Highlights
-
•
An epilepsy prevalence of 4.4% was observed in villages in Maridi.
-
•
Living close to the Maridi river is a major risk factor for epilepsy.
-
•
Persons with nodding seizures and with other forms of epilepsy live in the same areas.
-
•
Ivermectin coverage needs to increase to prevent onchocerciasis associated epilepsy.
Keywords: Onchocerciasis, Epilepsy, Nodding syndrome, Prevalence, Incidence, Ivermectin, Community directed treatment, Maridi, South Sudan
Abstract
Purpose
To determine the prevalence and incidence of epilepsy in an onchocerciasis endemic region of South Sudan.
Methods
In May 2018, a door-to-door household survey was conducted in 8 study sites in an onchocerciasis endemic area in Maridi County.
Results
A total of 2511 households agreed to participate in the study, corresponding to 17,652 individuals. An epilepsy screening questionnaire identified 799 persons suspected to have epilepsy (4.5%); in 736 of the 766 persons (96.1%) seen by a clinical officer the diagnosis of epilepsy was confirmed. Adding 38 persons who were not seen but with a positive answer to a combination of screening questions, 774 persons (4.4%) had epilepsy. Epilepsy prevalence was highest in the 11–20 age group (10.5%); 66 persons with epilepsy (PWE) developed their first seizures in the year preceding the survey (annual incidence = 373.9/100.000). Neurocysticercosis cannot explain the high epilepsy prevalence since no pigs are kept in the area. Independent risk factors for epilepsy included male gender, belonging to a “permanent household” and a farming family, and living in a village bordering the Maridi River. Only 7209 (40.8%) of the population took ivermectin in 2017.
Conclusion
A very high prevalence and incidence of epilepsy was observed in several villages in Maridi County located close to the Maridi River and the Maridi dam. Urgent action is needed to prevent children in Maridi County from developing OAE by strengthening the onchocerciasis elimination program.
1. Introduction
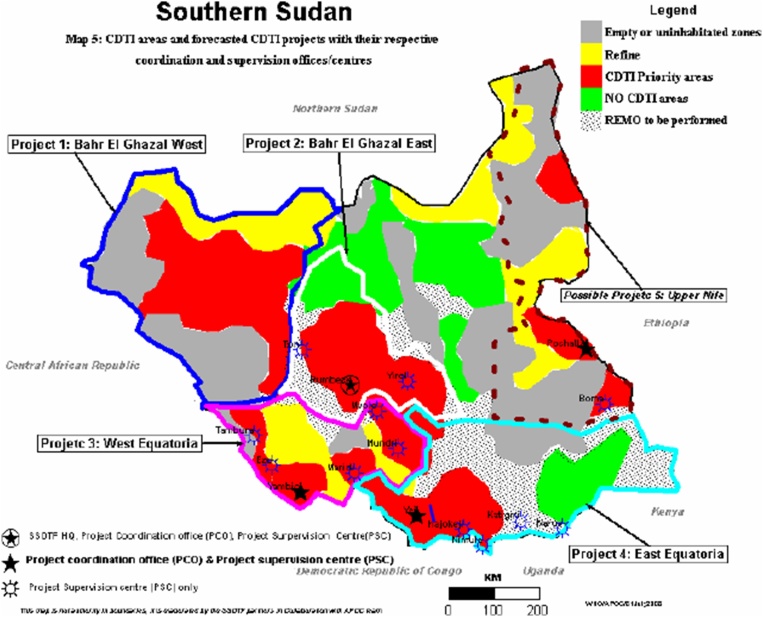
South Sudan is amongst the highly endemic countries for onchocerciasis in Africa, with the disease endemic in around half (49%) the country [1]. The most highly endemic foci of onchocerciasis in South Sudan are in Western Equatoria, and Northern and Western Bahr el Ghazal regions. However, in 2006 only 26% of the eligible population received ivermectin in five community directed treatment with ivermectin (CDTi) targeted areas [2] (Fig. 1).
Fig. 1.
South Sudan CDTi areas and distribution of onchocerciasis [2].
A high prevalence of epilepsy has been reported in many onchocerciasis meso- and hyper-endemic regions (>20% prevalence of onchocerca nodules) in Africa [[3], [4], [5], [6], [7], [8], [9], [10], [11]]. High numbers of persons with nodding syndrome (NS) and other forms of epilepsy have been reported in the Western Equatoria region of South Sudan [8,[12], [13], [14], [15], [16], [17]]. Since the first cases of NS were reported in 1990 in this region of South Sudan, a high rate of new NS cases and other forms of epilepsy have been reported [14]. In 2001, the World Health Organization (WHO) estimated the prevalence of NS at 4.6% among a small population in Western Equatoria region, which was considered to have the highest burden of the disease [18]. During a small household survey conducted in 2013 in the same area (Mvolo), one in six children had epilepsy, and one in two households had at least one child with epilepsy [14]. The South Sudan Relief and Rehabilitation Commission reported an epilepsy prevalence in the Mvolo area of 8.4% [14].
However, a population-based study to determine the prevalence of epilepsy including NS in an onchocerciasis-endemic region in South Sudan has never been conducted. Moreover, no interventions to prevent children from developing NS and other forms of epilepsy have been undertaken. In May 2018, in response to requests by the Ministry of Health of South Sudan and Maridi State, and as a baseline for a study to evaluate the effect of a biannual CDTi programme to decrease the incidence of NS and other forms of epilepsy, we performed a population-based survey in onchocerciasis endemic villages in Maridi County located close to the Maridi River.
In this paper we present the prevalence and incidence of epilepsy and potential risk factors for epilepsy in onchocerciasis-endemic villages in Maridi County. Detailed information concerning the clinical spectrum of epilepsy observed in these villages was recently published in this journal [19].
2. Methods
2.1. Study setting
The study was conducted in selected villages in 8 study sites, in Maridi County in Western Equatoria region of South Sudan (Fig. 2).
Fig. 2.
Map with the localisation of the selected study sites in the Maridi area.
