Abstract
Mineral and organic fertilization can be optimized by using rhizobacteria which increases dry matter, yield, and nutrients in the soil and plant, among the other biological inputs. However, the discovery of single microbes or a consortium that can benefit plants has been a challenge. In this context, this study aimed to evaluate the effects of Bacillus subtilis and Bacillus pumilus combined with mineral fertilization and sugar and alcohol industry by‐products in presprouted and the initial growth phase of sugar cane seedlings. The study was carried out in two phases. Phase 1 included presprouted seedlings with T1 = untreated control, T2 = B. subtilis, T3 = B. pumilus, and T4 = B. subtilis + B. pumilus treatments. Phase 2 included the same treatments with four types of fertilization: F1 = mineral fertilization, F2 = mineral fertilization + vinasse, F3 = mineral fertilization + filter cake, and F4 = mineral fertilization + filter cake compost. Of the phase 1 treatments, T2 (B. subtilis) was the best promoter of root growth and the total dry matter compared to the control with an increase of 23.0% compared to the control. In phase 2, B. pumilus application, increased the total dry matter by 13%, the number of tillers by 37%, and the diameter of the tillers by 48% when combined with mineral fertilization. The combined application of B. subtilis and B. pumilus increased the phosphorus content by 13% in soil treated with mineral fertilization and filter cake compost. The results of the this study strongly suggest that the use of B. subtilis and B. pumilus together with these by‐products can improve soil fertility parameters and decrease adverse effects associated with vinasse fertilization, in addition to providing shoot and root growth and providing collective synergy for a high yield of sugarcane production with environmental benefits.
Keywords: Bacillus pumilus, Bacillus subtilis, filter cake, plant growth promotion, sugar cane, vinasse
1. INTRODUCTION
Brazil is the world′s largest producer of sugarcane (Saccharum spp.), an important crop of the biofuels sector, with the potential for ethanol production and the generation of by‐products of industrial interest (De Abastecimento‐Conab, 2016). There is a high level of investment in cane production, including the generation of presprouted seedlings (PSS), the multiplication of plants in the nursery, uniform planting, and maintaining high standards of phytosanitation, etc., (Gazola, Cipola Filho, & Júnior, 2017).
In addition to using the PSS system, the use of bacterial inoculants is an alternative to achieve sustainable plant production, with less use of mineral fertilizers (Lesueur, Deaker, Herrmann, Bräu, & Jansa 2016. Studies have shown the benefits of using plant growth‐promoting rhizobacteria (PGPR) in sugarcane to increase the dry matter yield and productivity (de Oliveira, de Canuto, Urquiaga, Reis, & Baldani, 2006; Schultz et al., 2012).
Several isolates of Bacillus, especially Bacillus subtilis directly affect plant growth promotion, by the mechanisms such as acting as nitrogen fixers, as well as producers of indol butyric acid (IBA) (Araujo, Henning, & Hungria, 2005), indol acetic acid (IAA), and siderophores (Puente, Bashan, Li, & Lebsky, 2004), in addition to biocontrol activity (Ali, Charles, & Glick, 2014; Hanif et al., 2015; Oslizlo et al., 2015) and activities of protein expression relevant to the pharmaceutical and industrial sector (Guan et al., 2015). Bacillus subtilis has such varied functional capacities, which suggests that it has a versatile potential to be used as a plant growth promoter.
Another species with the potential to be used as plant growth promoter is Bacillus pumilus, which is a gibberellic acid producer, and has been shown to stimulate the growth and development of tree species such as Alnus glutinosa (GutiérreziMañero et al., 2001) and Pinus pinea (Probanza, Garcıa, Palomino, Ramos, & Mañero, 2002). It is additionally a chitinase producer, which makes it a promising antagonist to strong, economically important agricultural pathogens such as Fusarium solani and Aspergillus niger (Gurav, Tang, & Jadhav, 2017; Rishad, Rebello, Shabanamol, & Jisha, 2017).
The capability of Bacillus to produce durable endospores, make this genre more promising in the biofertilization business, competing with current widely used agrochemicals (Wu, Cao, Li, Cheung, & Wong, 2005).
Sugar and ethanol production generate high amounts of by‐products as waste. Filter cake, vinasse, boiler ash, and cane straw are considered the main by‐products because they have a high added value, a high potential for use in agriculture, and can be used as alternative fertilizers. Vinasse is a potential source of organic matter, with nutrients such as K, N, Ca, and Mg. It also improves water retention and can serve to increase sugarcane yield (Prado, Caione, & Campos, 2013). Filter cake is rich in organic phosphorus, which is released slowly by mineralization and the action of soil microorganisms (Almeida Júnior, do Nascimento, Sobral, da Silva, & Gomes, 2011). Compost production by combining filter cake with plaster, boiler ash, and straw adds value to the by‐product by improving the nutrient concentration and reducing the water content (da Silva, Teles, & Júnior, 2016).
Every year, the cane industry accumulates millions of tons of by‐products such as vinasse and filter cake. Moreover, because these by‐products are rich in phosphorus and potassium, they are returned to cane production as biofertilizers. Additionally, PGPR has been used in sugarcane production to reduce the necessity for mineral fertilization, thus reducing the cost of production. However, there is a dearth of studies comparing and combining the use of by‐products and bacterial inoculants on cane growth. The purpose of this study was to optimize the possibilities for the use of cane by‐products with bacterial inocula for sugarcane production.
2. EXPERIMENTAL PROCEDURES
This study has been conducted in two phases: Phase 1 was carried out in the greenhouse with PSS to evaluate the respective action of B. subtilis and B. pumilus on growth promotion. Phase 2 was performed outdoors by planting PSS from phase 1 in vases with a 50‐liter capacity. This served to evaluate the synergistic effect of the same bacterial inocula and cane by‐products such as vinasse, filter cake, and filter cake compost for sugarcane crop production using cane SP80‐3280 PSS.
