Abstract
The essential ingredient of Centella asiatic is asiatic acid (AA). There are a lot of biological activities in AA, such as anti-oxidant, anti-diabetic. However, so far, there have been no reports on the underlying protective mechanism of AA on podocytes. In this research, we observed the morphological changes of podocytes in diabetic rats by optics microscope and transmission electron microscopy and the protective effect of AA. Additionally, we investigated the expressions of nephrin, desmin and p-JNK, JNK in podocytes of diabetic rats and the influence of AA on podocytes and JNK signaling pathway. The results showed that AA could reduce renal function and urinary albumin. It could attenuate abnormal pathological findings of podocytes in kidney tissue of diabetic rats. Besides, treatment with AA could significantly improve the expression of nephrin and decrease expression of desmin. The ratio of p-JNK protein to JNK protein in podocytes was reduced considerably by AA. With the treatment dose of AA increased, the renal protective effect of AA was gradually improved. These results indicate that asiatic acid has a significant protective effect on diabetic nephropathy. Potential mechanisms include inhibiting the production of oxidants effectively, protection of podocytes, and suppression of the JNK signaling pathway activation. Therefore, there is an excellent prospect of using AA to treat diabetic nephropathy.
Keywords: Diabetic nephropathy, podocytes, asiatic acid, desmin, nephrin, JNK signaling pathway
Introduction
All over the world, diabetes mellitus (DM) has grown up to be a worldwide epidemic disease. The prevalence of diabetes mellitus will increase substantially from 2010. One of the most common chronic complications to diabetes is diabetic nephropathy. Nearly half of these patients with diabetes mellitus will be affected by diabetic nephropathy.
Podocytes locate on the outside of the glomerular basement membrane. It is one of highly differentiated cell in the kidney. Podocytes are the most critical components in the glomerular filtration barrier [1]. Besides, nephrin is an essential protein in podocytes of kidney. Nephrin is not only crucial to the slit membrane structure of podocytes but also can preserve glomerular ultrafiltration intact. Nephrin functions as cell adhesion and signaling, and regulates the structure and function of podocytes [2]. When podocytes are damaged, the desmin protein is also affected as an intermediate vector protein [3]. Desmin protein is one of the sensitive signs of rat podocytes injury.
In the MAPK superfamily, the c-Jun amino-terminal kinase (JNK) signaling pathway is one of the most critical members. It is activated by a variety of cellular stress pressures as well as plays an essential role in inflammation, cell proliferation, cell death and so on [4]. The characteristic feature of most kidney injury patients is that the JNK signaling pathway is activated. It is pronounced that similar JNK pathways are activated in animal models of chronic kidney injury. Underlining the pathological process of diabetic nephropathy (DN), oxidative stress results from the link with the majority of molecular events. In DN, as an increase in reactive oxygen species (ROS), the imbalance between pro-oxidant process and anti-oxidant process always exists [5]. The excessive production of ROS can reduce the level of the antioxidant enzyme.
Asiatic acid (AA) has been used widely in Chinese medicine [6]. However, the renal protective effect of AA on diabetic nephropathy remains unknown. Therefore, the present study assesses the protective effect of AA on diabetic nephropathy, probes the possible action of AA on podocytes by regulation of the JNK signaling pathway.
Materials and methods
Animals and management
40 male Sprague-Dawley rats weighing 180-200 g were purchased from the animal center of Jiangsu University in China and housed in the Animal Laboratory of Jiangsu University. Room temperature and humidity were controlled. All experimental procedures were following the National Institutes of Health Guide to Use and Care of Medical Laboratory Animals. All rat received compassionate care. The study protocol was approved by the Ethics Committee of Jiangsu University. After overnight fasting, STZ was injected intraperitoneally to induce the model of diabetes mellitus at the dose of 65 mg/kg. After seven days, the random blood glucose of rat tail vein was detected. When blood sugar was more than 16.7 mmol/L, the diabetic model was considered successful. Diabetic rats were divided into four groups (n=8 per group): diabetic group (DM group), low dose AA treated-diabetic group (AA1 group), medium dose AA treated-diabetic group (AA2 group) and high dose AA treated-diabetic group (AA3 group). Eight normal rats were used as control group (NC group). The rats in the DM and NC group were given distilled water daily. Rats in AA treatment groups were given asiatic acid every day by gavage for eight weeks (10, 20, 40 mg/kg respectively).