The population of Maridi County is estimated at 101,065 [20]. The County is divided into five payams; each payam includes several bomas, each containing several villages. Farming is the main economic and livelihood activity. Water is mainly accessed from the Maridi River, which is fast flowing with several shallow rapids and a weir (Maridi dam) near the town providing breeding sites for the Simulium vector (blackfly) that transmits Onchocerca volvulus. The livestock are mainly goats and sheep; no pigs are kept in the Maridi villages.
2.2. Study design
The study was conducted in May 2018 prior to starting the biannual mass distribution of ivermectin programme. The study protocol to evaluate the effect of a biannual CDTi programme to decrease the incidence of NS and other forms of epilepsy has been published previously [21]. To obtain baseline information, all individuals in selected villages close to Maridi town and the Maridi River were included in a door-to-door survey. A two-step epilepsy survey was used to identify persons with epilepsy (PWE). For epilepsy screening, a pre-tested validated questionnaire comprising five specific questions was used [22]. This questionnaire was translated into the local language and back translated into English to ensure no loss of meaning, and pilot tested in 10 households. The questionnaire was administered by a trained team of 12 research assistants living in the area. All persons with suspected epilepsy identified by the research assistants were visited in their homes by one of 10 trained clinical officers who took a detailed medical history and performed a clinical examination to confirm or reject the diagnosis of epilepsy, using a structured pre-tested questionnaire. Prior to the study, the research assistants were trained for 2 days on use of the screening questionnaire for suspected epilepsy, and the clinical officers were trained on how to confirm the diagnosis of epilepsy; both groups pilot tested the data collection tools. The training was organized by two medical doctors (RC and JYC). During the home visits RC supervised the clinical officers and interviewed and examined selected persons with suspected epilepsy with the assistance of a translator. JYC and PCO supervised the work of the assistants.
The survey was carried out at the start of the rains when no major farming activities were ongoing. However, if at the time of the home visit no-one was at home, households were revisited later, at least once.
2.2.1. Definitions
A case of epilepsy was defined based on the International League Against Epilepsy as an individual with at least two unprovoked seizures with a minimum of 24 h separating the two episodes [23].
Active epilepsy was defined as a person who presented with two or more epileptic seizures in the last year.
A new case of epilepsy was defined as a person who developed a first seizure within the 12 months preceding the survey.
Nodding seizures were defined as the head dropping forward repeatedly in a person during a brief period of reduced consciousness.
Onchocerciasis-associated epilepsy (OAE) was defined as a person meeting the following six criteria: 1) a history of at least two unprovoked epileptic seizures at least 24 h apart; 2) living at least 3 years in an onchocerciasis endemic region; 3) living in a village with high epilepsy prevalence and with families with more than one child with epilepsy; 4) no other obvious cause of epilepsy; 5) onset of epilepsy between the ages of 3 and 18 years; 6) normal psychomotor development before the onset of epilepsy.
Nakalanga features were defined as an association of growth retardation without obvious cause, delay or absence of external signs of sexual development, mental retardation, epilepsy and often facial, thoracic, and spinal abnormalities [9].
As potential “obvious causes of epilepsy” we considered a history of severe measles, malaria, encephalitis or meningitis, and head injury with loss of consciousness in the 5 years preceding the onset of epileptic seizures.
A “permanent household” was defined as a family who had lived in the village for at least 20 years.
An “immigrant household” referred to a family who had lived in the village for less than 20 years.
2.3. Data collection, management
Screening of households by research assistants to identify persons with suspected OAE was performed using a paper-based questionnaire. For confirmation of epilepsy, clinical officers and medical doctors used a questionnaire deployed on Open Data Kit (ODK, https://opendatakit.org/) software installed on tablet computers. Persons suspected to have epilepsy were gps-localized with the tablet computers.
2.4. Data analysis
Means and standard deviations are presented for continuous outcome measures, and frequencies and percentages are presented for categorical variables. Chi-squared tests were used to compare any group differences for categorical variables and t-tests were used for continuous variables. We determined the positive predictive value of the 5 epilepsy screening questions separately and in combination for the diagnosis of clinically confirmed epilepsy. The prevalence of epilepsy was calculated by dividing the number of clinically confirmed epilepsy cases by the total number of individuals screened. The overall epilepsy incidence in the total population and incidence in the 3–18 age group was calculated by dividing the number of new cases of epilepsy in the previous 12 months, 2 and 5 years by the summed person-years of the population at risk in these years. Similarly, epilepsy related mortality was obtained by calculating the number of deaths among persons with epilepsy in 2017 and the first 4 months of 2018 in person-years of the total population. Ivermectin coverage was calculated as the percentage of the population that reported taking ivermectin in 2017. An 85% coverage is considered the goal, as pregnant women, children under 5 and the seriously ill who are ineligible for ivermectin treatment are considered to make up 15% of the population [24].
For univariate analysis, a logistic regression was fitted to measure the strength of association between epilepsy and age, gender, occupation, distance of the village to the river, residence status and ivermectin use. A multivariable logistic regression analysis was performed to assess which of these variable(s) were independent predictors for epilepsy. A Likelihood Ratio test was performed to identify potential interactions. As the interaction between gender and distance to the river was significant, a model reflecting the differential impact for that particular outcome was included.
As the Maridi dam (4.89422 N, 29.45792 E) was suspected to be the main blackfly breeding site at the study site we calculated the distance between the Maridi dam in relation to the categories of the study population (population with a history of nodding seizures, population with other types of epilepsy and population not confirmed to have epilepsy). The median distances between the dam and each cluster type were further examined using the Mann-Whitney U test [25] based on the alternative hypothesis that the median distance of each cluster from the dam were not equal. We used Ward’s method of hierarchical clustering to measure the distance between, two distributions–persons with nodding seizures and persons with other form of epilepsy by calculating the ‘merging cost’ that is how much the sum of squares increase when we merge them into a single cluster [26].
To identify the presence of clusters of persons with nodding syndrome and other forms of epilepsy, we also used the Kulldorff's scan statistic [27] as implemented in the SaTScan software (https://www.satscan.org/).