The isolates of B. subtilis and B. pumilus came from the collection of the laboratory of Soil Microbiology of the Universidade Estadual Paulista, Câmpus de Jaboticabal. Both strains were isolated from grassy soil of the Farm of Unesp. The strains were identified by automatic sequencing of the 16 S ribosomal gene and stored as lyophilized in Brain Heart Infusion (BHI) medium in the freezer at temperature of − 20°C. The strains were reconstituted by adding 25 ml of BHI medium to 2 g of each strain. The strains were kept in a microbiological incubator for 24 hr at 28°C and 150 rotations per minute, before using in subsequent assays.
2.1. Phase 1
Phase 1 was carried out in a greenhouse located in Pradópolis (21º 21′ 34″ S, 48º 03′ 56″ W and 538 m altitude). The PSS were generated in plastic planting cells, and the duration of the experiment was 60 days (from November 2015 to January 2016). The design included randomized blocks with four repetitions, and the treatments were T1 = no inoculum, T2 = B. subtilis, T3 = B. pumilus, and T4 = B. subtilis and B. pumilus.
Planting and the subsequent experiment were carried out by following the recommendations for minor changes outlined by Landell et al. (2012). Briefly, the buds were planted directly in the planting cells. There were 128 seedlings in total. Of these, 64 were used in phase 1, and another 64 were used in phase 2.
A microbial inoculum was prepared by individually adding B. subtilis and B. pumilus with an inoculation loop into Erlenmeyer flasks containing Brain Heart Infusion (BHI) broth. The inocula were maintained in an incubator shaker at 28°C and 150 rpm for 24 hr. Then the culture concentrations were measured and adjusted to 5.7 × 107 colony‐forming units (CFU) m/L for B. subtilis and 1.4 × 108 CFU m/L for B. pumilus. The concentrations were standardized according to the generation time of each bacterial species (Souza et al., 2016).
The inoculation was carried out after 15 days of PSS growth and applied to the soil via pipette by adding 2 ml of inoculum per cell for a total of 4 ml (1:1) per cell for the treatment using both bacterial species. The control plants received no inoculum.
After 60 days (at the end of phase 1), the total bacterial load present in the substrate was counted using Standard Methods Agar (SMA) (Acumedia Brazil) with the addition of 10 g of the substrate to 100 ml of sodium pyrophosphate saline solution. This was followed by plating 0.1 ml of a 10−4 dilution after one hour of agitation (Vieira & Nahas, 2005).
The plants were carefully harvested from the cells for the root dry matter (RDM) and shoot dry matter (SDM) measurements. The aerial and root parts were separated by cutting at the collar region and were washed to remove adhering substrate (soil medium along with by‐product treatments) in which the PSS had been cultivated. Then, the parts were placed in paper bags and dried in the green house at 65°C to constant weight. Finally, the individual RDM and SDM were weighed on a semianalytical balance, and the weight was recorded. To obtain the total dry matter (TDM), the RDM and SDM were summed.
2.2. Phase 2
Phase 2 was conducted for 60 days (from January to March 2016) outdoors in Jaboticabal city—Sao Paulo State (21º 14′ 05″ S, 48º 17′ 09″ W, and 615 m altitude) by transferring the PSS from phase 1 to vases. Therefore, at the end of phase 2, the plants were 120 days old.
The experimental design included randomized blocks with four repetitions as a 4 × 4 factorial using 64 pots. Both factors were composed of four treatments. The factor I treatments included bacterial inoculum: T1 = no inoculum, T2 = B. subtilis, T3 = B. pumilus, and T4 = B. subtilis + B. pumilus. The factor II treatments included cane by‐products and mineral fertilizers viz: F1 = mineral fertilization (MF), F2 = fertilization + vinasse (FV), F3 = MF + filter cake (MF+FC), and F4 = MF+ filter cake compost (MF+FCC).
Bacterial inoculation for factor 1 utilized the same concentrations and methodology as described for phase 1.
Six inoculations were performed in total; the first four were weekly following the transfer of the PSS to the vases, and the last two were performed biweekly. Inoculants were applied to soil with a pipette. The inoculum (5 ml) was transferred at respective concentrations of 5.7 × 107 and 1.4 × 108 CFU m/L for B. subtilis and B. pumilus. The inoculum quantity was higher in phase 2 than in phase 1 because the vases were large, and the soil volume was greater, as well as because of precipitation, irrigation, and the plant growth stage during this phase.
Plant biometric data were obtained by counting all of the tillers per vase, measuring the height of the tillers using a graduated ruler from plant base up to leaf+1, according to Kuijper systems, and measuring tiller diameter near the soil using a pachymeter. Because there was more than one tiller per vase, the values of the height and diameter were summed, and the means were calculated. The RDM, SDM, and TDM were measured using methodology described previously.
For the soil analysis, samples were collected from the plant rhizosphere. The total bacterial CFU were measured using SMA medium (Buchbinder, Baris, & Goldstein, 1953) by dilution plating. The ammonium and nitrate levels were measured according to Keeney and Nelson (1982) in which the extraction was carried out with the aid of potassium chloride (KCl), followed by distillation with magnesium oxide (MgO) for the ammonium and sulfamic acid (H3NSO3), and by the method of Devarga for the determination of nitrate content. The titration sulfuric acid was used for ammonium and nitrate. Soluble phosphorus was measured by following the protocol developed by Murphy and Riley in 1986 (modified from Watanabe & Olsen, 1965).
