Abstract
GC is associated with over expression of epidermal growth factor receptor (EGRF), Cyclooxygenase-2 (COX-2) and 5-Lipoxygenase (5-LOX). We postulated that targeting these pathways will result in better treatment efficacy than using a single agent with higher dose which may cause toxicity and resistance. We evaluated Tepoxalin (TPX) a dual 5-LOX-COX inhibitor and Erlotinib (ERB) an EGFR inhibitor alone and combination in MGC-803 injected tumor xenografts mice. Female nude mice were selected and injected subcutaneously with MGC-803 GC cells and were grouped after the tumor model was formed. The treatment of TPX, ERB and their combination was given for 21 days. After treatment protocol proliferating index was measured, expression of apoptosis related proteins, 5-LOX, COX-2, EGFR, vascular endothelial growth factor-C (VEGF-C) and density of lymphatic vessel density was evaluated in tumor tissues. TUNEL assay was done for apoptosis. The outcomes of study revealed that TPX and ERB alone inhibited the growth of tumor but their combination showed a synergistic antitumor activity. TPX and ERB alone resulted in apoptosis and antiproliferative effect, whereas their combination showed highly significant results (P<0.01). TPX alone and its combination with ERB suppressed 5-LOX, COX-2, EGFR and VEGF-C and caused inhibition of lymphangiogenesis, however ERB alone was unable to affect expression of VEGF-C and lymphangiogenesis. The results confirmed combination of TPX and ERB produced a synergistic anticancer and antitumor activity, possibly by promoting apoptosis and antiproliferative effect on tumor cells via suppressing expression of COX-2, 5-LOX, EGFR and VEGF-C.
Keywords: Gastric cancer, tepoxalin, erlotinib, cyclooxygenase-2, 5-lipoxygenase
Introduction
Gastric cancer (GC) is identified to be 4th most common among all the type of cancers [1]. The treatment protocol for GC includes chemotherapy both prior and after surgical intervention to prevent the chances of recurrence. A study has concluded an increase in overall chances of survival by 6% in cases receiving chemotherapy along with surgery compared to those undergoing a chemo less surgical process [2], hence creating a need for discovering effective chemotherapeutic agents [3]. Herbs show potential therapeutic effects, hence are evaluated in process of discovering new drug entities. However, among the identified plant species on earth only 15% have been screened for there phyto-constituents and 6% for their activity in biological models [3].
The release of Arachidonic acid (AA) is mediated by phospholipase from membrane phospholipids. The two enzymes cyclooxygenase (COX) and LOX (Lipoxigenase) are involved in catalysis of AA. COX is responsible for converting AA to prostaglandins (PGs). The expression of COX-2 is uncommon in normal tissues but is over expressed after an inflammatory response. COX-2 is reported to be over expressed in number of cancers with gastric cancer being one of them [4]. COX-2 has been discovered to be a carcinogenic enzyme responsible for progression of cancer by encouraging cell proliferation, promoting angiogenesis, inhibiting apoptosis and immune response [5-9]. Selective COX-2 inhibitor Celecoxib has been proved to be effective against number of human cancers such as colon cancer [10], gastric cancer [11] and cancer of head and neck [12].
Along with COX-2, LOX is found to responsible for converting AA into hydroxyeicosatetraenoic acids (HETEs) and leukotrienes (LTs). Reports have conformed elevated levels of 5-lipoxygenase (5-LOX) in many types of cancers, hence COX-2 and 5-LOX the two enzymes are found to play an important role in proliferation and cell signaling in cancer [13,14]. It is now identified that the metabolites generated by COXs and LOXs are not only responsible for inflammation but also are involved in apoptosis and proliferation, hence along with COX the studies have also suggested inhibition of LOX as important target in treating cancer [15]. 5-LOX has been identified to as the key rate limiting enzyme responsible for synthesis 5-HETE and accompanying LTs [16,17]. In addition to this the activity of 5-LOX is found to be elevated in number of tumors such as brain, lung, prostate and colon [18].
Vascular endothelial growth factor-C (VEGF-C) has been found to accelerate lymphangiogenesis via activating VEGFR-3 and through its expression on lymphatic endothelial cells. In study involving breast cancer cell lines Timoshenko AV et al [19] found that COX-2 as one of the factor responsible for up-regulation of VEGF-C via EP1-/EP4-pathway, confirming a positive correlation between COX-2,VEGF-C and lymphangiogenesis.
Epidermal growth factor receptor (EGFR) is a tyrosine kinase receptor from ErbB family. EGFR serves an important factor causing cell division, inhibiting apoptosis, angiogenesis and migration [20]. Reports recently have indicated association of EGFR with development and progression of gastric cancer, making it as potential marker as well as target for anticancer therapy [21,22]. A study involving pancreatic cancer reported earlier established both COX-2 and EGFR as possible targets and found that the phosphorylation and transcription of EGFR was mediated by COX-2 cascade [9]. A study also reported, activation of EGFR cascade leads to gene transcription of COX-2.