Reagents and antibodies
Streptozotocin was purchased from Sigma Company (St. Louis, USA). Asiatic acid was procured from PuRuiFa Technology Development Co. Ltd (Chengdu, China). The rabbit anti-rat nephrin antibody and rabbit anti-rat desmin antibody were purchased from Abcam (Cambridge, UK). The rabbit anti-rat p-JNK antibody and rabbit anti-rat JNK antibody were purchased from Cell Signaling Technology (Danvers, USA).
Blood sample collection
The experiment finished after asiatic acid was given eight weeks. Then we measured the weight of each rat. After rats were anesthetized with ketamine (100 mg/kg), blood was collected from the abdominal aorta. As described previously [7] the serum was processed for detection of fasting blood glucose (FBG), blood urea nitrogen (BUN) and serum creatinine (SCR) using automatic biochemistry analyzer (Roche, Switzerland).
Urinary albumin excretion rate (UAER)
Before being sacrificed, rats had collected urine samples of 24 hours for measurement of albumin concentration. After centrifugation, the supernatants were frozen at -80°C refrigerator. Urinary albumin concentration was measured with an ELISA Kit according to the manufacturer’s protocol (Jiancheng Company, Jiangsu, China). As previously described [8], 24 h UAER was calculated by urinary albumin concentration multiplying 24 h urine volumes.
Kidney tissue hematoxylin-eosin staining
All kidneys were removed from rats. Changes in the weight of kidneys were recorded. After removal, kidney tissues were excised and fixed in 10% neutral formalin immediately. They were dehydrated, embedded in paraffin, cut into 4 μm sections and stained with hematoxylin-eosin for histological examination. As previously described [9], histopathologic evaluation was performed by two observers blind to the protocol.
The transmission electron microscopy detection of kidney tissue
1 mm3 of kidney tissue was obtained and immediately prefix in 2.5% glutaraldehyde, then fixed with 1% osmium tetroxide. Subsequently, tissues were dehydrated and embedded. Ultrastructural changes of podocytes were observed under JEM 1010 electron microscope (JEOL company, Japan), as previously described [10].
Assessment activities of SOD and contents of MDA
Kidney tissues were rinsed, weighed, and homogenized for ten minutes. After centrifugation at 4000 r/min 4 degrees for ten minutes, the supernatants were collected and used immediately for detection the activities of SOD and contents of MDA (Jiancheng Limited Company, Nanjing, Jiangsu, China).
Immunohistochemistry
Kidney tissues were fixed in 10% neutral formalin, embedded in paraffin, cut into 4 μm sections. As previously described [11], sections were dewaxed and quenched endogenous peroxidase for ten mins using 3% hydrogen peroxides. Immunohistochemical staining was performed for nephrin protein using rabbit anti-rat monoclonal antibody at the dilution of 1:2000 (#ab216341) overnight at 4°C. Immunohistochemical staining was performed for desmin protein using rabbit anti-rat desmin monoclonal antibody at the dilution of 1:2000 (#ab32362) overnight at 4°C. Later, the sections were incubated with HRP-connected secondary antibodies for 30 mins at room temperature. Then sections were stained with the DAB kit and counterstained with hematoxylin. Finally, stained images were captured with optics microscope, and all images were acquired on the Leica microscope.
Reverse transcription-quantitative PCR (RT-qPCR)
RNA from the renal cortex was isolated using Trizol (Thermo Fisher Scientific, USA). RNA was reverse transcribed using the GoScript Reverse Transcription kit (Promega Corporation, USA), according to the suppliers’ protocol. The mRNA levels were measured by RT-qPCR using SYBR-green (Takara, Japan) and normalized to housekeeping gene β-actin as described [12]. Relative quantification of RNA expression was calculated via the 2-ΔΔCt method. The primer sequences used to amplify mRNAs were shown in Table 1.
Table 1.