2.5. Ethics approval and consent to participate
Ethical approval was obtained from the ethics committee of the Ministry of Health of South Sudan and from the ethics committee of the University of Antwerp, Belgium. The study aims and procedures were explained to all participants in the language of their choice, and signed or finger-printed informed consent was obtained from participants, parents or carers, and assent obtained from adolescents (aged 12–18 years). All personal information was encoded and treated confidentially.
3. Results
The survey was performed in 8 study sites containing 44 villages. A total of 2511 households agreed to participate in the study, corresponding to 17,652 individuals. Households consisted of a median of 7 members (interquartile range (IQR) 5–9); 2060 (82%) were permanent and 451 (18%) were immigrant households. In 1732 (68.0%) households the main income generating activity was farming. The median age of study subjects was 17 years (IQR 8–30 years); 5826 (33%) were younger than 10 years; 9206 (52.1%) were females.
3.1. Prevalence of epilepsy
The screening questionnaire identified 799 (4.5%) individuals suspected to have epilepsy; 765 (95.7%) were seen by a clinical officer or medical doctor and in 736 (92.1%) the diagnosis of epilepsy was confirmed. In the 29 individuals (3.9%) for which the diagnosis of epilepsy was not confirmed the diagnoses included: 8 (27.6%) only one seizure, 7 (24.1%) febrile seizures, 5 (17.2%) psychiatric illness, 2 (6.9%) dizziness, 1 (3.5%) paroxysmal vertigo, 1 (3.5%) alcohol or drug abuse, and 5 (17.2%) other diagnoses.
The coding of study participants by clinical officers, medical doctors and household survey research assistants was inconsistent, therefore only data of 696 of the 765 persons (90.9%) suspected with epilepsy could be used to determine the positive predictive value (PPV) of the epilepsy screening questions to detect an individual with clinically confirmed epilepsy (Table 1). Considering the high PPV of a positive response (98.5%) of the combination Q1 + Q5 or Q2 + Q5 or Q3 + Q5 or Q4 + Q5 for the diagnosis of confirmed epilepsy and the high number of persons detected with confirmed epilepsy, this combination was used to decide whether a person suspected of having epilepsy who was not seen by a clinical officer or medical doctor had confirmed epilepsy. Considering all persons confirmed to have epilepsy by clinical officers or medical doctors and those who responded positively to Q1 + Q5 or Q2 + Q5 or Q3 + Q5 or Q4 + Q5, an additional 38 persons had epilepsy, increasing the total number of persons with clinically confirmed epilepsy to 736 + 38 = 774 cases, giving an epilepsy prevalence rate of 4.4%.
Table 1.
Number of persons suspected to have epilepsy identified by the five screening questions separately and in combination and its positive predictive value to detect persons with confirmed epilepsy.
| Questions | Number of persons suspected to have epilepsy identified in the total population (17,652 individuals) | Positive predictive value in a population of 696 persons suspected to have epilepsy |
|---|---|---|
| Q1 Lost consciousness and experienced loss of bladder control or foaming at the mouth | 662 | 98.5% (97.9%–98.9%) |
| Q2. Experienced absence or sudden loss of contact with the surroundings for a short time duration | 674 | 98.4% (97.9%–98.8%) |
| Q3. Experienced sudden, uncontrollable twitching or shaking of arms, legs or head for a few minutes | 690 | 98.6% (97.8%–99.1%) |
| Q4. Experienced sudden and brief bodily sensations, seen or heard things that were not there, or smelt strange odors | 649 | 98.2% (97.8%–98.5%) |
| Q5. Ever been diagnosed with epilepsy | 746 | 95% (93.3%–96.5%) |
| Q1 + Q5 or Q2 + Q5 or Q3 + Q5 or Q4 + Q5 | 740 | 98.5% (98.1%–98.9%) |
The epilepsy prevalence in the different study areas varied between 3.5–11.9%. The highest epilepsy prevalence was observed in the study area of Kazana-2 (Table 2)
Table 2.
Epilepsy prevalence by study site.
| Study Area | Number of subjects interviewed | Number of confirmed epilepsy cases | Epilepsy prevalence |
OR (90% CI) | P value |
|---|---|---|---|---|---|
| Kazana-1 | 2298 | 107 | 4.7 | ||
| Kazana-2 | 402 | 48 | 11.9 | 6 (1.8–3.7) | <0.001 |
| Kwanga | 273 | 16 | 5.9 | 1.4 (0.9–2.4) | 0.2 |
| Hai-Taiwara | 5619 | 205 | 3.6 | 0.8 (0.6–1.0) | 0.02 |
| Hai-Gabat | 4385 | 155 | 3.5 | 0.7 (0.6–0.9) | 0.01 |
| Mudubai | 970 | 44 | 4.5 | 0.9(0.6–1.3) | 0.6 |
| Hai-Matara | 2605 | 155 | 5.9 | 1.3 (1.0–1.6) | 0.6 |
| Ngabaka | 1100 | 42 | 3.8 | 0.8 (0.6–1.1) | 0.2 |
Kazana-2 is the study site located closest to Maridi dam (Fig. 3).
Fig. 3.
Picture of the Maridi dam.
3.2. Incidence of epilepsy
Two hundred and eighty four (38.6%) individuals developed their first seizures in the 5 years preceding the survey (annual incidence = 292.3/100.000), 85 (11.5%) in the two years before the survey (annual incidence = 240.8/100.000) and 66 (9%) in the year before the survey (annual incidence = 373.9/100.000). Fifty seven (86.4%) of the latter were 3–18 years old (annual incidence = 322.9/100.000).
3.3. Characteristics of the epilepsy
In 714 (97%) PWE information about the year of onset of seizures was obtained. The median age of onset of seizures was 10 years (IQR: 6–13); 647/714 (90.6%) developed their first seizures between the ages of 3–18 years. The most frequent seizure type was generalized tonic clonic seizures, reported in 511/736 (69.4%) of PWE. In 335/736 (45.4%) there was a history of nodding seizures and 98 of the 626 (15.7%) PWE above the age of 16 presented with Nakalanga features; 414 of the 486 (85.2%), for which data were available on all OAE criteria, met the criteria of OAE. All PWE reported at least 2 seizures 24 h apart during the previous 5 years, and 114 (16.2%) during the last year (active epilepsy).