As the studies confirm involvement of COX, LOX and EGFR cascades in inflammation and in tumor growth, selecting agents that on combining can obstruct these pathways simultaneously can produce a synergistic effect and lead to increased tumor inhibitory effect. Therefore, targeting both 5-LOX-COX and EGFR pathway simultaneously can be an effective strategy in arresting progression of cancer, leading to overall better therapeutic outcomes.
Till date there are no published reports involving both EGFR and 5-LOX-COX inhibitors as targets in treating gastric cancer. In present research, we evaluated the antitumor effect of Tepoxalin, a COX-2/5-LOX inhibitor and Erlotinib, an EGFR inhibitor. The effects of both of these agents as a single entity and in combination were assessed on MGC-803 tumor bearing nude mice along with any related molecular biological indicators.
Material and methods
Cell culture and chemicals
For the study gastric cancer cells MGC-803 were selected. The cell lines were obtained from American Type Culture Collection. The cell lines were authenticated from the department of microbiology and pathology, Affiliated Hospital of Jining Medical University. The use of cells received permission from institutional ethical committee of the Affiliated Hospital of Jining Medical University. The cells were subjected to culture in freshly prepared Dulbecco’s modified Eagle’s medium (DMEM) (Merck, USA) along with fetal bovine serum (FBS) supplemented with streptomycin and penicillin (1%) maintained in 5 CO2 at 37°C. TPX and ERB were procured from Sigma-Aldrich USA, both TPX and ERB were suspended in 0.5% CMC.
Animals
All the experimental animal protocols were sanctioned by institutional ethical committee Affiliated Hospital of Jining Medical University with approval number AS/JMU/2017/0076, adhering to ARRIVE guidelines. BALB/c nude mice (Females) ageing between 4 to 5 weeks were chosen having average weight between 14.5-15.5 g. The animals were housed under in pathogen-free conditions, fed with sterilized diet, water ad libitum.
The gastric tumor xenograft model and treatment of selected molecules
The nude mice were injected subcutaneously at right armpit site with injection containing 2 × 106/0.2 ml of MGC-803 cells to establish xenograft tumor model of GC. The mice were divided into 4 groups viz. 1. Control (Vehicle treated i.e. 0.5% CMC), 2. TPX (12 mg/kg/day) treated group, 3. ERB 50 mg/kg/day [23] treated group and the 4. TPX+ERB (12 mg+50 mg) treated group, each of the group comprised 10 mice. The mice were divided into groups only after the diameter of largest tumor reached 5 mm. All the groups received the treatment regimens for 21 days (3 weeks) by intra-gastric route. The tumor diameters were evaluated after every 3rd day using vernier caliper. Tumor growth curves were plotted by calculating the tumor volume (TV) using formula TV=1/2ab2. The body weight was also recorded both prior and post study for assessing any associated adverse effects of treatment. After 3 weeks (21 days) the mice were sacrificed by Ketamine anesthesia intraperitoneally (100 mg/kg body weight). The tumors were recovered and inhibition rate was measured, followed by fixing tumors in 10% formalin solution dehydrated before embedding them in paraffin for obtaining sections of 5 μM. TUNEL and immunohistochemistry analysis was done.
Immunohistochemical analysis
Immunohistochemistry analysis was done using IHC kit from Abcam USA, the procedure followed was as per manufacturer’s protocol. The obtained sections of tumors were deparaffinized using xylene followed by rehydration using alcohol and washing with phosphate buffer saline (PBS). The tumor sections were microwaved in citrate buffer for 15 min in order to retrieve antigen. The sections were treated with 3% hydrogen peroxide for 30 min in order to block endogenous peroxidase. To block the binding of nonspecific antibodies the sections were treated with goat serum at 37°C for 30 min. The tumor sections were incubated with Iry antibodies of COX-2, proliferating cell nuclear antigen (PCNA), pEGFR, 5-LOX and VEGF-C (Cell Signaling) for 12 h at 4°C in a hybridization chamber. After incubating with Iry antibodies the tissue sections were again incubated with anti-goat/anti-mouse/anti-rabbit IIry antibodies followed by staining with diaminobenzidine (DAB) for 30 min, the controls were devoid of Iry antibody.
Western blot analysis
For evaluating the expression of proteins, 50 ug of tumor lysates from gastric tumor from control group mice and drug-treated mice were subjected to SDS-PAGE separation and were transferred to a nitrocellulose membrane. The membranes were blocked using 5% nonfat milk (Biorad) in Tris-buffered saline (TBS), followed by incubation along with antibodies for COX-2, PCNA, 5-LOX, pEGFR, VEGF-C, Caspase-3, Bcl-2 and β-actin for 12 h at 4°C. After 12 h incubation the, membranes were washed and again incubated with secondary antibody for 45 min. The proteins were detected using BioMax MR films (Kodak) opting chemiluminescence (Super Signal, Pierce Biotechnology), Actin was used as loading control.