RT-qPCR primers’ sequence details
| Gene name | Forward primers (3’-5’) | Reverse primer (5’-3’) |
|---|---|---|
| Nephrin | GTCGTAGATTCCCCTTGGGT | GAGAGTCTATGGCCCACCTG |
| Desmin | CAACCTTCCTATCCAGACCTTCT | GTAGCCTCGCTGACAACCTCTC |
| β-actin | CGTTGACATCCGTAAAGACC | AACAGTCCGCCTAGAAGCAC |
Western blot analysis
The protein concentrations of p-JNK and JNK were quantified with the Bio-Rad protein colorimetric assay (Bio-Rad, USA). Kidney samples were homogenized, boiled and centrifuged. The protein content was measured by the BCA assay. Samples were separated by SDS-PAGE, proteins were transferred from the gel to PVDF membrane. At room temperature, membranes were immersed in TBST containing 5% BSA for one hour. Then membranes were incubated with primary antibodies at 4°C overnight. The primary antibodies which targeted p-JNK protein were at the dilution of 1:1000 (#4668) and targeted JNK were at the dilution of 1:1000 (#9252). Subsequently, at room temperature, membranes were incubated with secondary antibodies for one hour. The target bands of membranes were exposed to ECL kit. Band intensity was quantified using Image J software and normalized to the respective control. β-actin was used as a loading control at the dilution of 1:5000.
Statistical analysis
All statistical analyses were performed with SPSS 20.0. Experiments were performed at least three times. All data were presented as mean ± standard deviation. Differences in multiple groups were analyzed by one-way ANOVA with LSD multiple comparison post hoc. Two-tailed P<0.05 was considered statistically significant. *P<0.05 and **P<0.01 vs. NC group; #P<0.05 and ##P<0.01 vs. DM group. All figures were performed using Graph Pad Prism 5.0.
Results
The changes in clinical parameters
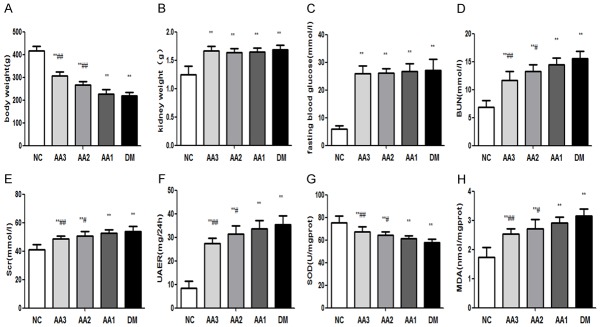
In the DM group, body weight was significantly reduced (P<0.05), however kidney weight, and fasting blood glucose were significantly increased when compared with the NC group (P<0.05). Besides, treatment for AA could attenuate body weight, kidney weight and fasting blood glucose when compared with DM group. There was no significant difference in three AA treatment groups (Figure 1A-C).
Figure 1.
The changes in clinical parameters and oxidative stress parameters. A. Asiatic acid treatment ameliorated body weight in diabetic nephropathy rats. B. Asiatic acid treatment ameliorated kidney weight in diabetic nephropathy rats. C. Asiatic acid treatment ameliorated fasting blood glucose. D. Asiatic acid treatment ameliorated blood urea nitrogen in diabetic nephropathy rats. E. Asiatic acid treatment ameliorated serum creatinine in diabetic nephropathy rats. F. Asiatic acid treatment ameliorated UAER in diabetic nephropathy rats. G. In DM group, it was decreased in the activity of SOD. However, AA treatment increased the activity of SOD. H. It was increased in the activity of MDA in the DM group, AA treatment decreased the content of MDA. *P<0.05 and **P<0.01 vs. NC group; #P<0.05 and ##P<0.01 vs. DM group.
The changes in blood and urine parameters
When compared with the NC group, the BUN, SCR, and UAER of DM group were significantly increased (P<0.05). However, in AA groups, the BUN, SCR, and UAER were decreased. These results indicated that AA could attenuate BUN, SCR, and UAER of diabetic rats (Figure 1D-F).
The changes in renal histopathology
In the NC group, the morphology and structure of renal tissues were normal. While in renal tissues of diabetic rats, under the optical microscope, glomerular hypertrophy, mesangial cell proliferation, as well as extracellular matrix accumulation were observed (magnification, 100×). In AA groups, these abnormal pathological findings in kidney tissues were improvement significantly (Figure 2A).
Figure 2.