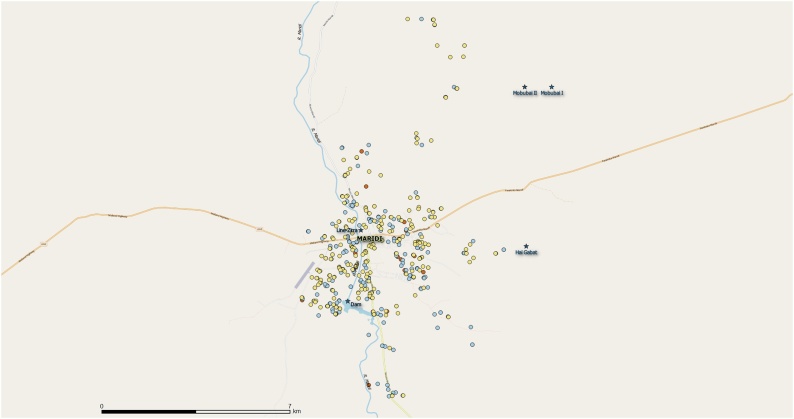
Geographic location data was available for 499 individuals of a total 765 persons suspected to have epilepsy examined by a clinical officer or medical doctor (65.1%); including 232/335 persons with a history of nodding seizures (69.3%), 246/401 persons with other types of epilepsy (61.3%) and 21/29 persons not confirmed to have epilepsy (724%) (Fig. 4).
Fig. 4.
Geographic location of persons with a history of nodding seizures (in blue), persons with other forms of epilepsy (in yellow) and persons not confirmed to have epilepsy (in red) (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Based on the high epilepsy prevalence in Kazana-2, located close to the Maridi dam (4.89422 N, 29.45792 E) we calculated the distance between the Maridi dam and the categories of population (i.e., population with history of nodding seizures, population with other types of epilepsy and population not confirmed to have epilepsy).Results showed that the cluster of persons with a history of nodding seizures was located closer to the dam compared to other types of epilepsy (p-value 0.001) and the cluster of persons not confirmed to have epilepsy (p-value 0.008). However, the distances from the dam to the cluster of other types of epilepsy and the cluster of persons not confirmed to have epilepsy were not significantly different. These findings were also confirmed using the SaTScan software (supporting file).
Using Ward’s method concerning the location of the persons with a history of nodding seizures and persons with other types of epilepsy, the merging cost was very low (0.007419386), which means that persons with a history of nodding seizures and persons with other forms of epilepsy were located in approximately in the same area. In fact, the geographic distance between the centroids of two distributions was only 1 km.
3.4. Risk factors for epilepsy
Epilepsy prevalence was higher in males than females and was highest (10.5%) in the 11–20 age group (Table 3).
Table 3.
Epilepsy prevalence by age group and gender.
| Persons with confirmed epilepsy N = 744 |
Total sample N = 17,652 |
Epilepsy prevalence (4.2%) [95% CI 3.9%- 4.5] |
Or (95% CI) | P value | |
|---|---|---|---|---|---|
| Gender | P value | ||||
| Female | 337 | 9206 | 3.7% | ||
| Male | 437 | 8445 | 5.2% | <0.001 | |
| Age groups in years | P value | ||||
| 0–5 | 20 | 2981 | 0.67 | 1 | |
| 6–10 | 58 | 2845 | 2.04 | 3.3 (2.0–5.5) | <0.001 |
| 11–20 | 513 | 4870 | 10.5 | 17.8 (11.4–27.9) | <0.001 |
| 21–30 | 150 | 2896 | 5.2 | 8.6 (5.4–13.7) | <0.001 |
| 31–40 | 18 | 1860 | 0.87 | 1.5 (0.8–2.9) | 0.2 |
| >40 | 13 | 2227 | 0.58 | 1.2 (0.6–2.3) | 0.6 |
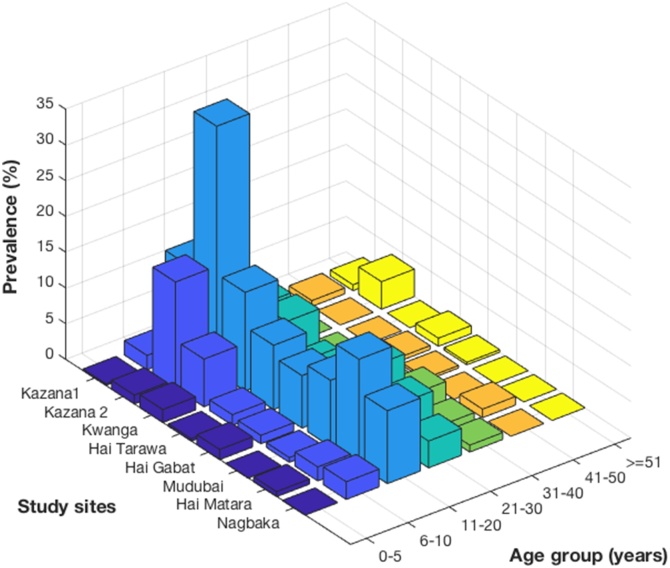
Age specific epilepsy prevalence was similar in each study site (Fig. 5).
Fig. 5.
Age-specific epilepsy prevalence in each study sites.
