Abstract
Posttraumatic stress disorder (PTSD) and alcohol use disorders (AUD) are two of the most common mental health disorders affecting civilians as well as military populations. If left untreated, individuals with co-occurring PTSD/AUD are at increased risk for developing other mental health problems (e.g., depression, anxiety), physical health problems, reduced resiliency and military readiness, and vocational and social impairment. Substantial gaps in the treatment of co-occurring PTSD/AUD exist and there is a critical need to develop more effective pharmacological treatments. The current study addresses this gap in the literature by testing the efficacy and safety of doxazosin, a long-acting and selective alpha-1 adrenergic antagonist, as compared to placebo in reducing PTSD and AUD severity among U.S. military veterans. Noradrenergic dysregulation has been implicated in the development and maintenance of PTSD and AUD, and pilot studies examining doxazosin in PTSD-only or AUD-only samples have shown promise. This is the first study, however, to evaluate doxazosin in a comorbid PTSD/AUD sample. This paper describes the rationale, design and methodology of a randomized, double-blind, placebo-controlled trial of doxazosin (16 mg/day) delivered over 12 weeks among military veterans with current PTSD and AUD. In addition, functional magnetic resonance imaging (fMRI) is applied at pre- and post-treatment to investigate the underlying pathophysiology of comorbid PTSD/AUD and identify prognostic indicators of treatment outcome. This study is designed to accelerate research on co-occurring PTSD/AUD and provide empirical evidence to inform clinical practice.
Keywords: Posttraumatic stress disorder, PTSD, Alcohol, Substance use disorder, Military, Veterans
1. Introduction
Military veterans are at increased risk of developing of posttraumatic stress disorder (PTSD) and substance use disorders (SUD) [56]. In comparison to the general population, rates of PTSD are almost 5 times higher, and rates of SUD are approximately twice as high among veterans [29,65,67]. Furthermore, extensive literature documents the frequent co-occurrence of PTSD and SUD [63,69,70]. Initial reports among military personnel focused on Vietnam veterans with PTSD, in which up to 84% met lifetime criteria for an alcohol use disorder (AUD) [27]. More recently, data from the Department of Veterans Affairs indicate that, among veterans serving in the Vietnam era or later (N = 1,001,996), 41.4% with SUD meet criteria for current PTSD [43]. A series of associated problems are common among individuals with dually diagnosed PTSD and SUD, including medical problems, family dysfunction, homelessness, HIV risk behavior, and poor treatment outcomes [6,34,36,39,45,46].
Despite the frequency and severity of co-occurring PTSD and addiction, there are substantial gaps in treatment, particularly pharmacotherapeutic treatment. The studies conducted to date have examined a variety of medications (e.g., sertraline, topiramate, naltrexone, nacetylcysteine) with modest therapeutic effects observed and significant room for improvement [4,9,14,44,63]. The noradrenergic system has been implicated in PTSD, withdrawal states from chronic substance use, and in response to substance-related cues [21,25,31,61], suggesting that therapeutic interventions targeting the noradrenergic system may represent a promising avenue for the treatment of comorbid PTSD and SUD. Previous studies have examined the use of prazosin, an alpha-1 noradrenergic blocker approved by the U.S. Food and Drug Administration (FDA) for hypertension and benign prostatic hyperplasia, in the treatment of PTSD, AUD and comorbid AUD/PTSD. The findings have been mixed with some studies showing significant reduction in AUD symptoms [15,59,60] and reduction in PTSD symptoms, particularly nightmares, sleep disruption and daytime hyperarousal symptoms [18,50,52], while other studies find no significant differences in prazosin vs. placebo for AUD or PTSD symptoms [41,49]. Several studies suggest that pre-treatment blood pressure may represent a biomarker to help identify who will respond favorably to prazosin, or potentially other alpha-1 blockers [48,74].
Doxazosin is another alpha-1 noradrenergic antagonist that is approved by the FDA for the treatment of hypertension and benign prostatic hyperplasia. Doxazosin and prazosin have the same chemical structure, with the central element being a piperazine ring. However, there are several advantages of doxazosin in comparison to prazosin [28]. For example, doxazosin has a significantly longer half-life of approximately 22 h (vs. 2–3 h half-life of prazosin). The longer half-life allows for a once-per-day dosing, rather than twice or thrice daily dosing, which is generally preferred by patients and promotes medication adherence [73]. The slower onset of action also reduces the risk of first-dose postural hypotension as compared to prazosin [17,30] and doxazosin has no significant effect on blood pressure among normotensive patients, which further reduces the risk of hypotensive side effects [26]. Unlike other alpha-1 blockers, doxazosin can be taken at any time during the day, with or without food, which further promotes medication adherence [30].
Two small studies provide initial support for doxazosin’s safety and therapeutic effects on PTSD. In a 12-week, open-label pilot trial (N = 12 civilians and veterans) of doxazosin (8 mg/day) in individuals with PTSD, De Jong et al. [13] found significant pre-to post-treatment reductions (baseline = 77 vs. end of treatment = 53) on the Clinician Administered PTSD Scale (CAPS; [72]). Depression severity, as measured by the Montogmery-Asberg Depression Scale [77] also significantly decreased from baseline to end of treatment (score at baseline = 25 vs. end of treatment = 19). More recently, a small randomized controlled study (N = 8 male veterans) demonstrated that doxazosin (16 mg/day) was effective in significantly reducing self-report PTSD symptoms as measured by the PTSD Checklist – Military Version (PCL-5; [7,71]) and a trend for reduction on the CAPS hyperarousal scale was observed [54,55]. However, both studies are limited by small samples sizes and require replication with larger samples.
Several early trials have also examined doxazosin in individuals with SUD. In a 10-week, randomized controlled trial of 41 individuals with AUD, Kenna et al. [28] found that doxazosin (16 mg/day) was associated with significantly lower reductions in drinks per week, heavy drinking days, and craving among individuals with high family history density of alcoholism. In a secondary analysis of the same data set (N = 41), Haass-Koffler et al. [20] examined the role of pre-treatment standing blood pressure (BP) as a moderator of doxazosin’s effect on AUD severity and found that patients with high pre-treatment BP had the most favorable AUD outcomes. Other clinical studies in patients with cocaine use disorder [58] as well as preclinical studies [37] suggest that doxazosin may help reduce SUD severity.
The current study is the first study to evaluate doxazosin among individuals with co-occurring PTSD/AUD. This paper describes the design and methodology of an ongoing randomized controlled trial (RCT) to evaluate doxazosin in reducing PTSD and AUD severity among a sample of military veterans. While the primary focus of the study is the medication trial, we are also employing functional magnetic resonance imaging (fMRI) at pre- and post-treatment to further investigate the underlying pathophysiology of PTSD/AUD and identify potential prognostic indicators of treatment outcome.