After drying in a plant oven at 65°C (Tedesco, Gianello, Bissani, Bohnen, & Volkweiss, 1995), the nitrogen content was measured according to Keeney and Nelson (1982), and nitrogen distillation was performed as outlined by Kjeldahl, using CuSO4 as a catalyst.
The phosphorus content was measured according to Sarruge and Haag (1974), in which the plant samples were digested with nitric acid (65%) and perchloric acid (70%). A volume of 5.0 ml of digestion extract was pipetted, and 20.0 ml of water was added plus 4 ml of a mixture of reagents (ammonium molybdate to 5% with ammonium vanadate to 0.25%). After 5 min, a spectrometer reading was performed at 420 nm.
The data were analyzed in an analysis of variance (F test), and the means were compared by Duncan's 5% probability test using the Agro Estat 1.0 program (Barbosa & Maldonado Júnior, 2010).
3. RESULTS
In phase 1, inoculation of B. subtilis (5.84 g), B. pumilus (5.30 g) and a mixture of B. subtilis and B. pumilus (5.84 g) increased the plant root dry matter content compared to the control (4.74 g) (p > .05). The increase in the total dry matter content by applied microbial treatments of B. subtilis (6.57 g), B. pumilus (7.22 g), and a mixture of both microorganisms (7.7 g) were not significantly different from the control (6.57 g) (Figure 1).
Figure 1.
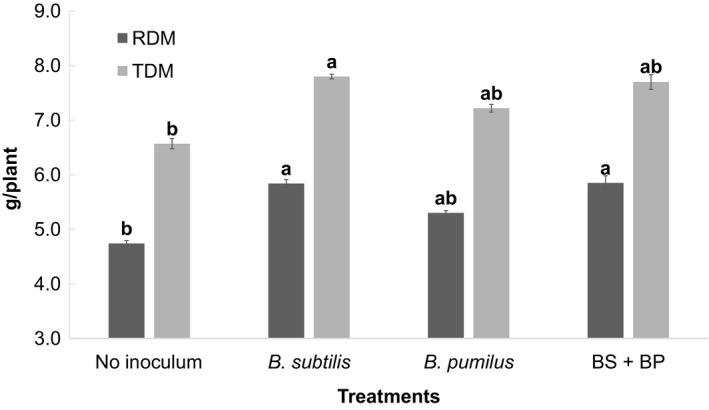
Means of root dry matter (RDM) and total dry matter (TDM) of presprouted seedlings (PSS) of sugarcane after 60 days of growth. Means followed by the same letter did not differ according to a Duncan test at 5% probability
In phase 2, B. pumilus (73.7 g) promoted higher root dry matter than B. subtilis (59.39 g) (p > .05). The total dry matter content of B. pumilus‐treated plants was significantly higher (102.3 g) (p > .05), as well as B. subtilis (87.5 g)‐treated plants, compared to the control (86.5 g) (Figure 2). Other than this, there was no other noticeable difference in the root dry matter content of plants treated or untreated with the microorganisms.
Figure 2.
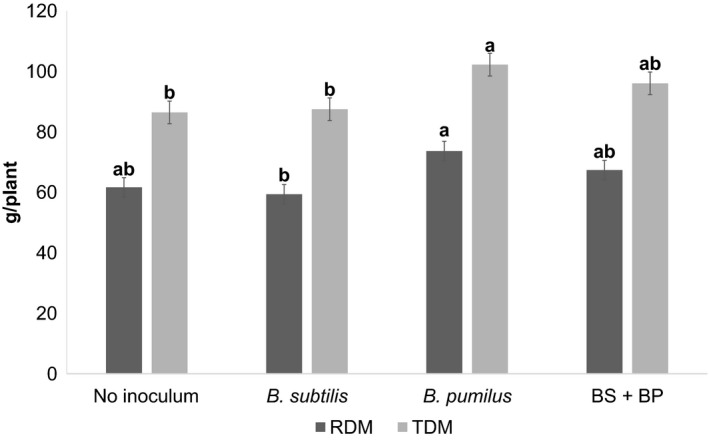
Means of root dry matter (RDM) and total dry matter (TDM) of sugarcane plants cultivated in vases 120 after planting. Means followed by the same letter did not differ according to a Duncan test at 5% probability
Bacillus pumilus combined with mineral fertilization produced highest number of tillers per vase (6.50) compared to the control (4.75). Bacillus subtilis (4.50) and a mixture of B. subtilis and B. pumilus (4.50) combined with mineral fertilization + vinasse also produced significantly more tillers than the control (3.00) (Figure 3).
Figure 3.
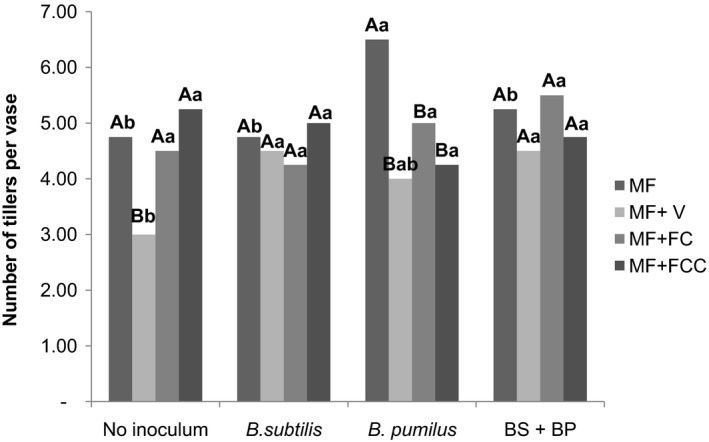
Interactions between the inoculum and the fertilization regime based on the number of tillers per vase. Uppercase letters compare means within the inocula and lowercase letters compare means within the fertilization regimes (Duncan p < .05). V: vinasse. FC: filter cake. FCC: filter cake compost. BS+BP= B. subtilis + B. pumilus
The diameter of the cane tillers was significantly increased with the application of B. pumilus combined with mineral fertilization (1.18 cm) compared to the control (0.80 cm) (p > .05) (Figure 4).