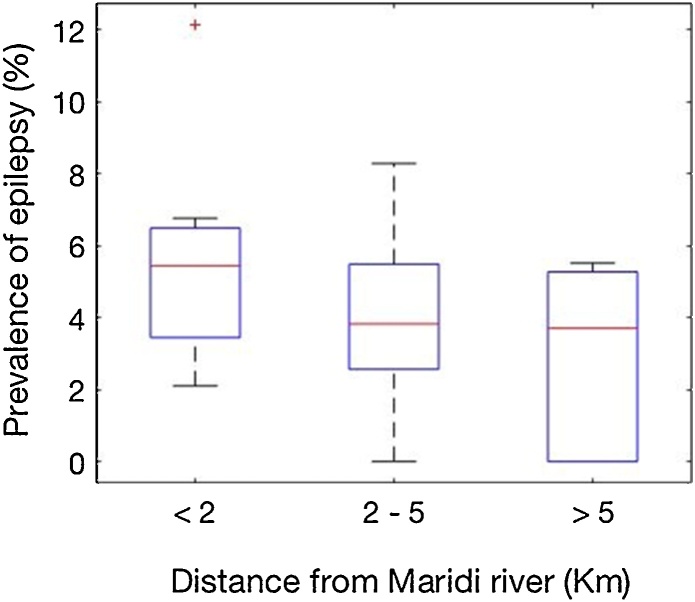
Epilepsy prevalence in villages <2 km from the Maridi River was significantly higher than in villages more distant from the river (p < 0.001) (Fig. 6).
Fig. 6.
Prevalence of epilepsy and distance of villages to the Maridi River.
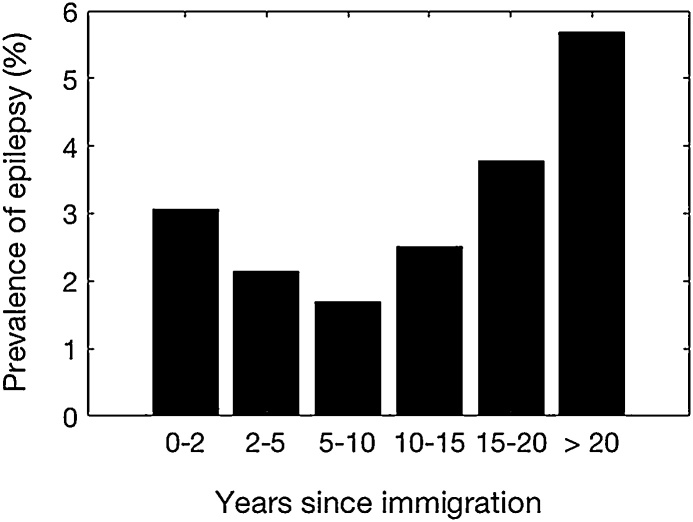
Six hundred ninety two (4.7%) of 4619 permanent households members were found to have epilepsy compared with 82 (2.7%) of 3033 immigrant households members (p < 0.001). Epilepsy prevalence among the latter increased with increasing numbers of years of residence in the village (Fig. 7).
Fig. 7.
Epilepsy prevalence in immigrant households according to their duration of stay in onchocerciasis endemic villages.
Five hundred and eighty eight (4.9%) of 12,045 members of farming households had epilepsy compared with 186 (3.3%) of 5614 members of households with another main income generating activity, such as employee, soldier, policeman, business man, and social worker (p < 0.001).
In 545 (21.7%) households there was one person with epilepsy, in 167 two persons with epilepsy (6.6%), in 55 three persons with epilepsy (2.2%) and at least four persons with epilepsy in 10 households (0.4%).
Only 7209 (40.8%) of the total population took ivermectin in 2017. There was no gender difference in ivermectin coverage but there was less ivermectin intake in the 5–10 year old age group (Table 4). There was low intake of ivermectin in certain study sites with high epilepsy prevalence (Kazana-2 and Kwanga) (Table 5).
Table 4.
Ivermectin coverage by gender and age group.
| Age | Male | Female | OR (90% CI) | P value |
|---|---|---|---|---|
| 5–10 | 432/1756 (26.2%) | 472/1729 (29.3%) | 9.8 (8.1–12.0) | <0.001 |
| 11–20 | 1268/2485 (51%) | 1182/2387 (49.5%) | 23.(19.5–28.4) | |
| 21–30 | 641/1210 (53%) | 841/1661 (50.6%) | 24.8 (20.4–30.1) | |
| 31–40 | 453/787 (57.6%) | 600/1073 (55.9%) | 30.3 (24.7–37.1) | |
| >40 | 612/1035/ (59.1%) | 699/1193 (58.6%) | 33.2 (27.2–40.6) |
Table 5.
Epilepsy prevalence and ivermectin coverage per study area.
| Study Area | Epilepsy prevalence (%) | Ivermectin coverage (%) | OR (90% CI) | P value |
|---|---|---|---|---|
| Kazana-1 | 4.7 | 719/2298 (31.3%) | ||
| Kazana-2 | 11.9 | 101/402 (25.1%) | 0.7 (0.6–0.9) | 0.01 |
| Kwanga | 5.9 | 54/219 (19.8%) | 0.5 (0.4–0.7) | <0.001 |
| Hai-Taiwara | 3.6 | 2502/5618 (44.4%) | 1.8 (1.6–2.0) | <0.001 |
| Hai-Gabat | 3.5 | 1863/4385 (42.6%) | 1.6 (1.5–1.8) | <0.001 |
| Mudubai | 4.5 | 498/472 (51.3%) | 2.3 (2.0–2.7) | <0.001 |
| Hai-Matara | 5.9 | 1169/2605 (44.9%) | 1.8 (1.6–2.0) | <0.001 |
| Ngabaka | 3.8 | 352/1100 (32%) | 1.1 (0.9–1.2) | 0.5 |
In a multivariate regression model, age group, male gender, being a permanent household family member, being a member of a farming household, and living in a village bordering the river remained independently associated with epilepsy (Table 6).
Table 6.