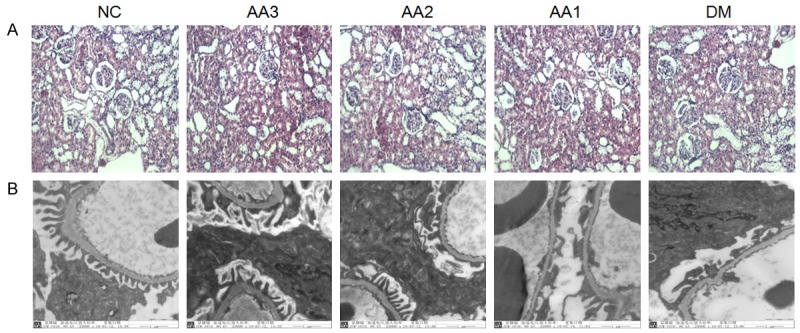
The changes in renal histopathology and podocytes under the optical microscope and transmission electron microscopy. A. The results of renal histopathology under the optical microscope. Hematoxylin-Eosin Staining of kidney in Control rats, DM rats and three AA treatment groups (Magnifications ×100). B. The results of podocytes under transmission electron microscopy (Magnifications ×25000). The foot process of podocytes were effacement in the DM group. However, AA treatment significantly attenuated the foot process of podocytes fusion.
The changes in podocytes under transmission electron microscopy
Transmission electron microscopy showed that foot process of podocytes effacement in DM group compared with NC group (magnification, 25000×). However, AA treatment significantly attenuated the foot process of podocytes fusion (Figure 2B).
The changes in SOD activities and MDA contents
In kidneys of DM group, the content of MDA was increased significantly (P<0.05), while it was decreased in the activity of SOD (P<0.05). Besides, treatment with AA could reduce the content of MDA and increase the activity of SOD. Moreover, as the treatment dose of AA increased, the activity of SOD in AA groups showed an increasing trend; however, the content of MDA in AA groups showed a downward trend (Figure 1G, 1H).
The changes in the expression of nephrin protein
Nephrin protein expression was corroborated by immunohistochemistry. Compared with the NC group, nephrin protein in kidneys of DM group showed a significant decline (P<0.01). However, AA treatment improved the expression of nephrin protein compared with DM group. As the treatment dose of AA increased, the expression of nephrin in AA groups showed an increasing trend (Figure 3A).
Figure 3.
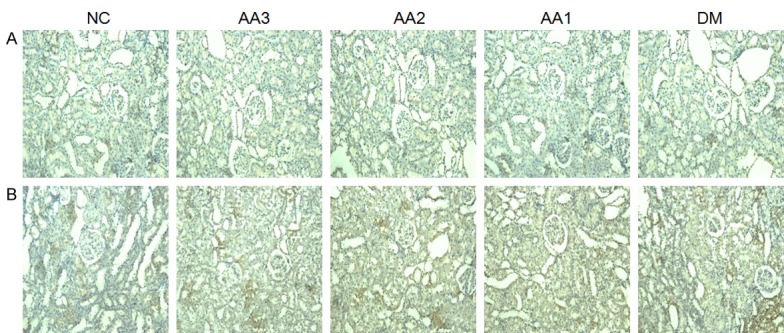
Representative photomicrographs of kidney sections immunostain for nephrin and desmin protein. A. The changes in expressions of nephrin protein (Magnifications ×100). In the DM group, the nephrin protein showed a significant decline. However, AA treatment improved the expression of nephrin protein. B. The changes in expressions of desmin protein in kidney (Magnifications ×100). The desmin protein in DM group showed significant increased. However, AA treatment decreased the expression of desmin protein.
The changes in the expression of desmin protein
Compared with the NC group, desmin protein in kidneys of DM group showed a significant increase (P<0.01). Compared with DM group, AA treatment decreased the expression of desmin protein. As the treatment dose of AA increased, the expression of desmin in AA groups showed a decreasing trend (Figure 3B).
The changes in the expressions of nephrin and desmin mRNA levels
Compared with the NC group, nephrin mRNA level in DM group showed significant decline and the desmin mRNA level in DM group showed a significant increase (P<0.01). However, compared with DM group, treatment with AA improved the nephrin mRNA levels and decreased desmin mRNA levels (P<0.01). Besides, as the treatment dose of AA increased, the expression of nephrin in AA groups showed an increasing trend, but the expression of desmin showed a decreasing trend (Figure 4).
Figure 4.