TUNEL staining assay
Apoptosis detection kit (Sigma-Aldrich USA) was used for TUNEL assay. The obtained tumor sections were dewaxed using xylene and rehydrated using ethanol and distilled water. The sections were incubated with proteinase K at 37°C for 15 min and were then washed twice with PBS. The TUNEL reagent supplied with kit was added to the sections and incubated at 37° for 60 min in humid atmospheric condition followed by washing with PBS for 3 times. The sections of tumor were colored with diaminobenzidine followed by staining with hematoxylin. The TUNEL quantification was done by selecting 5 random sites from each of the section opting magnification of 200 ×.
Statistical analysis
Graphpad Prism software was used for the statistical calculation of results. All the results expressed are mean ± SD. One-way ANOVA was used to establish differences between the groups. The changes in weight followed by treatment in every group were done analyzed by student’s t-test, values of P<0.05 were considered as significant statistically.
Results
Tumor xenograft model of gastric cancer in nude mice
The mice were divided into study groups on observing tumor size. The tumor diameter in selected MGC-803 injected nude mice was measured and the time required for attaining diameter of 5 mm was found to be 18 days after inoculation, the tumor formation rate was found to be 85%. The tumor volumes were measured before the treatment regimen, the volume were found to be control group 81.4±23.4 mm3, TPX treated group 81.45±21.1 mm3, ERB treated 80.25±19.2 mm3 and TPX+ERB combination treated 80.1±31.5 mm3 (Table 1). The tumor weight before the treatment do not varied significantly. The mice during the treatment duration do not showed any signs of variation in mental state, diet consumption, adverse effects and activity. The body weights noticed after treatment protocol were on higher side compared to before treatment regimen (Table 2). The tumor volumes in all the groups after the treatment regimen were found to be as follows: control group 1670.70±350.2 mm3, TPX treated group 1150.4±240.5 mm3, ERB treated 1040.40±264.5 mm3 and TPX+ERB combination treated 510.4±71.8 mm3 (Figure 1; Table 1). The outcomes showed that the volumes of tumor decreased in all treatment groups compared to control group (P<0.05), the combination group showed outstanding effects compared to their individual treatments (P<0.01). However, no significant differences in tumor volumes was observed for TPX and ERB treated group of mice (P>0.05). On evaluating the effect of treatments on tumor inhibition rates calculated as stated in study earlier [25] the outcomes were as follows: TPX treated group 42.8%, ERB treated 44.8% and TPX+ERB combination treated showed 75.4% inhibition suggesting synergistic effect of combination.
Table 1.
Changes in tumor volumes in treated group of tumor xenografts mice before and after treatment
| Group treatment | Tumor volume (mm3) Before treatment (mm3, mean ± SD) (n=6) | Tumor volume (mm3) After treatment (mm3, mean ± SD) (n=6) |
|---|---|---|
| Control group (0.5% CMC treated) | 81.4±23.4 | 1670±350.2a |
| TPX treated (12 mg/kg/day) | 81.45±21.1 | 1150±240.5a |
| ERB treated (50 mg/kg/day) | 80.25±19.2 | 1040.4±264.5a |
| TPX (12 mg)+ERB (50 mg) | 80.1±31.5 | 510.4±71.8a |
P<0.001, compared to volume before treatment.
Table 2.
Changes in weight of tumor xenografts mice in each group before and after treatment
| Group (Treatment) | Before treatment (g, mean ± SD) (n=6) | After treatment (g, mean ± SD) (n=6) |
|---|---|---|
| Negative Control (0.5% CMC treated) | 17.95±0.52 | 20.74±1.24a |
| TPX treated (12 mg/kg/day) | 18.01±0.78 | 19.41±0.68a |
| ERB treated (50 mg/kg/day) | 17.98±0.65 | 19.65±0.45b |
| TPX (12 mg)+ERB (50 mg) | 18.02±0.75 | 19.02±0.89b |
P<0.01 compared to weight before treatment;
P<0.05 compared to weight before treatment.
Figure 1.
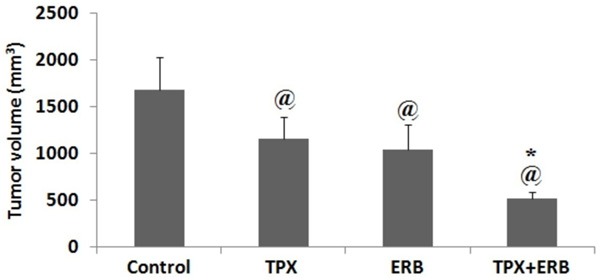
Tumor volume of subcutaneous gastric cancer xenograft nude mice. @P<0.05 compared to control group. The presented data is mean ± SEM, *P<0.01 compared to TPX and ERB treated group.
Effect of TPX and ERB on apoptosis and proliferation of each treatment group of mice
To study the effect of TPX, ERB and combination on apoptosis and proliferation in each treated group TUNEL and PCNA assay was done. After qualitative microscopy, the TUNEL positive cells in treated groups were as follows: control group 8.7%±1.1%, TPX treated 24.9%±0.9%, and ERB treated 27.1%±1.1% and combination of TPX+ERB (P<0.01) showing 51.2%±1.9% (Figure 2A). The % PCNA positive cells for the treated groups were as follows: control group 81.4%±2.1%, TPX treated 61.4%±2.7%, and ERB treated 55.8±2.4% and combination of TPX+ERB (P<0.01) showing 31.4%±1.4% (Figure 2B).