Multivariate regression model showing risk of epilepsy by age group, gender, permanent or immigrant household, family income generating activity, distance of the village to the river, and ivermectin intake.
| Variable | Univariate analysis | P value | Multivariate analysis | P value |
|---|---|---|---|---|
| Age | ||||
| 0–5 | Baseline | Baseline | ||
| 6–10 | 3.3 (2.0–5.5) | <0.001 | 3.2 (1.9–5.4) | <0.001 |
| 11–20 | 17.8 (11.4–27.9) | <0.001 | 17.3 (11.0–27.3) | <0.001 |
| 21–30 | 8.6 (5.4–13.7) | <0.001 | 8.4 (5.2–13.5) | <0.001 |
| 31–40 | 1.5 (0.8–2.9) | 0.2 | 1.5 (0.8–2.8) | 0.2 |
| 41–80 | 1.2 (0.6–2.3) | 0.6 | 1.2 (0.6–2.2) | 0.6 |
| Gender | <0.001 | |||
| Male | Baseline | 0.6* | <0.001 | |
| Female | 0.7 (0.6–0.8) | <0.001 | 0.3 ** | <0.001 |
| Occupation | ||||
| Farmer | Baseline | Baseline | ||
| Not farmer | 0.8 (0.6–0.9) | 0.002 | 0.8 (0.6–0.9) | 0.004 |
| Distance to river | <0.001 | |||
| <2 km | Baseline | |||
| >=2 km | 0.9 (0.8–1.0) | 0.08 | 0.6 (0.5–0.7) * | <0.001 |
| Residence status | 0.6 (0 .4–0.7) | <0.001 | ||
| Immigrant | Baseline | |||
| Local | 0.6 (0 .4–0.7) | <0.001 | 0.6 (0.4–0.7) | <0.001 |
| Ivermectin | ||||
| Ivermectin intake | Baseline | Baseline | ||
| No Ivermectin | 0.7 (0.6–8.6) | <0.001 | 1.0 (0.8–1.1) | 0.7 |
Male > 2 km.
Female >2 km.
3.5. Epilepsy related mortality
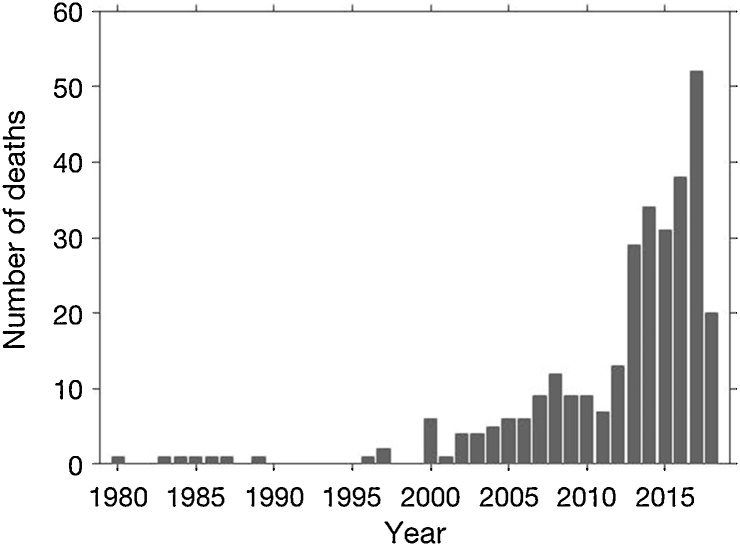
Two hundred and fifty seven (10.2%) households reported that a family member with epilepsy died. Three hundred twenty five PWE were reported to have died, 187 (57.5%) died between the ages 6–20 years and 306 (94.2%) before the age of 30 years. Starting in early 2000, an increasing number of PWE who died were reported (Fig. 8).
Fig. 8.
Number of persons with epilepsy reported to have died per year.
During the four months of 2018, 20 PWE died (mortality = 229.9 per 100,000 person years) and in 2017, 52 PWE died (mortality = 294.6 per 100,000 person years).
4. Discussion
This is the first population-based study to determine the prevalence and incidence of epilepsy in an onchocerciasis endemic region in South Sudan. High prevalence and incidence of epilepsy were observed in several villages in Maridi County located close to the Maridi River and in particular the Maridi dam. The fact that epilepsy prevalence and the risk for developing epilepsy in onchocerciasis-endemic regions increases with distance to the river was previously reported in Cameroon and the Democratic Republic of the Congo [9,28]. Moreover, in a recent a retrospective cohort study in the Mbam Valley, Cameroon, cumulative incidence of epilepsy was shown to increase with increasing microfilarial density in skin snips during childhood [29]. Our study provides additional evidence for the association between epilepsy and high ongoing O volvulus transmission.
The study showed a high peak prevalence of >10% in the 10–20 year age group. A high epilepsy prevalence in this age group has been observed in other onchocerciasis endemic areas before the implementation of CDTi (8.3% in western Uganda [30]. Epilepsy prevalence was significantly higher in males than females. This was also observed in other onchocerciasis endemic regions [7] and is expected in epilepsy associated with O volvulus infection as onchocerciasis is also generally more prevalent in males [31]. Epilepsy prevalence was also higher in permanent than immigrant households, and increased in immigrant households with increasing years of residence in the village. This suggests that several years of living in an onchocerciasis endemic village are required before a sufficiently high threshold of O volvulus infection is reached to result in epilepsy. Epilepsy prevalence was also more prevalent among farming families. This could be explained by the fact that the best farming land is located close to the river.
Our study shows a low intake of ivermectin among the population of Maridi. This is explained by many years of limited ivermectin distribution as a consequence of the insecurity in the area. Most people had taken ivermectin only in 2017 and many never took ivermectin. The very low intake among 5–10 year old children is a concern as ivermectin could protect them for developing OAE [28]. However, some 5 year olds may have been 4 years old at the time of ivermectin distribution and therefore were not eligible for ivermectin treatment according to WHO guidelines.
Our study also shows that persons with a history of nodding seizures and with other forms of epilepsy live in the same areas close to the Maridi River, confirming that nodding syndrome is only one of the phenotypic presentations of OAE.
Finally this study shows that epilepsy is an important cause of death in the Maridi area. The estimated 2.9 per 10,000 death rate because of epilepsy is 39.4% of the 2017 South Sudanese crude death rate of 7.36 deaths per 1000 population [32]. Such a high mortality because of epilepsy was also previously reported in other onchocerciasis endemic regions. In a study in the Mbam Valley, Cameroon the relative risk of dying during the follow-up among the group of people with epilepsy, compared with the controls, was 6.2 (95% CI, 2.7–14.1) [33].