Figure 4.
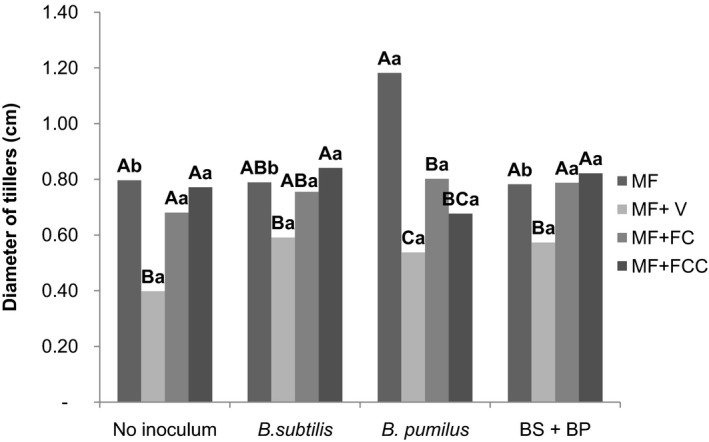
Interactions between inoculum and fertilization regime based on the diameter of tillers per vase. Uppercase letters compare means within the inocula and lowercase letters compare means within the fertilization regimes (Duncan p < .05). V: vinasse. FC: filter cake. FCC: filter cake compost. BS+BP= B. subtilis + B. pumilus
The highest phosphorus content was found in soil which received a mixture of B. subtilis and B. pumilus (45.45 μg of P dry soil g−1) and B. pumilus alone (27.17 μg of P dry soil g−1) combined with mineral fertilization + filter cake compost applied to the plants. No significant differences were found in comparisons among the other treatments (Figure 5).
Figure 5.
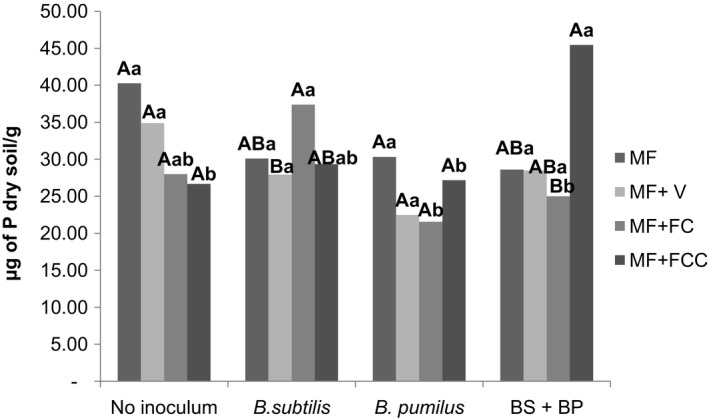
Interactions between inoculum and fertilization regime based on the soluble phosphorus concentration. Uppercase letters compare means within the inocula and lowercase letters compare means within the fertilization regimes (Duncan p < .05). V: vinasse. FC: filter cake. FCC: filter cake compost. BS+BP = B. subtilis + B. pumilus
Inoculation of B. pumilus combined with mineral fertilization + filter cake decreased the P content in the roots (1.22 μg of P root dry matter g−1) compared to the control (1.78 μg of P root dry matter g−1). Interestingly, the effect was stronger in plants which received a mixture of B. subtilis and B. pumilus combined with both mineral fertilization (0.99 μg of P root dry matter g−1) and mineral fertilization + vinasse (1.26 μg of P root dry matter g−1) compared to the respective controls (1.50 μg of P root dry matter g−1 and 1.82 μg of P root dry matter g−1) (Figure 6).
Figure 6.
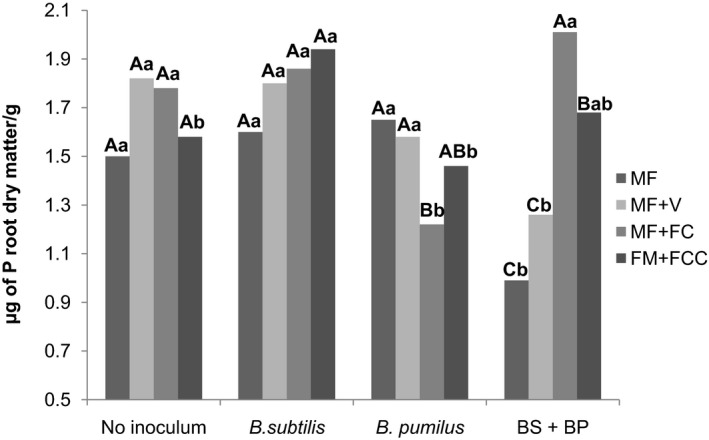
Interactions between inoculum and fertilization regime based on the phosphorus content of sugarcane roots 120 days after planting. Uppercase letters compare means within the inoculum and lowercase letters compare means within the fertilization regimes (Duncan p < .05). V: vinasse. FC: filter cake. FCC: filter cake compost. BS+BP= B. subtilis + B. pumilus
Figure 7 depicts the nitrogen content of the plant shoots. Inoculation of a mixture of B. subtilis and B. pumilus increased the nitrogen content of the plants (7.7 μg of N shoot dry matter) compared to the control (5.3 μg of N shoot dry matter) in the mineral fertilization + filter cake treatments. Additionally, a higher nitrogen content was recorded in the mineral fertilized plants inoculated with B. pumilus (8.0 μg of N shoot dry matter) and a mixture of B. subtilis and B. pumilus (8.1 μg of N shoot dry matter) than in the control (6.5 μg of N shoot dry matter).
