Abstract
Background:
Necrotizing enterocolitis (NEC) in premature infants is often a devastating surgical condition with poor outcomes. GYY4137 is a long-acting donor of hydrogen sulfide (H2S), a gasotransmitter that is protective against intestinal injury in experimental NEC, likely through protection against injury secondary to ischemia. We hypothesized that administration of GYY4137 would improve mesenteric perfusion, reduce intestinal injury, and reduce inflammatory responses in experimental NEC and ischemia-reperfusion injury, and that these benefits would be mediated through endothelial nitric oxide synthase-dependent pathways.
Methods:
NEC was induced in C57BL/6 wild type (WT) and endothelial nitric oxide synthase (eNOS) knockout (eNOSKO) pups via maternal separation, formula feeding, enteral lipopolysaccharide, and intermittent hypoxic and hypothermic stress. Pups received daily intraperitoneal injections of 50mg/kg GYY4137 or PBS vehicle. In separate groups, adult male WT and eNOSKO mice underwent superior mesenteric artery occlusion for 60 minutes. Prior to abdominal closure, 50mg/kg GYY4137 or PBS vehicle was administered into the peritoneal cavity. Laser Doppler Imaging was used to assess mesenteric perfusion of pups at baseline and on P9, and the adult mice at baseline and 24 hours post-ischemic insult. After euthanasia, the terminal ileum of each animal was fixed, paraffin embedded, sectioned, and stained with H&E. Sections were blindly graded using published injury scores. Intestinal tissue was homogenized and cytokines measured by ELISA. Data were compared using Mann-Whitney, and p-values <0.05 were significant.
Results:
After NEC and I/R injury, GYY4137 improved perfusion in WT mice compared to vehicle, but this effect was lost in the eNOSKO animals. Histologic injury followed a similar pattern with reduced intestinal injury in WT mice treated with GYY4137, and no significant improvement in the eNOSKO group. Cytokine expression after GYY4137 administration was altered by the ablation of eNOS in both NEC and I/R injury groups, with significant differences noted in IL-6 and VEGF.
Conclusion:
GYY4137, a long-acting donor of H2S, has potential as a therapeutic compound for NEC. It improves mesenteric perfusion and intestinal injury in experimental NEC and intestinal I/R injury, and these benefits appear to be mediated through eNOS-dependent pathways.
Keywords: animal model, necrotizing enterocolitis, hydrogen sulfide, ischemia-reperfusion, intestine
INTRODUCTION:
Necrotizing enterocolitis (NEC) remains a morbid condition seen in premature infants. Few therapeutic options are currently available, and patients often require extensive surgical resection of diseased bowel [1]. Its cause is multifactorial, but ischemia and intestinal necrosis are felt to be a common final pathway. Mortality rates are estimated to be as high as 40% in these patients [2], and therefore, novel therapies are desperately needed.
A growing body of evidence points to hydrogen sulfide (H2S) as a compound with potential benefit during ischemia. Both in vivo and in vitro studies have demonstrated that tissue injury, especially secondary to ischemia, can be rescued by H2S [3-6]. Most experimental animal models of NEC involve some sort of intermittent systemic hypoxia, coupled with formula feeding and enteral inoculation with bacterial antigens [7-9]. While it is clear that ischemic insult alone does not result in NEC, further investigation into the salvage effects of H2S can be bolstered by evaluation of treatment of intestinal ischemia reperfusion (I/R) injury, a much more exact and reproducible model. Previous work has shown that sodium hydrosulfide (NaHS), a H2S salt, is protective against intestinal I/R injury in adult mice [10], and similar benefits have been demonstrated in experimental NEC with other hydrogen sulfide donors [11].
In an intestinal I/R model, the benefit conferred by the NaHS was shown to be dependent on endothelial nitric oxide [10]. Endothelial cells constitutively express the enzyme endothelial nitric oxide synthase (eNOS), which produces the gasotransmitter nitric oxide (NO) [12]. This molecule acts as a vasodilator, and is especially important in the perinatal period [12, 13]. In our previous work, animals that were homozygous knockouts for eNOS did not demonstrate the same improvement after injury as wild type mice did with NaHS therapy [10].
NaHS and its counterpart sodium sulfide (Na2S) are both salts at room temperature and are short acting and highly volatile. They are not stable in aqueous solution at all, and their H2S production decreases quickly over time as soon as they are in solution. Additionally, they are malodorous and therefore may be less desirable as treatments for future clinical work. The synthetic options include AP39 [10-oxo-10-(4-(3-thioxo-3H-1,2-dithiol-5yl)phenoxy)decyl) triphenylphosphonium bromide] and GYY4137 (morpholin-4-ium 4-methoxyphenyl(morpholino) phosphinodithioate). AP39 is thought to directly target the mitochondria, and at while it is commercially available at this time, some labs synthesize it independently, and its molecular weight varies based on its source. Therefore it is likely not as reliable as the long-established GYY4137. GYY4137 was chosen for use because it is a synthetic, long-acting H2S donor that provides stable gas concentrations in aqueous solution over 24 hours, and is much more predictable than the salt-type donors [14]. GYY4137 is well-characterized, stable, has a longstanding history and has potential for clinical use for the treatment of necrotizing enterocolitis.