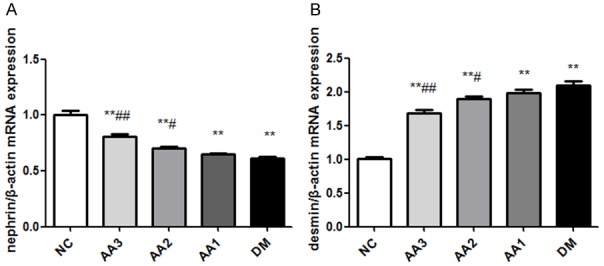
The effect of asiatic acid on nephrin and desmin mRNA levels in diabetic nephropathy rats. A. The changes were in nephrin mRNA expression in kidney. B. The changes were in desmin mRNA expression. Total RNA was extracted from a piece of kidney, mRNA levels were determined by RT-qPCR using β-actin as a housekeeping gene. *P<0.05 and **P<0.01 vs. NC group; #P<0.05 and ##P<0.01 vs. DM group.
The changes in the expressions of p-JNK and JNK protein in renal cortex
Podocytes in NC group expressed a lower level of p-JNK/JNK. However, podocytes in DM group and AA group showed a higher level of p-JNK/JNK (P<0.05). Besides, compared with the DM group, p-JNK/JNK in AA3 group was significantly decreased (P<0.05). As the treatment dose of AA increased, p-JNK/JNK in AA groups showed a decreasing trend (Figure 5). It was demonstrated that the JNK signaling pathway was involved in the protective effect of AA on diabetic nephropathy.
Figure 5.
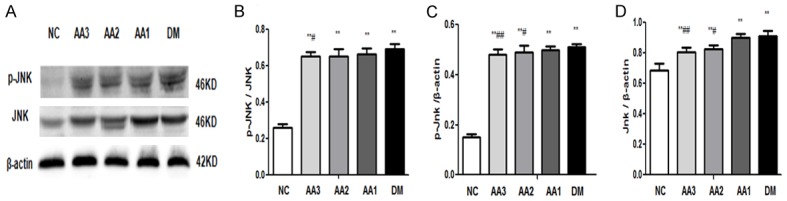
Immunoblots of p-JNK and JNK protein expressions in diabetic nephropathy rats. A. Western blot assay examination for p-JNK and JNK proteins expression. B. The ratio in phosphorylated JNK protein to total JNK protein. C. Statistical analysis for the p-JNK expression. D. Statistical analysis for the JNK expression. *P<0.05 and **P<0.01 vs. NC group; #P<0.05 and ##P<0.01 vs. DM group.
Discussion
The state of chronic oxidative stress which is caused by long-term hyperglycemia can further aggravate the diabetic state. Oxidative stress is thought to be one of the crucial factors in the pathogenesis of DN. Abnormal metabolism can result in the overproduction of free radicals such as hydroxyl and superoxide radicals, which are known as reactive oxygen species (ROS) [13]. The accumulation of ROS can lead to oxidative stress. It damages the tissue in and around kidney.
In diabetic patients and animal models, decreasing plasma antioxidant concentrations lead to activated of oxidative stress responses [14]. Malondialdehydes (MDA) is one of the most important secondary products of hydro peroxides degradation. It is also a widely used marker for lipid peroxidation. It has been raised in erythrocytes of type 2 diabetic patients. There are a large number of enzymatic and non-enzymatic components in the antioxidant defense system. Among them, SOD is the first line of defense against oxidative stress, which contributes to the development of DN [15]. Besides, SOD is also widespread in high-oxygen tissues.
The present state of renal dysfunction was demonstrated for the diabetic rat. Our data suggested that BUN, SCR, and UAER were significantly increased in diabetic rats. At the same time, there was an apparent pathological morphological abnormality in the kidney of diabetic rat. Above results were consistent with the previous research results, which stated that the nephropathy model of the diabetic rat was successful [16]. Our experimental results showed that the content of MDA increased while the activity of SOD decreased in the kidney of diabetic rats. It indicated that the products of oxidative damage increased and the activity of enzyme in the antioxidant system decreased. The balance of oxidation - antioxidant system was broken, and the state of oxidative stress in the body was aggravated significantly.
Podocytes are terminally differentiated specialized pericyte-like cells that encase the exterior basement membranes of glomerular capillary [17]. The basal side contains several types of integrins that help podocytes connect to glomerular basement membrane. The function of slit diaphragm as a size barrier is critical to successful retention of albumin. It has been established that mutations in slit diaphragm proteins lead to podocytes dysfunction and foot process effacement, resulting in defective glomerular filtration along with the onset of proteinuria [18]. So podocytes represent a vital cell type of the glomerulus which plays a significant role in physiological glomerular filtration function. The derangement in the pathological state of podocytes leads to proteinuria [19].