Figure 7.
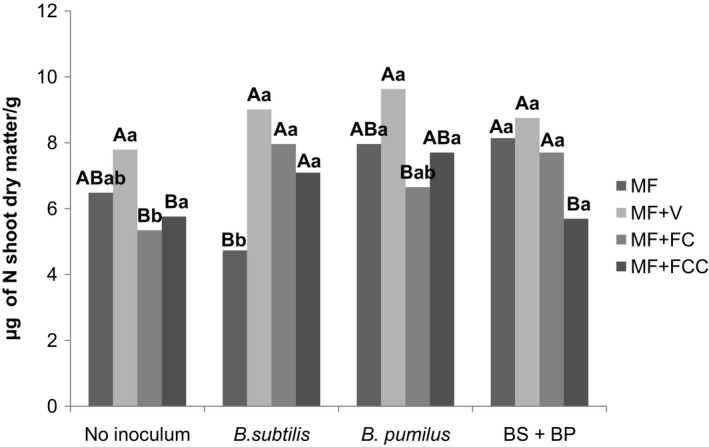
Interactions between inoculum and fertilization regime based on the nitrogen content of sugarcane roots 120 days after planting. Uppercase letters compare means within the inoculum and lowercase letters compare means within the fertilization regimes (Duncan p < .05). V: vinasse. FC: filter cake. FCC: filter cake compost. BS+BP= B. subtilis + B. pumilus
4. DISCUSSION
In phase 1, the inoculation of B. subtilis, B. pumilus and a mixture of both microorganisms promoted an increase of root dry matter, the total dry matter content of the plants compared to the control. In phase 2, B. pumilus promoted the highest root and total plant dry matter content compared to B. subtilis and B. pumilus combinations and the control.
The highest number of tillers was produced by B. pumilus combined with mineral fertilization. The combined application of B. pumilus + B. subtilis also significantly increased the number of tillers when mineral fertilization + vinasse was added.
The diameter of the tillers was increased when the plants received B. pumilus compared to the control with mineral fertilizer added.
The application of B. pumilus provided major benefits to the plants and the soil. Although reports on the enhancement of plant growth via PGPR are widely available, information about the use of B. pumilus and B. subtilis in the presence of different fertilization regimes that use cane waste for sugarcane production is scarce. Many studies regarding B. pumilus report that enzymes such as endoglucanase (Ariffin et al., 2008); xylanases (Bajaj, Khajuria, & Singh, 2012; Kapilan & Arasaratnam, 2011; Kapoor, Nair, & Kuhad, 2008); and chitinase (Gurav et al., 2017) are produced. A few studies report their capacity for plant growth promotion. According to our knowledge, this study is the first report about the use of B. pumilus as plant growth promoter in sugarcane production along under different fertilization regimes that mainly use cane waste for sugarcane production.
Phytohormones produced by B. pumilus and B. subtilis greatly contribute to the enhancement of plant development. Phytohormones are organic substances that can promote, inhibit, or modify the growth and development of plants at low concentrations (Damam, Kaloori, Gaddam, & Kausar, 2016). Phytohormones promote root cell proliferation by overproducing lateral roots and root hairs with a concomitant increase in nutrient and water uptake (Sureshbabu, Amaresan, & Kumar, 2016). The results of the present study show that increased root and shoot dry matter and tiller number and diameter could be due to the phytohormones produced by these bacteria.
Mineral fertilization enhances plant development because the plants are nourished and rescued from a nutritional deficiency. However, approximately 40%–70% N, 80%–90% P, 50%–70% K of the total applied conventional fertilizer are lost to the environment due to variations in the soil dynamics (Fageria, 2014), and the use of B. pumilus and B. subtilis improved the efficiency of nutrient uptake by plants fertilized with mineral fertilizers, thereby promoting enhanced plant growth.
(Breedt, Labuschagne, & Coutinho, 2017) verified the use of B. pumilus to promote maize growth, and they recorded significant enhancement of plant growth and development and reported that the commercialization of these bacteria is a viable option. Moon, Asif, and Basharat (2017) reported that the use of B. pumilus increased root and shoot dry matter, as also reported in this study. Auxin produced by different Bacillus strains is associated with enhanced vegetative growth parameters of grasses.
A significant portion of sugarcane industry uses PSS for sugarcane cultivation. This procedure significantly reduces the seedling cane volume spend per hectare, increases the multiplication rate, improves seedling sanitation and planting uniformity, and the use of a smaller volume of material in the field with an increase in field operability (Landell et al., 2012). The positive effects of B. pumilus and B. subtilis in growth promotion and plant development in phase 1 indicate that both species could be used to reduce the time taken to produce PSS. Thus, the sugarcane industry can increase its profitability by increasing the number of seedlings formed while maintaining the same structure used to produce these PSS seedlings.
The negative effect of application of mineral fertilization + vinasse, which significantly reduced the number of tillers produced per plant, has been reversed by the application of a mixture of B. subtilis + B. pumilus. This result suggests a dual role for microbial inoculation; the simultaneous promotion of plant growth while decreasing the adverse effects of vinasse application. Certainly, the microbial mixture had a strong impact on the ability of the plants to cope with the stress caused by the vinasse. In the this study, the amount of vinasse applied to the soil followed the recommendation by Prado et al. (2013). The vinasse used in this study was analyzed and was found to contain a microbiota with a high number of microorganisms, a high K content, and no alcohol content (data not shown). Interestingly, it reduced the number of tillers produced per plant. So, further detailed studies are needed to understand the composition of the vinasse, and the causes of its adverse effect on the sugarcane seedlings.
