the levels of proinflammatory factors such as TNF-α and IL-6 were significantly elevated compared with the H/R+FGF21 group (P<0.05) (Figure 5B). FGF21 promoted cell migration after H/R, and this effect was reversed by miR-145 inhibitor (Figure 5D and 5E), suggesting miR-145 inhibitor inhibited the FGF21 potentiation of cell apoptosis anti-inflammatory function after H/R. So, miR-145 was involved in FGF21 protection against I/R injury via regulation of cell apoptosis and cell migration.
Figure 5.
Downregulation of miR-145 rescued FGF21-inhibited cell apoptosis and inhibited FGF21-promoted cell migration. H9c2 cells were transfected with miR-145 inhibitor and underwent H/R injury and FGF21 treatment. A. Evaluation of cell apoptosis by Hoechst 33258. LSM magnification = 120×. B. Levels of proinflammatory factors TNF-α and IL-6, as measured by ELISA. **P<0.01 vs. H/R. #P<0.05; ##P<0. 01 vs. H/R+FGF21. C. Determination of cell apoptosis by flow cytometry. D. Quantification of apoptosis rate. **P<0.01 vs. H/R. ##P<0.01 vs. H/R+FGF21. E. Assessment of cell migration by Transwell assay. Magnification = 200×. F. Quantification of migratory cells per field. Each group randomly selected six fields for statistical analysis of migration. **P<0.01 vs. H/R.
Discussion
Ischemia-reperfusion (I/R) injury causes structural and functional changes in at the cellular, tissue, and organ level, culminating in cell death. Apoptosis is the main cause of cell death following I/R injury [2,4]. MiRNA plays a very important role in the occurrence and development of many diseases including cardiovascular disease [13]. In the present study, we identified the important role of miR-145 in the protective effects of FGF21 against myocardial I/R injury.
As previously demonstrated, H9c2 cardiomyocytes are a recognized cell model of heart ischemia-reperfusion injury and treatment [21]. We established an in vitro model of I/R with H9c2 and determined the effect of FGF21/miR-145 signalling on H9c2 migration. Previous evidence showed that the G-actin sequestering peptide thymosin β4 promotes myocardial cell migration in the embryonic heart and retains this property in postnatal cardiomyocytes, suggesting that cell migration may be important for cardiomyocytes in a damaged heart [22]. FGF21 is an endocrine factor that is mainly expressed in adipose and liver tissues [23]. It has been demonstrated that FGF21 improves cell survival and protects cells from cytokine-induced apoptosis through activation of extracellular signal-regulated kinase 1/2 and Akt pathways [24]. Endocrine FGF21 may inhibit cell death and reduce myocardial infarction in I/R injury through the fibroblast-growth factor receptor 1 (FGFRR 1)-PI3K-AKT-BAD-caspase-3 signaling pathway in cardiomyocytes [25]. A recent study reported that FGF21 protected cardiomyocytes against I/R injury through inhibition of Angpt2 [5]. Consistent with that study, we also found that FGF21inhibits H9c2 cell apoptosis and myocardial I/R injury and that FGF21 downregulates Angpt2.
It has been demonstrated that Angpt2 plays important role in heart injury and may be a potential predictor of heart disease [26,27]. Angpt2 inhibition prevents transplant-related I/R injury in rat cardiac allograft [26]. Angpt2 may compete with Angpt1 for binding and impede the activation of TEK tyrosine kinase [28-30]. Angpt2 also contributes to regulation of the inflammatory response and apoptosis [28]. It was reported that Angpt2 was downregulated in the model of H/R+FGF21, which promoted energy metabolism in cardiomyocytes and consequently led to more efficient cardioprotective effects of FGF21 [5]. In our study, we observed that FGF21 inhibited TNF-α and IL-6 levels after myocardial H/R injury; it is possible that this event is associated with Angpt2 downregulation. In tumors, miR-145 acts as tumor suppressor via regulation of Angpt2 [17,18]. Our results also suggested that Angpt2 was a direct target of miR-145. Collectively, findings showed that miR-145 was involved in myocardial I/R injury and that miR-145 was significantly decreased in the rat/mouse heart transplantation-induced I/R injury and myocardial H/R cell models [15,16]. This is consistent with our observation of downregulation of miR-145 in rat and cell myocardial I/R injury models. FGF21 administration increased the miR-145 levels following I/R injury, suggesting the involvement of miR-145/Angpt2 pathway in the protective effect of FGF21 from myocardial I/R injury.