1.1. Research objectives and hypotheses
This project is one of 11 nationwide research projects supported by the Consortium to Alleviate PTSD (CAP), which is part of a National Research Action Plan jointly issued in 2013 by the U.S. Department of Defense, Department of Veterans Affairs (VA), Department of Health and Human Services, and Department of Education. The overall aims of the CAP are: (1) develop and evaluate effective treatments for PTSD and comorbid conditions, such as alcohol and drug use disorders, in military service members and post-9/11 veterans; and (2) identify the biological causes of PTSD and changes in those biomarkers that are associated with treatment outcomes. More information about the CAP is available at www.ConsortiumToAlleviatePTSD.org.
The primary research objective of the current study is to address the gap in the evidence base regarding pharmacologic treatment of co-occurring PTSD and AUD by comparing doxazosin (16 mg/day) versus placebo in reducing PTSD symptomatology and alcohol use severity in veterans with co-occurring PTSD/AUD. There are three main hypotheses that pertain to changes in outcomes of interest during the treatment phase (weeks 1–12). Hypothesis 1 proposes that participants who receive doxazosin, as compared to placebo, will evidence significantly greater reductions in PTSD severity at week 12, as measured by the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) ([72]a) and the PTSD Checklist for DSM-5 (PCL-5) ([72]b). Hypothesis 2 is that participants who receive doxazosin, as compared to placebo, will evidence significantly greater reductions in AUD severity at week 12, as measured by the Timeline Follow Back (TLFB; [62]) and biological tests of alcohol consumption (e.g., breathalyzer, ethyl glucuronide). Hypothesis 3 centers on the neuroimaging component of the project and predicts that (a) connectivity between the prefrontal cortex and amygdala at rest and in response to trauma versus neutral cues will predict amount of change in PTSD symptoms as measured by the CAPS-5 at week 12 [72]; and (b) connectivity between prefrontal cortex and the amygdala at rest and in response to alcohol versus neutral cues will predict time to first use of alcohol (TLFB) during the treatment phase.
2. Materials and methods
2.1. Research design
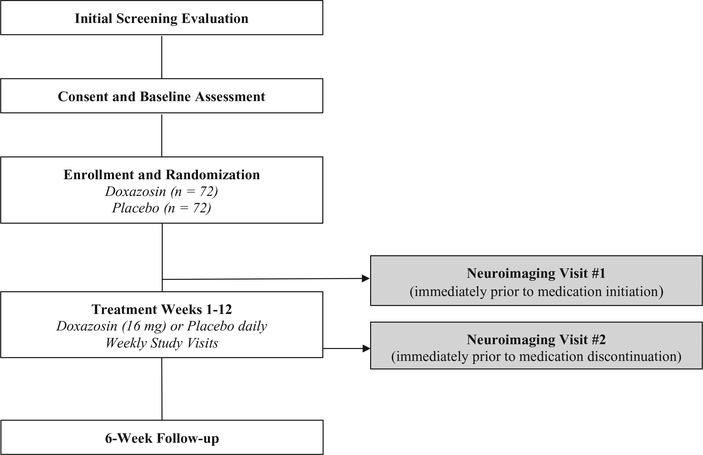
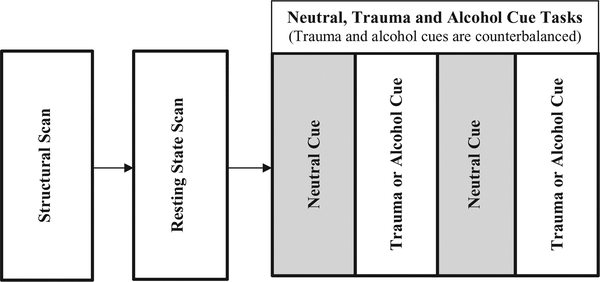
This study is a phase II, double-blind, 12-week RCT to evaluate the efficacy of the alpha-1 adrenergic antagonist doxazosin (16 mg/day, immediate release formulation), as compared to placebo in reducing PTSD and AUD severity in veterans. A follow-up visit is included at 6-weeks post treatment. If eligible and interested, participants may also complete a neuroimaging scan before and after the treatment phase. The project will last for approximately four years and is illustrated in Fig. 1 (clinical trial) and Fig. 2 (neuroimaging visit).
Fig. 1.
Study design overview.
Fig. 2.
Neuroimaging procedures design overview.
2.2. Participants
Participants (N = 144) are U.S. military veterans, ages 18–65, with current PTSD and AUD. Inclusion criteria include: a veteran of the U.S. military (any branch), male or female, any race or ethnicity, able to comprehend English, meet DSM-5 criteria for current (i.e., last 6 months) alcohol use disorder as assessed by the Mini International Neuropsychiatric Interview (MINI; [57], meet DSM-5 criteria for current (i.e., last month) PTSD as assessed by the CAPS-5 (Weathers et al., 2013), and enrolled for services at the Ralph H. Johnson VA Medical Center or affiliated community-based outpatient clinic. Subjects taking psychotropic medications are required to be maintained on a stable dose for at least 4 weeks before study medication initiation. Exclusion criteria include the following: previous treatment with doxazosin; women who are pregnant, nursing or not practicing an effective form of birth control; individuals with a history of adverse reactions to quinazolines or other alpha-1-antagonists; individuals currently taking alpha blockers (e.g., prazosin) or any other medication that may have a hazardous interaction if taken with doxazosin; and individuals with clinically significant medical or psychiatric conditions that in the opinion of the investigators may adversely affect safety or study participation. Individuals presenting with significant withdrawal symptoms as evidenced by a score on the revised Clinician Institute Withdrawal Assessment of Alcohol (CIWA-Ar) [66] of ≥10 will be referred for medically supervised detoxification and may be re-assessed for study eligibility after detoxification. Individuals taking medications thought to influence alcohol consumption or craving (e.g., naltrexone) will be excluded. MRI exclusions include claustrophobia; cardiac pacemaker; metal fragments in eye, skin, or body; heart valve replacement; brain clips; venous umbrella; history of aneurysm surgery; intracranial bypass, renal, or aortic clips; joint replacements; non-removable hearing aid, neurostimulator or insulin pump; shunts/stents; metal mesh/coil implants; metal plate/pin/screws/wires; or any other metal implants.
2.3. Procedures
This study was reviewed and approved by the Institutional Review Boards (IRB) of the Medical University of South Carolina (MUSC), the Ralph H. Johnson VA Medical Center, and the University of Texas Health Science Center at San Antonio. Potential participants are given a full description of the study and asked to read and sign IRB-approved informed consent forms before any study procedures take place. All participants are enrolled in the VA and have the option to receive weekly cognitive-behavioral therapy to ensure that all participants receive adequate psychosocial support and monitoring, regardless of medication arm. Interested individuals are screened for eligibility by telephone or in person. Individuals who meet inclusion/exclusion criteria are invited to come into the office for a comprehensive baseline assessment (see Table 1 for measures). Ineligible individuals are referred clinically for treatment.