The highest phosphorus content was found in soil which received a mixture of B. subtilis and B. pumilus combined with mineral fertilization + filter cake compost.
Phosphorus is the second most essential nutrient required by plants in adequate quantities for optimum growth. It plays an important role in almost all major metabolic processes, including energy transfer, signal transduction, respiration, macromolecular biosynthesis, and photosynthesis (Anand, Kumari, & Mallick, 2016). However, 95%–99% of phosphorus present in the soil is in insoluble, immobilized, or precipitated. Therefore, it is difficult for plants to absorb it directly. Solubilization and mineralization of phosphorus by phosphate solubilizing bacteria such as B. pumilus and B. subtilis has been reported by, Kaushal, Kumar, and Kaushal, (2017), and this study shows that it is an important state that can be achieved. Filter cake compost has been shown to be an effective type of fertilization that promotes phosphate (PO4) solubilization and increases P availability in the soil compared to other types of fertilization. Filter cake has been singled out for its great potential for agricultural use and has been utilized with good results as a substitute for PO4 mineral fertilizers in field crop production (Prado et al., 2013). Our results suggest that filter cake compost can be best employed when applied in conjunction with B. pumilus and B. subtilis microbial mixtures.
A decreased phosphorus content was recorded in the current study in sugarcane roots when both B. subtilis and a mixture of B. subtilis + B. pumilus were applied. This result may have occurred due to a negative feedback mechanism by high concentrations of indole acetic acid (IAA), because B. subtilis and B. pumilus are strong IAA producers, which is known to increase root area and biomass, facilitating microbial carbon uptake. Consequently, it increases the capacity of the roots to take up water and minerals as well as root development. Additionally, some studies have shown that many genes involved in phosphorus transport are downregulated by the presence of IAA in the roots.
Talboys, Owen, Healey, Withers, and Jones (2014) reported a low phosphorus content in the soil when B. amyloliquefaciens was used as an inoculant with mineral fertilization + filter cake compost, which supports the results of the current study, but this negative effect did not occur when other types of fertilization were used. It may be that the reduced phosphorus content in the roots was essential at the initial stage for positive bacterial behavior and their establishment in the roots. Further studies are needed to verify the effect and to strongly support the mechanism of action.
Biological nitrogen fixation is a process that accounts for approximately two‐thirds of the nitrogen fixed globally. This biological process is carried out either by symbiotic or nonsymbiotic bacteria, and it is mediated by nitrogenase Wu et al. 2005. Nitrogenase activity in the soil depends on ecological conditions in association with the specific nitrogen fixing capabilities of certain microorganisms and plant genotypes and various climatic conditions such as moisture, oxygen concentration, and the supply of organic C substrates (Shridhar, 2012). In the present study, the mineral fertilization + filter cake compost probably provided the best ecological conditions mainly because of the supply of organic C substrates to B. pumilus and B. subtilis, which allowed the expression of their potential to fix nitrogen in the roots, compared to other fertilization regimes.
Plant growth‐promoting bacteria are free‐living soil, rhizosphere, rhizoplane or phylosphere bacteria, and they are beneficial to plants under certain conditions. The participation of these bacteria in beneficial activities is associated with their enzymatic activity and their establishment in specific niches. Such favorable conditions are strongly influenced by the type of fertilization supplied. Some by‐products of the sugarcane industry are produced in high amounts, and attempts have been constantly made to use them for cane production, as a way to improve soil fertility, decrease the need for chemical fertilization, and avoid environmental pollution.
5. CONCLUSION
This study strongly suggests that the use of B. subtilis and B. pumilus improves the quality of cane by‐products, enhances soil fertility, and decreases the adverse effects of vinasse fertilization in addition to plant growth promotion, which is a strong evidence for the combination of microbes and cane by‐products to produce high yield and productivity in sugarcane production.
CONFLICT OF INTEREST
The authors declare to have no conflict of interest.
ACKNOWLEDGMENTS
The authors are grateful to CAPES for providing scholarships and Fapesp for financial support (Process Number 2014/18313‐8). Additionally, the authors thank the São Martinho group Padropolis Unit for its authorization to use their facility to conduct the first phase of this experiment.