Figure 2.
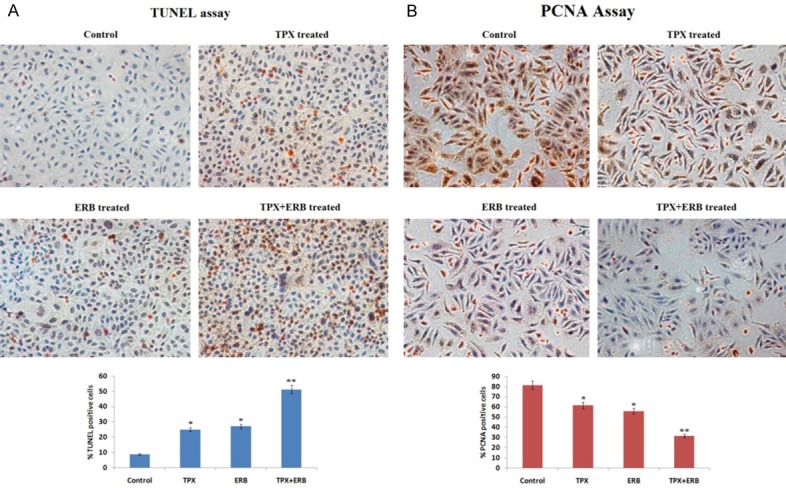
A: TUNEL staining of tumor tissues. Brown staining was shown by nuclei apoptotic cells. The apoptotic rate increased in all treatments compared to control *P<0.05, the brown staining was highly significant in combination treated group compared to control **P<0.01 (All the data presented is mean ± SEM). B: The expression of PCNA in tumors obtained from tumor xenografts nude mice by immunohistochemical staining. The brown staining was observed in nucleus of tumor cells (PCNA positive). The extent of proliferation i.e. proliferation index decreased in all treated groups compared to control (*P<0.05), whereas significant results were shown by combination group compared to control (**P<0.01) (All the data presented is mean ± SEM). (Magnification at 200 ×).
The effect of TPX and ERB on levels of apoptosis proteins
Western blot studies and IHC staining were done to detect the expression of apoptosis proteins in order to establish the effect of treatments on apoptosis. The expression levels of Bcl-2, PCNA, 5-LOX, COX-2, β-catenin, caspase-3, p21 were evaluated. The Immunoblotting studies showed decreased expression of PCNA, Bcl-2, Cox-2 and 5-LOX in all treatments compared to control, significant suppression was observed in lysates obtained from mice treated with TPX+ERB (P<0.01). The expression of p21 and caspase-3 increased whereas β-catenin decreased significantly in combination group compared to control suggesting involvement of apoptosis (Figure 3). The proteins were normalized against loading standard β-Actin.
Figure 3.
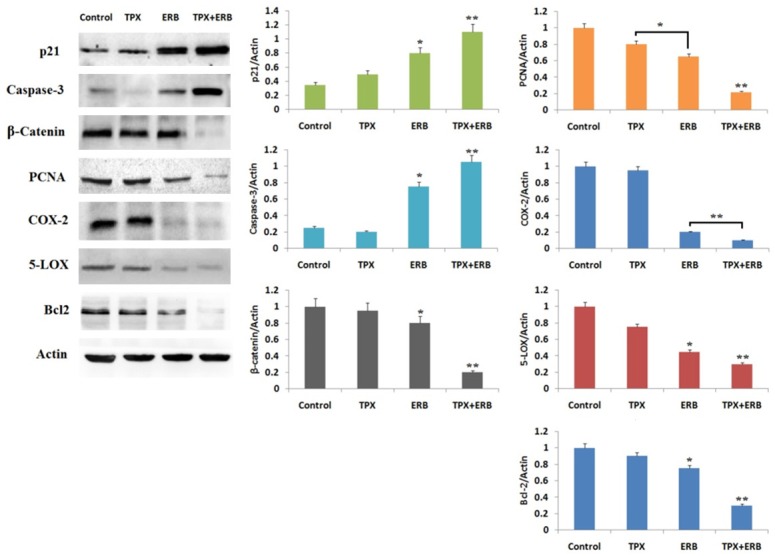
Effect of TPX, ERB and combination of TPX+ERB treatment on expression of proteins p21, caspase-3, β-Catenin, PCNA, COX-2, 5-LOX and Bcl2. The combination of TPX+ERB significantly increased expression of p21 and caspase-3, whereas the expression of β-Catenin, PCNA, COX-2, 5-LOX and Bcl2 decreased significantly compared to control. Actin was used as loading standard. (The data presented is mean ± SEM, *P<0.05 and **P<0.01 compared control).
IHC staining showed expression of pEGFR, VEGF-C, COX-2 and 5-LOX increased in control group whereas the levels decreased in treated groups, significant decrease was observed in combination treated group compared to control suggesting synergistic role of combination (Figure 4A).
Figure 4.