Table 1.
Core Assessment Measures.
| Measurements | Timeline (WEEK) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −1 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 18 | |
| Demographic, medical and diagnosic assessments | |||||||||||||||
| Demographic form | X | ||||||||||||||
| MINI diagnostic interview | X | ||||||||||||||
| History & physical examination | X | ||||||||||||||
| Substance-related assessments | |||||||||||||||
| Timeline follow back (TLFB) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Obsessive compulsive drinking scale (OCDS) | X | X | X | X | |||||||||||
| Alcohol use disorders identification test (AUDIT) | X | ||||||||||||||
| Clinician institute withdrawal assessment of alcohol – revised (CIWA-Ar) | X | ||||||||||||||
| Ethyl glucuronide | X | X | X | X | X | X | |||||||||
| Visual analog scales | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| Breathalyzer | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Urine drug screen | X | X | X | X | X | X | |||||||||
| Fagerstrom test for nicotine dependence | X | X | X | ||||||||||||
| Addiction Severity index – family history | X | ||||||||||||||
| Quick drinking screen | X | X | X | X | |||||||||||
| Brief addiction monitor (BAM) | X | X | X | X | |||||||||||
| Trauma and PTSD assessments | |||||||||||||||
| Clinician administered PTSD Scale for DSM-5 (CAPS-5) | X | X | X | X | |||||||||||
| PTSD checklist for DSM-5 (PCL-5) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Life events checklist (LEC) | X | X | X | X | |||||||||||
| Childhood trauma questionnaire (CTQ) | X | ||||||||||||||
| Deployment risk & resiliency inventory-2 (DRRI) | X | ||||||||||||||
| Revised conflicts tactics scale (CTS2) | X | X | X | X | X | ||||||||||
| Posttraumatic cognitions inventory (PTCI) | X | X | X | X | X | X | X | X | |||||||
| Frequency of nightmares questionnaire | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| Health & safety monitoring | |||||||||||||||
| Adverse events | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Vital signs | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Concomitant medications form | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Patient health questionnaire-9 (PHQ-9) | X | X | X | X | X | X | X | X | |||||||
| Pregnancy test (Female subjects) | X | X | X | ||||||||||||
| Veterans RAND 12 item health survey (VR-12) | X | X | X | X | |||||||||||
| History of head injuries | X | X | X | X | |||||||||||
| Treatment adherence | |||||||||||||||
| Riboflavin test | X | X | X | X | |||||||||||
| Medication adherence log | X | X | X | X | X | X | X | X | X | X | X | X | |||
| Other measures | |||||||||||||||
| Neuroimaging procedures | X | X | |||||||||||||
| Self-injurious thoughts and behaviors interview (SITBI) | X | X | X | X | |||||||||||
| Insomnia severity index (ISI) | X | X | X | X | X | X | X | X | |||||||
| Difficulties in emotion regulation scale (DERS) | X | X | X | X | |||||||||||
Note. Baseline occurs at Week −1. Medication initiation occurs at Week 0. Follow-up assessment occurs at Week 18.
2.4. Study medication
Participants are randomly assigned in a 1:1 manner to receive doxazosin (16 mg/day) or placebo. The dose was selected based on previous research [32,54,55]. Study medication and matching placebo are encapsulated in standard-sized gelatin capsules and are of sufficient opacity and color so as not to reveal contents inside or disclose any possible difference between placebo and active capsules. Active study medication capsules consist of USP-grade doxazosin and 25 mg riboflavin. All study capsules contain dextrose as the inert filling agent and are brought to proper packing level in color-matched, opaque, identically sized capsules. Participants who wish to take a multivitamin during the treatment phase of the study are given a multivitamin (TriVi-Sol) that does not contain riboflavin. Treatment assignment follows a prearranged randomization scheme and is carried out by a research pharmacist not involved in clinical management of participants in order to preserve the double-blind design. Doxazosin is initiated at 1 mg/day and then titrated up as follows: 2 mg at Week 2; 4 mg at Week 3; 8 mg at Week 4; and then 16 mg during Weeks 5–12. A downward titration for safety occurs at the end of Week 12 [28] and participants are titrated down to 8 mg on Day 1; 6 mg on Day 2; 4 mg on Day 3; 2 mg on Day 4; and 1 mg on Day 5.
2.5. Primary outcome measures
Participants complete the CAP Common Data Elements battery, which includes demographic and military history information, personality, deployment stress, trauma, psychiatric symptoms, traumatic brain injury, substance use, functional impairment, sources of support, sleep impairment, pain, relationship functioning, and treatment credibility expectancy [3]. The CAP Assessment Core has carefully reviewed and selected these measures to ensure that only measures with the highest levels of validity and reliability are included as part of the CAP Common Data Elements battery.
The primary outcome measures for this study are: (1) the CAPS-5 [72], (2) the PCL-5 [71], and (3) the TLFB [62]. The CAPS-5 is a semistructured interview used to assess PTSD diagnosis and symptom severity. The CAPS-5 is rated on a 5-point scale (0 = absent to 4 = extreme/incapacitating) with a total score ranging from 0 to 80. In this study, the CAPS-5 is administered by independent evaluators (IE) trained and certified by the CAP Assessment Core and blind to treatment status. The PCL-5 is a 20-item, self-report measure that assesses PTSD severity using a Likert scale (0 = not at all to 4 = extremely). Finally, the TLFB obtains retrospective self-report of substance use by using a calendar and memory prompts to stimulate recall. Quantity and frequency assessments are made using this instrument (e.g., total number of standard drink units, percent of days using) as well as abstinence (yes/no). TLFB yields consistently high test-retest correlations and correlates well with other self-reports and collateral reports. The TLFB assesses frequency and amount of substance use for 60 days prior to study entry, during the 12 weeks of treatment, and at follow-up.
2.6. Secondary outcome measures
Secondary outcomes related to alcohol craving and characterization of AUD are measured by using the Obsessive Compulsive Drinking Scale (OCDS; [2]), Brief Addiction Monitor (BAM; [10]), ethyl glucuronide (a conjugated alcohol metabolite biomarker of recent alcohol consumption), and family history of addiction using the Addiction Severity Index family history section [35]. Symptoms of depression, suicidality, psychosocial functioning, and emotional regulation will be assessed using the well-validated instruments shown in Table 1. Related aspects of PTSD severity such as sleep disturbance, frequency of nightmares, and insomnia will also be regularly monitored (see Table 1).