Nephrin is one of the essential molecules in podocytes which can maintain regular slit diaphragm structure of podocytes. Not only nephrin can interact with other slit diaphragm protein in podocytes, but also it can mediate a critical cell signaling pathway to podocytes. Reduction of nephrin expression is often observed in adult kidney diseases such as diabetic nephropathy and so on [20]. Besides the important role in scaffolding of slit diaphragms, nephrin serves as a “signaling node” in slit diaphragms. It can transmit extracellular domain from slit diaphragms to the intracellular actin cytoskeleton [21]. Desmin is a kind of cytoskeleton protein, which can be expressed in small amounts of the glomerular membrane cells frequently. However, desmin has no apparent expression in podocytes. When podocytes are damaged because of a variety of reasons, the expressions of desmin protein increase significantly, and the phenotypic of desmin transformations occur. The research of Herrmann A found that [22], in the puromycin aminonucleoside nephrosis (PAN) rat model, which was a model for acute podocytes injury, desmin staining was enhanced in podocytes. Enhanced desmin staining in podocytes was also observed in diabetic nephropathy [23]. On the tensile glomerular capillary wall, the up-regulation of desmin protein may increase the mechanical ability of cells, and enable podocytes to undergo morphological changes [24].
The JNK signaling pathway can be activated by a lot of stress response, such as oxidative stress, osmotic stress [25]. In the long-term hyperglycemia of diabetes, there are too many ROS in the body so that the JNK signaling pathway can be activated. The activating JNK signaling pathway can induce the dysfunction, injury, even apoptosis of podocytes, lead to the destruction of the integrity of the glomerular filtration barrier, resulting in the occurrence of proteinuria. Kim OS et al. [26] found that hydrogen peroxide can induce a large number of reactive oxygen species in glomerular mesangial cells, further activating the JNK signal pathway and increasing the expression of caspase-3. Our results suggested that the ratio of P-JNK protein to JNK protein increased, so the JNK signaling pathway was activated in diabetic nephropathy rats. Further lead to the expression of nephrin RNA and protein decreased, and the expression of desmin RNA and protein increased. It can result in injury of podocytes and lead to the occurrence of proteinuria.
Asiatic acid is a kind of widely used herbal medicine in China. There are many biological activities in AA, such as anti-oxidant [27], anti-diabetic [28], lipid-lowering [29], anticancer [30], liver-protecting [31] and so on. Ramachandran V [32] also found that, through anti-inflammatory action, regulation of glucose metabolism enzymes, and anti-fibrotic action in the pancreatic islets AA could reduce glucose levels. In conclusion, we indicate that asiatic acid can effectively inhibit the production of oxidation products and inhibit the JNK signaling pathway activated. Thus it can further protect podocytes and delay the progress of diabetic nephropathy. Asiatic acid has the potential application in the treatment of diabetic nephropathy.
Acknowledgements
This study was supported by the Natural Science Foundation of Jiangsu province of China (H200830).
Disclosure of conflict of interest
None.