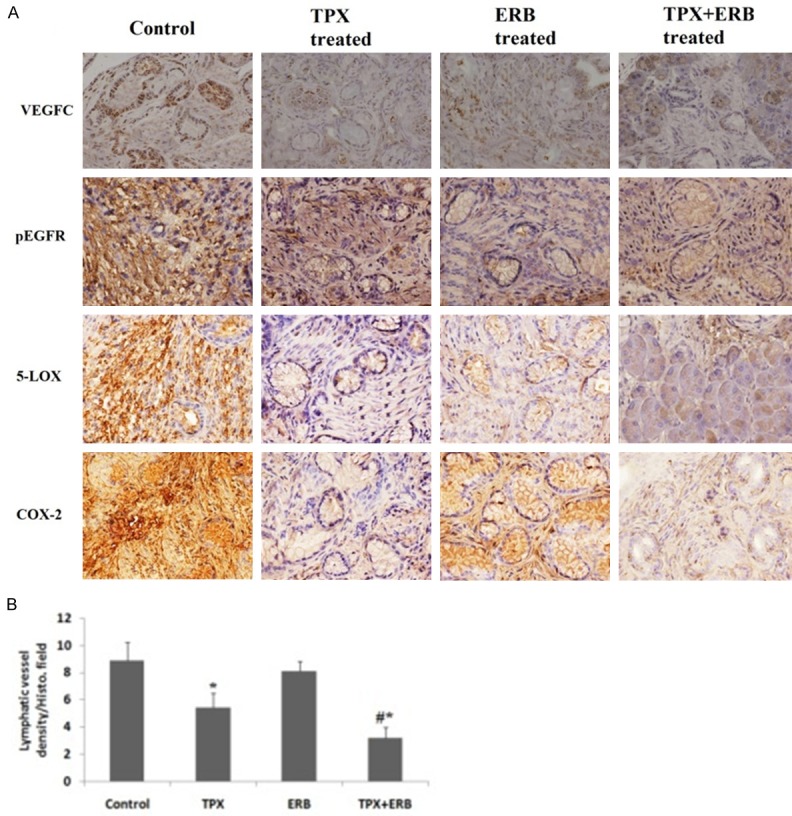
A: Immunohistochemistry (VEGFC, pEGFR, 5-LOX and COX-2) revealed decreased expression of COX-2, VEGFC, 5-LOX and p-EGFR in both individual treatment of TPX and ERB as well as in combination. B: Effect of TPX, ERB and its combination on lymphatic vessel density/histological field selected. The lympathic density decreased in all treatment groups compared to control with TPX treated showing *P<0.05 compared to control. The combination group showed significant reduction (#P<0.01 compared to control) (All the data presented is mean ± SEM).
The effect of TPX and ERB on the expression of COX-2 and VEGF-C proteins and lymphangiogenesis
Lymphatic vessel density (LVD) was done to interpret the effect of TPX, ERG and there combination on lymphangiogenesis. The lymphatic densities for selected histological fields of studied groups were as follows: control group 8.9±1.3/HF (Histological Field), TPX treated 5.4±1.1/HF, ERB treated 8.1±0.7/HF and combination of TPX+ERB showing 3.2±0.8/HF. The LVD of all the treated groups were lower than the control group. TPX alone and in combination with ERG decreased LVD, whereas ERG alone was unable to suppress it (Figure 4B).
Discussion
Many studies have confirmed COX-2 and 5-LOX contributing in development of tumors [24]. Enzyme COX-2 is found to be carcinogenic and leads to progression of cancer via encouraging cell proliferation, promoting angiogenesis, inhibiting apoptosis and immune response [25-27]. LOX the other important enzyme is responsible for conversion of Arachidonic acid to HETEs and LTs. In a study Hong et al established that LOX inhibitors are more valuable compared to COX inhibitors in suppressing proliferation of cancer cells, the study also identified 5-LOX as a crucial enzyme in synthesis of 5-HETE and LTs [15]. Epidermal growth factor receptor which is a tyrosine kinase receptor from ErbB family plays a valuable role in process of cell division, angiogenesis, cell apoptosis and migration [20]. EGFR is very closely associated with gastric cancer and is found to be over expressed making it as a important target for treating gastric cancer [21,22].
In the present study we selected two agents TPX and ERB. TPX is an anti-inflammatory agent and is reported to have inhibitory effect on both COX-2 and 5-LOX [28], whereas ERB is a EGFR inhibitor. Here we evaluated the effects of these agents individually as well as in combination on gastric cancer xenografts mice model. To our knowledge, this is first study of its kind evaluating efficacy of a dual 5-LOX-COX-2 inhibitor combined with an EGFR inhibitor in gastric cancer. In the present research we assessed changes in biological indicators associated with gastric cancer, PCNA an important indicator of cell proliferation was the first to be studied for its expression in each treatment group. We found that TPX, ERB and there combination inhibited the proliferation. The % PCNA cells in tumor sections in group of mice treated with TPX+ERB was on lower side compared to their individual treatments. ERB may have interfered with EGFR signaling of cancer cells whereas TPX may have caused suppression via inhibiting activity of both COX-2 and 5-LOX.