2.7. Neuroimaging component
In addition to testing the safety and efficacy of doxazosin, this study will investigate neural circuitry underlying comorbid PTSD/AUD. This information may help inform the selection of treatment targets and novel therapeutic agents in future research. To that end, neuroimaging data is acquired immediately prior to medication initiation and medication discontinuation on all eligible participants. As illustrated in Fig. 2, following the resting state scans, participants are exposed to neutral, trauma, and alcohol cues. To minimize potential carry-over effects, the trauma and alcohol cues are counterbalanced. For both resting state and task runs, T2*-weighted gradient-echo planar images (EPI) are acquired with the following parameters: TR = 1100 ms, TE = 30 ms, flip angle = 65°, ipat factor = 3, matrix 64 × 64, field of view = 19.2 cm, slice thickness = 3.0 mm with no gap, with 48 slices to cover the entire brain. All scans are conducted at the MUSC Center for Biomedical Imaging, which houses a Siemens 3 T Prisma MRI scanner (Siemens Medical, Erlangen, Germany).
2.8. Data analytic plan
2.8.1. General considerations and power
Analyses will be performed on the intent-to-treat (ITT) sample consisting of all randomized participants who provide any outcome data. Baseline clinical and demographic characteristics are collected, and contrasts will be performed between treatment groups. This study is powered to estimate the effects of doxazosin on significant reduction in PTSD symptoms at the end of treatment (see Hypothesis 1), and increased abstinence rates at the end of treatment (Hypothesis 2). Placebo rates of reduction in CAPS scores are conservatively estimated to be 20% [76]. In a small pilot study, De Jong et al. [13] found ~31% reduction in CAPS total scores using doxazosin. A sample of 100 participants will provide 80% power with a 0.05 type 1 error rate to detect a difference in the reduction of CAPS scores between doxazosin (31 ± 20%) and placebo (20 ± 20%). Placebo rates of abstinence are estimated from established double-blind, placebo-controlled trials. Continuous abstinence from weeks 9–12 is observed in 10–15% of treatment seekers without co-occurring PTSD [58] so we anticipate an attenuated rate of 5–10% in the placebo group. Although there is limited information on the effectiveness of doxazosin in AUD, a sample of 100 participants (50 in each arm) will provide adequate power to detect an abstinence rate as low as 32% in the doxazosin group when the placebo cessation rate is 10%, and as low as 23% when the placebo rate is 5%. Due to the nature of the study population, we anticipate ~30% dropout rate; thus, the number of subjects randomized is inflated to 144 with 72 participants randomized to each treatment arm of the study.
2.8.2. Clinical outcomes
To test hypothesis 1 that participants who receive doxazosin, as compared to placebo, will evidence significantly greater reductions in PTSD severity, restricted maximum likelihood (REML) methods will be used to estimate the fixed effects and variance components, and modelbased treatment effect estimates will be used to construct group level tests over time. To test hypothesis 2 that participants who receive doxazosin, as compared to placebo, will evidence significantly greater reductions in AUD severity, logistic regression analysis will be used to examine rates of abstinence. Weekly point prevalence abstinence will be examined using generalized linear models under a generalized estimating equations (GEE) framework. Weekly abstinence (yes/no) as well as amounts of alcohol use (standard drink units) will be examined over time to determine if treatment is effective in reducing alcohol consumption when abstinence is not achieved.
2.8.3. Neuroimaging outcomes
We will measure functional connectivity using a psychophysiological interaction (PPI) seed-based approach [16]. PPI is a method for investigating task-specific changes in the relationship between activity in different brain areas [38]. Following acquisition, preprocessing of the imaging data will correct for geometric distortion, head motion or other artifacts. A mask of the two seed regions will be made using a 12-mm diameter sphere located in the center of the left and right amygdala using the Montreal Neurological Institute coordinates (x, y, z = ± 22, 0, −22). For each participant, the mean corrected and high pass temporal filtered time series of the blood-oxygen-level dependent (BOLD) signal in the amygdala will be extracted using ‘fslmeants’ and used in a single subject whole brain PPI analysis. Statistical analysis will be performed at the individual-subject level using FEAT (FMRI Expert Analysis Tool). The interactions of interest are the trauma (v. neutral) × (left or right) amygdala time series and the alcohol (v. neutral) × (left or right) amygdala. A group-level analysis will use a mixed effects approach, with separate group analyses for left and right amygdala. For resting state fMRI analysis, a seed-based approach will also be used with the same amygdala seed regions used in the PPI analysis. Group level analyses and extraction of parameter estimates from prefrontal cortex (PFC) regions will be the same as for the PPI analysis.
To test hypotheses 3A and 3B that connectivity between the PFC and the left or right amygdala in response to cues at baseline will predict change in PTSD and AUD severity due to treatment, separate linear regression tests will be used. Changes in PTSD and AUD severity will be regressed against the parameter estimate obtained from the voxel with the maximum Z-score from each PFC cluster that exhibited a significant association with the amygdala time series at rest, and a significant task × seed interaction with the amygdala in response to the trauma cue (Hypothesis 3A) and alcohol cue (Hypothesis 3B).
3. Discussion
This paper presents the research design and methodology for an ongoing RCT that is part of the national Consortium to Alleviate PTSD and is the first to investigate doxazosin in the treatment of comorbid PTSD/AUD. PTSD and AUD are chronic, debilitating and common psychiatric conditions observed in civilian and military populations. The co-occurrence of PTSD and AUD is also highly prevalent, and military veterans incur a uniquely high risk of developing this complex dual diagnosis [19,47].
While mental health services are in place for U.S. military personnel and veterans, there are substantial gaps in the evidence base to guide the pharmacological treatment of co-occurring PTSD/AUD. Recent findings regarding several medications to treat AUD are encouraging [5,11,33]. However, the only FDA-approved medications to treat substance use problems target relapse prevention. While many medications have been investigated to treat PTSD, only selective serotonin reuptake inhibitors (SSRI) have received FDA approval. Just 20–30% of patients achieve PTSD remission with SSRI treatment [8,12,24,64]. Few investigations have examined pharmacotherapies for co-occurring PTSD/ SUD, and those that have been conducted suggest only a modest response [4,9,14,42,63]. Therefore, identifying effective medications to treat co-occurring PTSD and SUD, particularly among military veterans, is a national public health priority.