In recent studies, autophagy was found to be involved in inhibition of cell apoptosis in myocardial I/R injury. In myocardial I/R injury, chloramphenicol treatment induced autophagy and significantly reduced infarct size and creatine kinase release [31]. Exercise-induced cardiac autophagy contributed to cardioprotection from I/R injury [32]. N-acetylcysteine (NAC) reduced diabetic myocardial I/R injury via inhibiting excessive autophagy [10]. Recent evidence indicated that autophagy was involved in the protective effect of ischemic post-conditioning against myocardial I/R injury [11]. Post-conditioning with berbamine inhibited I/R-induced impairment of autophagosome in cardiomyocytes [9]. Moreover, I/R elicited inflammatory responses [12]. Although autophagy is a survival mechanism for I/R cells, it also augments their production of chemokines including IL-6 [12]. In agreement with previous findings, our data also revealed high levels of TNF-α and IL-6 in the I/R model and alleviation of those increases by FGF21. Autophagy was regulated by miRNA. A recent study showed that miR-34a protects myocardial cells against I/R injury via modulation of TNF-α expression [33]. Our results indicated that FGF21 induced autophagy, which validates the previous study. However, our study is limited by the need for further investigation regarding the contribution of autophagy as well as the role of Angpt2 on cardiomyocyte apoptosis and inflammatory response by using autophagy and Angpt2 inhibitors.
In summary, FGF21 protects myocardial cells against I/R injury through the reduction of miR-145-mediated autophagy, along with the increase of miR-145 and suppression of Angpt2 expression. These results may suggest a novel therapeutic approach for reducing myocardial I/R injury.
Disclosure of conflict of interest
None.
References
- 1.Kunecki M, Plazak W, Podolec P, Golba KS. Effects of endogenous cardioprotective mechanisms on ischemia-reperfusion injury. Postepy Hig Med Dosw (Online) 2017;71:20–31. doi: 10.5604/17322693.1228267. [DOI] [PubMed] [Google Scholar]
- 2.Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. 2015;6:524–551. doi: 10.1016/j.redox.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou T, Chuang CC, Zuo L. Molecular characterization of reactive oxygen species in myocardial ischemia-reperfusion injury. Biomed Res Int. 2015;2015:864946. doi: 10.1155/2015/864946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu D, Wang J, Wang H, Ji A, Li Y. Protective roles of bioactive peptides during ischemia-reperfusion injury: from bench to bedside. Life Sci. 2017;180:83–92. doi: 10.1016/j.lfs.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Hu S, Cao S, Liu J. Role of angiopoietin-2 in the cardioprotective effect of fibroblast growth factor 21 on ischemia/reperfusion-induced injury in H9c2 cardiomyocytes. Exp Ther Med. 2017;14:771–779. doi: 10.3892/etm.2017.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Neblina F, Toledo AH, Toledo-Pereyra LH. Molecular biology of apoptosis in ischemia and reperfusion. J Invest Surg. 2005;18:335–350. doi: 10.1080/08941930500328862. [DOI] [PubMed] [Google Scholar]
- 7.Machado NG, Alves MG, Carvalho RA, Oliveira PJ. Mitochondrial involvement in cardiac apoptosis during ischemia and reperfusion: can we close the box? Cardiovasc Toxicol. 2009;9:211–227. doi: 10.1007/s12012-009-9055-1. [DOI] [PubMed] [Google Scholar]