We further studied the antitumor effect of TPX and ERB by evaluating apoptosis. Outcomes of the experiment suggested that both TPX and ERB resulted in apoptosis of cancer cells in tumor xenografts mice. However the combination of TPX+ERB turned to be more effective resulting in higher level of apoptotic index compared to their individual treatments (P<0.01). It was observed that expression of Bcl-2 decreased and that of caspase-3 was upregulated in all the treatment groups and more significantly in combination group (P<0.01) compared to control mice. Bcl-2 is a protein which inhibits apoptosis and caspase 3 is a important enzyme responsible for apoptosis signaling causing apoptosis [29]. A study established prognostic value of caspase-3 in cancers of digestive tract [30]. Caspase-3 along with surviving are associated with gastric cancer [31]. Wang et al established caspase-3 dependent pyroptosis in gastric cancer [32].
Lymphatic metastasis is identified to be one of the prime mechanisms of gastric cancer metastasis which helps in deciding the treatment and also the prognosis [33-35]. The action of Lymphangiogenesis is established as a process encouraging metastasis of lymph node in tumors, it is a process which leads to formation of new lymphatic vessels from the existing [36]. COX-2 and LOX-5 are the lymphatic factors reported earlier [37]. In a study Zhang et al [38] reported expression of COX-2 was linked to metastasis of lymph node and lymphangiogenesis. In our research, TPX alone and in combination with ERB inhibited expression of COX-2, 5-LOX and EGFR. The treatment of TPX and its combination with ERB inhibited the expression of VEGF-C decreasing the density of lymphatic vessels; however ERB alone was unable to suppress VEGF-C. In a study earlier, Celecoxib halted the growth of implanted tumors, inhibited generation of lymphatic vessels and resulted in inhibition of VEGF expression in cancer cells [39,40]. Furthermore, studies have provided evidence that COX-2 inhibitor suppressed the expression of VEGF-C and lymphangiogenesis, the possible mechanism may be COX-2 mediated regulation of VEGF-C via PGE-2 pathway [41,42]. The outcomes of our study were in accordance to results of these studies suggesting involvement of COX-2 in VEGF-C and lymphangiogenesis.
Though both TPX and ERB resulted in antitumor effects when given alone compared to control mice; a synergistic response was observed on combining the two agents which resulted in antitumor response in MGC-803 injected xenografts tumor model of GC. The combination of TPX and ERB inhibited progression of tumor growth in mice mainly via inhibiting the growth of cancer cells and encouraging apoptosis. TPX and ERB in combination may suppress expression of COX-2, which in turn may have lead to down regulation expression of VEGF-C, thereby curbing lymphangiogenesis.
Acknowledgements
We are extremely thankful to the staff and management of Department of Gastrointestinal Surgery, Affiliated Hospital of Jining Medical University, Jining for providing necessary facilities.
Disclosure of conflict of interest
None.
References
- 1.American Cancer Society. Global Cancer Facts & Figures. 2nd edition. Atlanta: American Cancer Society; 2011. [Google Scholar]
- 2.GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research International Collaboration) Group. Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M, Van Cutsem E, Buyse M. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729–1737. doi: 10.1001/jama.2010.534. [DOI] [PubMed] [Google Scholar]
- 3.Verpoorte R. Pharmacognosy in the new millennium: leadfinding and biotechnology. J Pharm Pharmacol. 2000;52:253–262. doi: 10.1211/0022357001773931. [DOI] [PubMed] [Google Scholar]
- 4.Joo YE, Chung IJ, Park YK, Koh YS, Lee JH, Park CH, Lee WS, Kim HS, Choi SK, Rew JS, Park CS, Kim SJ. Expression of cyclooxygenase-2, p53 and Ki-67 in gastric cancer. J Korean Med Sci. 2006;21:871–876. doi: 10.3346/jkms.2006.21.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudnick DA, Perlmutter DH, Muglia LJ. Prostaglandins are required for CREB activation and cellular proliferation during liver regeneration. Proc Natl Acad Sci U S A. 2001;98:8885–8890. doi: 10.1073/pnas.151217998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Souza RF, Shewmake K, Beer DG, Cryer B, Spechler SJ. Selective inhibition of cyclooxygenase-2 suppresses growth and induced apoptosis in human esophageal adenocarcinoma cells. Cancer Res. 2000;60:5767–5772. [PubMed] [Google Scholar]