Before widespread clinical use, it is important to elucidate the mechanisms by which GYY4137 protects the intestine. In addition to histologic injury, NEC frequently causes alterations in inflammatory response and cytokine cascade in intestinal tissue [15]. Interleukin 6 (IL-6) has both inflammatory and anti-inflammatory properties, is modulated by NO in some tissues, and may protect intestine from injury [16, 17]. Interleukin 10 (IL-10) is a counterregulatory cytokine that inhibits propagation of inflammation, but is typically elevated in NEC models [18]. Interferon gamma-induced protein 10 (IP-10, also known as CXCL10) is a chemokine that acts as a chemoattractant for immune cells during times of injury [10, 19]. VEGF is an endothelium-specific angiogenic factor, released in endothelial cell injury [20]. These signaling molecules and many others are altered in intestinal injury and recovery and are important to evaluate with any potential treatment.
We hypothesized that GYY4137 as a hydrogen sulfide donor protects the intestine in experimental NEC through an eNOS-dependent pathway. Further, we hypothesized that this benefit is due to protection against injury specifically related to intermittent I/R injury, similar to our adult model.
MATERIALS AND METHODS
Animal Use
Indiana University Institutional Animal Care and Use Committee approved the experimental protocols and animal use. Male adult wild-type (WT) (C57BL/6J, Stock No: 00664, 8-12 weeks/20-30g, Jackson Labs, Bar Harbor, ME) and eNOS knockout (eNOSKO) mice (B6.129P20Nos3tm1Unc/J, Stock No: 002684, 8-12 weeks/20-30g; Jackson Laboratory, Bar Harbor, ME) used in this study underwent 48 hours of acclimation after arrival prior to participating in experimentation. Normal chow and water were provided to the animals.
WT and eNOSKO mouse pups were bred in house from these animals and same-strain females. Experimental pups were separated from their mothers while controls remained with mothers to breastfeed. Experimental pups were kept in approved satellite housing for the duration of the experiment in a neonatal incubator with humidity 40% and temperature 32°C.
Experimental NEC Model:
A previously validated model of experimental NEC was utilized [8, 11, 21]. Briefly, experimental groups, both WT and eNOSKO (n=10), were permanently separated from their mother on postnatal day 5 (P5) through the end of the protocol on postnatal day 9 (P9). Both control groups (n=10) remained with their mother and breastfed ad libitum. Experimental groups were gavage fed with a 2 French catheter three times daily with 300 kcal/kg/day of hyperosmolar formula with 8 mg/kg lipopolysaccharide (lipopolysaccharides from Escherichia coli O111:B4, Sigma-Aldrich Company LLC, Dorset, UK). Formula was prepared using 4g of Esbilac canine supplement and 6g Similac with 20 mL of nanopure-filtered water (Barnstead Nanopure, APS Water Services Inc., Van Nuys, CA). Prior to each feeding, pups were placed in a chamber with 5% O2 and 95% N2 for 10 minutes. After the morning and evening feed, pups were placed in the 4°C refrigerator for 10 minutes.
Each morning, experimental groups received intraperitoneal injections of either phosphate buffered saline (PBS) vehicle or a 50 mg/kg of GYY4137 in PBS. Total volume of each injection was 10µL. Control groups did not receive any injections. The GYY4137 dose was based on our previous work and other animal models of ischemia and reperfusion injury [22]. Pups were excluded if they died within 24 hours of the beginning of the study because death was likely secondary to a technical error of gavage feeding rather than NEC itself. If they died later than 24 hours and were identified immediately, they were still included in analysis for all data points including tissue evaluation.
Clinical Assessment:
Control groups were assessed daily, while experimental groups were assessed with each feed. Pups were assessed in a systematic fashion with a clinical assessment score to ensure consistency [8]. This is performed in a semi-blinded fashion as the observer is not aware of the pup’s treatment before scoring the pup, but then must find out the group before recording the data. The reported clinical assessment score was the pup’s last score prior to death or euthanasia.
Ischemia-Reperfusion (I/R) Injury Model
The I/R model was carried out on separate adult mice as previously described [10, 23, 24]. Adult mice (n=6 per group) were anesthetized with 3% isoflurane and maintained under anesthesia with 1.5% isoflurane in oxygen. Hair was removed and the abdomen was prepped in a sterile fashion. Subcutaneous 0.9% saline (1mL) was injected pre-operatively to compensate for fluid losses. Analgesia was provided pre-operatively with 1mg/kg buprenorphine and 5mg/kg carprofen via subcutaneous injection.
A midline laparotomy was performed and the intestines were eviscerated. The base of the mesentery was temporarily occluded with an atraumatic vascular clamp. The intestines were replaced and the abdomen was temporarily closed with silk suture to prevent fluid losses. After 60 minutes, the abdomen was reopened and the microvascular clamp was removed. The abdomen was then closed with a two-layer technique with silk suture. Prior to abdominal closure, animals received intraperitoneal administration of PBS (vehicle control), or 50 mg/kg GYY4137 in PBS. Total volume of each injection was 250µL. Animals were awakened from anesthesia, allowed to recover, and returned to animal housing.
Perfusion Analysis:
For NEC pups, intestinal perfusion was analyzed transcutaneously with a Laser Doppler Perfusion Imager (LDI; Moor Instruments, Wilmington, DE) as previously described [11]. This was performed on P5 and P9, and perfusion was expressed as a percentage of baseline perfusion on P5. For the adult mice, perfusion images were acquired during laparotomy at baseline, at initial clamping (to ensure successful occlusion), and after 24 hours of recovery [10]. Perfusion was expressed as a percentage of baseline.
Histological Injury Score:
Terminal ileum was resected following euthanasia of experimental groups and fixed for 24 hours in 4% paraformaldehyde at 4°C. Tissue was subsequently dehydrated with 70% ethanol, paraffin-embedded, sectioned, and stained with hematoxylin and eosin. Histologic scoring was performed by two blinded observers using previously described methods [10, 11].