- 8.Hamacher-Brady A, Brady NR, Gottlieb RA. The interplay between pro-death and pro-survival signaling pathways in myocardial ischemia/reperfusion injury: apoptosis meets autophagy. Cardiovasc Drugs Ther. 2006;20:445–462. doi: 10.1007/s10557-006-0583-7. [DOI] [PubMed] [Google Scholar]
- 9.Zheng Y, Gu S, Li X, Tan J, Liu S, Jiang Y, Zhang C, Gao L, Yang HT. Berbamine postconditioning protects the heart from ischemia/reperfusion injury through modulation of autophagy. Cell Death Dis. 2017;8:e2577. doi: 10.1038/cddis.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang S, Wang C, Yan F, Wang T, He Y, Li H, Xia Z, Zhang Z. N-acetylcysteine attenuates diabetic myocardial ischemia reperfusion injury through inhibiting excessive autophagy. Mediators Inflamm. 2017;2017:9257291. doi: 10.1155/2017/9257291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao M, Zhu S, Hu L, Zhu H, Wu X, Li Q. Myocardial ischemic postconditioning promotes autophagy against ischemia reperfusion injury via the activation of the nNOS/AMPK/mTOR Pathway. Int J Mol Sci. 2017;18:e614. doi: 10.3390/ijms18030614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solhjou Z, Uehara M, Bahmani B, Maarouf OH, Ichimura T, Brooks CR, Xu W, Yilmaz M, Elkhal A, Tullius SG, Guleria I, McGrath MM, Abdi R. Novel application of localized nanodelivery of anti-interleukin-6 protects organ transplant from ischemia-reperfusion injuries. Am J Transplant. 2017;17:2326–2337. doi: 10.1111/ajt.14266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makhdoumi P, Roohbakhsh A, Karimi G. MicroRNAs regulate mitochondrial apoptotic pathway in myocardial ischemia-reperfusioninjury. Biomed Pharmacother. 2016;84:1635–1644. doi: 10.1016/j.biopha.2016.10.073. [DOI] [PubMed] [Google Scholar]
- 14.Gottlieb RA, Pourpirali S. Lost in translation: miRNAs and mRNAs in ischemic preconditioning and ischemia/reperfusion injury. J Mol Cell Cardiol. 2016;95:70–77. doi: 10.1016/j.yjmcc.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao ZH, Hao W, Meng QT, Du XB, Lei SQ, Xia ZY. Long non-coding RNA MALAT1 functions as a mediator in cardioprotective effects of fentanyl in myocardial ischemia-reperfusion injury. Cell Biol Int. 2017;41:62–70. doi: 10.1002/cbin.10701. [DOI] [PubMed] [Google Scholar]
- 16.Li R, Yan G, Li Q, Sun H, Hu Y, Sun J, Xu B. MicroRNA-145 protects cardiomyocytes against hydrogen peroxide (H(2)O(2))-induced apoptosis through targeting the mitochondria apoptotic pathway. PLoS One. 2012;7:e44907. doi: 10.1371/journal.pone.0044907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu R, Ji Z, Li X, Zhai Q, Zhao C, Jiang Z, Zhang S, Nie L, Yu Z. miR-145 functions as tumor suppressor and targets two oncogenes, ANGPT2 and NEDD9, in renal cell carcinoma. J Cancer Res Clin Oncol. 2014;140:387–397. doi: 10.1007/s00432-013-1577-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, Hang C, Ou XL, Nie JS, Ding YT, Xue SG, Gao H, Zhu JX. MiR-145 functions as a tumor suppressor via regulating angiopoietin-2 in pancreatic cancer cells. Cancer Cell Int. 2016;16:65. doi: 10.1186/s12935-016-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takagawa J, Zhang Y, Wong ML, Sievers RE, Kapasi NK, Wang Y, Yeghiazarians Y, Lee RJ, Grossman W, Springer ML. Myocardial infarct size measurement in the mouse chronic infarction model: comparison of area- and length-based approaches. J Appl Physiol (1985) 2007;102:2104–2111. doi: 10.1152/japplphysiol.