- 7.Pockaj BA, Basu GD, Pathangey LB, Gray RJ, Hemandez JL, Gendler SJ, Mukherjee P. Reduced T-cell and dendritic cell function is related to cyclooxygenase-2 overexpression and prostaglandin E(2) secretion in patients with breast cancer. Ann Surg Oncol. 2004;11:328–339. doi: 10.1245/aso.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 8.Patel S, Chiplunkar S. Role of cyclooxygenase-2 in tumor progression and immune regulation in lung cancer. Indian J Biochem Biophys. 2007;44:419–428. [PubMed] [Google Scholar]
- 9.Ozuysal S, Bilgin T, Ozgur T, Celik N, Evrensel T. Expression of cyclooxygenase-2 in ovarian serous carcinoma: correlation with angiogenesis, nm23 expression and survival. Eur J Gynaecol Oncol. 2009;30:640–645. [PubMed] [Google Scholar]
- 10.Wang L, Chen W, Xie X, He Y, Bai X. Celecoxib inhibits tumor growth and angiogenesis in an orthotopic implantation tumor model of human colon cancer. Exp Oncol. 2008;30:42–51. [PubMed] [Google Scholar]
- 11.Kim N, Kim CH, Ahn DW, Lee KS, Cho SJ, Park JH, Lee MK, Kim JS, Jung HC, Song IS. Antigastric cancer effects of celecoxib, a selective COX-2 inhibitor, through inhibition of Akt signaling. J Gastroenterol Hepatol. 2009;24:480–487. doi: 10.1111/j.1440-1746.2008.05599.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim YY, Lee EJ, Kim YK, Kim SM, Park JY, Myoung H, Kim MJ. Anti-cancer effects of celecoxib in head and neck carcinoma. Mol Cells. 2010;29:185–194. doi: 10.1007/s10059-010-0026-y. [DOI] [PubMed] [Google Scholar]
- 13.Funahashi H, Satake M, Dawson D, Huynh NA, Reber HA, Hines OJ, Eibl G. Delayed progression of pancreatic intraepithelial neoplasia in a conditional Kras (G12D) mouse model by a selective cyclooxygenase-2 inhibitor. Cancer Res. 2007;67:7068–7071. doi: 10.1158/0008-5472.CAN-07-0970. [DOI] [PubMed] [Google Scholar]
- 14.Mohammed A, Janakiram NB, Madka V, Brewer M, Ritchie RL, Lightfoot S, Kumar G, Sadeghi M, Patlolla JM, Yamada HY, Cruz-Monserrate Z, May R, Houchen CW, Steele VE, Rao CV. Targeting pancreatitis blocks tumor-initiating stem cells and pancreatic cancer progression. Oncotarget. 2015;6:15524–15539. doi: 10.18632/oncotarget.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong SH, Avis I, Vos MD, Martinez A, Treston AM, Mulshine JL. Relationship of arachidonic acid metabolizing enzyme expression in epithelial cancer cell lines to the growth effect of selective biochemical inhibitors. Cancer Res. 1999;59:2223–2228. [PubMed] [Google Scholar]
- 16.Werz O, Klemm J, Samuelesson B, Radmark O. 5-lipoxygenase is phosphorylated by p38 kinase-dependent MAPKAP kinase. Proc Natl Acad Sci U S A. 2000;97:5261–5266. doi: 10.1073/pnas.050588997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szekeres CK, Tang K, Trikha M, Honn KV. Eicosanoid activation of extracellular signalregulated kinase 1/2 in human epidermoid carcinoma cells. J Biol Chem. 2000;275:38831–38841. doi: 10.1074/jbc.M002673200. [DOI] [PubMed] [Google Scholar]
- 18.Avis IM, Jett M, Boyle T, Burdick M, Strieter R, Peters-Golden M. Constitutive activation of 5-lipoxygenase in the lungs of patients with idiopathic pulmonary fibrosis. J Clin Invest. 1996;97:1827–1836. doi: 10.1172/JCI118612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Timoshenko AV, Chakraborty C, Wagner GF, Lala PK. COX-2-mediated stimulation of the lymphangiogenic factor VEGF-C in human breast cancer. Br J Cancer. 2006;94:1154–1163. doi: 10.1038/sj.bjc.6603067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J. Clin. Oncol. 2003;21:2787–2799. doi: 10.1200/JCO.2003.01.504. [DOI] [PubMed] [Google Scholar]
- 21.Ciardiello F, Tortora G. A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res. 2001;7:2958–2970. [PubMed] [Google Scholar]
- 22.Galizia G, Lieto E, Ferrarraccio F, De Vita F, Castellano P, Orditura M, Imperatore V, La Mura A, La Manna G, Pinto M, Catalano G, Pignatelli C, Ciardiello F. Prognostic significance of epidermal growth factor receptor expression in colon cancer patients undergoing curative surgery. Ann Surg Oncol. 2006;13:823–835. doi: 10.1245/ASO.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 23.Friess T, Scheuer W, Hasmann M. Erlotinib antitumor activity in non-small cell lung cancer models is independent of HER1 and HER2 overexpression. Anticancer Res. 2006;26:3505–3512. [PubMed] [Google Scholar]
- 24.Jin ZJ. Addition in drug combination. Acta Pharmacologica Sinica. 1980;1:70–76. [PubMed] [Google Scholar]