For pup tissue, scores ranged from 0 to 4 [11]. 0 = normal intestine; 1 = some disarrangement of villus enterocytes, villus-core separation; 2 = significant disarrangement of villus enterocytes, villus-core separation down sides of villi, blunting of villi; 3 = epithelial sloughing of villi, loss of villi; 4= necrosis. A score of 2 or higher indicated presence of NEC, with 3 or higher indicating severe NEC.
The adult mouse tissue was scored using a well established adult scoring system as follows [10]: 0 = no damage; 1 = subepithelial space at the villous tip; 2 = loss of mucosal lining at the villous tip; 3 = loss of less than half of the villous structure; 4 = loss of more than half of the villous structure.
Intestinal Cytokine Analysis:
Terminal ileum was acquired and processed for proteins as previously described [10, 11, 24, 25]. Tissue was snap frozen in liquid nitrogen and stored at −80°C. Tissue was thawed and homogenized with the Bullet Blender (Next Advance, Averill Park, NY) in RIPA buffer (Sigma, St. Louis, MO) with 1:100 dilutions of both phosphatase and protease inhibitors (Sigma, St. Louis, MO). After homogenization, samples were centrifuged at 12,000 rpm and supernatants were collected for further analysis. Total protein was quantified with the Bradford Assay using a spectrophotometer (VersaMax microplate reader, Molecular Devices, Sunnyvale, CA).
Murine IL-6, IL-10, IP-10, and VEGF were measured using ELISA (R&D Systems, Bio-Techne Corporation, Minneapolis, MN) for the NEC pup samples and a Bio-Plex multiplex beaded assay system (Bio-Rad, Hercules, CA) for the adult mouse tissue. All ELISAs and multiplex assays were repeated. ELISAs were performed at 1:20 dilution and multiplex assays at 1:25 dilution. To account for variations in individual plates and assays, cytokines were normalized to their respective vehicle controls for both WT and eNOSKO intestinal samples.
Statistical Analysis:
Ordinal data was reported using median and interquartile range. Continuous variables were reported as mean ± standard error of the mean (SEM). All non-parametric data was compared using the Mann-Whitney U test. GraphPad Prism 7 (GraphPad Software, La Jolla, CA) was used for all statistical analysis and figures. P values less than 0.05 were considered statistically significant.
RESULTS
GYY4137 reduces clinical severity of experimental NEC through an endothelial nitric oxide-dependent mechanism.
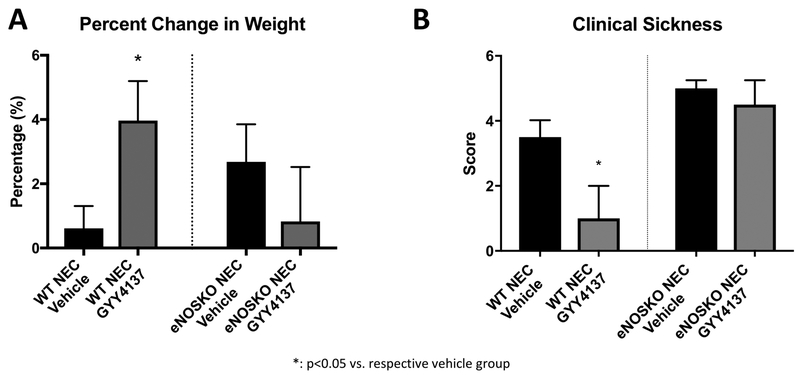
In the WT pups, those treated with GYY4137 gained significantly more weight (4.0% ± 1.2) over the protocol than those in the vehicle group (0.6% ± 0.7, p=0.0280), while in the eNOSKO groups there was no difference between those treated with GYY4137 (0.8% ± 1.7) and vehicle (2.7 ± 1.17, p=0.3657). Clinical sickness scores demonstrated a similar result. The WT group treated with GYY4137 had a median score of 1 (95% CI= 0-2), while the vehicle group had a median score of 3 (2.75-5.25, p=0.0002). In the eNOSKO mice, there was no difference between the group treated with GYY4137 (4.5, 3-5.25) and vehicle (5, 3-5.25, p=0.8259, Figure 1).
Figure 1: GYY4137 improves weight gain and clinical status in WT animals, but not in eNOSKO animals.
A) The WT NEC group experienced significantly higher percent weight gain than its vehicle control, while this effect was not noted in eNOSKO mice. B) The clinical sickness score was improved in the WT NEC group treated with GYY4137, but this effect was not seen with ablation of eNOS (*: p<0.05 vs. vehicle group).
GYY4137 improves intestinal perfusion and reduces histologic injury through an endothelial oxide-dependent mechanism in experimental NEC.
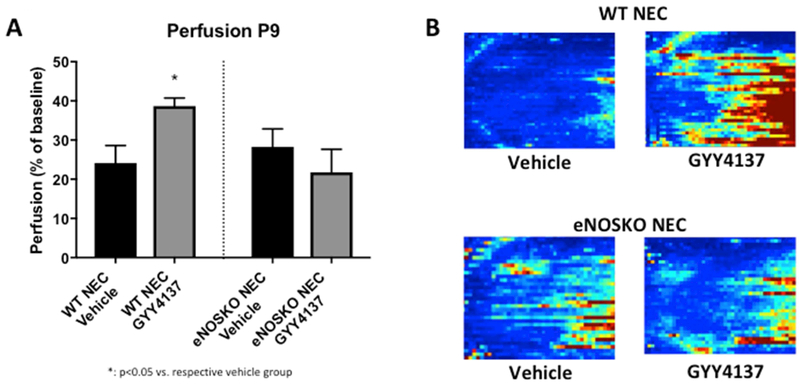
WT pups treated with GYY4137 had a mean perfusion of 38.69% ± 2.02 compared to 24.16% ± 4.45 in the vehicle group (p=0.0079). This same improvement was not noted in the eNOSKO mice, as similar perfusion in the GYY4137-treated (21.77% ± 5.89) and vehicle (28.26% ± 4.60) groups (p=0.6409) were noted (Figure 2).