Our study has several limitations: the diagnosis of epilepsy was mainly made by clinical officers and medical doctors but was not confirmed by a neurologist; and several individuals suspected to have epilepsy were not seen by a clinical officer or medical doctor. In addition, no laboratory investigations were performed. Therefore it was not possible to confirm the possible causes of epilepsy. Meeting the OAE criteria does not mean that in all these individuals epilepsy was caused by onchocerciasis. Other causes of epilepsy were only excluded by medical history. However given the absence of pigs in the Maridi villages, neurocysticercosis cannot explain the high prevalence of epilepsy. We only had 6 tablets for 10 clinical officers. Therefore certain clinical officers were unable to collect GPS data and this may have created a bias. However, given the fact that clinical officers with and without tablets moved together when visiting the villages and that a similar percentage of individuals with a history of nodding and other seizures and with a non-confirmed diagnosis of epilepsy were geo-located, it is unlikely that bias influenced our results.
5. Conclusion
A high prevalence and incidence of epilepsy was observed in several villages in Maridi County located close to the Maridi River and the Maridi dam. For hundred and ninety two (94.3%) PWE met the criteria for OAE. Urgent action is needed to prevent new OAE cases appearing, to treat all PWE with appropriate anti-epileptic drugs and provide supportive care. To achieve this goal a CDTi programme with ivermectin distribution every 6 months is being instituted by the Ministry of Health and a decentralized system for the comprehensive treatment and care of PWE is planned for Maridi County. Moreover it is planned to repeat this survey in two years to evaluate the effect of this intervention.
Competing interests
No conflict of interest declared
Funding
R Colebunders received funding from the European Research Council (ERC 671051)
Authors’ contributions
RC, GA and MYL wrote the protocol. RC, JYC, PCO, and KP MO were involved in performing the survey and data collection, SB, SM and CE analyzed the data, RL and MYL were involved in preparing the study and provided support from the Ministry of Health of South Sudan, RC wrote the first draft.
Acknowledgements
We are grateful to the clinical officers and research assistants who performed the study; the Maridi Health Sciences Institute, Amref Health Africa for use of its facilities, equipment and vehicles; the Maridi State government for their support; and the population of the Maridi villages for their participation. SB thanks SERB, India and ERC, European Union for supporting the research visit to University of Antwerp. We thank Evy Lenaerts for her assistance with Fig. 4.
Contributor Information
Robert Colebunders, Email: robert.colebunders@uantwerpen.be.
Jane Y Carter, Email: jcarter@iconnect.co.ke.
Peter Claver Olore, Email: Peter.Claver@Amref.org.
Kai Puok, Email: kaipuokbiel5@gmail.com.
Samit Bhattacharyya, Email: samitb@snu.edu.in.
Sonia Menon, Email: sonia.menon@uantwerpen.be.
Gasim Abd-Elfarag, Email: gasim4u83@gmail.com.
Morrish Ojok, Email: Morrish.Ojok@amref.org.
Chellafe Ensoy-Musoro, Email: chellafeensoy@uhasselt.be.
Richard Lako, Email: richardlako@yahoo.com.
Makoy Yibi Logora, Email: morrelogora@yahoo.com.
References
- 1.Noma M., Zouré H.G., Tekle A.H., Enyong P.A., Nwoke B.E., Remme a J.H. The geographic distribution of onchocerciasis in the 20 participating countries of the African Programme for Onchocerciasis Control: (1) priority areas for ivermectin treatment. Parasit Vectors. 2014;7(325):1–15. doi: 10.1186/1756-3305-7-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ministry of Health Republic of South Sudan . 2008. Neglected tropical diseases and their control in Southern Sudan. Situation analysis, intervention options, appraisal and gap analysis.https://www.malariaconsortium.org/userfiles/file/NTD%20Resources/ntds_southern_sudan%5B1%5D 2018 pAJ. (Accessed 3 November 2018) [Google Scholar]
- 3.Colebunders R., Nelson Siewe F.J., Hotterbeekx A. Onchocerciasis-associated epilepsy, an additional reason for strengthening onchocerciasis elimination programs. Trends Parasitol. 2017 doi: 10.1016/j.pt.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Pion S.D., Kaiser C., Boutros-Toni F., Cournil A., Taylor M.M., Meredith S.E. Epilepsy in onchocerciasis endemic areas: systematic review and meta-analysis of population-based surveys. PLoS Negl Trop Dis. 2009;3(6):e461. doi: 10.1371/journal.pntd.0000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prischich F., De Rinaldis M., Bruno F., Egeo G., Santori C., Zappaterreno A. High prevalence of epilepsy in a village in the Littoral Province of Cameroon. Epilepsy Res. 2008;82(2-3):200–210. doi: 10.1016/j.eplepsyres.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Kaiser C., Asaba G., Leichsenring M., Kabagambe G. High incidence of epilepsy related to onchocerciasis in West Uganda. Epilepsy Res. 1998;30(3):247–251. doi: 10.1016/s0920-1211(98)00007-2. [DOI] [PubMed] [Google Scholar]
- 7.Levick B., Laudisoit A., Tepage F., Ensoy-Musoro C., Mandro M. High prevalence of epilepsy in onchocerciasis endemic regions in the Democratic Republic of the Congo. PLoS Negl Trop Dis. 2017;11(7) doi: 10.1371/journal.pntd.0005732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tumwine J.K., Vandemaele K., Chungong S., Richer M., Anker M., Ayana Y. Clinical and epidemiologic characteristics of nodding syndrome in Mundri County, southern Sudan. Afr Health Sci. 2012;12(3):242–248. doi: 10.4314/ahs.v12i3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boussinesq M., Pion S.D., Demanga N., Kamgno J. Relationship between onchocerciasis and epilepsy: a matched case-control study in the Mbam Valley, Republic of Cameroon. Trans R Soc Trop Med Hyg. 2002;96(5):537–541. doi: 10.1016/s0035-9203(02)90433-5. [DOI] [PubMed] [Google Scholar]