Santos RM, Kandasamy S, Rigobelo EC. Sugarcane growth and nutrition levels are differentially affected by the application of PGPR and cane waste. MicrobiologyOpen. 2018;7:e617 10.1002/mbo3.617
REFERENCES
- Ali, S. , Charles, T. C. , & Glick, B. R. (2014). Amelioration of high salinity stress damage by plant growth‐promoting bacterial endophytes that contain ACC deaminase. Plant Physiology and Biochemistry, 80, 160–167. 10.1016/j.plaphy.2014.04.003 [DOI] [PubMed] [Google Scholar]
- Almeida Júnior, A. B. D. , do Nascimento, C. W. , Sobral, M. F. , da Silva, F. B. , & Gomes, W. A . (2011). Soil fertility and uptake of nutrients by sugarcane fertilized with filter cake. Revista Brasileira de Engenharia Agrícola e Ambiental, 15, 1004–1013. 10.1590/S1415-43662011001000003 [DOI] [Google Scholar]
- Anand, K. , Kumari, B. , & Mallick, M. (2016). Phosphate solubilizing microbes: An effective and alternative approach as biofertilizers. Journal of Pharmacy and Pharmaceutical Sciences, 8, 37–40. [Google Scholar]
- Araujo, F. F. , Henning, A. A. , & Hungria, M. (2005). Phytohormones and antibiotics produced by Bacillus subtilis and their effects on seed pathogenic fungi and on soybean root development. World Journal of Microbiology and Biotechnology, 21, 1639–1645. 10.1007/s11274-005-3621-x [DOI] [Google Scholar]
- Ariffin, H. , Hassan, M. A. , Shah, U. K. M. , Abdullah, N. , Ghazali, F. M. , & Shirai, Y. (2008). Production of bacterial endoglucanase from pretreated oil palm empty fruit bunch by bacillus pumilus EB3. Journal of Bioscience and Bioengineering, 106, 231–236. 10.1263/jbb.106.231 [DOI] [PubMed] [Google Scholar]
- Bajaj, B. K. , Khajuria, Y. P. , & Singh, V. P. (2012). Agricultural residues as potential substrates for production of xylanase from alkali‐thermotolerant bacterial isolate. Biocatalysis and Agricultural Biotechnology, 1, 314–320. 10.1016/j.bcab.2012.05.001 [DOI] [Google Scholar]
- Barbosa, J. , & Maldonado Júnior, W . (2010). AgroEstat: Sistema para análises estatísticas de ensaios agronômicos. Jaboticabal: Faculdade de Ciências Agrárias e Veterinárias, Unesp. [Google Scholar]
- Breedt, G. , Labuschagne, N. , & Coutinho, T. A. (2017). Seed treatment with selected plant growth‐promoting rhizobacteria increases maize yield in the field. Annals of Applied Biology, 171, 229–236. 10.1111/aab.12366 [DOI] [Google Scholar]
- Buchbinder, L. , Baris, Y. , & Goldstein, L. (1953). Further studies on new milk‐free media for the standard plate count of dairy products. American Journal of Public Health and the Nations Health, 43, 869–872. 10.2105/AJPH.43.7.869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva, B. , Teles, M. G. , & Júnior, G. G. (2016). Growth of eucalyptus seedlings irrigated with different vinasse concentrations. Australian Journal of Basic and Applied Sciences, 10, 115–121. [Google Scholar]
- Damam, M. , Kaloori, K. , Gaddam, B. , & Kausar, R. (2016). Plant growth promoting substances (phytohormones) produced by rhizobacterial strains isolated from the rhizosphere of medicinal plants. International Journal of Pharmaceutical Sciences Review and Research, 37, 130–136. [Google Scholar]
- De Abastecimento‐Conab, C. N . (2016). Acompanhamento da safra brasileira grãos. Acesso em 1.
- de Oliveira, A. L. M. , de Canuto, E. L. , Urquiaga, S. , Reis, V. M. , & Baldani, J. I. (2006). Yield of micropropagated sugarcane varieties in different soil types following inoculation with diazotrophic bacteria. Plant and Soil, 284, 23–32. 10.1007/s11104-006-0025-0 [DOI] [Google Scholar]
- Fageria, N. (2014). Yield and yield components and phosphorus use efficiency of lowland rice genotypes. Journal of Plant Nutrition, 37, 979–989. 10.1080/01904167.2014.888735 [DOI] [Google Scholar]
- Gazola, T. , Cipola Filho, M. L. , & Júnior, N. C. F. (2017). Avaliação de mudas pré‐brotadas de cana‐de‐açúcar provenientes de substratos submetidos a adubação química e orgânica. Científica, 45, 300–306. 10.15361/1984-5529.2017v45n3p300-306 [DOI] [Google Scholar]
- Guan, C. , Cui, W. , Cheng, J. , Zhou, L. , Guo, J. , Hu, X. , … Zhou, Z. (2015). Construction and development of an auto‐regulatory gene expression system in Bacillus subtilis . Microbial Cell Factories, 14, 150 10.1186/s12934-015-0341-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurav, R. , Tang, J. , & Jadhav, J. (2017). Novel chitinase producer Bacillus pumilus RST25 isolated from the shellfish processing industry revealed antifungal potential against phyto‐pathogens. International Biodeterioration & Biodegradation, 125, 228–234. 10.1016/j.ibiod.2017.09.015 [DOI] [Google Scholar]
- GutiérreziMañero, F. J. , Ramos‐Solano, B. , Probanza, A. , Mehouachi, J. , Tadeo, F. R. , & Talon, M. (2001). The plant growth promoting rhizobacteria Bacillus pumilus and Bacillus licheniformis produce high amounts of physiologically active gibberellins. Physiologia Plantarum, 111, 206–211. 10.1034/j.1399-3054.2001.1110211.x [DOI] [Google Scholar]
- Hanif, K. , Hameed, S. , Imran, A. , Naqqash, T. , Shahid, M. , & Van Elsas, J. D. (2015). Isolation and characterization of a β‐propeller gene containing phosphobacterium Bacillus subtilis strain KPS‐11 for growth promotion of potato (Solanum tuberosum L.). Frontiers in Microbiology, 6, 583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapilan, R. , & Arasaratnam, V. (2011). Paddy husk as support for solid state fermentation to produce xylanase from Bacillus pumilus . Rice Science, 18, 36–45. 10.1016/S1672-6308(11)60006-1 [DOI] [Google Scholar]
- Kapoor, M. , Nair, L. M. , & Kuhad, R. C. (2008). Cost‐effective xylanase production from free and immobilized Bacillus pumilus strain MK001 and its application in saccharification of Prosopis juliflora. Biochemical Engineering Journal, 38, 88–97. 10.1016/j.bej.2007.06.009 [DOI] [Google Scholar]
- Kaushal, M. , Kumar, A. , &. Kaushal, R. (2017). Bacillus pumilus strain YSPMK11 as plant growth promoter and bicontrol agent against Sclerotinia sclerotiorum. 3 Biotech 7, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney, D. R. , & Nelson, D. W . (1982). Nitrogen—Inorganic Forms 1. Methods of soil analysis. Part 2. Chemical and microbiological properties, 643‐698.