Figure 2: Intestinal perfusion was improved after treatment with GYY4137 in the WT animals, but this effect was not seen in the eNOSKO group.
A) Perfusion on P9, expressed as a percentage of baseline perfusion (P5) was improved in the WT group treated with GYY4137, but there was no difference in the eNOS groups (*: p<0.05 vs. vehicle group). B) Representative images obtained with the LDI – the left side of each image is the pelvis and the right is the diaphragm. Red indicates areas of better perfusion and blue indicates poor perfusion.
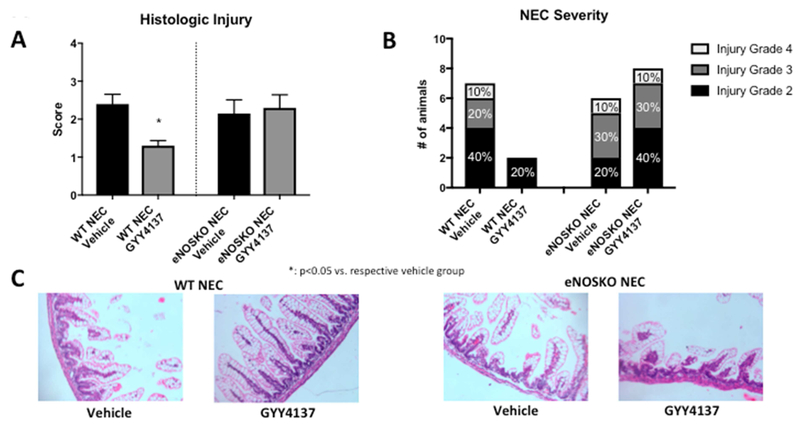
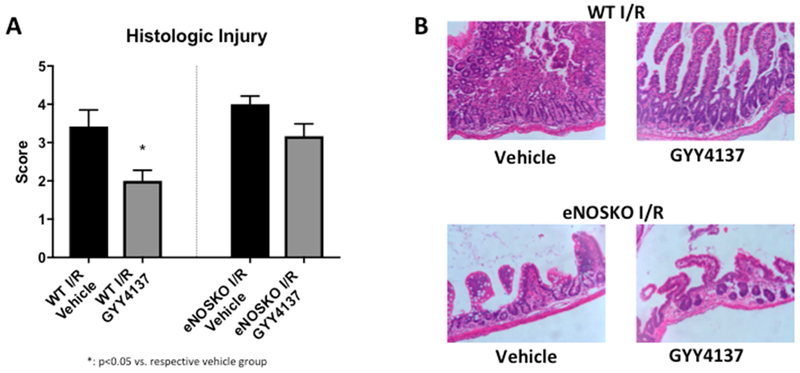
A complementary pattern was noted in histologic injury scores, with a significant improvement in the WT pups after treatment with GYY4137 (1, IQR 1-1.625) compared to vehicle (2.5, IQR 1.5-3.0, p=0.0011). The eNOSKO group did not have the same results, with both the vehicle and GYY4137 groups having median scores of 2.25 (IQR vehicle: 1.0-3.0, GYY4137: 1.9-3.0, p=0.7494). In terms of NEC severity, GYY4137 decreased the proportion of severe NEC from 30% to 0% in the WT group, but had no change on the 40% rate of severe NEC in the eNOSKO group (Figure 3).
Figure 3: Intestinal injury is less severe in WT NEC animals treated with GYY4137, but there is no change with GYY4137 treatment in the eNOSKO animals.
A) Histologic injury scores are improved in the WT NEC group treated with GYY 4137 (*: p<0.05 vs. vehicle group). B) The proportion of severe NEC is improved in the GYY4137-treated WT animals from 30% to 0%, while there is no change in the eNOSKO animals. C) Representative histology images demonstrating sloughing villi and destructed architecture in the vehicle groups and the eNOSKO group treated with GYY4137, with relatively normal villous structure in the GYY4137-treated WT group only.
GYY4137 improves intestinal perfusion and reduces histologic injury in an adult intestinal I/R injury model.
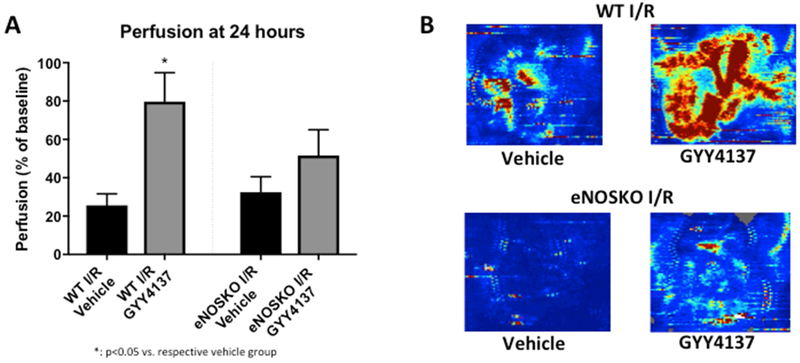
WT adult mice treated with GYY4137 after I/R injury had a mean perfusion of 79.74% ± 15.07 compared to 25.59% ± 6.05 in the vehicle-treated group (p=0.0221). In the eNOSKO mice, there was no significant difference in intestinal perfusion between those treated with GYY4137 (51.62% ± 13.41) and vehicle (32.48% ± 8.08, p=0.2403, Figure 4).