References
- 1.Ying Q, Wu G. Molecular mechanisms involved in podocyte EMT and concomitant diabetic kidney diseases: an update. Ren Fail. 2017;39:474–483. doi: 10.1080/0886022X.2017.1313164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ristola M, Lehtonen S. Functions of the podocyte proteins nephrin and Neph3 and the transcriptional regulation of their genes. Clin Sci (Lond) 2014;126:315–328. doi: 10.1042/CS20130258. [DOI] [PubMed] [Google Scholar]
- 3.Huang G, Lv J, Li T, Huai G, Li X, Xiang S, Wang L, Qin Z, Pang J, Zou B, Wang Y. Notoginsenoside R1 ameliorates podocyte injury in rats with diabetic nephropathy by activating the PI3K/Akt signaling pathway. Int J Mol Med. 2016;38:1179–1189. doi: 10.3892/ijmm.2016.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grynberg K, Ma FY, Nikolic-Paterson DJ. The JNK signaling pathway in renal fibrosis. Front Physiol. 2017;8:829. doi: 10.3389/fphys.2017.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miranda-Díaz AG, Pazarín-Villaseñor L, Yanowsky-Escatell FG, Andrade-Sierra J. Oxidative stress in diabetic nephropathy with early chronic kidney disease. J Diabetes Res. 2016;2016:7047238. doi: 10.1155/2016/7047238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orhan IE. Centella asiatica (L.) urban: from traditional medicine to modern medicine with neuroprotective potential. Evid Based Complement Alternat Med. 2012;2012:946259. doi: 10.1155/2012/946259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Wu CG, Fang CQ, Gao J, Liu YZ, Chen Y, Chen YN, Xu ZG. The protective effect of alpha-Lipoic acid on mitochondria in the kidney of diabetic rats. Int J Clin Exp Med. 2013;6:90–97. [PMC free article] [PubMed] [Google Scholar]
- 8.Chen S, Chen H, Liu Q, Ma Q. Effect of simvastatin on the expression of nephrin, podocin, and vascular endothelial growth factor (VEGF) in podocytes of diabetic rat. Int J Clin Exp Med. 2015;8:18225–18234. [PMC free article] [PubMed] [Google Scholar]
- 9.Yousefi H, Karimi P, Alihemmati A, Alipour MR, Habibi P, Ahmadiasl N. Therapeutic potential of genistein in ovariectomy-induced pancreatic injury in diabetic rats: the regulation of MAPK pathway and apoptosis. Iran J Basic Med Sci. 2017;20:1009–1015. doi: 10.22038/IJBMS.2017.9269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boels MG, Avramut MC, Koudijs A, Dane MJ, Lee DH, van der Vlag J, Koster AJ, van Zonneveld AJ, van Faassen E, Grone HJ, van den Berg BM, Rabelink TJ. Atrasentan reduces albuminuria by restoring the glomerular endothelial glycocalyx barrier in diabetic nephropathy. Diabetes. 2016;65:2429–2439. doi: 10.2337/db15-1413. [DOI] [PubMed] [Google Scholar]
- 11.Jiang P, Huang R, Ma N, Jiang F. The expression of calcium sensing receptor in normal and diabetic rat eyes. Med Sci Monit. 2018;24:706–710. doi: 10.12659/MSM.905657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Groot T, Damen L, Kosse L, Alsady M, Doty R, Baumgarten R, Sheehan S, van der Vlag J, Korstanje R, Deen PMT. Lithium reduces blood glucose levels, but aggravates albuminuria in BTBR-ob/ob mice. PLoS One. 2017;12:e0189485. doi: 10.1371/journal.pone.0189485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johar DR, Bernstein LH. Biomarkers of stress-mediated metabolic deregulation in diabetes mellitus. Diabetes Res Clin Pract. 2017;126:222–229. doi: 10.1016/j.diabres.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 15.Li C, Miao X, Li F, Wang S, Liu Q, Wang Y, Sun J. Oxidative stress-related mechanisms and antioxidant therapy in diabetic retinopathy. Oxid Med Cell Longev. 2017;2017:9702820. doi: 10.1155/2017/9702820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu HE, Jian CH, Chen SF, Chen TM, Lee ST, Chang CS, Weng CF. Hypoglycaemic effects of fermented mycelium of Paecilomyces farinosus (G30801) on high-fat fed rats with streptozotocin-induced diabetes. Indian J Med Res. 2010;131:696–701. [PubMed] [Google Scholar]
- 17.Kawakami T, Ren S, Duffield JS. Wnt signalling in kidney diseases: dual roles in renal injury and repair. J Pathol. 2013;229:221–231. doi: 10.1002/path.4121. [DOI] [PubMed] [Google Scholar]