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng Y, Liu XH, Tarbell J, Fu B. Sphingosine 1-phosphate induced synthesis of glycocalyx on endothelial cells. Exp Cell Res. 2015;339:90–95. doi: 10.1016/j.yexcr.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Law CH, Li JM, Chou HC, Chen YH, Chan HL. Hyaluronic acid-dependent protection in H9C2 cardiomyocytes: a cell model of heart ischemia-reperfusion injury and treatment. Toxicology. 2013;303:54–71. doi: 10.1016/j.tox.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Bock-Marquette I, Saxena A, White MD, Dimaio JM, Srivastava D. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432:466–472. doi: 10.1038/nature03000. [DOI] [PubMed] [Google Scholar]
- 23.Fukumoto S. Actions and mode of actions of FGF19 subfamily members. Endocr J. 2008;55:23–31. doi: 10.1507/endocrj.kr07e-002. [DOI] [PubMed] [Google Scholar]
- 24.Wente W, Efanov AM, Brenner M, Kharitonenkov A, Koster A, Sandusky GE, Sewing S, Treinies I, Zitzer H, Gromada J. Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes. 2006;55:2470–2478. doi: 10.2337/db05-1435. [DOI] [PubMed] [Google Scholar]
- 25.Liu SQ, Roberts D, Kharitonenkov A, Zhang B, Hanson SM, Li YC, Zhang LQ, Wu YH. Endocrine protection of ischemic myocardium by FGF21 from the liver and adipose tissue. Sci Rep. 2013;3:2767. doi: 10.1038/srep02767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Syrjala SO, Tuuminen R, Nykanen AI, Raissadati A, Dashkevich A, Keranen MA, Arnaudova R, Krebs R, Leow CC, Saharinen P, Alitalo K, Lemstrom KB. Angiopoietin-2 inhibition prevents transplant ischemia-reperfusion injury and chronic rejection in rat cardiac allografts. Am J Transplant. 2014;14:1096–1108. doi: 10.1111/ajt.12672. [DOI] [PubMed] [Google Scholar]
- 27.Poss J, Ukena C, Kindermann I, Ehrlich P, Fuernau G, Ewen S, Mahfoud F, Kriechbaum S, Bohm M, Link A. Angiopoietin-2 and outcome in patients with acute decompensated heart failure. Clin Res Cardiol. 2015;104:380–387. doi: 10.1007/s00392-014-0787-y. [DOI] [PubMed] [Google Scholar]
- 28.Scholz A, Plate KH, Reiss Y. Angiopoietin-2: a multifaceted cytokine that functions in both angiogenesis and inflammation. Ann N Y Acad Sci. 2015;1347:45–51. doi: 10.1111/nyas.12726. [DOI] [PubMed] [Google Scholar]
- 29.Zeng Y. Endothelial glycocalyx as a critical signalling platform integrating the extracellular haemodynamic forces and chemical signalling. J Cell Mol Med. 2017;21:1457–1462. doi: 10.1111/jcmm.13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng Y. Endothelial glycocalyx: novel insight into atherosclerosis. J Biomed. 2017;2:109–116. [Google Scholar]
- 31.Giricz Z, Varga ZV, Koncsos G, Nagy CT, Gorbe A, Mentzer RM Jr, Gottlieb RA, Ferdinandy P. Autophagosome formation is required for cardioprotection by chloramphenicol. Life Sci. 2017;186:11–16. doi: 10.1016/j.lfs.2017.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee Y, Kwon I, Jang Y, Song W, Cosio-Lima LM, Roltsch MH. Potential signaling pathways of acute endurance exercise-induced cardiac autophagy and mitophagy and its possible role in cardioprotection. J Physiol Sci. 2017;67:639–654. doi: 10.1007/s12576-017-0555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shao H, Yang L, Wang L, Tang B, Wang J, Li Q. MicroRNA-34a Protect Myocardial Cells against Ischemia-Reperfusion Injury through Inhibiting Autophagy via Regulating TNFalpha Expression. Biochem Cell Biol. 2018;96:349–354. doi: 10.1139/bcb-2016-0158. [DOI] [PubMed] [Google Scholar]