Figure 4: Mesenteric perfusion is improved after GYY4137 treatment in an WT adult mice after I/R, but this effect is not seen in eNOSKO mice.
A) Perfusion, expressed as a percentage of baseline perfusion, is significantly improved in WT mice treated with GYY4137 compared to vehicle, but this effect is lost with eNOS ablation (*: p<0.05 vs. vehicle group). B) Representative images from the LDI demonstrating improved perfusion only in the WT group treated with GYY4137. The intestines are eviscerated for these photos, and a region of interest is created around the loops. Red indicates good perfusion, and blue indicates poor perfusion.
Similar results were seen in intestinal histologic injury. The WT mice demonstrated significantly improved mucosal injury scores after treatment with GYY4137 (2, IQR 1.0-2.8) as compared to vehicle (4, IQR 2.0-5.0, p=0.0214). This benefit was not noted in the eNOSKO animals, with the GYY4137 group scoring 3 (IQR 2.0-4.0) and the vehicle-treated group scoring 4 (IQR 3.3-4.6, p=0.0591, Figure 5).
Figure 5: GYY4137 reduces intestinal injury in WT animals treated with GYY4137 after I/R, but does not affect injury in eNOSKO animals.
A) Histologic injury scores were significantly improved in the WT I/R group treated with GYY4137, but no similar effect was noted in eNOSKO animals (*: p<0.05 vs. vehicle group). B) Representative histology sections of the 4 groups, demonstrating very deranged villous architecture in both vehicle groups and the eNOSKO group treated with GYY4137. Only the WT animals treated with GYY4137 retain a semblance of normal bowel structure.
GYY4137 alters the inflammatory cascade in experimental NEC and intestinal I/R injury models.
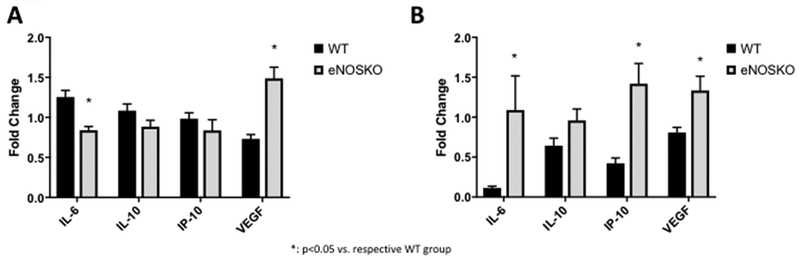
In the GYY4137-treated NEC groups, IL-6 was significantly higher in the WT animals (1.254 ± 0.083 folds of control) than the eNOSKO animals (0.839 ± 0.047 folds of control, p<0.0001). VEGF was significantly lower in the WT pups (0.733 ± 0.053 folds of control) compared to the eNOSKO animals (1.488 ± 0.140 folds of control, p<0.0001). Neither IL-10 (WT: 1.084 ± 0.083 folds of control, eNOSKO: 0.884 ± 0.080 folds of control, p=0.0859) nor IP-10 (WT: 0.985 ± 0.073 folds of control, eNOSKO: 0.837 ± 0.134 folds of control, p=0.3306) were significantly different between the NEC groups.
For the I/R model, the GYY4137-treated groups had significantly lower IL-6 levels in the WT animals (0.115 ± 0.022 folds of control) compared to the eNOSKO animals (1.089 ± 0.428 folds of control). IP-10 was significantly lower in the WT animals as well (0.4231 ± 0.066 folds of control) compared to the eNOSKO animals (1.419 ± 0.254, folds of control, p<0.0001). VEGF similarly was decreased in the WT group (0.807 ± 0.065 folds of control) compared to the eNOSKO group (1.334 ± 0.180 folds of control, p=0.0090). IL-10 was not significantly different between the groups (Figure 6).
Figure 6: Cytokine and growth factor aberrations are affected by treatment with GYY4137 after experimental NEC and I/R injury models.
Cytokine and growth factor changes with eNOS ablation in GYY4137 treatment of experimental NEC (A) and ischemia reperfusion injury model (B). Black bars represent WT mice and grey bars represent eNOSKO mice. Values are expressed as fold change of the vehicle treated group to normalize among different plates and animal strains for comparison.
DISCUSSION
Hydrogen sulfide donors like GYY4137 have been shown to have benefit in preventing tissue injury from ischemia [5, 10, 22, 26, 27]. There are multiple theorized mechanisms for this benefit including H2S modifying the eNOS protein through sulfhydration and possibly other actions [28]. Increases in eNOS activity would lead to increased nitric oxide production, vasodilation, and protection from injury secondary to tissue ischemia. Herein, we have demonstrated loss of the beneficial effects of GYY4137 in eNOSKO mice, indicating that the compound works through the eNOS pathway.
In the NEC model, GYY4137 conveyed clinical benefit as demonstrated by increased weight gain and decreased sickness scores in the WT animals, but this effect was lost in the eNOSKO mice. It is likely that GYY4137 reduces bowel injury and allows for better nutrient absorption in the WT animals. Improved weight gain was lost in the eNOSKO animals, indicating that this protein plays a critical role in overall animal health during NEC. Clinical sickness scores were similar in the vehicle groups, which is confirmation of our previous work [29], but improvement after GYY4137 was only seen in the WT groups.