- Landell, M. D. A. , Campana, M. , Figueiredo, P. , Xavier, M. , Anjos, I. D. , Dinardo‐Miranda, L. , … Silva, D. D . (2012). Sistema de multiplicação de cana‐de‐açúcar com uso de mudas pré‐brotadas (MPB), oriundas de gemas individualizadas. Ribeirão Preto: Instituto Agronômico de Campinas. [Google Scholar]
- Lesueur, D. , Deaker, R. , Herrmann, L. , Bräu, L. , & Jansa, J . (2016). The production and potential of biofertilizers to improve crop yields In Arora N.K. et al. (Eds.), Bioformulations: for Sustainable Agriculture (pp. 71‐92). India: Springer; 10.1007/978-81-322-2779-3_4 [DOI] [Google Scholar]
- Moon, S. , Asif, R. , & Basharat, A. (2017). Phylogenetic diversity of drought tolerant Bacillus spp. and their growth stimulation of Zea mays L. under different water regimes. Research Journal of Biotechnology, 12, 38–46. [Google Scholar]
- Oslizlo, A. , Stefanic, P. , Vatovec, S. , Beigot Glaser, S. , Rupnik, M. , & Mandic‐Mulec, I. (2015). Exploring ComQXPA quorum‐sensing diversity and biocontrol potential of Bacillus spp. isolates from tomato rhizoplane. Microbial Biotechnology, 8, 527–540. 10.1111/1751-7915.12258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado, R. D. M. , Caione, G. , & Campos, C. N. S . (2013). Filter cake and vinasse as fertilizers contributing to conservation agriculture. Applied and Environmental Soil Science, 2013, 8. [Google Scholar]
- Probanza, A. , Garcıa, J. L. , Palomino, M. R. , Ramos, B. , & Mañero, F. G. (2002). Pinus pinea L. seedling growth and bacterial rhizosphere structure after inoculation with PGPR Bacillus (B. licheniformis CECT 5106 and B. pumilus CECT 5105). Applied Soil Ecology, 20, 75–84. 10.1016/S0929-1393(02)00007-0 [DOI] [Google Scholar]
- Puente, M. , Bashan, Y. , Li, C. , & Lebsky, V. (2004). Microbial populations and activities in the rhizoplane of rock‐weathering desert plants. I. Root colonization and weathering of igneous rocks. Plant Biology, 6, 629–642. 10.1055/s-2004-821100 [DOI] [PubMed] [Google Scholar]
- Rishad, K. , Rebello, S. , Shabanamol, P. , & Jisha, M. (2017). Biocontrol potential of halotolerant bacterial chitinase from high yielding novel Bacillus Pumilus MCB‐7 autochthonous to mangrove ecosystem. Pesticide Biochemistry and Physiology, 137, 36–41. 10.1016/j.pestbp.2016.09.005 [DOI] [PubMed] [Google Scholar]
- Sarruge, J. R. , & Haag, H. P . (1974). Análises químicas em plantas. Esalq Piracicaba.
- Schultz, N. , De Morais, R. F. , da Silva, J. A. , Baptista, R. B. , Oliveira, R. P. , Leite, J. M. , … Baldani, J. I. (2012).Avaliação agronômica de variedades de cana‑de‑açúcar inoculadas com bactérias diazotróficas e adubadas com nitrogênio. Pesquisa Agropecuária Brasileira, 47, 261–268. 10.1590/S0100-204X2012000200015 [DOI] [Google Scholar]
- Shridhar, B. S. (2012). RNitrogen fixing microorganisms. International Journal of Microbiology Research, 3, 46–52. [Google Scholar]
- Souza, G. , Nietsche, S. , Xavier, A. A. , Costa, M. R. , Pereira, M. C. T. , & Santos, M. A. (2016). Triple combinations with PGPB stimulate plant growth in micropropagated banana plantlets. Applied Soil Ecology, 103, 31–35. 10.1016/j.apsoil.2016.03.001 [DOI] [Google Scholar]
- Sureshbabu, K. , Amaresan, N. , & Kumar, K. (2016). Amazing multiple function properties of plant growth promoting rhizobacteria in the rhizosphere Soil. International Journal of Current Microbiology Applied Sciences, 5, 661–683. 10.20546/ijcmas [DOI] [Google Scholar]
- Talboys, P. J. , Owen, D. W. , Healey, J. R. , Withers, P. J. , & Jones, D. L. (2014). Auxin secretion by Bacillus amyloliquefaciens FZB42 both stimulates root exudation and limits phosphorus uptake in Triticum aestivum. BMC Plant Biology, 14, 51 10.1186/1471-2229-14-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco, M. J. , Gianello, C. , Bissani, C. A. , Bohnen, H. , & Volkweiss, S. J . (1995). Análises de solo, plantas e outros materiais. Porto Alegre: Ufrgs. [Google Scholar]
- Vieira, F. , & Nahas, E. (2005). Comparison of microbial numbers in soils by using various culture media and temperatures. Microbiological Research, 160, 197–202. 10.1016/j.micres.2005.01.004 [DOI] [PubMed] [Google Scholar]
- Watanabe, F. , & Olsen, S. (1965). Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from soil 1. Soil Science Society of America Journal, 29, 677–678. 10.2136/sssaj1965.03615995002900060025x [DOI] [Google Scholar]
- Wu, S. , Cao, Z. , Li, Z. , Cheung, K. , & Wong, M. (2005). Effects of biofertilizer containing N‐fixer, P and K solubilizers and AM fungi on maize growth: A greenhouse trial. Geoderma, 125, 155–166. 10.1016/j.geoderma.2004.07.003 [DOI] [Google Scholar]