- 18.Saurus P, Kuusela S, Lehtonen E, Hyvönen ME, Ristola M, Fogarty CL, Tienari J, Lassenius MI, Forsblom C, Lehto M, Saleem MA, Groop PH, Holthöfer H, Lehtonen S. Podocyte apoptosis is prevented by blocking the toll-like receptor pathway. Cell Death Dis. 2015;6:e1752. doi: 10.1038/cddis.2015.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bose M, Almas S, Prabhakar S. Wnt signaling and podocyte dysfunction in diabetic nephropathy. J Investig Med. 2017;65:1093–1101. doi: 10.1136/jim-2017-000456. [DOI] [PubMed] [Google Scholar]
- 20.Li X, He JC. An update: the role of nephrin inside and outside the kidney. Sci China Life Sci. 2015;58:649–657. doi: 10.1007/s11427-015-4844-1. [DOI] [PubMed] [Google Scholar]
- 21.New LA, Martin CE, Jones N. Advances in slit diaphragm signaling. Curr Opin Nephrol Hypertens. 2014;23:420–430. doi: 10.1097/01.mnh.0000447018.28852.b6. [DOI] [PubMed] [Google Scholar]
- 22.Herrmann A, Tozzo E, Funk J. Semi-automated quantitative image analysis of podocyte desmin immunoreactivity as a sensitive marker for acute glomerular damage in the rat puromycin aminonucleoside nephrosis (PAN) model. Exp Toxicol Pathol. 2012;64:45–49. doi: 10.1016/j.etp.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Benardeau A, Verry P, Atzpodien EA, Funk JM, Meyer M, Mizrahi J, Winter M, Wright MB, Uhles S, Sebokova E. Effects of the dual PPARalpha/gamma agonist aleglitazar on glycaemic control and organ protection in the Zucker diabetic fatty rat. Diabetes Obes Metab. 2013;15:164–174. doi: 10.1111/dom.12006. [DOI] [PubMed] [Google Scholar]
- 24.Park MJ, Bae CS, Lim SK, Kim DI, Lim JC, Kim JC, Han HJ, Moon JH, Kim KY, Yoon KC, Park SH. Effect of protopanaxadiol derivatives in high glucose-induced fibronectin expression in primary cultured rat mesangial cells: role of mitogen-activated protein kinases and Akt. Arch Pharm Res. 2010;33:151–157. doi: 10.1007/s12272-010-2237-3. [DOI] [PubMed] [Google Scholar]
- 25.Ma FY, Liu J, Nikolic-Paterson DJ. The role of stress-activated protein kinase signaling in renal pathophysiology. Braz J Med Biol Res. 2009;42:29–37. doi: 10.1590/s0100-879x2008005000049. [DOI] [PubMed] [Google Scholar]
- 26.Kim OS, Kim YS, Jang DS, Yoo NH, Kim JS. Cytoprotection against hydrogen peroxide-induced cell death in cultured mouse mesangial cells by erigeroflavanone, a novel compound from the flowers of erigeron annuus. Chem Biol Interact. 2009;180:414–420. doi: 10.1016/j.cbi.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 27.Pakdeechote P, Bunbupha S, Kukongviriyapan U, Prachaney P, Khrisanapant W, Kukongviriyapan V. Asiatic acid alleviates hemodynamic and metabolic alterations via restoring eNOS/iNOS expression, oxidative stress, and inflammation in diet-induced metabolic syndrome rats. Nutrients. 2014;6:355–370. doi: 10.3390/nu6010355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Lu Q, Yu DS, Chen YP, Shang J, Zhang LY, Sun HB, Liu J. Asiatic acid mitigates hyperglycemia and reduces islet fibrosis in Goto-Kakizaki rat, a spontaneous type 2 diabetic animal model. Chin J Nat Med. 2015;13:529–534. doi: 10.1016/S1875-5364(15)30047-9. [DOI] [PubMed] [Google Scholar]
- 29.Ramachandran V, Saravanan R, Senthilraja P. Antidiabetic and antihyperlipidemic activity of asiatic acid in diabetic rats, role of HMG CoA: in vivo and in silico approaches. Phytomedicine. 2014;21:225–232. doi: 10.1016/j.phymed.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Ai L, Lv T, Jiang X, Liu F. Asiatic acid, a triterpene, inhibits cell proliferation through regulating the expression of focal adhesion kinase in multiple myeloma cells. Oncol Lett. 2013;6:1762–1766. doi: 10.3892/ol.2013.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Yang F, Yuan M, Jiang L, Yuan L, Zhang X, Li Y, Dong L, Bao X, Yin S. Synthesis and evaluation of asiatic acid derivatives as anti-fibrotic agents: structure/activity studies. Steroids. 2015;96:44–49. doi: 10.1016/j.steroids.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Ramachandran V, Saravanan R. Efficacy of asiatic acid, a pentacyclic triterpene on attenuating the key enzymes activities of carbohydrate metabolism in streptozotocin-induced diabetic rats. Phytomedicine. 2013;20:230–236. doi: 10.1016/j.phymed.2012.09.023. [DOI] [PubMed] [Google Scholar]