We noted that the WT NEC group treated with GYY4137 experienced improved mesenteric perfusion, and this effect was lost in the eNOSKO group. Based on previous research, we thought it likely that GYY4137 was working through eNOS to reduce injury to ischemic tissue [10, 26, 27, 30]. GYY4137, acting as an H2S donor, likely sulfyhdrates eNOS, resulting in upregulation of NO production, vasodilation, and improvement of blood flow to threatened tissue [31]. Previous studies have demonstrated that endogenous NO is required for the vasorelaxant action of H2S to occur, which may explain why this benefit was not seen in the eNOSKO mice. [32]
NEC is a complex pathology with many factors at play, including, but not limited to gut immaturity, bacterial colonization and translocation, and hypothermic injuries. Because of this complexity, we isolated the intestinal ischemia aspect by using an intestinal I/R model to evaluate GYY4137. We noted the same findings of improved perfusion after treatment with GYY4137 in WT mice, but not in the eNOSKO mice. These findings support our theory that GYY4137 works to protect the intestine during NEC, at least in part, through eNOS-dependent vasorelaxation and improved intestinal perfusion [32, 33].
In addition to improved perfusion, intestinal mucosal injury was limited in WT pups and adults treated with GYY4137. This beneficial effect was lost with genetic ablation of eNOS. Improved perfusion likely played a role in improved mucosal injury. However, H2S donors such as GYY4137 have antioxidant, anti-inflammatory, and anti-apoptotic properties [10, 34, 35]. Therefore, it is possible that GYY4137 may also provide secondary protection by limiting oxidative stress and tissue injury at the mucosal level.
Interestingly, the cytokine and growth factor responses to GYY4137 were not identical in experimental NEC and the I/R model. In the I/R model, the WT animals treated with GYY4137 had significantly reduced levels IL-6, IP-10, and VEGF compared to the eNOSKO animals. In contrast, the NEC model had varying results, with significantly lower IL-6 and higher VEGF in the eNOSKO group compared to WT.
IL-6 is overall thought to be pro-inflammatory, but does have some noted protective and anti-inflammatory effects during NEC [15, 16]. In fact, our previous work in NEC has demonstrated decreases in IL-6 in experimental NEC, and a return to higher levels after treatment, suggesting that IL-6 may serve in a protective role during NEC [11]. In the I/R model, we have previously shown increases in IL-6 after injury and associated reductions in IL-6 levels after H2S donor therapy, suggesting that IL-6 may be detrimental in the I/R model. These previous findings are consistent with our results presented here [10]. The paradoxical response reinforces the fact that there are other factors involved in the development of NEC; intestinal I/R injury does not fully elicit the pathology. Additionally, the significant differences between the WT and eNOSKO animals in both models supports the theory that eNOS is involved in the action of GYY4137.
IL-10 was not found to be different in either the NEC model or the I/R model after treatment with GYY4137. This is likely because IL-10 acts as an anti-inflammatory cytokine, and in fact has been used in animal models to reduce intestinal injury [36]. While this is typically increased in NEC and other forms of intestinal inflammation [37], it does not appear to be dampened by GYY4137 in comparison to vehicle in either model for either strain. It is likely that this is because GYY4137 is acting through modulation of mesenteric perfusion via endothelial nitric oxide, and that its anti-inflammatory and antioxidant effects are less important in these models.
IP-10 is a chemoattractant for macrophages and other immune cells, and therefore it is understandable that levels would increase in an intestinal injury state [38]. In the I/R model, treatment with GYY4137 led to the WT mice having a much lower concentration of IP-10 than the eNOSKO mice, but in the NEC model there was no difference between these groups. These findings reinforce the idea that while the I/R injury model is clean and precise, experimental NEC in neonates has a more complex pathophysiology. In previous animal NEC experiments, macrophages have been shown to dominate neonatal intestinal infiltrates, while in mature mice the recruited leukocytes are more varied [39]. This could potentially contribute to the reason that IP-10 does not decrease in response to GYY4137 in experimental NEC.
Finally, VEGF has an intimate relationship with intestinal inflammation and eNOS. This growth factor induces vascular permeability and angiogenesis in response to inflammation, but its effects are dependent on the presence of endothelial NO [20]. We noted significantly higher VEGF in the setting of eNOS ablation after GYY4137 treatment, and this is likely from upregulation of VEGF in the setting of injury without eNOS. This also supports our theory that GYY4137 is acting predominantly through eNOS to support vasodilation and improve mesenteric perfusion, rather than through direct anti-inflammatory and antioxidant effects of the compound.
GYY4137 has promise as a stable, long-acting H2S donor that works through eNOS to protect against intestinal injury. It likely works at least partially through direct protection against ischemia and reperfusion injury, but other factors are involved as well, as indicated by differing aberrations in the inflammatory response. While the involvement of eNOS is clear, the exact nature of the substances’ interactions warrants further investigation.
LIMITATIONS
Our previous work has demonstrated some baseline differences in the WT and eNOSKO mice, which may affect results [29]. For this reason, values are normalized to their respective controls as much as possible to reduce variability among strains. Additionally, the NEC model approximates human NEC, but is certainly not a true representation of this complex disease process in humans. The model is especially difficulty in that the diagnosis cannot be established until after euthanasia. Our rate of NEC is about 70%, as demonstrated in our previous work, and this is consistent with the published model that we are emulating [8]. Despite these shortfalls, it is the best studied animal model available for the study of NEC.
CONCLUSION
GYY4137 is a beneficial treatment option in experimental NEC, where it works through an eNOS-dependent pathway. Given that GYY4137 was seen to improve intestinal perfusion in both the NEC pup model as well as the adult I/R model, it is likely that this compound works through eNOS to promote vasodilation of the mesenteric vessels. Improved perfusion likely portrays less mucosal injury and improved intestinal inflammation following injury. Further investigation is needed to determine the specific molecular interactions of GYY4137 and eNOS prior to widespread clinical use.