One potential medication for the treatment of PTSD and co-occurring SUD is the alpha-1 adrenergic antagonist prazosin. Several RCTs with varying sample sizes of veterans and civilians demonstrate safety, tolerability and efficacy of prazosin to reduce nightmares, sleep impairment, and overall PTSD symptom severity [1,18,51–53,59,68], as well as AUD severity [59,60]. However, the largest RCT to date examining prazosin in the treatment of PTSD (N = 304) [49] resulted in null findings. Veterans in this study were recruited from 13 VA hospitals across the nation. Participants completed a 16-week treatment phase following a 10-week maintenance phase in which participants were stabilized on their current new medications and continued to participate in ongoing psychotherapy, but no new interventions were added during the study apart from prazosin or placebo. The lengthy stabilization phase in this trial is a substantial distinguishing factor between this and previous smaller prazosin trials. The authors hypothesize that the null findings might be attributable, in part, to an a priori inclusion criterion requiring participants to demonstrate psychiatric stability prior to the treatment phase. It is necessary for future studies to explore the possibility that some Veterans might benefit from initiating prazosin earlier in their course of treatment or sooner after PTSD onset. In the [49] study, approximately one third of participants with similar distributions between medication conditions received individual or group psychotherapy (n = 43; 28%) and the majority of participants were prescribed antidepressants (95% and 89% in the prazosin vs. placebo group, respectively). Thus, an additional consideration posed by the authors is that prazosin is less efficacious in the context of an established course of evidence-supported medications and/or psychotherapy to treat PTSD.
Because hyperactivity of the noradrenergic system is also identified as a neurobiological mechanism underlying AUD [21,31,40], the findings from the collective prazosin literature lend support to the notion that alpha-1 adrenergic antagonists may also be effective in the treatment of co-occurring PTSD and AUD. One limitation of prazosin is its relatively short half-life, which commonly results in challenges to medication adherence in order to obtain a therapeutic dose. Some literature has also demonstrated substantial and persistent geographic differences in the effective prescribing of prazosin [22,23].
In order to address these gaps in the literature and add to the ongoing investigation of the clinical utility and efficacy of alpha-1 blockers in the treatment of PTSD and SUD, the current study will examine the ability of doxazosin versus placebo in reducing co-occurring PTSD and AUD symptom severity among U.S. military veterans. Previous clinical studies, described in more detail in the Introduction section, support the ability of doxazosin to reduce PTSD symptoms [13,54,55], although they are based on small sample sizes. A proof-ofconcept study found preliminary support for the ability of doxazosin to reduce alcohol consumption among individuals with AUD and high (versus low) family history density of alcoholism [28]. This effect was also moderated by higher pretreatment BP such that participants with higher pretreatment BP yielded greater reduction in alcohol consumption during treatment [20]. Both family history and BP are being measured in the current study. Future research in this area should include measurements of pre-treatment BP, family history of AUD, as well as genetic data [75].
The current study is designed and adequately powered to examine the effects of treatment group (doxazosin vs. placebo) on PTSD and AUD severity at the end of treatment. The findings may inform future investigations of doxazosin and its potential translation to treatment settings. Adverse events are monitored, recorded, and reviewed by the investigative team, study prescribers, and safety monitor at regular intervals. Other important considerations being examined include medication adherence and the effects of medication on secondary outcomes such as depression, sleep, and anxiety. Notably, participants in the current study are permitted to maintain or initiate behavioral intervention as needed in order to maximize generalizability of study findings. Concurrent treatment engagement is monitored closely by the study team at weekly visits. The neuroimaging component of this study will allow us to identify possible neurobiological mechanisms underlying treatment response (or nonresponse). Considering the significant heterogeneity in the etiology and course of PTSD and AUD, as well as the complexity of individual treatment needs, the findings from this study might allow us to maximize treatment outcomes and more effectively identify and treat individuals with this complex and detrimental dual diagnosis. Another important aspect of this study’s design is the rigorous coordination of our assessment procedures under the guidance of the Consortium to Alleviate PTSD (CAP). Joining our study’s findings with those of other ongoing CAP projects not only ensures reliable and valid assessment of primary and secondary outcomes; it also improves the ability of this study to contribute to the larger literature examining PTSD and SUD comorbidity among military veterans.
Acknowledgments
Funding
This research was supported by Consortium to Alleviate PTSD award numbers W81XWH-13-2-0065 from the U.S. Department of Defense, Defense Health Program, Psychological Health and Traumatic Brain Injury Research Program (PH/TBI RP), and I01CX001136-01 from the U.S. Department of Veterans Affairs, Office of Research & Development, Clinical Science Research & Development Service. Additional support was provided from the National Institute on Drug Abuse (K02 DA039229) and the National Institute on Alcohol Abuse and Alcoholism (K23 AA023845).
Role of the funding source
The funding sources had no involvement in the study design, the collection, analysis and interpretation of data, the writing of this manuscript, or the decision to submit this manuscript for publication.
Abbreviations:
- AMY
amygdala
- AUD
alcohol use disorder
- BAM
Brief Addiction Monitor
- BP
blood pressure
- CAP
Consortium to Alleviate PTSD
- CAPS-5
Clinician-Administered PTSD Scale for DSM-5
- CIWA
ArRevised Clinician Institute Withdrawal Assessment of Alcohol
- DSM-5
Diagnostic and Statistical Manual of Mental Disorders Fifth Edition
- EPI
gradient-echo planar images
- FDA
U.S. Food and Drug Administration
- fMRI
functional magnetic resonance imaging
- HIV
human immunodeficiency virus
- IRB
Institutional Review Board
- MINI
Mini International Neuropsychiatric Interview
- MPRAGE
magnetization-prepared rapid gradientecho
- MRI
magnetic resonance imaging
- MUSC
Medical University of South Carolina
- OCDS
Obsessive Compulsive Drinking Scale
- PCL-5
PTSD Checklist for DSM-5
- PFC
prefrontal cortex
- PPI
psychophysiological interaction
- PTSD
posttraumatic stress disorder
- RCT
randomized controlled trial
- SSRI
selective serotonin reuptake inhibitors
- TLFB
Timeline Follow Back
- USP
grade, meets or exceeds requirements of the United States Pharmacopeia
- VA
U.S. Department of Veterans Affairs
- U.S.
United States
Footnotes
Disclaimer
The views expressed herein are solely those of the authors and do not reflect an endorsement by or the official policy or position of the Ralph H. Johnson VA, the U.S. Army Office of the Surgeon General, the Department of the Army, the Department of the Air Force, the Department of Defense, the Department of Veterans Affairs, or the U.S. Government.