- 10.Gerrits C. A West African epilepsy focus. Lancet. 1983;1(8320):358. doi: 10.1016/s0140-6736(83)91663-x. [DOI] [PubMed] [Google Scholar]
- 11.Ovuga E., Kipp W., Mungherera M., Kasoro S. Epilepsy and retarded growth in a hyperendemic focus of onchocerciasis in rural western Uganda. East Afr Med J. 1992;69(10):554–556. [PubMed] [Google Scholar]
- 12.Centers for disease Control and prevention. Nodding syndrome - South Sudan, 2011. MMWR Morb Mortal Wkly Rep Surveill Summ. 2012;61(3):52–54. [PubMed] [Google Scholar]
- 13.de Polo G., Romaniello R., Otim A., Benjamin K., Bonanni P., Borgatti R. Neurophysiological and clinical findings on nodding Syndrome in 21 South Sudanese children and a review of the literature. Seizure. 2015;31:64–71. doi: 10.1016/j.seizure.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Colebunders R., Hendy A., Mokili J.L., Wamala J.F., Kaducu J., Kur L. Nodding syndrome and epilepsy in onchocerciasis endemic regions: comparing preliminary observations from South Sudan and the Democratic Republic of the Congo with data from Uganda. BMC Res Notes. 2016;9:182. doi: 10.1186/s13104-016-1993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lacey M. Nodding disease: mystery of southern Sudan. Lancet Neurol. 2003;2:714. doi: 10.1016/s1474-4422(03)00599-4. [DOI] [PubMed] [Google Scholar]
- 16.Nyungura J.L.A.T., Lako A., Abe G., Lejeng L., William G. Investigation into nodding Syndrome in Witto Payam, Western Equatoria state. South Sudan Med J. 2011;4(1):3. [Google Scholar]
- 17.Reik L.A.A., Opoka M., Mindra G., Sejvar J., Dowell S.F. Nodding syndrome South Sudan 2011. Morb Mortal Wkly Rep Surveill Summ. 2012;61(3):52. [PubMed] [Google Scholar]
- 18.World Health Organization; Geneva: WHO: 2017. WHO. Nodding syndrome (NS)http://www.who.int/onchocerciasis/symptoms/nodding_syndrome/en/ Contract No.: March 1st 2017 (Accessed 3 September 2018) [Google Scholar]
- 19.A-EG Colebunders R., Carter J.Y., Olore P.C., Puok K., Menon S., Fodjo Joseph Nelson. Clinical characteristics of onchocerciasis-associated epilepsy in villages in Maridi County, Republic of South Sudan. Seizure. 2018 doi: 10.1016/j.seizure.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 20.National Bureau of Statistics . Vol. 2. National Bureau of Statistics; 2013. National Bureau of Statistics: population dustribution by age group by sex by payam; p. 2013.http://www.ssnbss.org/home/document/census/population-distribution-by-age-group-sex-and-payam-greater-bahr-el-ghazal (Population distribution by age group by sex by payam. Edited by release M). (Accessed 3 September 2018) [Google Scholar]
- 21.Abd-Elfarag G., Logora M.Y., Carter J.Y., Ojok M., Songok J., Menon S., Wit F., Lako R., Colebunders R. The effect of bi-annual community-directed treatment with ivermectin on the incidence of epilepsy in onchocerciasis endemic villages in South Sudan: a study protocol. Infect Dis Poverty. 2018;7(Nov (1)):112. doi: 10.1186/s40249-018-0496-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diagana M., Preux P.M., Tuillas M., Ould Hamady A., Druet-Cabanac M. Depistage de l’epilepsie en zones tropicales: validation d’un questionnaire en Mauritanie. Bull Soc Pathol Exot. 2006;99(2):103–107. [PubMed] [Google Scholar]
- 23.Fisher R.S., Acevedo C., Arzimanoglou A., Bogacz A., Helen Cross J., Elger C.E. A practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 24.Burnham G., Mebrahtu T. Review: The delivery of ivermectin (Mectizan (R)) Trop Med Int Health. 2004;9(4):A26–A44. doi: 10.1111/j.1365-3156.2004.01211.x. [DOI] [PubMed] [Google Scholar]
- 25.Gibbons J.D., Chakraborti S. 5th ed. CRC Press; 2010. Nonparametric statistical interference. [Google Scholar]
- 26.Ward J.H. Hierarchical grouping to optimize an objective function. J Am Stat Assoc. 1963;58:236–244. [Google Scholar]
- 27.Kulldorff M. A spatial scan statistic. Commun Stat Theory Methods. 1997;26:1487–1496. [Google Scholar]
- 28.Colebunders R., Tepage F., Rood E., Mandro M., Abatih E.N., Musinya G. Prevalence of River Epilepsy in the Orientale Province in the Democratic Republic of the Congo. PLoS Negl Trop Dis. 2016;10(5) doi: 10.1371/journal.pntd.0004478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chesnais C., Nana-Djeunga H.C., Njamnshi A.K., Lenou-Nanga C.G. The temporal relationship between onchocerciasis and epilepsy: a prospective population-based cohort study. Lancet Infect Dis. 2018;(September 26) doi: 10.1016/S1473-3099(18)30425-0. pii: S1473-3099(18)30425-0. [DOI] [PubMed] [Google Scholar]
- 30.Kaiser C., Kipp W., Asaba G., Mugisa C., Kabagambe G., Rating D. The prevalence of epilepsy follows the distribution of onchocerciasis in a west Ugandan focus. Bull World Health Organ. 1996;74(4):361–367. [PMC free article] [PubMed] [Google Scholar]
- 31.Brabin L. Factors affecting the differential susceptibility of males and females to onchocerciasis. Acta Leiden. 1990;59(1-2):413–426. [PubMed] [Google Scholar]
- 32.World Atlas Data. Sudan crude date rate. https://knoema.com/atlas/Sudan/topics/Demographics/Mortality/Crude-death-rate. (Accessed 5 November 2018).
- 33.Kamgno J., Pion S.D., Boussinesq M. Demographic impact of epilepsy in Africa: results of a 10-year cohort study in a rural area of Cameroon. Epilepsia. 2003;44(7):956–963. doi: 10.1046/j.1528-1157.2003.59302.x. [DOI] [PubMed] [Google Scholar]