Acknowledgments
This work was made possible with support from:
-
1)
KL2TR001106 (A. Shekhar, PI) from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award
-
2)
Indiana University Health, Indianapolis, IN
Footnotes
NAD and ARJ performed animal care and experiments, NAD drafted the manuscript, NAD, ARJ, and JTW performed histological grading and statistical analysis, NAD, ARJ and JTW performed protein isolation and tissue analysis, TAM contributed critical ideas, assistance and manuscript advice. All authors provided critical revisions to the manuscript and assisted with its final preparation.
No disclosures to report.
This work was presented at the American Surgical Congress in 2018.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Lin HC, Wu SF, Underwood M. Necrotizing enterocolitis. The New England journal of medicine 2011;364(19):1878–9; author reply 9. [DOI] [PubMed] [Google Scholar]
- [2].Neu J, Walker WA. Necrotizing enterocolitis. The New England journal of medicine 2011;364(3):255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Li B, Zani A, Martin Z, Lee C, Zani-Ruttenstock E, Eaton S, et al. Intestinal epithelial cell injury is rescued by hydrogen sulfide. Journal of pediatric surgery 2016;51(5):775–8. [DOI] [PubMed] [Google Scholar]
- [4].Jensen AR, Drucker NA, Khaneki S, Ferkowicz MJ, Yoder MC, DeLeon ER, et al. Hydrogen Sulfide: A Potential Novel Therapy for the Treatment of Ischemia. Shock 2017. [DOI] [PubMed] [Google Scholar]
- [5].Sodha NR, Clements RT, Feng J, Liu Y, Bianchi C, Horvath EM, et al. Hydrogen sulfide therapy attenuates the inflammatory response in a porcine model of myocardial ischemia/reperfusion injury. The Journal of thoracic and cardiovascular surgery 2009;138(4):977–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yin J, Tu C, Zhao J, Ou D, Chen G, Liu Y, et al. Exogenous hydrogen sulfide protects against global cerebral ischemia/reperfusion injury via its anti-oxidative, anti-inflammatory and anti-apoptotic effects in rats. Brain research 2013;1491:188–96. [DOI] [PubMed] [Google Scholar]
- [7].Yang J, Watkins D, Chen CL, Bhushan B, Zhou Y, Besner GE. Heparin-binding epidermal growth factor-like growth factor and mesenchymal stem cells act synergistically to prevent experimental necrotizing enterocolitis. J Am Coll Surg 2012;215(4):534–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zani A, Cordischi L, Cananzi M, De Coppi P, Smith VV, Eaton S, et al. Assessment of a neonatal rat model of necrotizing enterocolitis. Eur J Pediatr Surg 2008;18(6):423–6. [DOI] [PubMed] [Google Scholar]
- [9].Chen Y, Koike Y, Miyake H, Li B, Lee C, Hock A, et al. Formula feeding and systemic hypoxia synergistically induce intestinal hypoxia in experimental necrotizing enterocolitis. Pediatric surgery international 2016;32(12):1115–9. [DOI] [PubMed] [Google Scholar]
- [10].Jensen AR, Drucker NA, Khaneki S, Ferkowicz MJ, Markel TA. Hydrogen sulfide improves intestinal recovery following ischemia by endothelial nitric oxide-dependent mechanisms. Am J Physiol Gastrointest Liver Physiol 2017;312(5):G450–G6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Drucker NA, Jensen AR, Ferkowicz MJ, Markel TA. Hydrogen Sulfide Provides Intestinal Protection During A Murine Model of Experimental Necrotizing Enterocolitis. Journal of pediatric surgery 2018. [DOI] [PubMed] [Google Scholar]
- [12].Yazji I, Sodhi CP, Lee EK, Good M, Egan CE, Afrazi A, et al. Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS-NO-nitrite signaling. Proceedings of the National Academy of Sciences of the United States of America 2013;110(23):9451–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fan WQ, Smolich JJ, Wild J, Yu VY, Walker AM. Nitric oxide modulates regional blood flow differences in the fetal gastrointestinal tract. The American journal of physiology 1996;271(4 Pt 1):G598–604. [DOI] [PubMed] [Google Scholar]
- [14].Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, et al. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation 2008;117(18):2351–60. [DOI] [PubMed] [Google Scholar]
- [15].Markel TA, Crisostomo PR, Wairiuko GM, Pitcher J, Tsai BM, Meldrum DR. Cytokines in necrotizing enterocolitis. Shock 2006;25(4):329–37. [DOI] [PubMed] [Google Scholar]
- [16].Jin X, Zimmers TA, Zhang Z, Pierce RH, Koniaris LG. Interleukin-6 is an important in vivo inhibitor of intestinal epithelial cell death in mice. Gut 2010;59(2):186–96. [DOI] [PubMed] [Google Scholar]
- [17].Siednienko J, Nowak J, Moynagh PN, Gorczyca WA. Nitric oxide affects IL-6 expression in human peripheral blood mononuclear cells involving cGMP-dependent modulation of NF-kappaB activity. Cytokine 2011;54(3):282–8. [DOI] [PubMed] [Google Scholar]
- [18].Lane JS, Todd KE, Lewis MP, Gloor B, Ashley SW, Reber HA, et al. Interleukin-10 reduces the systemic inflammatory response in a murine model of intestinal ischemia/reperfusion. Surgery 1997;122(2):288–94. [DOI] [PubMed] [Google Scholar]
- [19].Markel TA, Crafts TD, Jensen AR, Hunsberger EB, Yoder MC. Human mesenchymal stromal cells decrease mortality after intestinal ischemia and reperfusion injury. The Journal of surgical research 2015;199(1):56–66. [DOI] [PubMed] [Google Scholar]