References
- [1].Ahmadpanah M, Sabzeiee P, Hosseini SM, Torabian S, Haghighi M, Jahangard L, ... Brand S, Comparing the effect of prazosin and hydroxyzine on sleep quality in patients suffering from posttraumatic stress disorder, Neuropsychobiology 69 (4) (2014) 235–242. [DOI] [PubMed] [Google Scholar]
- [2].Anton RF, Moak DH, Latham P, The Obsessive Compulsive Drinking Scale: a self-rated instrument for the quantification of thoughts about alcohol and drinking behavior, Alcohol. Clin. Exp. Res 19 (1) (1995) 92–99. [DOI] [PubMed] [Google Scholar]
- [3].Barnes JB, Presseau C, Jordan AH, Kline NK, S, Y. M., Peterson AL,…, and The Consortium to Alleviate PTSD. (under review). Common data elements for military-related PTSD research applied in the consortium to alleviate PTSD. [DOI] [PubMed] [Google Scholar]
- [4].Batki SL, Pennington DL, Lasher B, Neylan TC, Metzler T, Waldrop AE, ... Herbst E, Topiramate treatment of alcohol use disorder in veterans with posttraumatic stress disorder: a randomized controlled pilot trial, Alcohol. Clin. Exp. Res 38 (8) (2014) 2169–2177, https://doi.org/10.1111/acer.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Berglund M, Thelander S, Salaspuro M, Franck J, Andréasson S, Öjehagen A, Treatment of Alcohol Abuse: an Evidence-based Review, Alcohol. Clin. Exp. Res 27 (10) (2003) 1645–1656. [DOI] [PubMed] [Google Scholar]
- [6].Blanco C, Xu Y, Brady KT, Pérez-Fuentes G, Okuda M, Wang S, Comorbidity of posttraumatic stress disorder with alcohol dependence among US adults: results from National epidemiological survey on alcohol and related conditions, Drug Alcohol Depend. 132 (3) (2013) 630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bovin MJ, Marx BP, Weathers FW, Gallagher MW, Rodriguez P, Schnurr PP, Keane TM, Psychometric properties of the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders–Fifth Edition (PCL-5) in veterans, Psychol. Assess. 28 (2016) 1379–1391, https://doi.org/10.1037/pas0000254. [DOI] [PubMed] [Google Scholar]
- [8].Brady KT, Pearlstein T, Asnis GM, Baker DG, Rothbaum BO, Sikes CR, Farfel GM, Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial, J. Am. Med. Assoc 283 (14) (2000) 1837–1844. [DOI] [PubMed] [Google Scholar]
- [9].Brady KT, Sonne SC, Anton RF, Randall CL, Back SE, Simpson K, Sertraline in the treatment of co-occurring alcohol dependence and posttraumatic stress disorder, Alcoholism 29 (3) (2005) 395–401. [DOI] [PubMed] [Google Scholar]
- [10].Cacciola JS, Alterman AI, Dephilippis D, Drapkin ML, Valadez C, Fala NC, ... McKay JR, Development and initial evaluation of the Brief Addiction Monitor (BAM), J. Subst. Abus. Treat 44 (3) (2013) 256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chick J, Lehert P, Landron F, Does acamprosate improve reduction of drinking as well as aiding abstinence? J. Psychopharmacol 17 (4) (2003) 397–402. [DOI] [PubMed] [Google Scholar]
- [12].Davidson JT, Rothbaum BO, van der Kolk BA, Sikes CR, Farfel GM, Multicenter, double-blind comparison of sertraline and placebo in the treatment of posttraumatic stress disorder, Arch. Gen. Psychiatry 58 (5) (2001) 485–492. [DOI] [PubMed] [Google Scholar]
- [13].De Jong J, Wauben P, Huijbrechts I, Oolders H, Haffmans J, Doxazosin treatment for posttraumatic stress disorder, J. Clin. Psychopharmacol 30 (1) (2010) 84–85. [DOI] [PubMed] [Google Scholar]
- [14].Foa EB, Yusko DA, McLean CP, Suvak MK, Bux DA, Oslin D, ... Volpicelli J, Concurrent naltrexone and prolonged exposure therapy for patients with comorbid alcohol dependence and PTSD: a randomized clinical trial, J. Am. Med. Assoc 310 (5) (2013) 488–495. [DOI] [PubMed] [Google Scholar]
- [15].Fox HC, Anderson GM, Tuit K, Hansen J, Kimmerling A, Siedlarz KM, ... Sinha R, Prazosin effects on stress-and cue-induced craving and stress response in alcohol-dependent individuals: preliminary findings, Alcohol. Clin. Exp. Res 36 (2) (2012) 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ, Psychophysiological and modulatory interactions in neuroimaging, NeuroImage 6 (3) (1997) 218–229, https://doi.org/10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- [17].Fulton B, Wagstaff AJ, Sorkin EM, Doxazosin, Drugs 49 (2) (1995) 295–320. [DOI] [PubMed] [Google Scholar]
- [18].Germain A, Richardson R, Moul DE, Placebo-controlled comparison of prazosin and cognitive-behavioral treatments for sleep disturbances in US military veterans, J. Psychosom. Res 72 (2012) 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, ... Huang B, Epidemiology of DSM-5 alcohol use disorder: results from the national epidemiologic survey on alcohol and related conditions III, JAMA psychiatry 72 (8) (2015) 757–766, https://doi.org/10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Haass-Koffler CL, Goodyear K, Zywiak WH, Magill M, Eltinge SE, Wallace PM, Kenna GA, Higher pretreatment blood pressure is associated with greater alcohol drinking reduction in alcohol-dependent individuals treated with doxazosin, Drug Alcohol Dependence 177 (2017) 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Haass-Koffler CL, Swift RM, Leggio L, Noradrenergic targets for the treatment of alcohol use disorder, Psychopharmacology (2018) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Harpaz-Rotem I, Rosenheck RA, Tracing the flow of knowledge: geographic variability in the diffusion of prazosin use for the treatment of posttraumatic stress disorder nationally in the Department of Veterans Affairs, Arch. Gen. Psychiatry 66 (4) (2009) 417–421. [DOI] [PubMed] [Google Scholar]
- [23].Hermes E, Harpaz-Rotem I, Rosenheck R, Diffusion of prazosin treatment for PTSD, Am. J. Psychiatr 171 (1) (2014) 117. [DOI] [PubMed] [Google Scholar]
- [24].Ipser JC, Stein DJ, Evidence-based pharmacotherapy of post-traumatic stress disorder (PTSD), Int. J. Neuropsychopharmacol 15 (6) (2012) 825–840. [DOI] [PubMed] [Google Scholar]
- [25].Kalk NJ, Nutt DJ, Lingford-Hughes AR, The role of central noradrenergic dysregulation in anxiety disorders: evidence from clinical studies, J. Psychopharmacol 25 (1) (2011) 3–16. [DOI] [PubMed] [Google Scholar]
- [26].Kaplan SA, Soldo KA, Olsson CA, Terazosin and doxazosin in normotensive men with symptomatic prostatism: a pilot study to determine the effect of dosing regimen on efficacy and safety, Eur. Urol 28 (1995) 223–228. [DOI] [PubMed] [Google Scholar]