- 25.Schneider C, Pozzi A. Cyclooxygenases and lipoxygenases in cancer. Cancer Metastasis Rev. 2011;30:277–294. doi: 10.1007/s10555-011-9310-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao CV, Janakiram NB, Mohammed A. Lipoxygenase and cyclooxygenase pathways and colorectal cancer prevention. Curr Colorectal Cancer Rep. 2012;8:316–324. doi: 10.1007/s11888-012-0146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menter DG, Schilsky RL, DuBois RN. Cyclooxygenase-2 and cancer treatment: understanding the risk should be worth the reward. Clin Cancer Res. 2010;16:1384–1390. doi: 10.1158/1078-0432.CCR-09-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Breazna A, Kim K, Tang J, Rosenstein RB, Umar A, Bagheri D, Collins NT, Burn J, Chung DC, Dewar T, Foley TR, Hoffman N, Macrae F, Pruitt RE, Saltzman JR, Salzberg B, Sylwestrowicz T, Hawk ET Adenoma Prevention with Celecoxib Study Investigators. Five-year efficacy and safety analysis of the adenoma prevention with Celecoxib trial. Cancer Prev Res. 2009;2:285–287. doi: 10.1158/1940-6207.CAPR-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergmann F, Moldenhauer G, Herpel E, Gaida MM, Strobel O, Werner J, Esposito I, Müerköster SS, Schirmacher P, Kern MA. Expression of L1CAM, COX-2, EGFR, c-KIT and Her2/neu in anaplastic pancreatic cancer: putative therapeutic targets. Histopathology. 2010;56:440–448. doi: 10.1111/j.1365-2559.2010.03499.x. [DOI] [PubMed] [Google Scholar]
- 30.Chen H, Yang X, Feng Z, Tang R, Ren F, Wei K, Chen G. Prognostic value of caspase-3 expression in cancers of digestive tract: a meta-analysis and systematic review. Int J Clin Exp Med. 2015;8:10225–10234. [PMC free article] [PubMed] [Google Scholar]
- 31.Kania J, Konturek SJ, Marlicz K, Hahn EG, Konturek PC. Expression of survivin and caspase-3 in gastric cancer. Dig Dis Sci. 2003;48:266–71. doi: 10.1023/a:1021915124064. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Yin B, Li D, Wang G, Han X, Sun X. GSDME mediates caspase-3-dependent pyroptosis in gastric cancer. Biochem Biophys Res Commun. 2018;495:1418–1425. doi: 10.1016/j.bbrc.2017.11.156. [DOI] [PubMed] [Google Scholar]
- 33.Akagi T, Shiraishi N, Kitano S. Lymph node metastasis of gastric cancer. Cancers (Basel) 2011;3:2141–2159. doi: 10.3390/cancers3022141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng J, Biang H. Clinical significance of lymph node metastasis in gastric cancer. World J Gastroenterol. 2014;20:3967–3975. doi: 10.3748/wjg.v20.i14.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maruyama K, Gunvén P, Okabayashi K, Sasako M, Kinoshita T. Lymph node metastases of gastric cancer. General pattern in 1931 patients. Ann Surg. 1989;210:596–602. doi: 10.1097/00000658-198911000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fung-Leung WP, Pope BL, Chourmouzis E, Panakos JA, Lau CY. Tepoxalin, a novel immunomodulatory compound, synergizes with CsA in suppression of graft-versus-host reaction and allogeneic skin graft rejection. Transplantation. 1995;60:362–368. doi: 10.1097/00007890-199508270-00011. [DOI] [PubMed] [Google Scholar]
- 37.Dandekar DS, Lopez M, Carey RI, Lokeshwar BL. Cyclooxygenase-2 inhibitor celecoxib augments chemotherapeutic drug-induced apoptosis by enhancing activation of caspase-3 and -9 in prostate cancer cells. Int J Cancer. 2005;115:484–492. doi: 10.1002/ijc.20878. [DOI] [PubMed] [Google Scholar]
- 38.Flis S, Splwinski J. Inhibitory effects of 5-fluorouracil and oxaliplatin on human colorectal cancer cell survival are synergistically enhanced by sulindac sulfide. Anticancer Res. 2009;29:435–41. [PubMed] [Google Scholar]
- 39.He Y, Karpanen T, Alitalo K. Role of lymphangiogenic factors in tumor metastasis. Biochim Biophys Acta. 2004;1654:3–12. doi: 10.1016/j.bbcan.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J, Ji J, Yuan F, Zhu L, Yan C, Yu YY, Liu BY, Zhu ZG, Lin YZ. Cyclooxygenase-2 expression is associated with VEGF-C and lymph node metastases in gastric cancer patients. Biomed Pharmacother Suppl. 2005;59:S285–288. doi: 10.1016/s0753-3322(05)80047-2. [DOI] [PubMed] [Google Scholar]
- 41.Barnes NL, Warnberg F, Farnie G. Cyclooxy-genase-2 inhibition: effects on tumour growth, cell cycling and lymphangiogenesis in a xenograft model of breast cancer. Br J Cancer. 2007;96:575–582. doi: 10.1038/sj.bjc.6603593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu H, Yang Y, Xiao J, Lv Y, Liu Y, Yang H, Zhao L. COX-2-mediated regulation of VEGF-C in association with lymphangiogenesis and lymph node metastasis in lung cancer. Anat Rec (Hoboken) 2010;293:1838–1846. doi: 10.1002/ar.21240. [DOI] [PubMed] [Google Scholar]