- [20].Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, et al. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proceedings of the National Academy of Sciences of the United States of America 2001;98(5):2604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jilling T, Simon D, Lu J, Meng FJ, Li D, Schy R, et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. Journal of immunology 2006;177(5):3273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chatzianastasiou A, Bibli SI, Andreadou I, Efentakis P, Kaludercic N, Wood ME, et al. Cardioprotection by H2S Donors: Nitric Oxide-Dependent and Independent Mechanisms. The Journal of pharmacology and experimental therapeutics 2016;358(3):431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jensen AR, Manning MM, Khaneki S, Drucker NA, Markel TA. Harvest tissue source does not alter the protective power of stromal cell therapy after intestinal ischemia and reperfusion injury. The Journal of surgical research 2016;204(2):361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jensen AR, Doster DL, Hunsberger EB, Manning MM, Stokes SM, Barwinska D, et al. Human Adipose Stromal Cells Increase Survival and Mesenteric Perfusion Following Intestinal Ischemia and Reperfusion Injury. Shock 2016;46(1):75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jensen AR, Manning MM, Khaneki S, Drucker NA, Markel TA. Harvest tissue source does not alter the protective power of stromal cell therapy after intestinal ischemia and reperfusion injury. The Journal of surgical research 2016;204(2):361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pavoni V, Nicoletti P, Benemei S, Materazzi S, Perna F, Romagnoli S, et al. Effects of hydrogen sulfide (H2S) on mesenteric perfusion in experimental induced intestinal ischemia in a porcine model. Heart, lung and vessels 2015;7(3):231–7. [PMC free article] [PubMed] [Google Scholar]
- [27].Liu H, Bai XB, Shi S, Cao YX. Hydrogen sulfide protects from intestinal ischaemia-reperfusion injury in rats. The Journal of pharmacy and pharmacology 2009;61(2):207–12. [DOI] [PubMed] [Google Scholar]
- [28].Altaany Z, Ju Y, Yang G, Wang R. The coordination of S-sulfhydration, S-nitrosylation, and phosphorylation of endothelial nitric oxide synthase by hydrogen sulfide. Science signaling 2014;7(342):ra87. [DOI] [PubMed] [Google Scholar]
- [29].Drucker NA, Jensen AR, te Winkel JP, Ferkowicz MJ, Markel TA. Loss of Endothelial Nitric Oxide Synthase Exacerbates Intestinal and Lung Injury in Experimental Necrotizing Enterocolitis. Journal of Pediatric Surgery. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gheibi S, Aboutaleb N, Khaksari M, Kalalian-Moghaddam H, Vakili A, Asadi Y, et al. Hydrogen sulfide protects the brain against ischemic reperfusion injury in a transient model of focal cerebral ischemia. Journal of molecular neuroscience : MN 2014;54(2):264–70. [DOI] [PubMed] [Google Scholar]
- [31].Paul BD, Snyder SH. H2S: A Novel Gasotransmitter that Signals by Sulfhydration. Trends in biochemical sciences 2015;40(11):687–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Modis K, Panopoulos P, et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proceedings of the National Academy of Sciences of the United States of America 2012;109(23):9161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Beltowski J, Jamroz-Wisniewska A. Hydrogen sulfide and endothelium-dependent vasorelaxation. Molecules 2014;19(12):21183–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Henderson PW, Weinstein AL, Sohn AM, Jimenez N, Krijgh DD, Spector JA. Hydrogen sulfide attenuates intestinal ischemia-reperfusion injury when delivered in the post-ischemic period. J Gastroenterol Hepatol 2010;25(10):1642–7. [DOI] [PubMed] [Google Scholar]
- [35].Zuidema MY, Peyton KJ, Fay WP, Durante W, Korthuis RJ. Antecedent hydrogen sulfide elicits an anti-inflammatory phenotype in postischemic murine small intestine: role of heme oxygenase-1. American journal of physiology Heart and circulatory physiology 2011;301(3):H888–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ozturk H, Dokucu AI, Ogun C, Buyukbayram H. Protective effects of recombinant human interleukin-10 on intestines of hypoxia-induced necrotizing enterocolitis in immature rats. Journal of pediatric surgery 2002;37(9):1330–3. [DOI] [PubMed] [Google Scholar]
- [37].Edelson MB, Bagwell CE, Rozycki HJ. Circulating pro- and counterinflammatory cytokine levels and severity in necrotizing enterocolitis. Pediatrics 1999;103(4 Pt 1):766–71. [DOI] [PubMed] [Google Scholar]
- [38].Yu Y, Klemann C, Feng X, Ginzel M, Vieten G, Lacher M, et al. Increased inflammatory reaction to intestinal ischemia-reperfusion in neonatal versus adult mice. European journal of pediatric surgery : official journal of Austrian Association of Pediatric Surgery [et al] = Zeitschrift fur Kinderchirurgie 2015;25(1):46–50. [DOI] [PubMed] [Google Scholar]
- [39].MohanKumar K, Kaza N, Jagadeeswaran R, Garzon SA, Bansal A, Kurundkar AR, et al. Gut mucosal injury in neonates is marked by macrophage infiltration in contrast to pleomorphic infiltrates in adult: evidence from an animal model. Am J Physiol Gastrointest Liver Physiol 2012;303(1):G93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]