- [27].Keane TM, Kaloupek DG, Kolb LC, VA Cooperative Study# 334: Summary of findings on the psychophysiological assessment of PTSD, PTSD Res. Quarterly 9 (1998) 1–6. [Google Scholar]
- [28].Kenna GA, Haass-Koffler CL, Zywiak WH, Edwards SM, Brickley MB, Swift RM, Leggio L, Role of the α1 blocker doxazosin in alcoholism: a proof-ofconcept randomized controlled trial, Addict. Biol 21 (4) (2016) 904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kessler RC, Demler O, Frank RG, Olfson M, Pincus HA, Walters EE, ... Zaslavsky AM, Prevalence and treatment of mental disorders, 1990 to 2003, N. Engl. J. Med 352 (24) (2005) 2515–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kirby RS, Chapple CR, Sethia K, Flannigan M, Milroy EJG, Abrams P, Morning vs evening dosing with doxazosin in benign prostatic hyperplasia: efficacy and safety, Prostate Cancer Prostatic Dis. 1 (3) (1998) 163. [DOI] [PubMed] [Google Scholar]
- [31].Lanteri C, Salomon L, Torrens Y, Glowinski J, Tassin JP, Drugs of abuse specifically sensitize noradrenergic and serotonergic neurons via a non-dopaminergic mechanism, Neuropsychopharmacology 33 (2008) 1724–1734. [DOI] [PubMed] [Google Scholar]
- [32].Le AN, Haass-Koffler CL, Zywiak WH, Brickley MB, Edwards SM, Swift RM, ... Leggio L, Doxazosin for Alcohol Dependence: a pilot Double-blind Placebocontrolled Trial, Alcoholism 39 (2015) (78A). [Google Scholar]
- [33].Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW, Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction 108 (2) (2013) 275–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Marx BP, Brailey K, Proctor SP, MacDonald HZ, Graefe AC, Amoroso P, ... Vasterline JJ, Association of time since deployment, combat intensity, and posttraumatic stress symptoms with neuropsychological outcomes following Iraq war deployment, Arch. Gen. Psychiatry 66 (2009) 996–1004. [DOI] [PubMed] [Google Scholar]
- [35].McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, ... Argeriou M, The fifth edition of the Addiction Severity Index, J. Subst. Abus. Treat 9 (3) (1992) 199–213. [DOI] [PubMed] [Google Scholar]
- [36].Monson CM, Taft CT, Fredman SJ, Military-related PTSD and intimate relationships: from description to theory-driven research and intervention development, Clin. Psychol. Rev 29 (8) (2009) 707–714, https://doi.org/10.1016/j.cpr.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].O’Neil ML, Beckwith LE, Kincaid CL, Rasmussen DD, The α1-Adrenergic Receptor Antagonist, Doxazosin, Reduces Alcohol Drinking in Alcohol-Preferring (P) Rats, Alcohol. Clin. Exp. Res 37 (2) (2013) 202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].O’Reilly JX, Woolrich MW, Behrens TE, Smith SM, Johansen-Berg H, Tools of the trade: psychophysiological interactions and functional connectivity, Soc. Cogn. Affect. Neurosci 7 (5) (2012) 604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ouimette PC, Moos RH, Finney JW, PTSD treatment and 5-year remission among patients with substance use and posttraumatic stress disorders, J. Consult. Clin. Psychol 71 (2) (2003) 410. [DOI] [PubMed] [Google Scholar]
- [40].Patkar AA, Gopalakrishnan R, Naik PC, Murray HW, Vergare MJ, Marsden CA, Changes in plasma noradrenaline and serotonin levels and craving during alcohol withdrawal, Alcohol 38 (2003) 224–231. [DOI] [PubMed] [Google Scholar]
- [41].Petrakis IL, Desai N, Gueorguieva R, Arias AJ, O’Brien E, Jane JS, ... Ralevski E, Prazosin for veterans with Posttraumatic stress disorder and comorbid alcohol dependence: a clinical trial, Alcohol. Clin. Exp. Res 40 (1) (2016) 178–186. [DOI] [PubMed] [Google Scholar]
- [42].Petrakis IL, Ralevski E, Desai N, Trevisan L, Gueorguieva R, Rounsaville B, Krystal JH, Noradrenergic vs serotonergic antidepressant with or without naltrexone for veterans with PTSD and comorbid alcohol dependence, Neuropsychopharmacology 37 (4) (2012) 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Petrakis IL, Rosenheck R, Desai R, Substance Use Comorbidity among Veterans with Posttraumatic stress Disorder and Other Psychiatric Illness, Am. J. Addict 20 (3) (2011) 185–189, https://doi.org/10.1111/j.1521-0391.2011.00126.x. [DOI] [PubMed] [Google Scholar]
- [44].Petrakis IL, Simpson TL, Posttraumatic stress disorder and alcohol use disorder: a critical review of pharmacologic treatments, Alcohol. Clin. Exp. Res 41 (2) (2017) 226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pietrzak RH, Goldstein MB, Malley JC, Johnson DC, Southwick SM, Subsyndromal posttraumatic stress disorder is associated with health and psychosocial difficulties in veterans of Operations Enduring Freedom and Iraqi Freedom, Depression Anxiety 26 (8) (2009) 739–744, https://doi.org/10.1002/da.20574. [DOI] [PubMed] [Google Scholar]
- [46].Pietrzak RH, Goldstein RB, Southwick SM, Grant BF, Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions, J. Anxiety Disord 25 (3) (2011) 456–465, https://doi.org/10.1016/j.janxdis.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Pietrzak RH, Goldstein RB, Southwick SM, Grant BF, Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions, J. Anxiety Disord 25 (3) (2011) 456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Raskind MA, Millard SP, Petrie EC, Peterson K, Williams T, Hoff DJ, ... Daniels C, Higher pretreatment blood pressure is associated with greater posttraumatic stress disorder symptom reduction in soldiers treated with prazosin, Biol. Psychiatry 80 (10) (2016) 736–742. [DOI] [PubMed] [Google Scholar]
- [49].Raskind MA, Peskind ER, Chow B, Harris C, Davis-Karim A, Holmes HA, ... Reist C, Trial of prazosin for post-traumatic stress disorder in military veterans, N. Engl. J. Med 378 (6) (2018) 507–517. [DOI] [PubMed] [Google Scholar]
- [50].Raskind MA, Peskind ER, Hoff DJ, Hart KL, Holmes HA, Warren D, ... Gross C, A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder, Biol. Psychiatry 61 (8) (2007) 928–934. [DOI] [PubMed] [Google Scholar]
- [51].Raskind MA, Peskind ER, Hoff DJ, Hart KL, Holmes HA, Warren D, ... Gross CA, A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder, Biol. Psychiatry 61 (8) (2007) 928–934. [DOI] [PubMed] [Google Scholar]
- [52].Raskind MA, Peskind ER, Kanter ED, Petrie EC, Radant A, Thompson CE, ... Straits-Tröster K, Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study, Am. J. Psychiatr 160 (2) (2003) 371–373. [DOI] [PubMed] [Google Scholar]
- [53].Raskind MA, Peterson K, Williams T, Hoff DJ, Hart KL, Holmes HA, ... Calohan J, A trial of prazosin for combat trauma PTSD with nightmares in activeduty soldiers returned from Iraq and Afghanistan, Am. J. Psychiatr 170 (9) (2013) 1003–1010. [DOI] [PubMed] [Google Scholar]
- [54].Rodgman C, Verrico CD, Holst M, Thompson-Lake D, Haile CN, De La Garza R, ... Newton TF, Doxazosin XL reduces symptoms of posttraumatic stress disorder in veterans with PTSD: a pilot clinical trial, The Journal of clinical psychiatry 77 (5) (2016) e561–e565. [DOI] [PubMed] [Google Scholar]
- [55].Rodgman C, Verrico CD, Holst M, Thompson-Lake D, Haile CN, Raskind MA, Newton TF, Doxazosin XL reduces symptoms of posttraumatic stress disorder in veterans with PTSD: a pilot clinical trial, The Journal of clinical psychiatry 77 (5) (2016) e561–e565. [DOI] [PubMed] [Google Scholar]
- [56].Seal KH, Bertenthal D, Miner CR, Sen S, Marmar C, Bringing the war back home: Mental health disorders among 103 788 US veterans returning from Iraq and Afghanistan seen at Department of Veterans Affairs Facilities, Arch. Intern. Med 167 (5) (2007) 476–482. [DOI] [PubMed] [Google Scholar]
- [57].Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, ... Dunbar GC, The mini-international neuropsychiatric interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSMIV and ICD-10, J. Clin. Psychiatry 59 (1998) 22–33. [PubMed] [Google Scholar]
- [58].Shorter D, Lindsay JA, Kosten TR, The alpha-1 adrenergic antagonist doxazosin for treatment of cocaine dependence: a pilot study, Drug Alcohol Dependence 131 (1) (2013) 66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Simpson TL, Malte CA, Dietel B, Tell D, Pocock I, Lyons R, ... A.J. Saxon, A pilot trial of prazosin, an alpha-1 adrenergic antagonist, for comorbid alcohol dependence and posttraumatic stress disorder, Alcohol. Clin. Exp. Res 39 (5) (2015) 808–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Simpson TL, Saxon AJ, Meredith CW, Malte CA, McBride B, Ferguson LC, ... Raskind MA, A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence, Alcohol. Clin. Exp. Res 33 (2) (2009) 255–263. [DOI] [PubMed] [Google Scholar]
- [61].Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM, Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals, Neuropsychopharmacology 34 (5) (2009) 1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sobell LC, Sobell MB, Litten RZ, Allen JP, Timeline follow-back: A technique for assessing self-reported alcohol consumption, Humana Press, Totowa, NJ, 1992. [Google Scholar]
- [63].Sofuoglu M, Rosenheck R, Petrakis IL, Pharmacological treatment of comorbid PTSD and substance use disorder: recent progress, Addict. Behav 39 (2) (2014) 428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Stein DJ, Ipser J, McAnda N, Pharmacotherapy of posttraumatic stress disorder: a review of meta-analyses and treatment guidelines, CNS Spectrums 14 (1) (2009) 25–31. [PubMed] [Google Scholar]
- [65].Substance Abuse, Mental Health Services Administration, Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings, (2013) (HHS Publication No (SMA) 13–4795. (Retrieved from Rockville, MD).
- [66].Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM, Assessment of Alcohol Withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar), Br. J. Addict 84 (11) (1989) 1353–1357, https://doi.org/10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- [67].Tanielian T, Haycox LH, Schell TL, Marshall GN, Burnam MA, Eibner C, ... Vaiana ME, Invisible Wounds of War. Summary and Recommendations for Addressing Psychological and Cognitive Injuries, (2008) (Retrieved from). [Google Scholar]
- [68].Taylor FB, Martin P, Thompson CE, Williams J, Mellman TA, Gross CA, ... Raskind MA, Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study, Biol. Psychiatry 63 (6) (2008) 629–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Torchalla I, Nosen L, Rostam H, Allen P, Integrated treatment programs for individuals with concurrent substance use disorders and trauma experiences: a systematic review and meta-analysis, J. Subst. Abus. Treat 42 (1) (2012) 65–77, https://doi.org/10.1016/j.jsat.2011.09.001. [DOI] [PubMed] [Google Scholar]
- [70].van Dam D, Vedel E, Ehring T, Emmelkamp P, Psychological treatments for concurrent posttraumatic stress disorder and substance use disorder: a systematic review, Clin. Psychol. Rev 32 (3) (2012) 202–214. [DOI] [PubMed] [Google Scholar]
- [71].Weathers FW, The PTSD checklist for DSM-5 (PCL-5): development and initial psychometric analysis, Paper Presented at the the 29th annual Meeting of the International Society for Traumatic Stress Studies Philadelphia, 2013. (PA). [Google Scholar]
- [72].Weathers FW, Blake DD, Schnurr PP, Clinician-Administered PTSD Scale for DSM-5 (CAPS-5), US Department of Veterans Affairs. PTSD: National Center for PTSD, 2013. [Google Scholar]
- [73].Weiss RD, Adherence to pharmacotherapy in patients with alcohol and opioid dependence, Addiction 99 (2004) 1382–1392. [DOI] [PubMed] [Google Scholar]
- [74].Wilcox CE, Tonigan JS, Bogenschutz MP, Clifford J, Bigelow R, Simpson TL, A randomized, placebo-controlled, clinical trial of prazosin for the treatment of alcohol use disorder, J. Addict. Med (2018), https://doi.org/10.1097/ADM.0000000000000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zhang X, Nielsen DA, Domingo CB, Shorter DI, Nielsen EM, Kosten TR, Pharmacogenetics of dopamine B-hydroxylase in cocaine depenence therapy with doxazosin, Addict. Biol (2018), https://doi.org/10.1111/adb.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Zohar J, Amital D, Miodownik C, Kotler M, Bleich A, Lane RM, Austin C, Double-blind placebo-controlled pilot study of sertraline in military veterans with posttraumatic stress disorder, J. Clin. Psychopharmacol 22 (2) (2002) 190–195. [DOI] [PubMed] [Google Scholar]
- [77].Montgomery SA, Asberg M, A new depression scale designed to be sensitive to change, Br. J. Psychiatry 134 (1979) 382–389. [DOI] [PubMed] [Google Scholar]